Abstract
Purpose
Recent studies have reported high frequencies of somatic mutations in the phosphoinositide-3-kinase, catalytic, alpha (PIK3CA) gene in several human solid tumors. Although gene amplifications of PIK3CA have been reported in head and neck squamous cell carcinoma (HNSCC), small mutation of the gene has not been evaluated in HNSCC previously. In this study, we examined the mutation frequency of PIK3CA in HNSCC.
Experimental Design
More than 75% of the somatic mutations of PIK3CA are clustered in the helical (exon 9) and kinase domains (exon 20). To investigate the possible role of PIK3CA in HNSCC tumorigenesis, exons 1, 4, 5, 6, 7, 9, and 20 of the gene were analyzed by direct genomic DNA sequencing in 38 HNSCC specimens.
Results
We identified four missense mutations in the seven exons of PIK3CA from 38 HNSCC specimens (11%). Three of the four mutations, named H1047R, E542K and E545K respectively, have been previously reported as hot-spot mutations. The remaining novel mutation, Y343C, is identified at exon 4 nucleotide 1028 A → G. Three of the four mutations were shown to be somatic, while the forth mutation (H1047R) was identified in a cell line. Interestingly, three of the four mutations identified were in pharyngeal cancer samples.
Conclusions
These data provide evidence that oncogenic properties of PIK3CA contributes to the carcinogenesis of human head and neck cancers, especially in pharyngeal cancer. A specific kinase inhibitor to PIK3CA may potentially be an effective therapeutic reagent against HNSCC or pharyngeal cancer in particular.
The abbreviations used are: HNSCC, Head and neck squamous cell carcinoma; PCR, polymerase chain reaction; PI3K, phosphatidylinositol 3-kinase; PIP3, phosphatidylinositol-3,4,5-triphosphate
INTRODUCTION
The phosphatidylinositol 3-kinase (PI3K) signaling pathway regulates many normal cellular processes, such as cell proliferation, survival and apoptosis (1–3). Dysregulation or genetic aberration of components of this pathway, including AKT, PTEN, and PIK3CA, has been associated with cancer development (4–12).
PIK3CA is located on chromosome 3q26.32 and encodes for the catalytic subunit p110α of class IA PI3-kinase. It has been implicated to function as an oncogene in human cancer because of its elevated kinase activity and genomic amplification in tumor samples (7–12). Recently high frequencies of somatic mutations in the PIK3CA gene have been reported in several human cancer types, including colon, brain, stomach, breast, and ovary (13–18). More than 75% of these mutations are clustered in the helical (exon 9) and kinase domains (exon 20) of the gene (13). The three most frequently reported mutation hot spots in PIK3CA, named E542K, E545K and H1047R, have been shown to elevate its lipid kinase activity and lead to the activation of the downstream Akt signaling pathway (13, 19). Interestingly, PIK3CA mutations and PTEN loss are nearly mutually exclusive, suggesting that the homeostasis of phosphatidylinositol-3,4,5-triphosphate regulated by both PIK3CA and PTEN is critical to carcinogenesis (20). This further evinced the importance of the PI3K pathway in the tumorigenesis of many cancer types.
Although the PIK3/AKT/PTEN pathway has been implicated in HNSCC (12, 21–23), no genetic mutation of PIK3CA has been described to date. To investigate whether PIK3CA activating mutation is a common mechanism involved in the tumorigenesis of HNSCC, we analyzed for genetic alterations of the PIK3CA gene in 38 HNSCC specimens including eight cell lines by direct genomic DNA sequencing. Only exons 1, 4, 5, 6, 7, 9, and 20 of the gene were sequenced in these specimens because they covered the most common PIK3CA mutations previously observed in human cancer (13–17, 24–26).
MATERIAL AND METHODS
Tissue samples and cell lines
Eight HNSCC cell lines, RPMI 2650, A-253, SW579, Detroit 562, FADU, CAL 27, SCC-15 and SCC-25, were purchased from American Type Culture Collection (Rockville, MD). The cell lines were maintained as recommended by ATCC.
Thirty frozen primary tumor samples and their corresponding match normal muscle specimens were obtained from the Tumor Bank facility of the Herbert Irving Comprehensive Cancer Center and Department of Otolaryngology/Head and Neck Surgery of the Columbia University Medical Center. Acquisition of the tissue specimens was approved by the Institutional Review Board of Columbia University Medical Center and performed in accordance with Health Insurance Portability and Accountability Act (HIPAA) regulations. Fresh-frozen tumor samples were dissected to ensure that the specimen contained at least 75% cancer cells. The cancer sites were nasal cavity (2), pharynx (6), larynx (10), oral cavity (8) and other sites (4). The patients’ ages ranged from 40 to 85 years, average 64.0 ± 14.5. The grades of the tumors were moderately to poorly differentiated.
PCR amplification and PCR product direct sequencing
Genomic DNAs were extracted from the cell lines and the frozen tissue samples using DNeasy tissue kit (Qiagen, CA). The procedures were performed according to the manufacturer’s instructions.
Exons 1, 4, 5, 6, 7, 9, and 20 of PIK3CA gene were analyzed by PCR amplification of genomic DNA and PCR product direct sequencing. Genomic DNAs (40 ng per sample) were amplified with primers covering the entire coding region and the exon/intron boundaries of the desired exons (PIK3CA-E9F:5’-ctgtgaatccagaggggaaa-3’; PIK3CA-E9R: 5’-gcatttaatgtgccaactacca-3’; PIK3CA-E9FS: 5’-tccagaggggaaaaatatgaca-3’, (13)). All gene sequencings were performed with ABI's 3100 capillary automated sequencers at the DNA facility of Columbia University Medical Center using previously published sequencing primers (13). All samples found to have a genetic alteration in the target were subsequently sequenced in the reverse direction to confirm the mutation using the reverse PCR primers (13). The mutation was then further verified by sequencing of a second PCR product derived independently from the original template.
RESULTS
A novel sequence identified similar to PIK3CA
While sequencing for mutations using primers we had designed for exon 9, we were surprised to find an alteration at nucleotide 1634 A → C (E545A) in all the cases (Figures 1, 2). However, this nucleotide change of PIK3CA A1634C (E545A) always co-existed with another alteration of G → C at nucleotide 1658 and a base deletion at nucleotide 1659 (Figure 1). Subsequent sequencing analyses of the matching normal tissue specimens revealed that the same nucleotide changes occurred in both tumor and normal tissues (data not shown). This unusual result led us to blast search this PCR fragment (410-bp long) in the GenBank. We found two genomic DNA clones that contain fragments that are 97% (401/410) homologous to the exon 9 and its flanking intronic sequences. These two clones are located at chromosome 22q11.2 cat eye syndrome region (gi 5931525) and at chromosome 16 (gi 28913054) (Figure 2 and data not shown). Further comparisons of the sequences using the BLAST search revealed that both genomic clones on chromosome 22 and chromosome 16 contain sequences highly homologous to the exons 9, 11–13 and partial exon 10 of the PIK3CA gene (data not shown). An automatic computational analysis using the GNOMON gene prediction method predicted a protein that can be transcribed and translated from the chromosome 22 clone. The predicted protein (gi 51475436) is similar to the helical domain of the PIK3CA protein. However, this sequence homolog is likely to be a pseudogene since no RNA transcripts of the predicted protein can be detected by RT-PCR (data not shown).
Figure 1. Identification of a sequence similar to PIK3CA on chromosomes 22 and 16.
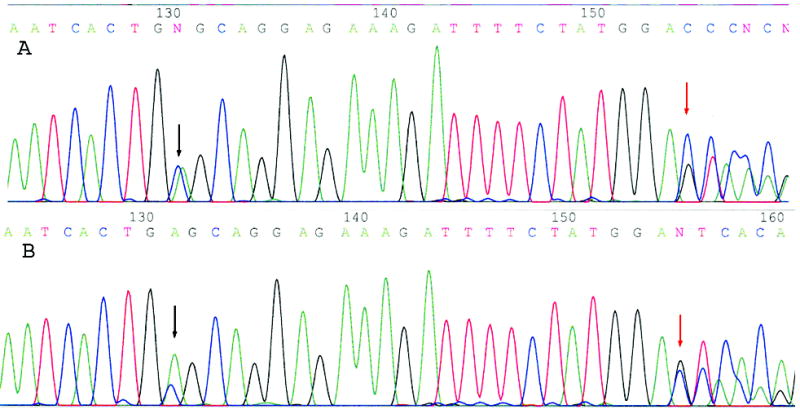
Both A and B represent the so-called A1634C (E545A) “mutation” of the PIK3CA gene detected in all of our tumor samples. In our study, this “mutation” (black arrow) always co-existed with G1658C (red arrow) and a deletion of nucleotide 1659T. This sequencing profile was also detected in the matching normal specimens. We subsequently concluded that this abnormal profile is caused by the interference of a DNA sequence that is located at chromosome 22q11.2 cat eye syndrome region and chromosome 16 that are 97% homologous to the exon 9 of the PIK3CA gene.
Figure 2. The alignment of a PCR fragment of the PIK3CA gene containing exon 9 and its flanking intronic sequences with a human genomic DNA clone located at chromosome 22q11.2 Cat Eye Syndrome region (gi 5931525).

The alignment shows that the homology between the two pieces of nucleotide sequences is 97% (401/410). Arrows mark the three nucleotide differences located inside the exon 9 coding region (in upper cases). The PCR primers designed by us (PIK3CA-E9F and PIK3CA-E9R) and Samuel et al. (hCT1640694-Ex9F and hCT1640694-Ex9R) are underlined.
This sequence homolog was probably not reported by previous publications because its detectability depends highly on primer designs. When we moved the PCR primer sites, used the primers published in the study by Samuels et al. (13), or increased the stringency of our PCR condition, all the nucleotide alterations including the so-called PIK3CA A1634C (E545A) “mutation” disappeared. We concluded that the A1634C (E545A) “mutation” observed in our hands was an artifact created by interferences from the sequence homolog.
PIK3CA is activated by small mutation in HNSCC
Four missense mutations of the PIK3CA gene were identified in the 38 HNSCC specimens (Figure 3 and Table 1)-Two of the mutations were in the exon 9 (E545K, E542K), one was in the exon 20 (H1047Y) and one was in the exon 4 (Y343C). None of these mutations was detected in the corresponding normal tissues except for the H1047Y mutation, which was identified in HNSCC cell line Detroit 562. Three of the four PIK3CA missense mutations (E545K, E542K, and H1047R) are previously described hot-spot mutations (13). Functional studies showed that PI3-kinase carrying any one of the three hot-spot mutations is able to induce transformation in cultures of chicken embryo fibroblasts, and that the transforming activity of the mutant is correlated with increased lipid kinase activity and activation of the Akt signaling pathway (13, 19). The mutation in the exon 4 nucleotide 1028 A → G, which leads to alteration at codon 343 TAC (Y) → TGC(C), has not been described before (Fig. 3).
Figure 3. PIK3CA mutations found in HNSCC.
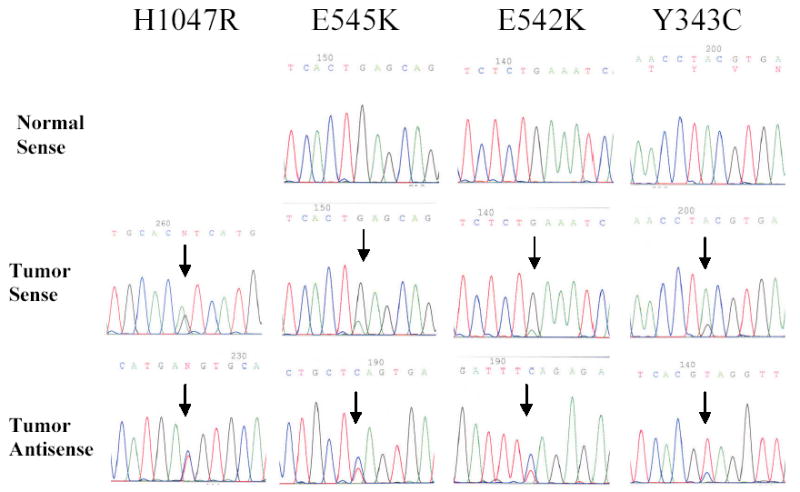
Three out of the four mutations (E545K, E542K, and Y343C) were confirmed to be somatic in sporadic HNSCC. The H1047R mutation was found in a HNSCC cell line.
Table 1.
Nucleotide alterations within the coding exons of PIK3CA identified in 38 HNSCC specimens
| Cases | Exon | Nucleotide | Amino acid | Present in normal tissue | Tumor site (number) |
|---|---|---|---|---|---|
| Detroit 562 | 20 | A3140G | H1047R | N/A | pharynx (1) |
| 102T | 9 | G1624A | E542K | no | oropharynx (1) |
| 109T | 9 | G1633A | E545K | no | hypopharynx (1) |
| 182T | 4 | A1028G | Y343C | no | tongue (1) |
| 80T | 5 | C1143G | P381P | yes | tongue (1) |
| 6 cases | 6 | A1173G | I391M | yes | pharynx (2) oral (2)
larynx(1) neck (1) |
The nucleotide alterations are described according to the cDNA sequence with GenBank accession number NM_006218.
Two other nucleotide alterations were also detected in the exonic regions of the PIK3CA gene (Table 1). One alteration, located at the exon 6 nucleotide 1173 A→G ( codon 391 ATA (ILe) → ATG (Met)), was found in six HNSCC tumor specimens. This nucleotide alteration was also detected in the six matching normal tissues. A search of the SNP database revealed that A1173G is a known SNP (rs2230461) that has been validated by multiple PCR reactions and genotype data. Thus, we conclude that this germline alteration represents a non-disease-causing polymorphism of PIK3CA. Another exonic alteration was also deemed a polymorphism because it occurred at exon 5 C1143G (P381P) without resulting in an amino acid change and was observed in both the tumor and normal samples of one tongue cancer patient. All of the polymorphisms observed in the intronic regions flanking the seven exons of PIK3CA examined in this study are listed in Table 2. These polymorphisms are unlikely to cause significant changes in the function of PIK3CA.
Table 2.
Polymorphisms of PIK3CA found in 38 HNSCC specimens
| Nucleotide position | Allele/allele frequency (number) | |||
|---|---|---|---|---|
| IVS 1 | +43 A>G | A/G (13) | A/A (14) | G/G (1) |
| +130 insert TAT | heterozygosity (13) | homozygosity (1) | ||
| IVS 4 | −69 G>T | G/T (15) | G/G (23) | T/T (0) |
| −17 A>T | A/T (15) | A/A (16) | C/C (7) | |
| +62 C>A | C/A (13) | C/C (13) | A/A (12) | |
| IVS 5 | −38 T>C | T/C (4) | T/T (34) | C/C (0) |
| +54 G>A | G/A(15) | G/G (14) | A/A (9) | |
| +307G>A | G/A (15) | G/G (14) | A/A (9) | |
| IVS 7 | +42 del TC | heterozygosity (1) | ||
| IVS 9 | +105 T>G | T/G (4) | T/T (34) | G/G (0) |
The nucleotide alterations are described according to the genomic DNA sequences of PIK3CA (gi 8705172).
DISCUSSION
The mutation frequency of PIK3CA has been reported at 32% in colon cancer, ~4–25% in gastric cancer, 8–40% in breast cancer, 5–27% in brain cancer, 4% in lung cancer, and 4–7% in ovarian cancer (13, 16–18, 25). In the present study we report 11% (4/38) of PIK3CA mutations in sporadic HNSCC. Interestingly, three out of the four cases with mutations are from the same organ site, pharynx (Table 1). Cancer of the pharynx is the 9th most common cancer worldwide (27). It is characterized as the following subsites: posterior pharynx, hypopharynx and lateral pharyngeal walls. A total of six pharyngeal squamous cell carcinoma cases were examined in this study, suggesting that as high as 50% (3/6) of pharyngeal tumor samples may harbor PIK3CA mutations. This data is supported by a previous report that showed chromosome 3q26 is amplified in 100% of nasopharyngeal carcinoma (22). However, from the present study we are unable to conclude the exact mutational frequency of PIK3CA in pharyngeal carcinomas and comment on which subtype of pharyngeal carcinomas (nasopharynx, oropharynx and hypopharynx) is targeted for PIK3CA mutation. Among our six pharyngeal samples, there was one oropharygneal cancer sample, one hypopharyngeal cancer, and four that were not subtyped. More studies with larger sample sizes and various pharyngeal subtypes are necessary to further investigate these potentials.
Gene amplification is a more commonly observed mechanism of oncogene activation in HNSCC than small genetic mutation. Cyclin D1 gene amplification has been observed in ~34–37% of HNSCC (28, 29). EGF receptor gene amplification has been reported in 7–19% of HNSCC (30–32). In contrast, RAS mutation is relatively rare in HNSCC in comparison to other solid cancers- less than 6% in HNSCC vs. 99% in pancreatic cancer and 37–47% in colorectal cancer (33–39). Amplification of chromosome 3q26 is frequently observed in HNSCC and is linked to tumor progression and negatively correlated with clinical outcome (40–42). Gene amplification and overexpression of PIK3CA are observed in low to moderate dysplasic cases, but their increased frequencies are associated with transition to invasive cancer (21, 23). Here we showed that gene amplification is not the only mechanism to activate PIK3CA in HNSCC. Small mutation and gene amplification both contribute to the activation of PIK3CA in HNSCC.
In summary, we report missense mutations of the PIK3CA gene in HNSCC (4/38, 11%). Among the four cases identified here, the Y343C mutation, which is located at PIK3CA exon 4 nucleotide 1028 A→ G, is novel and has not been described in previous studies. Although the physiological significance of the novel mutation Y343C, which is located within the PIK3CA C2 domain, is not known, it has been shown that the C2 domain in the Class IB PI3K interacts primarily with the helical domain, and also interacts with the linker segment before the Ras-binding domain and with the C-terminal lobe of the catalytic domain (43). The C2 domain is often involved in Ca2+-dependent or Ca2+-independent phospholipids membrane binding. By analogy with enzymes like protein kinase C and cytosolic phospholipase A2, the C2 domain of ClassIB PI3K might participate in Ca2+-independent phospholipids membrane binding (43). Since mutations found in the C2 domain account for 7% of total PIK3CA mutations found in a study of 396 cancer samples (13), it will be worthwhile to determine the exact function of the C2 domain of Class 1A PI3K in future studies. The other three are hot-spot mutations (E545K, E542K, and H1047R) and all were found in pharyngeal cancer patients. The smoking histories of the mutated patients are unknown. We did not find any significant correlation of the PIK3CA gene mutation to the gender or age of the patients.
Here we also report the discovery of a PIK3CA homolog. This homolog is almost identical to the exons 9, 11–13 and partial exon 10 of the PIK3CA gene and can be found on both chromosomes 16 and 22. However, we think that this sequence homolog is likely to be a pseudogene since no RNA transcripts of the predicted protein can be detected by RT-PCR. In our study, interferences from this sequence homolog had caused confusions by creating nucleotide alterations including the so-called PIK3CA A1634C (E545A) “mutation” (Figures 1, 2), which subsequently vanished with better primer designs and more stringent PCR conditions. Intriguingly, this A1634C (E545A) mutation has been previously reported in human cancers by two publications. One study described 11 cases with the A1634C (E545A) mutation out of 73 hepatocellular carcinomas (15). More recently this exact mutation was reported to contribute up to 88% (21/24) of the total PIK3CA mutations identified in ovarian cancer (24). This mutation was not described in other reports on PIK3CA mutation (13, 14, 16–18, 25, 26). In light of our discovery, it is important for future studies to be aware of the possible interference from the homologous sequences on chromosomes 22 and 16. Although we did not study exons 10–13 in our current study, potential artifacts there are also probable.
Our data confirm that PIK3CA is important to HNSCC tumorigenesis and provide evidence that small mutation can also contribute to oncogene activation of PIK3CA in HNSCC. Furthermore, our data suggest that PIK3CA gene mutations may be more involved in the carcinogenesis of a particular subset of human head and neck cancers (pharyngeal cancers) than others. The knowledge of the PIK3CA’s involvement in HNSCC is important because a specific kinase inhibitor could be considered as a future therapeutic option for HNSCC patients with PIK3CA mutations. Most HNSCC are diagnosed at advanced stage, and are usually unresectable despite significant surgical advances. Improvements in chemotherapy and radiotherapy in recent decades have not been translated into better prognosis of HNSCC patients (44). Recently kinase inhibitors such as Gleevec (Imatinib), Herceptin (Trastzumab), and Iressa (Gefitinib) have been successfully developed for therapies in some cancer types (45). Since amplification and overexpression of the PIK3CA gene locus is an early oncogenic event of HNSCC tumorigenesis and is also correlated to invasion (21, 23), abrogation of its oncogenic activities may conceivably slow or stop tumor progression. It is believed that such a selective small-molecule inhibitor against PIK3CA would have tremendous potential as a novel cancer chemotherapeutic for HNSCC (46). Our findings further supports PIK3CA as an important potential target in head and neck cancer for pathway-specific, kinase inhibitor based therapies.
Footnotes
This work was supported by the NCI Temin Award CA95434 and the NCI R01 CA109525.
References
- 1.Klippel A, Escobedo MA, Wachowicz MS, et al. Activation of phosphatidylinositol 3-kinase is sufficient for cell cycle entry and promotes cellular changes characteristic of oncogenic transformation. Mol Cell Biol. 1998;18(10):5699–711. doi: 10.1128/mcb.18.10.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang HW, Aoki M, Fruman D, et al. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276(5320):1848–50. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy SG, Wagner AJ, Conzen SD, et al. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11(6):701–13. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 4.Yuan ZQ, Sun M, Feldman RI, et al. Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene. 2000;19(19):2324–30. doi: 10.1038/sj.onc.1203598. [DOI] [PubMed] [Google Scholar]
- 5.Bellacosa A, de Feo D, Godwin AK, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64(4):280–5. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 6.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96(8):4240–5. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips WA, St Clair F, Munday AD, Thomas RJ, Mitchell CA. Increased levels of phosphatidylinositol 3-kinase activity in colorectal tumors. Cancer. 1998;83(1):41–7. doi: 10.1002/(sici)1097-0142(19980701)83:1<41::aid-cncr6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Benistant C, Chapuis H, Roche S. A specific function for phosphatidylinositol 3-kinase alpha (p85alpha-p110alpha) in cell survival and for phosphatidylinositol 3-kinase beta (p85alpha-p110beta) in de novo DNA synthesis of human colon carcinoma cells. Oncogene. 2000;19(44):5083–90. doi: 10.1038/sj.onc.1203871. [DOI] [PubMed] [Google Scholar]
- 9.Andrew S. PIK3CA: determining its role in cellular proliferation and ovarian cancer. Clin Genet. 1999;56(3):190–1. doi: 10.1034/j.1399-0004.1999.560302.2.x. [DOI] [PubMed] [Google Scholar]
- 10.Ma YY, Wei SJ, Lin YC, et al. PIK3CA as an oncogene in cervical cancer. Oncogene. 2000;19(23):2739–44. doi: 10.1038/sj.onc.1203597. [DOI] [PubMed] [Google Scholar]
- 11.Shayesteh L, Lu Y, Kuo WL, et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21(1):99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 12.Pedrero JM, Carracedo DG, Pinto CM, et al. Frequent genetic and biochemical alterations of the PI 3-K/AKT/PTEN pathway in head and neck squamous cell carcinoma. Int J Cancer. 2005;114(2):242–8. doi: 10.1002/ijc.20711. [DOI] [PubMed] [Google Scholar]
- 13.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 14.Bachman KE, Argani P, Samuels Y, et al. The PIK3CA Gene is Mutated with High Frequency in Human Breast Cancers. Cancer Biol Ther. 2004;3(8):772–5. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 15.Lee JW, Soung YH, Kim SY, et al. PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. Oncogene. 2004 doi: 10.1038/sj.onc.1208304. [DOI] [PubMed] [Google Scholar]
- 16.Campbell IG, Russell SE, Choong DY, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64(21):7678–81. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 17.Broderick DK, Di C, Parrett TJ, et al. Mutations of PIK3CA in anaplastic oligodendrogliomas, high-grade astrocytomas, and medulloblastomas. Cancer Res. 2004;64(15):5048–50. doi: 10.1158/0008-5472.CAN-04-1170. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Helland A, Holm R, Kristensen GB, Borresen-Dale AL. PIK3CA mutations in advanced ovarian carcinomas. Hum Mutat. 2005;25(3):322. doi: 10.1002/humu.9316. [DOI] [PubMed] [Google Scholar]
- 19.Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci U S A. 2005;102(3):802–7. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saal LH, Holm K, Maurer M, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65(7):2554–9. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 21.Estilo CL, P OC, Ngai I, et al. The role of novel oncogenes squamous cell carcinoma-related oncogene and phosphatidylinositol 3-kinase p110alpha in squamous cell carcinoma of the oral tongue. Clin Cancer Res. 2003;9(6):2300–6. [PubMed] [Google Scholar]
- 22.Or YY, Hui AB, Tam KY, Huang DP, Lo KW. Characterization of chromosome 3q and 12q amplicons in nasopharyngeal carcinoma cell lines. Int J Oncol. 2005;26(1):49–56. [PubMed] [Google Scholar]
- 23.Woenckhaus J, Steger K, Werner E, et al. Genomic gain of PIK3CA and increased expression of p110alpha are associated with progression of dysplasia into invasive squamous cell carcinoma. J Pathol. 2002;198(3):335–42. doi: 10.1002/path.1207. [DOI] [PubMed] [Google Scholar]
- 24.Levine DA, Bogomolniy F, Yee CJ, et al. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res. 2005;11(8):2875–8. doi: 10.1158/1078-0432.CCR-04-2142. [DOI] [PubMed] [Google Scholar]
- 25.Li VS, Wong CW, Chan TL, et al. Mutations of PIK3CA in gastric adenocarcinoma. BMC Cancer. 2005;5(1):29. doi: 10.1186/1471-2407-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuels Y, Velculescu VE. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle. 2004;3(10):1221–4. doi: 10.4161/cc.3.10.1164. [DOI] [PubMed] [Google Scholar]
- 27.Blanchaert RH., Jr Oral and oral pharyngeal cancer: an update on incidence and epidemiology, identification, advances in treatment, and outcomes. Compend Contin Educ Dent. 2002;23(12 Suppl):25–9. [PubMed] [Google Scholar]
- 28.Callender T, el-Naggar AK, Lee MS, Frankenthaler R, Luna MA, Batsakis JG. PRAD-1 (CCND1)/cyclin D1 oncogene amplification in primary head and neck squamous cell carcinoma. Cancer. 1994;74(1):152–8. doi: 10.1002/1097-0142(19940701)74:1<152::aid-cncr2820740124>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 29.Jares P, Fernandez PL, Campo E, et al. PRAD-1/cyclin D1 gene amplification correlates with messenger RNA overexpression and tumor progression in human laryngeal carcinomas. Cancer Res. 1994;54(17):4813–7. [PubMed] [Google Scholar]
- 30.Kearsley JH, Leonard JH, Walsh MD, Wright GR. A comparison of epidermal growth factor receptor (EGFR) and c-erbB-2 oncogene expression in head and neck squamous cell carcinomas. Pathology. 1991;23(3):189–94. doi: 10.3109/00313029109063564. [DOI] [PubMed] [Google Scholar]
- 31.Ishitoya J, Toriyama M, Oguchi N, et al. Gene amplification and overexpression of EGF receptor in squamous cell carcinomas of the head and neck. Br J Cancer. 1989;59(4):559–62. doi: 10.1038/bjc.1989.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonard JH, Kearsley JH, Chenevix-Trench G, Hayward NK. Analysis of gene amplification in head-and-neck squamous-cell carcinoma. Int J Cancer. 1991;48(4):511–5. doi: 10.1002/ijc.2910480406. [DOI] [PubMed] [Google Scholar]
- 33.Anderson JA, Irish JC, Ngan BY. Prevalence of RAS oncogene mutation in head and neck carcinomas. J Otolaryngol. 1992;21(5):321–6. [PubMed] [Google Scholar]
- 34.Yarbrough WG, Shores C, Witsell DL, Weissler MC, Fidler ME, Gilmer TM. ras mutations and expression in head and neck squamous cell carcinomas. Laryngoscope. 1994;104(11 Pt 1):1337–47. doi: 10.1288/00005537-199411000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Weber A, Langhanki L, Sommerer F, Markwarth A, Wittekind C, Tannapfel A. Mutations of the BRAF gene in squamous cell carcinoma of the head and neck. Oncogene. 2003;22(30):4757–9. doi: 10.1038/sj.onc.1206705. [DOI] [PubMed] [Google Scholar]
- 36.Almoguerra C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–54. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 37.Rozenblum E, Schutte M, Goggins M, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731–4. [PubMed] [Google Scholar]
- 38.Bos JL, Fearon ER, Hamilton SR, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327(6120):293–7. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- 39.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 40.Hashimoto Y, Oga A, Kawauchi S, et al. Amplification of 3q26 approximately qter correlates with tumor progression in head and neck squamous cell carcinomas. Cancer Genet Cytogenet. 2001;129(1):52–6. doi: 10.1016/s0165-4608(01)00425-3. [DOI] [PubMed] [Google Scholar]
- 41.Liehr T, Ries J, Wolff E, et al. Gain of DNA copy number on chromosomes 3q26-qter and 5p14-pter is a frequent finding in head and neck squamous cell carcinomas. Int J Mol Med. 1998;2(2):173–179. doi: 10.3892/ijmm.2.2.173. [DOI] [PubMed] [Google Scholar]
- 42.Singh B, Stoffel A, Gogineni S, et al. Amplification of the 3q26.3 locus is associated with progression to invasive cancer and is a negative prognostic factor in head and neck squamous cell carcinomas. Am J Pathol. 2002;161(2):365–71. doi: 10.1016/S0002-9440(10)64191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker EH, Perisic O, Ried C, Stephens L, Williams RL. Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nature. 1999;402(6759):313–20. doi: 10.1038/46319. [DOI] [PubMed] [Google Scholar]
- 44.Seiwert TY, Cohen EE. State-of-the-art management of locally advanced head and neck cancer. Br J Cancer. 2005;92(8):1341–8. doi: 10.1038/sj.bjc.6602510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Couzin J. Cancer drugs. Smart weapons prove tough to design. Science. 2002;298(5593):522–5. doi: 10.1126/science.298.5593.522. [DOI] [PubMed] [Google Scholar]
- 46.Rogers SJ, Box C, Harrington KJ, Nutting C, Rhys-Evans P, Eccles SA. The phosphoinositide 3-kinase signalling pathway as a therapeutic target in squamous cell carcinoma of the head and neck. Expert Opin Ther Targets. 2005;9(4):769–90. doi: 10.1517/14728222.9.4.769. [DOI] [PubMed] [Google Scholar]


