Short abstract
A popular method of RNA interference shows levels of toxicity in mice that could limit its therapeutic potential.
Abstract
Short hairpin RNAs can provide stable gene silencing via RNA interference. Recent studies have shown toxicity in vivo that appears to be related to saturation of the endogenous microRNA pathway. Will these findings limit the therapeutic use of such hairpins?
RNA interference (RNAi) has loomed large on the scientific radar screen since its discovery nearly a decade ago. Scientists have adopted RNAi as the standard tool for sequence-specific silencing of genes and investors have poured money into companies that aim to take advantage of its potential as a surrogate genetic tool and as a therapeutic modality. However, an article published by Mark Kay and colleagues [1] in Nature recently reported fatal side effects in tests of therapeutic RNAi in mice. While some are discouraged by the severity of the toxicity and argue that RNAi is not as promising as it used to be, we believe that these and other results that pinpoint RNAi's imperfections will instead improve our understanding of RNAi and strengthen the field.
The article by Grimm et al. [1] reported the results of experiments in which short hairpin RNAs (shRNAs) were expressed from vectors based on adeno-associated virus that were delivered by low-pressure intravenous injections. The first example of toxicity was seen when the researchers co-injected viral vectors that expressed firefly luciferase with five vectors that expressed different shRNAs against luciferase. While two of the shRNA vectors produced stable luciferase knockdown, several of the mice died less than one month after the injection. The authors then designed new shRNAs against a gene expressed by transgenic mice and experienced the same toxicity problems: of 49 vectors expressing 40 different shRNAs, 36 constructs were severely toxic and 23 resulted in lethality in the mice within two months.
Of course, this is not the only apparent setback that RNAi has encountered. The first came when both short interfering RNAs (siRNAs) [2] and shRNAs [3] were shown to trigger immune responses under certain conditions, and many asked whether the hype surrounding RNAi was finally over. Research showed, however, that some of the perceived problems with RNAi-induced nonspecific immune responses could be avoided with proper design - by refraining from using sequences containing certain motifs, for instance (reviewed in [4]). Another check came when several papers showed that RNAi off-target effects are widespread and may cause toxic phenotypes in vivo [5,6]. Unfortunately, it may never be possible to design a sequence that avoids the potential for such effects altogether [7,8], as only limited sequence complementarity to the target is enough to cause knockdown [9]. One group has, however, already proposed that chemical modifications may provide a remedy that significantly reduces or avoids off-target effects [10]. Even though immune reactions and off-target effects remain challenging to RNAi researchers, continued research into the mechanisms of RNAi produces potential solutions.
It appears, however, that neither immune responses nor off-target effects can be blamed for the toxicity in the recent paper by Grimm et al. [1] where mice died following injection of shRNA-expressing viral vectors. On the one hand, inflammatory cytokines were not present above normal levels in the mice, which rules out immune-stimulatory reactions. On the other hand, the fact that many different shRNAs caused lethality suggested that the phenotype was independent of sequence, thereby rendering off-target effects an unlikely cause. The adverse effects seem instead to be the consequence of competition with the endogenous microRNA pathway for post-transcriptional gene regulation.
Both siRNAs and shRNAs - the triggers of transient and stable RNAi, respectively - are similar to processing intermediates in the microRNA pathway and harness its cellular machinery. The availability of at least four distinct protein complexes is critical for appropriate function of microRNAs (reviewed in [11]). First, the Drosha-containing Microprocessor complex makes a cut at the non-closed end of primary stem-loop transcripts, which results in short hairpins (usually 60-80 nucleotides in humans) with a two-nucleotide overhang at the 3' end [12-14]. Second, the enzymes Exportin-5 and the small GTPase Ran are responsible for export of these precursors from the nucleus and their release into the cytoplasm [15-17]. Third, the RNAse III Dicer excises the hairpin loop from the precursors and leaves a duplex with characteristic two-nucleotide 3' overhangs on both sides [18-20]. Finally, the RNA-induced silencing complex (RISC) [21,22] incorporates one of the RNA duplex strands and uses it as a guide to target complementary messages for cleavage [23-25], degradation [26-28] or translational suppression [29-31]. The main microRNA processing intermediates are illustrated in Figure 1 and the processing pathway in Figure 2.
Figure 1.
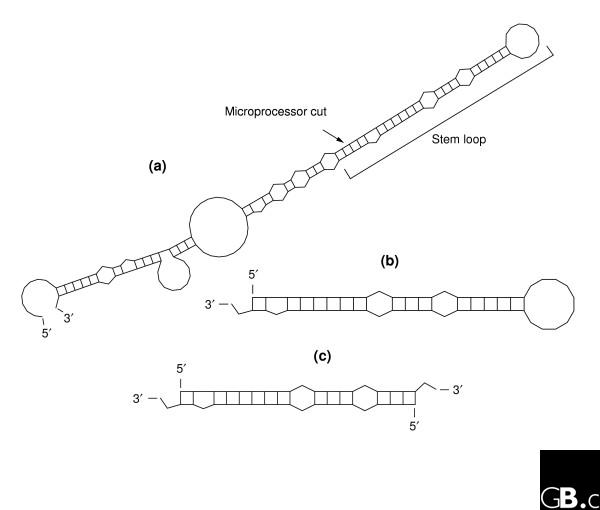
Characteristic intermediates in microRNA processing. (a) A typical example of primary microRNA transcript before the Microprocessor cut distal to the stem loop. The 5' and 3' ends of the primary transcripts are not generally known; this example was obtained by folding hsa-mir-23a with 50 nucleotides flanking the Microprocessor site, as defined by the ends of the mature microRNA [41]. (b) The precursor microRNA as transported from the nucleus to the cytoplasm. (c) A mature duplex microRNA after Dicer processing, but before incorporation into RISC. Note that shRNAs can be similar to primary microRNA transcripts or precursors, whereas siRNAs are made similar to the mature duplex.
Figure 2.
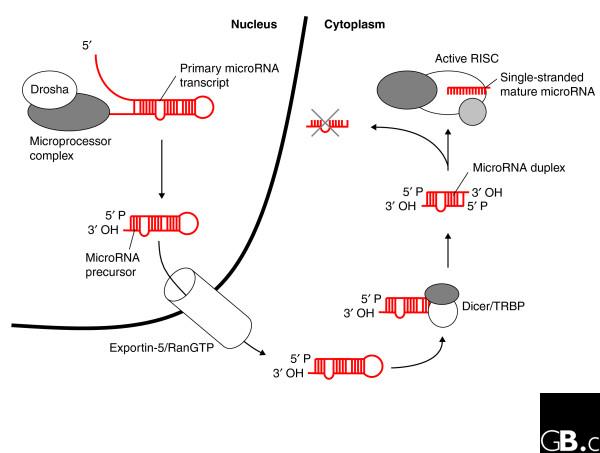
MicroRNA biogenesis. The protein Drosha, a member of the RNase III family, processes primary transcripts as part of the Microprocessor complex. The hairpins are exported to the cytoplasm via a complex of Exportin-5 and GTP-bound Ran (RanGTP). Once in the cytoplasm, the microRNA precursor is further processed by the RNase III Dicer in a complex with TAR RNA binding protein (TRBP) to give a mature double-stranded microRNA. A single-stranded microRNA is then handed over to the RISC. Ectopically expressed shRNAs can compete for various components of this pathway, and can thereby affect the levels of endogenous microRNAs that enter RISC.
So why did shRNAs kill the treated mice when in vivo siRNA studies have shown no adverse effects [32-34]? After all, previous results have suggested that both shRNAs and longer siRNAs may achieve increased potency at lower concentrations because they undergo some microRNA biogenesis [35,36]. That is, shRNAs may, depending on the length of the transcript, enter the microRNA pathway either before or after the Microprocessor step, whereas longer (approximately 27 basepairs) siRNAs are thought to enter the pathway before the Dicer step. Given the similar processing pathways that are used by microRNAs and shRNAs, the toxicity can probably be explained by saturation of one or more components of the endogenous RNAi machinery as a result of high doses of the shRNAs, leading to loss of microRNA function.
The downside of the potentially higher efficacy that comes from exploiting more of the microRNA pathway is the potential for expressed hairpins and longer duplexes to interfere with the endogenous function of microRNAs. Any of the molecular factors important for microRNA biogenesis and function could be saturated by overexpression of shRNAs, whereas siRNAs are less likely to do so as they are incorporated directly into RISC, although they could also compete with microRNAs at this step under certain conditions of siRNA excess. It has previously been reported that highly expressed shRNAs can compete with endogenous microRNAs to saturate the carrier protein, Exportin-5, that is necessary for nuclear export [37]. Indeed, Exportin-5 emerged as the prime suspect for the deaths of mice in the study by Grimm et al. [1], as overexpression of this protein improved silencing of the target gene, suggesting that Exportin-5 is a rate-limiting component of the miRNA pathway. As the authors remark, saturation of other cellular components cannot be disregarded on the basis of these experiments, but will have to be confirmed by inhibition studies for each of the critical factors. The results may even explain previous accounts of toxicity in the literature. For example, in an article [38] that studied shRNA-expressing transgenic mice, the authors suggested that immune stimulatory responses were to blame for a higher fetal and neonatal death rate among offspring that had inherited the shRNA gene compared with those that had not. Since microRNAs are involved in early development, however, it may be that saturation at this point is the worst possible time for the organism, and that perturbation of normal microRNA function induced the fatal phenotypes.
As expressed hairpins are being considered as therapeutic drugs, it is important to remember that the mice were treated with very high doses, and it should be noted that high doses of any drug are likely to cause severe toxicity. For example, overdoses of acetaminophen - the active chemical entity in many of the most common over-the-counter pain relievers - is the leading cause of drug-related acute liver failure in the US [39]. It is therefore not surprising that high doses of shRNAs will perturb cells, nor that this may in some cases have disastrous consequences for the organism. It should be noted that when mice transgenic for hepatitis B virus were treated with shRNA-expressing viral vectors at lower doses, no lethal phenotype was observed among these animals, suggesting that shRNAs transcribed using RNA polymerase III can be safe and effective when the dosing and target-site selection processes are carefully controlled.
There is no doubt that our understanding of RNAi mechanisms is still in its infancy and that additional surprises will be encountered as siRNAs and shRNAs are tested preclinically. It is important to note that the most serious types of problems reported for RNAi so far - that is, immune reactions, off-target effects and saturation - are all dependent on siRNA or shRNA concentration. In turn, this emphasizes the need to find the most potent target site and to work at the lowest concentrations possible [40]. Problems with saturation also strongly suggest that researchers should check for appropriate and efficient processing, and that the mature species resulting from expression in vivo are those that are expected. We believe that these recent reports on toxicity in vivo - most prominently the article by Grimm et al. [1] - will stimulate research that will ultimately contribute to an increased understanding of the microRNA pathway. Careful design may then be able to circumvent some of the problems we have seen recently. While it is still early days for RNAi, and more challenges are likely to emerge, the achievement of clinical therapeutic silencing will arguably still depend mainly on the development of safe and practical methods for in vivo delivery of the silencing constructs.
References
- Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- Sledz CA, Holko M, de Veer MJ, Silverman RH, Williams BR. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol. 2003;5:834–839. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- Bridge AJ, Pebernard S, Ducraux A, Nicoulaz AL, Iggo R. Induction of an interferon response by RNAi vectors in mammalian cells. Nat Genet. 2003;34:263–264. doi: 10.1038/ng1173. [DOI] [PubMed] [Google Scholar]
- Marques JT, Williams BR. Activation of the mammalian immune system by siRNAs. Nat Biotechnol. 2005;23:1399–1405. doi: 10.1038/nbt1161. [DOI] [PubMed] [Google Scholar]
- Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- Fedorov Y, Anderson EM, Birmingham A, Reynolds A, Karpilow J, Robinson K, Leake D, Marshall WS, Khvorova A. Off-target effects by siRNA can induce toxic phenotype. RNA. 2006;12:1188–1196. doi: 10.1261/rna.28106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AL, Burchard J, Schelter J, Chau BN, Cleary M, Lim L, Linsley PS. Widespread siRNA "off-target" transcript silencing mediated by seed region sequence complementarity. RNA. 2006;12:1179–1187. doi: 10.1261/rna.25706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham A, Anderson EM, Reynolds A, Ilsley-Tyree D, Leake D, Fedorov Y, Baskerville S, Maksimova E, Robinson K, Karpilow J, et al. 3' UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat Methods. 2006;3:199–204. doi: 10.1038/nmeth854. [DOI] [PubMed] [Google Scholar]
- Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS Biol. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AL, Burchard J, Leake D, Reynolds A, Schelter J, Guo J, Johnson JM, Lim L, Karpilow J, Nichols K, et al. Position-specific chemical modification of siRNAs reduces "off-target" transcript silencing. RNA. 2006;12:1197–1205. doi: 10.1261/rna.30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6:376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18:3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- Bohnsack MT, Czaplinski K, Gorlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA. 2004;10:185–191. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- Hutvagner G, McLachlan J, Pasquinelli AE, Balint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- Ketting RF, Fischer SE, Bernstein E, Sijen T, Hannon GJ, Plasterk RH. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001;15:2654–2659. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–2060. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- Mourelatos Z, Dostie J, Paushkin S, Sharma A, Charroux B, Abel L, Rappsilber J, Mann M, Dreyfuss G. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev. 2002;16:720–728. doi: 10.1101/gad.974702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Giraldez AJ, Cinalli RM, Glasner ME, Enright AJ, Thomson JM, Baskerville S, Hammond SM, Bartel DP, Schier AF. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308:833–838. doi: 10.1126/science.1109020. [DOI] [PubMed] [Google Scholar]
- Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci USA. 2006;103:4034–4039. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen PH, Ambros V. The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blockingLIN-14 protein synthesis after the initiation of translation. Dev Biol. 1999;216:671–680. doi: 10.1006/dbio.1999.9523. [DOI] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9:347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- Kim DH, Behlke MA, Rose SD, Chang MS, Choi S, Rossi JJ. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat Biotechnol. 2005;23:222–226. doi: 10.1038/nbt1051. [DOI] [PubMed] [Google Scholar]
- Siolas D, Lerner C, Burchard J, Ge W, Linsley PS, Paddison PJ, Hannon GJ, Cleary MA. Synthetic shRNAs as potent RNAi triggers. Nat Biotechnol. 2005;23:227–231. doi: 10.1038/nbt1052. [DOI] [PubMed] [Google Scholar]
- Yi R, Doehle BP, Qin Y, Macara IG, Cullen BR. Overexpression of exportin 5 enhances RNA interference mediated by short hairpin RNAs and microRNAs. RNA. 2005;11:220–226. doi: 10.1261/rna.7233305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Hunter R, Strnatka D, McQueen CA, Erickson RP. DNA constructs designed to produce short hairpin, interfering RNAs in transgenic mice sometimes show early lethality and an interferon response. J Appl Genet. 2005;46:217–225. [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature. 2004;431:371–378. doi: 10.1038/nature02870. [DOI] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]


