Abstract
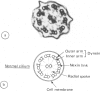
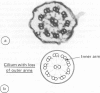
One hundred and sixty seven children, ranging in age from 5 weeks to 16 years, with chronic upper or lower respiratory tract problems, or both, were investigated for ciliary dyskinesia. Abnormal ciliary function was found in 18 cases all of whom had chronic lower respiratory disease and most of whom also had upper respiratory problems. Fifteen of the 18 cases had reduced ciliary beat frequencies (less than 10 Hz) associated with dyskinesia and the other three showed apparent absence of ciliated cells. Of the 15 cases with reduced ciliary beat frequencies, ciliary ultrastructure was normal in seven cases but abnormal with missing dynein arms and occasional abnormalities of microtubular arrangement in eight. Respiratory symptoms in the perinatal period were more common in children with abnormal ciliary function and present in all those with ultrastructural abnormalities or absence of ciliated cells compared with 34 (26%) of 132 children, in whom symptoms were recorded, with normal ciliary function. This study would suggest that all children with unexplained chronic respiratory disease, in particular those with symptoms starting in the perinatal period, should be investigated for ciliary dyskinesia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afzelius B. A. A human syndrome caused by immotile cilia. Science. 1976 Jul 23;193(4250):317–319. doi: 10.1126/science.1084576. [DOI] [PubMed] [Google Scholar]
- Carson J. L., Collier A. M., Hu S. S. Acquired ciliary defects in nasal epithelium of children with acute viral upper respiratory infections. N Engl J Med. 1985 Feb 21;312(8):463–468. doi: 10.1056/NEJM198502213120802. [DOI] [PubMed] [Google Scholar]
- Corbeel L., Cornillie F., Lauweryns J., Boel M., van den Berghe G. Ultrastructural abnormalities of bronchial cilia in children with recurrent airway infections and bronchiectasis. Arch Dis Child. 1981 Dec;56(12):929–933. doi: 10.1136/adc.56.12.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi L., Lungarella G., Palatresi R. Lack of kinocilia in the nasal mucosa in the immotile-cilia syndrome. Eur J Respir Dis. 1982 Nov;63(6):558–563. [PubMed] [Google Scholar]
- Greenstone M. A., Dewar A., Cole P. J. Ciliary dyskinesia with normal ultrastructure. Thorax. 1983 Nov;38(11):875–876. doi: 10.1136/thx.38.11.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstone M., Cole P. J. Ciliary function in health and disease. Br J Dis Chest. 1985 Jan;79(1):9–26. doi: 10.1016/0007-0971(85)90003-8. [DOI] [PubMed] [Google Scholar]
- Götz M., Stockinger L. Aplasia of respiratory tract cilia. Lancet. 1983 Jun 4;1(8336):1283–1283. doi: 10.1016/s0140-6736(83)92740-x. [DOI] [PubMed] [Google Scholar]
- Rossman C. M., Lee R. M., Forrest J. B., Newhouse M. T. Nasal ciliary ultrastructure and function in patients with primary ciliary dyskinesia compared with that in normal subjects and in subjects with various respiratory diseases. Am Rev Respir Dis. 1984 Jan;129(1):161–167. doi: 10.1164/arrd.1984.129.1.161. [DOI] [PubMed] [Google Scholar]
- Rott H. D. Genetics of Kartagener's syndrome. Eur J Respir Dis Suppl. 1983;127:1–4. [PubMed] [Google Scholar]
- Rutland J., Cox T., Dewar A., Cole P., Warner J. O. Transitory ultrastructural abnormalities of cilia. Br J Dis Chest. 1982 Apr;76(2):185–188. [PubMed] [Google Scholar]
- Rutland J., Dewar A., Cox T., Cole P. Nasal brushing for the study of ciliary ultrastructure. J Clin Pathol. 1982 Mar;35(3):357–359. doi: 10.1136/jcp.35.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallman L. A., Hill S. L., Stockley R. A. Reduction of ciliary beat frequency in vitro by sputum from patients with bronchiectasis: a serine proteinase effect. Thorax. 1984 Sep;39(9):663–667. doi: 10.1136/thx.39.9.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes D. A., Wilson R., Greenstone M., Currie D. C., Steinfort C., Cole P. J. Deleterious effects of purulent sputum sol on human ciliary function in vitro: at least two factors identified. Thorax. 1987 Apr;42(4):256–261. doi: 10.1136/thx.42.4.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw A., Evans A., Corrin B. Immotile cilia syndrome: a new cause of neonatal respiratory distress. Arch Dis Child. 1981 Jun;56(6):432–435. doi: 10.1136/adc.56.6.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Alton E., Rutman A., Higgins P., Al Nakib W., Geddes D. M., Tyrrell D. A., Cole P. J. Upper respiratory tract viral infection and mucociliary clearance. Eur J Respir Dis. 1987 May;70(5):272–279. [PubMed] [Google Scholar]
- Wilson R., Roberts D., Cole P. Effect of bacterial products on human ciliary function in vitro. Thorax. 1985 Feb;40(2):125–131. doi: 10.1136/thx.40.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Sykes D. A., Currie D., Cole P. J. Beat frequency of cilia from sites of purulent infection. Thorax. 1986 Jun;41(6):453–458. doi: 10.1136/thx.41.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]