Abstract
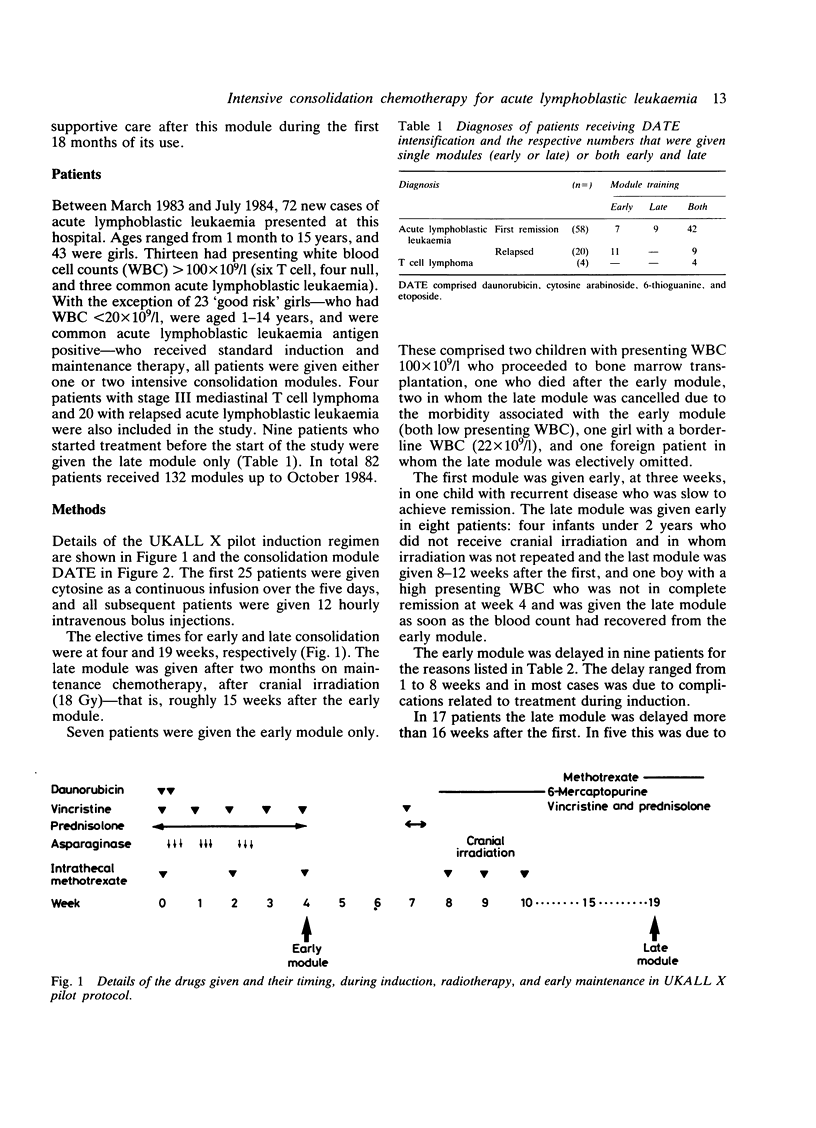
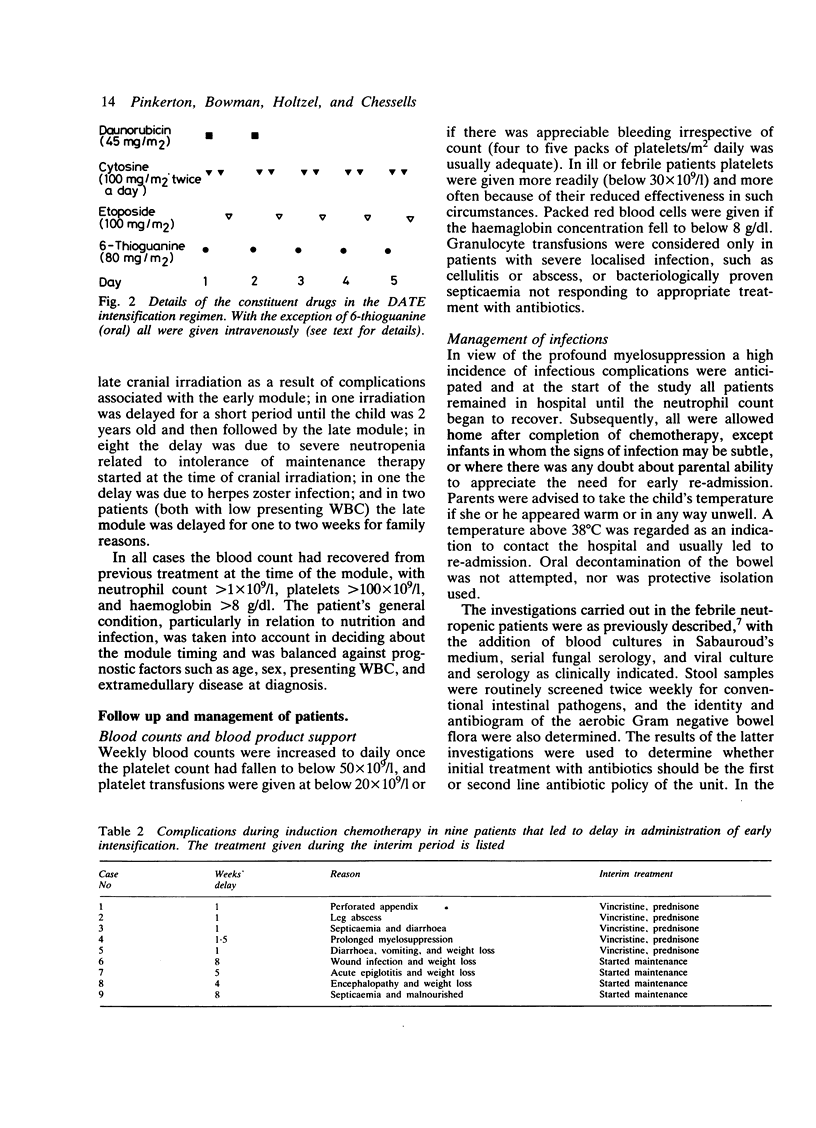
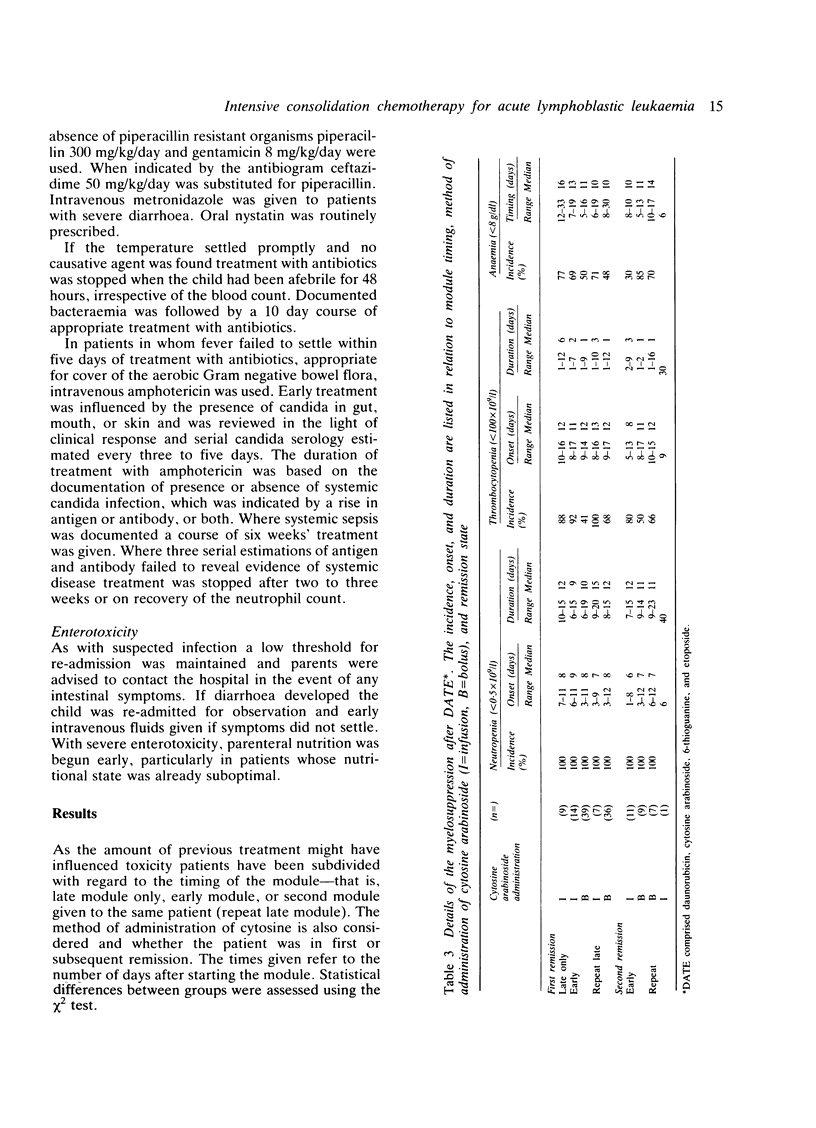
Eighty two children with acute lymphoblastic leukaemia presenting at this hospital received one or two modules of intensive chemotherapy to consolidate remission. Modules were given after four and roughly 19 weeks on treatment. Each included two doses of daunorubicin (45 mg/m2/day), cytosine arabinoside (100 mg/m2 twice daily X 5), etoposide (100 mg/m2/day X 5), and 6-thioguanine (80 mg/m2/day X 5). A total of 132 courses were given. This study included all new patients except girls aged 1-14 years with presenting leucocyte count less than 20 X 10(9)/l. Twenty patients with recurrent disease were also included. The first 32 patients were given cytosine as a 24 hour infusion, but combined with the other agents this was associated with severe intestinal toxicity, which necessitated a change to a less toxic 12 hourly bolus regimen. The complications of the module are reviewed in terms of myelosuppression, enterotoxicity, infection, and other clinical problems encountered. All patients became profoundly neutropenic and thrombocytopenic. The latter was significantly more severe after cytosine infusion. Overall, 64% received platelet transfusions and 85% were re-admitted with fevers requiring intravenous antibiotics for between four and 56 days. Gastrointestinal toxicity with the modified module occurred in 38% of patients and was severe in 13%. This intensification module has been adopted by the Medical Research Council Working Party on Childhood Leukaemia for use in a multicentre study (UKALL X) and the details of the problems encountered in the pilot study may be of value to other centres now using this protocol.
Full text
PDF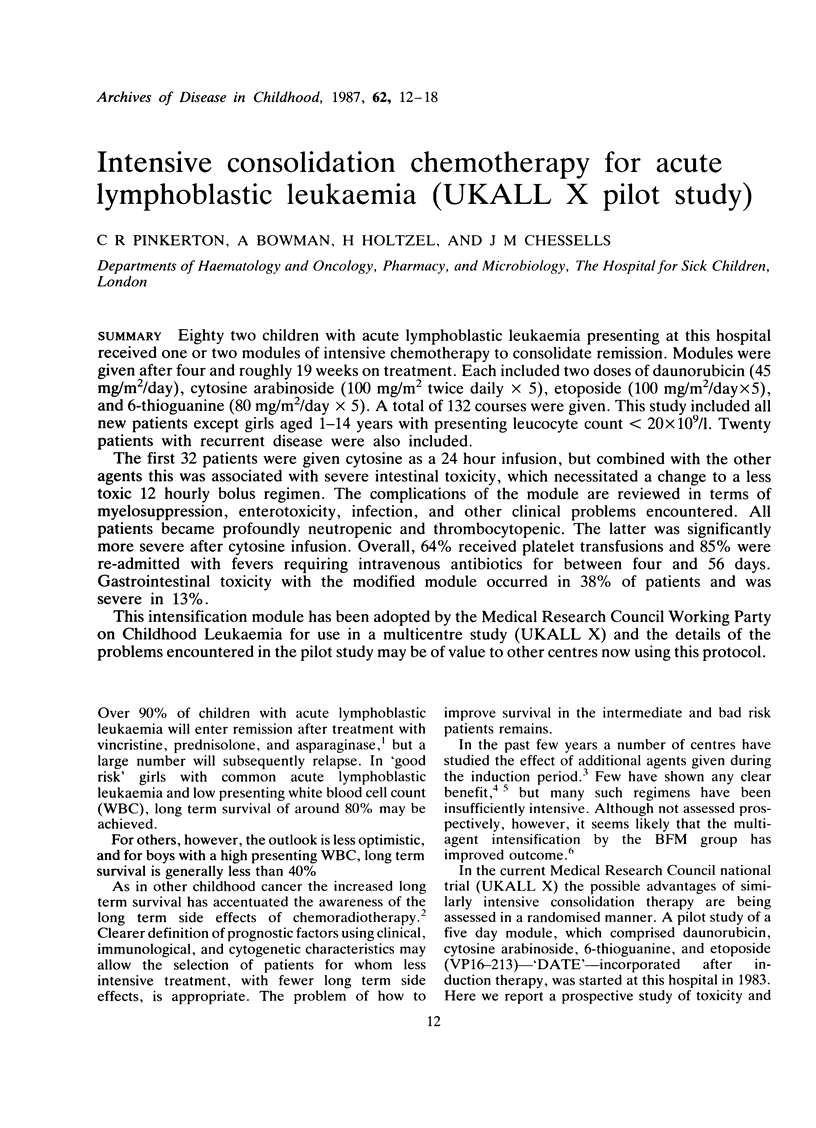


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chessells J. M. Childhood acute lymphoblastic leukaemia: the late effects of treatment. Br J Haematol. 1983 Mar;53(3):369–378. doi: 10.1111/j.1365-2141.1983.tb02037.x. [DOI] [PubMed] [Google Scholar]
- Chessells J. M., Leiper A. D. Infection during remission induction in childhood leukaemia. Arch Dis Child. 1980 Feb;55(2):118–123. doi: 10.1136/adc.55.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessells J. M. Risks and benefits of intensive treatment of acute leukaemia. Arch Dis Child. 1985 Mar;60(3):193–195. doi: 10.1136/adc.60.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Antifungal chemotherapy. Lancet. 1982 Sep 4;2(8297):532–537. doi: 10.1016/s0140-6736(82)90610-9. [DOI] [PubMed] [Google Scholar]
- Johnson H., Smith T. J., Desforges J. Cytosine-arabinoside-induced colitis and peritonitis: nonoperative management. J Clin Oncol. 1985 May;3(5):607–612. doi: 10.1200/JCO.1985.3.5.607. [DOI] [PubMed] [Google Scholar]
- Lampert F., Henze G., Langermann H. J., Schellong G., Gadner H., Riehm H. J. Acute lymphoblastic leukemia: current status of therapy in children. Recent Results Cancer Res. 1984;93:159–181. doi: 10.1007/978-3-642-82249-0_6. [DOI] [PubMed] [Google Scholar]
- Miller D. R., Leikin S., Albo V., Sather H., Karon M., Hammond D. Prognostic factors and therapy in acute lymphoblastic leukemia of childhood: CCG-141. A report from childrens cancer study group. Cancer. 1983 Mar 15;51(6):1041–1049. doi: 10.1002/1097-0142(19830315)51:6<1041::aid-cncr2820510612>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Ochs J., Sinkule J. A., Danks M. K., Look A. T., Bowman W. P., Rivera G. Continuous infusion high-dose cytosine arabinoside in refractory childhood leukemia. J Clin Oncol. 1984 Oct;2(10):1092–1097. doi: 10.1200/JCO.1984.2.10.1092. [DOI] [PubMed] [Google Scholar]
- Pizzo P. A., Robichaud K. J., Gill F. A., Witebsky F. G. Empiric antibiotic and antifungal therapy for cancer patients with prolonged fever and granulocytopenia. Am J Med. 1982 Jan;72(1):101–111. doi: 10.1016/0002-9343(82)90594-0. [DOI] [PubMed] [Google Scholar]
- Rai K. R., Holland J. F., Glidewell O. J., Weinberg V., Brunner K., Obrecht J. P., Preisler H. D., Nawabi I. W., Prager D., Carey R. W. Treatment of acute myelocytic leukemia: a study by cancer and leukemia group B. Blood. 1981 Dec;58(6):1203–1212. [PubMed] [Google Scholar]
- Sackmann-Muriel F., Svarch E., Eppinger-Helft M., Braier J. L., Pavlovsky S., Guman L., Vergara B., Ponzinibbio C., Failace R., Garay G. E. Evaluation of intensification and maintenance programs in the treatment of acute lymphoblastic leukemia. Cancer. 1978 Oct;42(4):1730–1740. doi: 10.1002/1097-0142(197810)42:4<1730::aid-cncr2820420411>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Schaller R. T., Jr, Schaller J. F. The acute abdomen in the immunologically compromised child. J Pediatr Surg. 1983 Dec;18(6):937–944. doi: 10.1016/s0022-3468(83)80050-5. [DOI] [PubMed] [Google Scholar]
- Slavin R. E., Dias M. A., Saral R. Cytosine arabinoside induced gastrointestinal toxic alterations in sequential chemotherapeutic protocols: a clinical-pathologic study of 33 patients. Cancer. 1978 Oct;42(4):1747–1759. doi: 10.1002/1097-0142(197810)42:4<1747::aid-cncr2820420413>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Slevin M. L., Piall E. M., Aherne G. W., Johnston A., Lister T. A. The pharmacokinetics of cytosine arabinoside in the plasma and cerebrospinal fluid during conventional and high-dose therapy. Med Pediatr Oncol. 1982;10 (Suppl 1):157–168. doi: 10.1002/mpo.2950100715. [DOI] [PubMed] [Google Scholar]


