Abstract
Slow-channel congenital myasthenic syndrome, caused by mutations in subunits of the endplate ACh receptor (AChR), results in prolonged synaptic currents and excitotoxic injury of the postsynaptic region by Ca2+ overloading. The Ca2+ overloading could be due entirely to the prolonged openings of the AChR channel or could be abetted by enhanced Ca2+ permeability of the mutant channels. We therefore measured the fractional Ca2+ current, defined as the percentage of the total ACh-evoked current carried by Ca2+ ions (Pf), for AChRs harbouring the αG153S or the αV249F slow-channel mutation, and for wild-type human AChRs in which Pf has not yet been determined. Experiments were performed in transiently transfected GH4C1 cells and human myotubes with simultaneous recording of ACh-evoked whole-cell currents and fura-2 fluorescence signals. We found that the Pf of the wild-type human endplate AChR was unexpectedly high (Pf∼7%), but neither the αV249F nor the αG153S mutation altered Pf. Fetal human AChRs containing either the wild-type or the mutated α subunit had a much lower Pf (2–3%). We conclude that the Ca2+ permeability of human endplate AChRs is higher than that reported for any other human nicotinic AChR, with the exception of α7-containing AChRs (Pf > 10%); and that neither the αG153S nor the αV249F mutations affect the Pf of fetal or adult endplate AChRs. However, the intrinsically high Ca2+ permeability of human AChRs probably predisposes to development of the endplate myopathy when opening events of the AChR channel are prolonged by altered AChR-channel kinetics.
Although the permeability of the endplate acetylcholine receptor (AChR) channel to Ca2+ ions was demonstrated around three decades ago (Kusano et al. 1975; Bregestovski et al. 1979), the physiological role of the ensuing Ca2+ influx has not been established. However, several lines of information suggest that the Ca2+ influx through fetal and adult AChRs (γ-AChR and ɛ-AChR, respectively) probably plays a role in formation and function of the normal endplate. Ca2+ influx at the endplate might contribute to shape muscle spiking activity, by regulating the activation of depolarizing voltage-gated Na+ channels and of repolarizing Ca2+-dependent K+-channels (Allard et al. 1996; Young & Caldwell, 2005). Ca2+ entry through fetal AChR channels has also been noted to promote myotube survival (Bandi et al. 2005).
The Ca2+ permeability of endplate AChRs also plays a role in endplate diseases. Specifically, cationic overloading of the endplate leads to pathological alterations of endplate structure (Salpeter et al. 1979); however, this can be prevented by chelation of Ca2+ in the extracellular fluid (Leonard & Salpeter, 1979). This endplate myopathy has been repeatedly described in the slow-channel congenital myasthenic syndrome (SCCMS), a disease that arises from dominant point mutations in AChR subunits that prolong opening events of the AChR channel and decay of the synaptic current (reviewed by Engel et al. 2003a, b). Consistent with the pathological role of Ca2+ in the SCCMS, millimolar levels of Ca2+ accumulation has been demonstrated at endplates of transgenic mice (Gomez et al. 2002) and SCCMS patients (Engel et al. 1982, 2003b; Vohra et al. 2004).
The cytotoxic effects of excessive Ca2+ entry (referred to as excitotoxicity) have been well characterized when glutamate receptors, in particular the highly Ca2+-permeable NMDA receptors, are over stimulated by excessive synaptic release of glutamate (Rothman & Olney, 1995; Arundine & Tymianski, 2003). Indeed, for these receptors, the percent current carried by Ca2+ ions, termed fractional Ca2+ current (Pf), is as large as 12% (Burnashev et al. 1995). In the case of SCCMS, Ca2+ enters the endplate through the ɛ-AChR, whose Pf in rodents is about 4% (for review see Fucile, 2004), which is much smaller than the Pf of NMDA receptors. This suggests that the excitotoxic events in the SCCMS would not be adequately explained by the prolonged synaptic response to ACh unless it was also augmented by increased Ca2+ permeability of the mutant channel. Consistent with this assumption, in animal models of the SCCMS, endplate myopathy was observed chiefly when the prolonged synaptic currents were associated with an increased Ca2+ permeability of the mutant AChR channels (Gomez et al. 2002).
We therefore wished to establish the Pf values of the wild-type human ɛ- and γ-AChRs, which have not been previously documented. We also measured the Pf of two well-characterized human slow-channel AChRs that harbour the αG153S or the αV249F mutation. In humans carrying αG153S or αV249F, single-channel recordings show that γ-AChR is also expressed at the endplate, accounting for 5–12% of all channel openings (Sine et al. 1995; Milone et al. 1997). Hence, we measured the fractional Ca2+ current carried by the mutant γ-AChR as well.
For the reliable determination of Pf (Zhou & Neher, 1993), the AChR channels must be the only pathway for Ca2+ entry because concomitant Ca2+ release from intracellular stores will lead to an overestimate of the Ca2+ signal and confound the results. Mature muscle fibres are not suitable for this purpose as full blockade of Ca2+ release from the sarcoplasmic reticulum is difficult to achieve. Moreover, the large size of mature muscle fibres is likely to prevent their successful voltage clamping, and activation of voltage-gated Ca2+ channels will invalidate the experiment. Small myotubes in culture are more amenable to Pf measurements, but they only express γ-AChR. We therefore performed experiments on transfected GH4C1 cells expressing either γ- or ɛ-AChRs and used cultured human myotubes to validate our expression system for γ-AChR.
We report that wild-type human ɛ-AChR has an unexpectedly high Pf, which is not affected by either the αG153S or the αV249F mutation. Thus, a constitutively high Pf of the mature human receptor predisposes the endplate to excitotoxic damage when the synaptic current is prolonged.
Methods
Cell cultures and transfection
Cultures were maintained in a humidified incubator with 5% CO2 at 37°C. All media and supplements were purchased from Gibco/Invitrogen, except for epidermal growth factor (Sigma). For experiments, cells were plated onto 35-mm Petri dishes at a density of 104 cells cm−2. Rat pituitary GH4C1 cells were grown in HAM F10 nutrient mixture plus 10% fetal calf serum (FCS) and 1% penicillin-streptomycin.
Different combinations of the human wild-type α, β, δ, γ or ɛ subunits, or α subunits harbouring the αG153S or αV249F mutation, were expressed in GH4C1 cells by transient transfection using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions, to yield γ- or ɛ-AChR containing either the wild-type α subunit or one of the mutant α subunits. The other cDNAs used (mouse α, β, δ, ɛ or human α7) were transfected by the same procedures. For each subunit, 1 μg cDNA was added to each 35-mm Petri dish, and experiments were carried out 48–72 h after transfection. Human myotubes were obtained from satellite cells derived from human muscle specimens (Broccolini et al. 2004). The muscle specimens were obtained with informed written consent from patients without neuromuscular disease undergoing surgery. Cells were allowed to proliferate on collagen-coated Petri dishes, in Dulbecco's modified Eagle's medium (DMEM) supplemented with 15% FCS, 2% penicillin-streptomycin, 1% insulin-transferrin-selenium and 10 ng ml−1 epidermal growth factor. Differentiation was induced at approximately 50% confluence by switching to a low-serum medium (DMEM plus 2% horse serum and penicillin-streptomycin). Due to the reduced physical contact between cells, many cells differentiated but remained mononucleated, as previously described (Krause et al. 1995).
Solutions and chemicals
Cells were superfused with a standard external medium containing (mm): NaCl 140, KCl 2.8, CaCl2 2, MgCl2 2, Hepes-NaOH 10 and glucose 10; pH 7.3. CaCl2 was omitted in the Ca2+-free solution. For cell-attached experiments, patch pipettes were filled the same solution plus ACh (100 nm). For whole-cell recordings, patch pipettes were filled with a solution containing (mm): N-methyl-d-glucamine (NMDG) 140, Hepes-HCl 10, Fura-2 pentapotassium salt 0.25 and thapsigargin 0.001; pH 7.3. Calibration measurements of F/Q, which require that current is only due to the flux of Ca2+ ions, were performed using the following extracellular solution (mm): NMDG 130, CaCl2 10 and Hepes-HCl 10; pH 7.3. All chemicals were purchased from Sigma (USA), except for Fura-2 pentapotassium salt and the acetoxylmethyl ester (AM) form of Fura-2 (Molecular Probes).
Electrophysiology
Whole-cell currents were recorded using borosilicate glass patch pipettes (3–6 MΩ tip resistance) connected to an Axopatch 200A amplifier (Molecular Devices, Union City, CA, USA). Data were recorded and analysed using pCLAMP8 software (Molecular Devices). Whole-cell capacitance and patch series resistance (5–15 MΩ) were estimated from slow-transient compensations, with a series-resistance compensation of 70–90%. Cells were voltage clamped at a holding potential (HP) of −70 mV (unless otherwise indicated) and continuously superfused using a gravity-driven perfusion system with independent tubes for standard and agonist-containing solutions, positioned 50–100 μm from the patched cell. A fast exchanger system (RSC-100, BioLogique, France) allowed complete solution exchange in less than 50 ms. Data are given as means ± s.e.m. Two data sets were considered statistically different when P > 0.05 (ANOVA test).
Single-channel data were recorded using Sylgard-coated borosilicate glass pipettes (tip resistance, 3–4 MΩ), with an Axopatch 200B amplifier. Data were filtered at 4 kHz and sampled at 20 kHz, using pClamp9 software (Molecular Devices). Analysis was performed with a 50% threshold criterion using pClamp9, omitting events shorter than 0.2 ms. Slope conductance was calculated by linear fitting of the unitary amplitudes recorded at different pipette potentials (at least three for each cell). The critical time used to identify a burst (determined for each cell from the closed-time distribution) ranged from 1 to 3 ms, as previously reported (Fucile et al. 2002). The distribution of burst durations was fitted (by pClamp 9 routines) with the sum of two (or three) exponential components, with time constraints τb1, τb2 (and τb3), respectively.
Pf determination
The methods to determine Pf (the proportion of whole-cell current carried by Ca2+) have been fully reported previously (Fucile et al. 2000). Fluorescence determinations were made using a conventional fluorescence microscopy system composed of an upright microscope (Axioskop 2, Zeiss, Germany), a digital 12-bit cooled camera (SensiCam, PCO, Germany) and a monochromator (Till, Germany). The system was driven by Axon Imaging Workbench software (Molecular Devices). All optical parameters and the setting of the digital camera (exposure time, 25 ms; 4 × 4 binning) were maintained throughout all measurements to avoid non-homogeneous data. Records of fluorescent signals and membrane currents were synchronized by a digital signal from the imaging computer triggering the start of electophysiological recording. Images were acquired and stored on a PC (Dell, USA), then analysed off-line. The changes in intracellular calcium ([Ca2+]i) were measured at a single excitation wavelength (380 nm), to achieve a higher time resolution, and expressed as the ratio of time-resolved fluorescence variation to the basal fluorescence (ΔF/F).
Petri dishes were incubated at 37°C with the membrane-permeant dye Fura-2 AM (4 μm) for 45 min in DMEM. The AChR-expressing cells were initially identified as those generating a fluorescence transient in response to a brief application of nicotine. These cells were selected for whole-cell recordings, and filled with Fura-2 pentapotassium salt through the patch pipette. As high basal [Ca2+]i alters Pf values (S.F. and F.E., unpublished observations), the ratiometric dye was chosen to carefully assess [Ca2+]i prior to Pf measurements. Cells with high basal [Ca2+]i (fluorescence at 340 nm (F340)/fluorescence at 380 nm (F380) ratio values, > 2) or low basal F380 values (< 200 arbitary units (a.u.)), indicating poor loading, were discarded. Determinations were carried out after obtaining a stable value of basal fluorescence, using the same a.u. setting throughout all the experiments. The ratio between the fluorescence increase (F, in a.u.) and the total charge entering the cell (Q, in nC) was defined as:
where INic was the nicotine-evoked whole-cell current. Each F/Q point was obtained by measuring the charge entering the cell at each fluorescence acquisition time. The F/Q ratio values (in nC−1) used in determining Pf were represented by the slopes of the linear regression best fitting the F versus Q plots. We used only F/Q points measured immediately after the onset of the ACh-induced response that exhibited a linear relationship, indicating that the Ca2+-buffering capability of Fura-2 was not saturated. Pf was determined by normalizing the F/Q ratio obtained in standard medium (F/Qstandard) to the ratio obtained in calibration medium (F/Qcalibration) containing Ca2+ as the only permeant ion (Ca2+ medium), according to the equation:
The F/Qcalibration values obtained in this work were 8.0 ± 0.4 nC−1 (mean ± s.e.m., n = 6 cells) for GH4C1 cells and 3.0 ± 0.4 nC−1 (n = 4) for human myotubes.
Results
Electrophysiological characterization
The pituitary cell line GH4C1 is a reliable mammalian expression system for neuronal AChRs (Fucile, 2004). To confirm that it also represents an adequate expression system for muscle γ- and ɛ-AChR, we first tested whether the kinetic properties of AChR channels expressed in these cells are comparable to those observed in muscle cells. As we aimed to measure the Ca2+ permeability of γ- and ɛ-AChRs containing either wild-type or mutant α subunits, we considered several subunit assemblies.
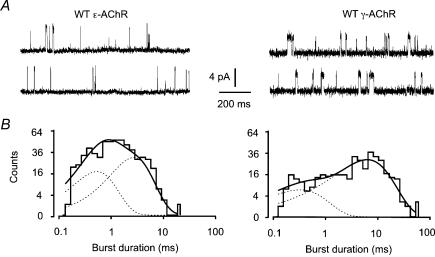
In cells expressing wild-type ɛ-AChR, ACh (100 nm) evoked unitary events with a slope conductance of 50.1 ± 3.5 pS (n = 3) and burst duration of 2.5 ms. Under our experimental conditions, most bursts consisted of single openings (see Fig. 1A left), although we cannot rule out the possibility that brief openings, below our detection threshold, were also present. The distribution of burst durations was adequately fitted by two exponential components in all cells (Fig. 1B left). For the wild-type γ-AChR, single-channel conductance was 30.5 ± 2.1 pS (n = 5) and the burst duration was about 6 ms. Again, most bursts comprised a single opening (Fig. 1A right), and the distribution of burst durations was fitted by two exponential components (Fig. 1B right).
Figure 1. Single-channel currents of wild-type human ɛ- and γ-AChRs.
A, typical examples of single-channel openings (upward) recorded in the cell-attached configuration from GH4C1 cells transiently transfected with wild-type αβɛδ or αβγδ AChR subunit cDNAs, as indicated. ACh concentration in the recording pipette was 100 nm. Channel conductance was 46.4 pS (ɛ-AChR) and 30.5 pS (γ-AChR). Traces filtered at 2 kHz for display purposes. B, distribution of burst durations (calculated with critical τ = 1 ms) from the same recordings, best-fitted by the sum of two exponential components (dotted lines). Time constants (weight) of the fitting distributions for ɛ-AChR were: τb1 = 0.55 ms (43%), τb2 = 2.7 ms (57%); and for γ-AChR were: τb1 = 0.4 ms (18%), τb2 = 6.8 ms (82%).
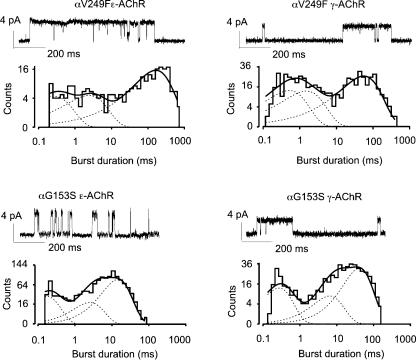
The αV249F or αG153S mutations had no significant effect on conductance of either the ɛ- or γ-AChR channel (n = 3 cells for each mutation), but significantly prolonged the burst duration and altered its distribution so that it was now best-fitted with three exponential components in all the tested cells. In agreement with previous reports (Sine et al. 1995; Milone et al. 1997), the burst duration of ɛ-AChRs containing the αG153S mutation was about 13 ms, and of ɛ-AChRs harbouring the αV249F mutation was between 35 and 80 ms (Fig. 2). For γ-AChRs, the burst duration was about 40 ms in AChRs carrying either the αG153S or the αV249F mutation (Fig. 2).
Figure 2. Single-channel currents of mutant human ɛ- and γ-AChRs.
Typical examples of single-channel openings recorded in the cell-attached configuration from GH4C1 cells transiently transfected with αV249Fβɛδ, αG153Sβɛδ, αV249Fβγδ or αG153Sβγδ AChR subunit cDNAs, as indicated. Under each trace is the corresponding distribution of burst durations, best-fitted by the sum of three exponential components. ACh concentration in the recording pipette was 100 nm. Channel conductance and time constants of the burst fitting components (weight) were: 45.4 pS, τb1 = 0.3 ms (18%), τb2 = 2.7 ms (19%), τb3 = 183 ms (62%) (αV249Fβɛδ; critical τ = 2 ms); 34.4 pS, τb1 = 0.6 ms (25%), τb2 = 1.8 ms (25%), τb3 = 64 ms (50%) (αV249Fβγδ; critical τ = 3 ms); 50.6 pS, τb1 = 0.2 ms (29%), τb2 = 2.9 ms (23%), τb3 = 16.8 ms (47%) (αG153Sβɛδ; critical τ = 1 ms); 26.3 pS, τb1 = 0.3 ms (30%), τb2 = 6.6 ms (23%), τb3 = 41.7 ms (47%) (αG153Sβγδ; critical τ = 1 ms). Traces filtered at 2 kHz for display purposes.
As Pf determinations are best performed using nicotine as an agonist (see below), we examined the properties of INic in GH4C1 cells transiently transfected with either human wild-type or mutant α subunits together with complementary wild-type β, δ and γ or ɛ subunits. No response was observed in non-transfected cells, indicating that GH4C1 native cells do not express functional nicotinic AChRs. Nicotine (100 μm) elicited inward currents with peak amplitudes between 0.3 and 4 nA for all the AChR isoforms examined, indicating equivalent expression levels (Table 1). Upon prolonged transmitter application, INic decayed exponentially as a result of receptor desensitization. The decay was best-fitted to a single-exponential function for wild-type γ- and ɛ-AChR (Table 1). Enhanced desensitization has been reported on the basis of single-channel data for ɛ-AChRs containing either the αV249F or the αG153S mutation (Sine et al. 1995; Milone et al. 1997). Consistent with this enhanced desensitization, the decay of INic was faster in cells expressing ɛ-AChR or γ-AChR that harboured the mutant α subunit (compare Figs 3 and 4 with Fig. 5). These data show that γ- and ɛ-AChRs containing the αV249F or αG153S mutations retain their main functional characteristics in GH4C1 cells.
Table 1.
Functional parameters of nicotine-evoked whole-cell responses
| AChR subunits | No. of cells | Peak amplitude (nA) | Decay (s) | Pf (%) |
|---|---|---|---|---|
| WT αβγδ | 8 | 1.2 ± 0.3 | 2.0 ± 0.5 | 2.9 ± 0.2 |
| WT αβɛδ | 8 | 1.2 ± 0.3 | 2.1 ± 0.8 | 7.2 ± 1.1 |
| αV249F βɛδ | 6 | 1.3 ± 0.5 | 0.48 ± 0.06* | 7.6 ± 0.6 |
| αG153S βɛδ | 7 | 1.3 ± 0.2 | 0.5 ± 0.1* | 7.1 ± 0.5 |
| αV249F βγδ | 7 | 1.7 ± 0.5 | 0.35 ± 0.05* | 3.3 ± 0.2 |
| αG153S βγδ | 5 | 2.3 ± 0.5 | 0.8 ± 0.2* | 2.9 ± 0.6 |
Peak amplitude of inward currents elicited by nicotine application (100 μm; duration, 1 s), expressed as mean ± s.e.m., in the indicated number of cells. Current decay was best-fitted by a single exponential with the indicated time constants.
Significantly different from the corresponding wild-type value (P < 0.01).
Figure 3. Ca2+ permeability of human γ-AChR.
A, left-hand traces show simultaneous recordings of whole-cell current (top) and Ca2+ transient (bottom), induced by nicotine (100 μm, horizontal thin line), in a GH4C1 cell transiently transfected with wild-type αβγδ AChR subunit cDNAs. Holding potential, −70 mV. At the excitation wavelength of 380 nm, [Ca2+]i increase corresponds to a downward deflection of fluorescence intensity. Traces are aligned and share the same temporal scale. Middle panel shows typical epifluorescence image of a GH4C1 cell dialysed with Fura-2 through the patch pipette (halo on the right). Contrast was adjusted to increase the visibility of nucleus. Graph on the right shows linear relationships between ΔF/F and Q obtained from the same cell. For this example, the Pf value, calculated by normalizing the slope obtained in A (standard medium) to the mean slope obtained from calibration experiments (dashed line), is 3.2%. B, left-hand traces show simultaneous recordings of whole-cell current (top) and Ca2+ transient (bottom), induced by nicotine (100 μm, horizontal thin line), from a human myotube. Holding potential, −70 mV. Traces are aligned and share the same temporal scale. Middle panel shows typical fluorescence image of a binucleated human myotube dialysed with Fura-2. Graph on the right shows linear relationship between ΔF/F and Q obtained from the cell shown in B. For this example, the Pf value, calculated by normalizing the slopes obtained in B (standard medium) with respect to the mean slope obtained from calibration experiments (3.07 nC−1; dashed line), is 2.9%.
Figure 4. Ca2+ permeability of human, mouse and chimeric ɛ-AChRs.
A, simultaneous recordings of whole-cell current (top) and Ca2+ transient (bottom) induced by nicotine (100 μm, horizontal thin line) in GH4C1 cells transiently transfected with wild-type human αβɛδ (left), mouse αβɛδ (middle) and human αβδ plus mouse ɛ (right) AChR subunit cDNAs. Holding potential, −70 mV. Traces are aligned and share the same temporal scale. B, linear relationships between ΔF/F and Q obtained from the cells shown in A. For these examples, the Pf values, calculated by normalizing the slopes obtained in A (standard medium) with respect to the mean slope obtained from calibration experiments (8.0 ± 0.4 nC−1, dashed line) are 7.5% for human αβɛδ (^), and 3.4% and 3.5% for mouse αβɛδ (▵) and for human αβδ-mouse ɛ (□), respectively.
Figure 5. Ca2+ permeability of human mutant AChRs.
A, simultaneous recordings of whole-cell current (top) and Ca2+ transient (bottom), induced by nicotine (100 μm, horizontal thin line), in GH4C1 cells transiently transfected with αV249Fβɛδ (left) and αG153Sβɛδ (right) AChR subunit cDNAs. Holding potential, −70 mV. Traces are aligned and share the same temporal scale. B, simultaneous recordings of whole-cell current (top) and Ca2+ transient (bottom), induced by nicotine, in GH4C1 cells transiently transfected with αV249Fβγδ (left) and αG153Sβγδ (right) AChR subunit cDNAs. Holding potential, −70 mV. Traces are aligned and share the same temporal scale. C, linear relationships between ΔF/F and Q obtained from the cells shown in A and B. For these examples, the Pf values, calculated by normalizing the slopes obtained in A and B (standard medium) with respect to the mean slope obtained from calibration experiments (8.0 ± 0.4 nC−1, dashed line), are 7.7% (αV249Fβɛδ, ▵), 7.6% (αG153Sβɛδ, □), 3.7% (αV249Fβγδ, ▴), and 3.4% (αG153Sβγδ, ▪).
Control measurements of Ca2+ mobilization
As the fractional Ca2+ current of AChRs describes the percentage of the ACh-evoked current carried by Ca2+ ions, it is crucial that Ca2+ ions enter the cytoplasm only through the AChR channel and not from any other source. Accordingly, in Pf measurements, we used nicotine instead of ACh to prevent the activation of putative native muscarinic AChRs that might mobilize Ca2+ from intracellular stores. Moreover, we depleted intracellular stores by intracellular dialysis (via the patch pipette) of the Ca2+-ATPAse inhibitor thapsigargin, and prevented their refill by omitting ATP from the intracellular solution. The small size of GH4C1 cells ensured adequate control of the intracellular milieu (Fig. 3A).
With these precautions, nicotine failed to evoke Ca2+ transients from transfected cells superfused with a Ca2+-free external solution or from non-transfected cells exposed to the standard medium (data not shown). Thus, the experimental conditions excluded release of residual Ca2+ from internal stores. In transfected cells in the presence of extracellular Ca2+, nicotine application evoked a Ca2+ transient (Figs 3–5). The delay between current onset and the beginning of fluorescence change was below the sampling interval of our optical system, ruling out a long-delayed metabotropic cause of Ca2+ influx. Our experimental conditions prevented Ca2+ extrusion out of the cytoplasm, into intracellular organelles or to the extracellular medium, so that no recovery of [Ca2+]i to the basal levels took place (see Figs 3–5). Considered together, these findings indicate that the enhanced [Ca2+]i was due to Ca2+ entry from the extracellular medium through bona fide exogenous AChRs.
Pf determinations for wild-type γ-AChRs
For Pf determinations, nicotine-evoked Ca2+ and current responses were simultaneously recorded, and the F/Q ratios calculated. In cells expressing the wild-type γ-AChR, the Pf was around 3% (Fig. 3A and Table 1), a value comparable to that found in rodent γ-AChR (Ragozzino et al. 1998). To validate our heterologous cell-expression system, we measured the Pf of human γ-AChR in cultured primary myotubes that express γ-AChR soon after differentiation is induced (Krause et al. 1995). To ensure a valid space clamp and a complete intracellular dialysis through the patch pipette, only small myotubes with one to three nuclei were selected (Fig. 3B). These cells had a Pf value of 2.7 ± 0.6% (n = 6; Fig. 3B), similar to that found in transfected cells expressing the wild-type γ-AChR (Table 1).
Pf determinations for wild-type ɛ-AChRs
In cells expressing human wild-type ɛ-AChR (see Fig. 4A), normalization to calibration values yielded Pf values of ∼7% (Table 1), a value much higher than in rodents (Fucile, 2004). To rule out experimental artifacts, we repeated the Pf measurement for mouse and human wild-type ɛ-AChR in parallel. Consistent with the initial data, we found a Pf of 4.1 ± 0.4% (n = 5) in cells expressing mouse ɛ-AChR (Fig. 4B), indicating that the high fractional Ca2+ current of human ɛ-AChR is genuine. To further confirm that our experimental conditions were similar to those of our previous work, we repeated the determination of the Pf for the homomeric human α7-AChR in GH4C1 cells and found a Pf value of 10.9 ± 1.9% (n = 3, data not shown), which is similar to that previously measured in GH4C1 cells (11.4%; Fucile et al. 2003), or in rat native neurons (9.5%; Fucile et al. 2005).
The human ɛ subunit appears to be a crucial determinant of the Pf value, as no difference was observed between mouse and human γ-AChRs. To test this point directly, we produced a chimeric receptor by transfecting the human α, β and δ subunits along with the mouse ɛ subunit in GH4C1 cells. In these cells, nicotine elicited inward currents with a mean amplitude of 640 ± 180 pA (n = 7). The measured Pf value was 4.1 ± 0.3% (n = 5, Fig. 4B), confirming that the major determinant of the increased Ca2+ permeability of human ɛ-AChRs is indeed the ɛ subunit.
Pf determinations for mutant ɛ- and γ-AChRs
To evaluate whether mutations leading to SCCMS could increase the Pf of muscle AChRs, the measurements of this parameter were repeated for ɛ- and γ-AChRs comprising the mutant αV249F or αG153S subunits. However, neither the αV249F nor the αG153S mutations altered the Pf values of ɛ-AChR or γ-AChR as compared to the corresponding wild-type receptor (Fig. 5 and Table 1). Therefore, these slow-channel mutations had no significant effect on the Ca2+ permeability of the AChR channels.
Discussion
We report in this work that the Pf of the wild-type human ɛ-AChR is above 7%, which is higher than that of neuronal AChRs, with the notable exceptions of α7-AChR and the α9-containing AChR (Fucile, 2004; Fucile et al. 2006). The Ca2+ permeability of the human ɛ-AChR is significantly higher that than of mouse ɛ-AChR, whereas the Ca2+ permeability of the human γ-AChR is slightly, but not significantly, higher than that of mouse γ-AChR (see Fucile, 2004). Concurrent control studies on mouse ɛ-AChR and α7-AChR yielded Pf values consistent with previously published results (Ragozzino et al. 1998; Fucile et al. 2003), confirming the validity of the determinations. Interestingly, these findings contradict the general view that the Ca2+ permeability of neuronal AChRs is much larger than that of the muscle-type AChRs (see for instance Vernino et al. 1992).
As our measurements were performed in transfected GH4C1 cells, it is questionable whether ɛ-AChR has the same high Ca2+ permeability in native muscle fibres. It was previously shown that Pf values of the rat α7-AChR, which is highly permeable to Ca2+, were similar in GH4C1 cells and in native preparations (Fucile et al. 2003, 2005). Here we show that the Pf for human γ-AChR is similar in cultured human muscle cells and in GH4C1 cells. Moreover, single-channel properties of the ɛ-AChR expressed in GH4C1 cells are similar to those in human muscle fibres (Sine et al. 1995; Milone et al. 1997), and the single-channel properties of wild-type γ-AChR expressed in GH4C1 cells are similar to those in human myotubes (F.G. and A. Di Castro, unpublished observations). Finally, the kinetic properties of the ɛ-AChR mutants harbouring the αG153S or the αV249F are similar in GH4C1 cells and muscle fibres (Sine et al. 1995; Milone et al. 1997). These data confirm that GH4C1 cells represent a faithful expression system for nicotinic receptors.
Ca2+ influx at the endplate is held responsible for the endplate myopathy of the SCCMS. This is a dominantly inherited disorder of neuromuscular transmission in which point mutations in genes encoding subunits of the AChR produce abnormally long-lasting synaptic currents, resulting in excessive Ca2+ entry and in excitotoxic damage of the postsynaptic region (Engel & Sine, 2005). Staining with dyes that are sensitive to Ca2+ in the millimolar range (Bodensteiner & Engel, 1978) has demonstrated Ca2+ accumulation at human slow-channel endplates (Engel et al. 1982, 2003b; Vohra et al. 2004) and in mouse models of the disease (Gomez et al. 2002). Caspase activation, triggered by enhanced [Ca2+]i has also been detected at the endplate of SCCMS patients (Vohra et al. 2004), providing a possible link between Ca2+ influx and focal damage at the endplate. Our data show that the Pf values observed for γ- or ɛ-AChRs harbouring the mutant αV249F or αG153S subunits were comparable to the corresponding control values, indicating that these mutations only affect channel opening kinetics, with endplate currents decaying up to 10 times more slowly in patients with SCCMS than in controls (Sine et al. 1995; Milone et al. 1997).
Although the details of the pathological process remain to be elucidated, the high Pf of human ɛ-AChR is a likely predisposing factor for the endplate myopathy, because in mice that have a lower Pf than humans the histological damage is only detected when the endplate currents are much more prolonged than in most SCCMS patients (Gomez et al. 2002). Further evidence that long openings of AChR channels must be coupled to a high Ca2+ influx to be toxic comes from the observation that developing mouse muscle fibres express γ-AChR at the endplate that has 3- to 5-fold longer openings than the ɛ-AChR (Grassi et al. 1998). The long-lasting γ-AChR openings are present for about 2 weeks postnatally (Villarroel & Sakmann, 1996; Grassi & Degasperi, 2000), although at a low and rapidly decreasing density. In this context, far from being toxic, γ-AChR channels are required for a correct synapse formation, a role in which they cannot be replaced by ɛ-AChR (Koenen et al. 2005). Thus, appearance of the endplate myopathy is dependent on strongly prolonged openings of the highly Ca2+-permeable mutant ɛ-AChR channels.
It is striking that human and mouse γ-AChRs are similar in their Ca2+ permeability, whereas that of the ɛ-AChR is markedly different between the two species. Here we unequivocally show that the relatively large Pf of the human ɛ-AChR is uniquely determined by the human ɛ subunit, as AChR containing human αβδ plus mouse ɛ subunits has a Pf identical to that of the mouse ɛ-AChR. The molecular determinants underlying the high Ca2+ permeability of the human wild-type ɛ-AChR await further investigation.
Acknowledgments
This work was supported by grants from Ministry of Education, University and Research to F.E and F.G. and by National Institutes of Health Grant NS6277 and a Muscular Dystrophy Association Grant to A.G.E. A.S. is supported by the PhD programme in Neurophysiology of Universita' La Sapienza.
References
- Allard B, Bernengo J-C, Rougier O, Jacquemond V. Intracellular Ca2+ changes and Ca2+-activated K+ channel activation induced by acetylcholine at the endplate of mouse skeletal muscle fibres. J Physiol. 1996;494:337–349. doi: 10.1113/jphysiol.1996.sp021496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Bandi E, Bernareggi A, Grandolfo M, Mozzetta C, Augusti-Tocco G, Ruzzier F, Lorenzon P. Autocrine activation of nicotinic acetylcholine receptors contributes to Ca2+ spikes in mouse myotubes during myogenesis. J Physiol. 2005;568:171–180. doi: 10.1113/jphysiol.2005.091439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodensteiner J, Engel AG. Intracellular calcium accumulation in Duchenne dystrophy and other myopathies: a study of 567,000 muscle fibers in 114 biopsies. Neurology. 1978;28:439–446. doi: 10.1212/wnl.28.5.439. [DOI] [PubMed] [Google Scholar]
- Bregestovski PD, Miledi R, Parker I. Calcium conductance of acetylcholine-induced end-plate channels. Nature. 1979;279:638–639. doi: 10.1038/279638a0. [DOI] [PubMed] [Google Scholar]
- Broccolini A, Ricci E, Pescatori M, Papacci M, Gliubizzi C, D'Amico A, Servidei S, Tonali P, Mirabella M. Insulin-like growth factor I in inclusion-body myositis and human muscle cultures. J Neuropathol Exp Neurol. 2004;63:650–659. doi: 10.1093/jnen/63.6.650. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Zhou Z, Neher E, Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J Physiol. 1995;485:403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AG, Lambert EH, Mulder DM, Torres CF, Sahashi K, Bertorini TE, Whitaker JN. A newly recognized congenital myasthenic syndrome attribute to a prolonged open time of the acetylcholine-induced ion channel. Ann Neurol. 1982;11:553–569. doi: 10.1002/ana.410110603. [DOI] [PubMed] [Google Scholar]
- Engel AG, Ohno K, Sine SM. Congenital myasthenic syndromes: progress over the past decade. Muscle Nerve. 2003a;27:4–25. doi: 10.1002/mus.10269. [DOI] [PubMed] [Google Scholar]
- Engel AG, Ohno K, Sine SM. Sleuthing molecular targets for neurological diseases at the neuromuscular junction. Nat Rev Neurosci. 2003b;4:339–352. doi: 10.1038/nrn1101. [DOI] [PubMed] [Google Scholar]
- Engel AG, Sine SM. Current understanding of congenital myasthenic syndromes. Curr Opin Pharmacol. 2005;5:308–321. doi: 10.1016/j.coph.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Fucile S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35:1–8. doi: 10.1016/j.ceca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Fucile S, Palma E, Martinez-Torres A, Miledi R, Eusebi F. The single-channel properties of human acetylcholine α7 receptors are altered by fusing α7 to the green fluorescent protein. Proc Natl Acad Sci U S A. 2002;99:3956–3961. doi: 10.1073/pnas.052699599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile S, Palma E, Mileo AM, Miledi R, Eusebi F. Human neuronal threonine-for-leucine-248 α7 mutant nicotinic acetylcholine receptors are highly Ca2+ permeable. Proc Natl Acad Sci U S A. 2000;97:3643–3648. doi: 10.1073/pnas.050582497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile S, Renzi M, Lax P, Eusebi F. Fractional Ca2+ current through human neuronal alpha7 nicotinic acetylcholine receptors. Cell Calcium. 2003;34:205–209. doi: 10.1016/s0143-4160(03)00071-x. [DOI] [PubMed] [Google Scholar]
- Fucile S, Sucapane A, Eusebi F. Ca2+ permeability of nicotinic acetylcholine receptors from rat dorsal root ganglion neurones. J Physiol. 2005;565:219–228. doi: 10.1113/jphysiol.2005.084871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile S, Sucapane A, Eusebi F. Ca2+ permeability through rat cloned α9-containing nicotinic acetylcholine receptors. Cell Calcium. 2006;39:349–355. doi: 10.1016/j.ceca.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Gomez MC, Maselli RA, Groshong J, Zayas R, Wollmann RL, Cens T, Charnet P. Active calcium accumulation underlies severe weakness in a panel of mice with slow-channel syndrome. J Neurosci. 2002;22:6447–6457. doi: 10.1523/JNEUROSCI.22-15-06447.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi F, Degasperi V. Modulation of fetal and adult acetylcholine receptors by Ca2+ and Mg2+ at developing mouse end-plates. Pflugers Arch. 2000;440:704–709. doi: 10.1007/s004240000354. [DOI] [PubMed] [Google Scholar]
- Grassi F, Epifano O, Mileo AM, Barabino B, Eusebi F. The open duration of fetal ACh receptor-channel changes during mouse muscle development. J Physiol. 1998;508:393–400. doi: 10.1111/j.1469-7793.1998.393bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen M, Peter C, Villarroel A, Witzemann V, Sakmann B. Acetylcholine receptor channel subtype directs the innervation pattern of skeletal muscle. EMBO Rep. 2005;6:570–576. doi: 10.1038/sj.embor.7400429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause RM, Hamann M, Bader CR, Liu J-H, Baroffio A, Bernheim L. Activation of nicotinic acetylcholine receptors increases the rate of fusion of cultured human myoblasts. J Physiol. 1995;489:779–790. doi: 10.1113/jphysiol.1995.sp021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K, Miledi R, Stinnakre J. Post-synaptic entry of calcium induced by transmitter action. Proc R Soc Lond B Biol Sci. 1975;189:38–47. doi: 10.1098/rspb.1975.0040. [DOI] [PubMed] [Google Scholar]
- Leonard JP, Salpeter MM. Agonist-induced myopathy at the neuromuscular junction is mediated by calcium. J Cell Biol. 1979;82:811–819. doi: 10.1083/jcb.82.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milone M, Wang HL, Ohno K, Fukudome T, Pruitt JN, Bren N, Sine SM, Engel AG. Slow-channel myasthenic syndrome caused by enhanced activation, desensitization, and agonist binding affinity attributable to mutation in the M2 domain of the acetylcholine receptor alpha subunit. J Neurosci. 1997;17:5651–5665. doi: 10.1523/JNEUROSCI.17-15-05651.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino D, Barabino B, Fucile S, Eusebi F. Ca2+ permeability of mouse and chick nicotinic acetylcholine receptors expressed in transiently transfected human cells. J Physiol. 1998;507:749–757. doi: 10.1111/j.1469-7793.1998.749bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Olney JW. Excitotoxicity and the NMDA receptor – still lethal after eight years. Trends Neurosci. 1995;18:57–58. doi: 10.1016/0166-2236(95)93869-y. [DOI] [PubMed] [Google Scholar]
- Salpeter MM, Kasprzak H, Feng H, Fertuck H. End-plates after esterase inactivation in vivo: correlation between esterase concentration, functional response and fine structure. J Neurocytol. 1979;8:95–115. doi: 10.1007/BF01206461. [DOI] [PubMed] [Google Scholar]
- Sine SM, Ohno K, Bouzat C, Auerbach A, Milone M, Pruitt JN, Engel AG. Mutation of the acetylcholine receptor alpha subunit causes a slow-channel myasthenic syndrome by enhancing agonist binding affinity. Neuron. 1995;15:229–239. doi: 10.1016/0896-6273(95)90080-2. [DOI] [PubMed] [Google Scholar]
- Vernino S, Amador M, Luetje CW, Patrick J, Dani JA. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 1992;8:127–134. doi: 10.1016/0896-6273(92)90114-s. [DOI] [PubMed] [Google Scholar]
- Villarroel A, Sakmann B. Calcium permeability increase of endplate channels in rat muscle during postnatal development. J Physiol. 1996;496:331–338. doi: 10.1113/jphysiol.1996.sp021688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohra BPS, Groshong JS, Maselli RA, Verity MA, Wollmann RL, Gomez CM. Focal caspase activation underlies the endplate myopathy in slow-channel syndrome. Ann Neurol. 2004;55:347–352. doi: 10.1002/ana.10823. [DOI] [PubMed] [Google Scholar]
- Young KA, Caldwell JH. Modulation of skeletal and cardiac voltage-gated sodium channels by calmodulin. J Physiol. 2005;565:349–370. doi: 10.1113/jphysiol.2004.081422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Neher E. Calcium permeability of nicotinic acetylcholine receptor channels in bovine adrenal chromaffin cells. Pflugers Arch. 1993;425:511–517. doi: 10.1007/BF00374879. [DOI] [PubMed] [Google Scholar]