Abstract
Aminoglycoside antibiotics induce sensorineural hearing loss by destroying hair cells of the organ of Corti, causing progressive secondary degeneration of primary auditory or spiral ganglion neurons (SGNs). Recent studies show that the p75 neurotrophin receptor (NTR) is aberrantly up-regulated under pathological conditions when the neurotrophin receptor tyrosine kinases (Trks) are presumptively down-regulated. We provide in vivo evidence demonstrating that degenerating SGNs induced an augmented p75NTR expression and a coincident reduction of TrkB expression in their peripheral processes. Nuclear transcription factors c-Jun and cyclic AMP response element-binding protein phosphorylated by p75NTR- and TrkB-activated signal pathways, respectively, also showed a corresponding differential modulation, suggesting an activation of apoptotic pathways, coupled to a loss of pro-survival neurotrophic support. Our findings identified brain-derived neurotrophic factor (BDNF) expression in hair and supporting cells of the adult cochlea, and its loss, specifically the mature form, would impair TrkB-induced signaling. The precursor of BDNF (pro-BDNF) is differentially cleaved in aminoglycoside-deafened cochleae, resulting in a predominant up-regulation of a truncated form of pro-BDNF, which colocalized with p75NTR-expressing SGN fibers. Together, these data suggest that an antagonistic interplay of p75NTR and TrkB receptor signaling, possibly modulated by selective BDNF processing, mediates SGN death in vivo.
Hearing loss is one of the most common disabilities in modern society and is exacerbated by aging and our extensive exposure to loud noise or ototoxic drugs such as aminoglycoside antibiotics.1 In most cases, the hearing impairment results from damage to the cochlear sensory hair cells of the inner ear. Because mammalian hair cells are incapable of regeneration, their loss inevitably and irreversibly causes hearing loss.2 Sensory hair and supporting cells in the organ of Corti provide neurotrophic support to primary auditory (spiral ganglion) neurons,3–5 and their loss can trigger a secondary degeneration of these neurons.6–8
Spiral ganglion neurons (SGNs) express the neurotrophin receptors tropomyosin-related kinase (Trk) receptor tyrosine kinase and the p75 neurotrophin receptor (NTR), a member of the tumor necrosis factor receptor superfamily.9,10 The Trk receptors bind different members of the neurotrophin family, with TrkA showing a preference for nerve growth factor, TrkB selectively binding brain-derived neurotrophic factor (BDNF) and neurotrophin 4/5, and TrkC preferentially interacting with neurotrophin 3 (NT-3).11 The p75NTR, however, can bind all neurotrophins and also interacts with Trk receptors to modulate ligand binding specificity, affinity, and functionality within certain cell types.12,13 TrkA is not known to be present in SGNs as assessed by both immunohistochemical and in situ hybridization analyses,10,14 whereas TrkB, TrkC, and p75NTR mRNAs have been detected in SGNs.9,10 Studies of mutant mice with deletions in TrkB and TrkC reveal significant loss of SGNs and innervation defects in the cochlea during development,15 whereas adult mutant mice with severely reduced TrkB signaling have been associated with a significant hearing loss.16
At least two signaling pathways, notably the phosphoinositide 3-kinase and the mitogen-activated protein kinase cascades, mediate Trk-activated survival response in neurons.17,18 In contrast, the role of p75NTR in the cochlea remains elusive, but it has been suggested to play a role in the formation of the inner sulcus during cochlear development, presumptively through apoptotic events and the differentiation of Pillar cells to form the tunnel of Corti.9,19 Recently, p75NTR has been shown to be aberrantly up-regulated under pathological and inflammatory conditions,20–22 when Trk receptors may have been presumptively down-regulated, suggesting that an imbalance of neurotrophin receptor signaling may be involved in diseases of the nervous system.23 Furthermore, certain precursors of neurotrophins (pro-neurotrophins) have been shown to mediate cell apoptosis by binding to p75NTR.24–26
Because p75NTR and Trk receptors are frequently coexpressed in the same neuron, we sought to establish to what extent each individual receptor is associated with neuronal death in degenerating SGNs using an in vivo model, relevant to deafness-induced pathological changes in the cochlea. We used aminoglycoside antibiotics to destroy sensory hair and supporting cells in the organ of Corti of rats and analyzed the expression of these neurotrophin receptors in SGNs after a deafness period ranging from 6 weeks to 4 months. The data show an augmentation of p75NTR expression and a reduced TrkB expression in degenerating SGNs, concomitant with a temporal decline of SGN density in the Rosenthal’s canal where these molecular changes occur. Coincidentally, the proportion of degenerating neurons expressing phosphorylated c-Jun, a target of p75NTR-mediated pathway,27,28 is increased, whereas there is a converse decline in the proportion of neurons expressing phosphorylated cyclic AMP response element binding protein (CREB), a target of TrkB-mediated pathway.29 Our studies also identify an elevation of a truncated form of pro-BDNF and a reduction of mature BDNF in amino-glycoside-deafened cochleae, reflecting a differential processing of BDNF under pathological conditions. These findings not only provide insights into the antagonistic interplay of p75NTR and TrkB receptor signaling as a key event in SGN degeneration, but they also have general implications in the design of pharmacological agents to target specific growth factor signaling pathway to ameliorate deafness.
Materials and Methods
Evaluation of Hearing Function
Healthy adult rats weighing approximately 200 g were used in this study under approval by the Royal Victorian Eye and Ear Hospital’s Animal Research and Ethics Committee and conformed to the guidelines of the National Health and Medical Research Council of Australia. Normal hearing was determined by the presence of Preyer’s reflex in response to a clap startle and confirmed with click-evoked auditory brainstem response (ABR) measurement. ABRs of deafened rats were evaluated at least 2 to 3 weeks after aminoglycoside administration. Before the ABR evaluation, rats were anesthetized with intraperitoneal injections of ketamine (75 mg/kg body weight; Parnell Laboratories, Alexandria, NSW, Australia) and xylazil (7.5 mg/kg body weight; Troy Laboratories, Smithfield, NSW, Australia). Procedures for ABR measurements have been previously described.6 Normal hearing rats register a threshold reading of less than 43 decibels peak equivalent sound pressure level whereas deafened rats display a permanent threshold shift of >50 decibels.
Deafening with Aminoglycoside Antibiotics
A total of 15 rats were deafened and analyzed in this study, whereas 8 age-matched normal hearing rats were used as controls. Before deafening, rats were anesthetized as described above. Gentamicin sulfate (420 mg/kg body weight; Sigma, St. Louis, MO) and frusemide (200 mg/kg body weight; Troy Laboratories) were prepared separately in 2 ml of saline solution and delivered subcutaneously in the skin folds on the lateral abdominal side and the dorsal neck area, respectively. The animals’ temperature was maintained at 37°C by using a heating pad. After deafening, the animals were kept in the colony for a period between 6 weeks to 4 months before sacrifice.
Immunohistochemistry
A total of five normal hearing and seven deafened rats were sacrificed either with an overdose of carbon dioxide or anesthetized with ketamine/xylazil (described above) followed by intracardial perfusion with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). These two different methods of sacrifice were used to harvest either fresh brains or fixed brains for future analysis, but in each experiment, normal hearing and deafened animals were prepared equally using one of the two methods. For BDNF immunohistochemistry in the cochlea, we also sacrificed one postnatal day-7 rat pup and two normal hearing 6-week-old mice (C57BL6) with an overdose of carbon dioxide. For euthanasia involving carbon dioxide, decapitation was rapidly performed after the animal showed the last sign of breathing. Sagittal halves of the head were placed in ice before the cochleae were dissected. These steps ensured the preservation of tissue integrity.
Cochleae were rapidly removed and fixed for 2 hours with either 2 or 4% PFA/PBS. Cochleae from control and deafened animals in the same experiment were fixed using an identical PFA concentration to allow equal comparison within a single experiment. All of the antibodies tested worked equally well regardless of the PFA concentration. Therefore, fixation with either 2 or 4% PFA/PBS did not affect the antigenic sites for the antibodies tested. After fixation, they were decalcified in 10% ethylene diamine tetra-acetic acid (BDH Laboratory, Darmstadt, Germany) formulated in PBS. Next, they were incubated overnight in 25% sucrose in PBS before embedding in OCT compound (Sakura, Tokyo, Japan). Cochleae were cryo-sectioned in 12 μm thickness and mounted on SuperFrost Plus microscopic slides. Cerebellar tissue used to illustrate phosphorylated CREB (pCREB) immunostaining was fixed for 2 hours in 2% PFA/PBS, incubated overnight in 25% sucrose in PBS, and sectioned at 12 μm. Cochlear and cerebellar sections were stored at −20°C before use.
Cochlear sections were thawed, permeabilized for 10 minutes at room temperature with 0.1% Triton-X, blocked with 10% normal goat serum (Vector Laboratories, Burlingame, CA) in 0.1% Triton-X for 2 hours, and incubated overnight at 4°C with primary antibodies diluted in blocking solution. On the following day, sections were washed 3× with PBS for 10 minutes, before incubating for 2 hours at room temperature with the appropriate secondary antibodies. At the end of the incubation, sections were rinsed three times in PBS for 20 minutes, before mounting in Vectorshield (Vector Laboratories) containing the nuclear stain 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) to help identify specific cell populations. All sections were viewed with a Zeiss Axioplan 2 microscope.
Affinity-purified, polyclonal (rabbit) antibodies against the Trk neurotrophin receptors, brain-derived neurotrophic factor, and phosphorylated c-Jun were obtained from Santa Cruz: pan-TrkB (sc-8316; 1:100) targeting the extracellular domain of TrkB identifying both full-length and truncated isoforms, fl-TrkB (sc-12; 1:100) targeting the catalytic C terminus identifying the full-length isoform, t-TrkB [TK-] (sc-119; 1:100) targeting the truncated T1 isoform, BDNF (sc-546; 1:100) targeting the N terminus of mature BDNF, and p c-Jun (sc-16312; 1:200) raised against phosphorylated Ser 63 and 73 of human c-Jun. Affinity-purified polyclonal (rabbit) antibodies against phosphorylated CREB and the pro-domain of BDNF were obtained from Upstate (06-519; 1:150) and Alomone (ANT-006; 1:400), respectively. Affinity-purified polyclonal (goat) antibody against the growth-associated protein GAP43 was obtained from Santa Cruz (sc-7457; 1:100). To control for the specificity of the antibody, blocking peptide recommended by the manufacturer was used when available, whereas sc-547P neurotrophin-3 blocking peptide was chosen as an irrelevant peptide for BDNF antibody specificity tests. Negative immunoreactivity was also performed using affinity-purified control immunoglobulin IgG from naïve rabbits from Santa Cruz (sc-2027, 1:100). Two different antibodies against the p75NTR were used. The polyclonal antibody (Ab 9651; 1:200) was a generous gift from Moses Chao (Skirball Institute for Biomolecular Medicine, New York City) and Simon Murray (Howard Florey Institute, Melbourne, Australia), whereas the purified, monoclonal antibody (AN-170; 1:100) was obtained from Alomone Labs. Purified monoclonal antibody against the 200-kd neurofilament (MAB5266; 1:500) and synaptophysin (MAB368; 1:500) were obtained from Chemicon. The following fluorescein-conjugated secondary antibodies from Molecular Probes were used at a 1:500 dilution: highly cross-adsorbed Alexa Fluor 488 goat anti-mouse IgG (A-11029), highly cross-adsorbed Alexa Fluor 594 goat anti-rabbit IgG (A-11037), and Alexa Fluor 594 chicken anti-goat (A-21468).
Western Blotting
A total of four normal hearing and eight deafened rats were euthanized with an overdose of carbon dioxide. Cochleae and hippocampal tissues were rapidly dissected and snap frozen in liquid nitrogen. Cochleae from each animal were homogenized for 15 seconds with a pestle (Kontes, NJ) before extracting the cytoplasmic proteins with lysis buffer (78833) from Pierce Technologies. Cytoplasmic proteins were enriched using PAGEprep (26800; Pierce Technologies), and concentrations were determined with a Bradford reagent (B6916; Sigma). The same procedure was performed for hippocampal tissues but with the additional extraction of nuclear proteins for pCREB antibody specificity test. A 12% Bis-Tris gel (3450117; BioRad) was used to separate the proteins using a reducing XT-MOPS buffer system (1610793; BioRad) and a running condition of 200 V, ∼1 hour. At the end of the run, separated proteins were transferred to polyvinylidene difluoride membranes (1620238; BioRad) using a running condition of 30 V, 1 hour 20 minutes and a 1× Tris/glycine buffer (1610771; BioRad) with 20% methanol. Membranes were subsequently blocked for 90 minutes in 5% milk powder (1706404; BioRad) before incubating overnight at 4°C with primary antibodies. Antibodies against the Trk neurotrophin receptors GAP43, pCREB, and BDNF were as described as above and used in a 1:250 dilution. For the p75NTR Western blot, we used a rabbit polyclonal antibody (Ab 9992), generously provided by Moses Chao and Simon Murray at a 1:4000 dilution. As a loading control for cytoplasmic proteins, we used a rabbit polyclonal antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from Abcam (ab9485) at a 1:20,000 dilution. On the following day, blots were washed three times for 20 minutes in a 0.1% Tween 20/PBS before incubating for 1 hour with horseradish peroxidase-conjugated secondary antibodies: goat anti-rabbit IgG at a 1:10,000 dilution (1706515; BioRad) or donkey anti-goat IgG at 1:7500 dilution (sc2056; Santa Cruz). The blots were next washed four times for 30 minutes in 0.1% Tween 20/PBS, and substrate development was performed using ECL Plus Western blotting detection reagents (RPN2132; Amersham Biosciences).
We used the public domain NIH image program developed at the National Institutes of Health and the Alpha Imager (Alpha Innotech, San Leandro, CA) for our densitometry measurements. Statistical analyses between normal hearing and deafened groups were determined using the two-tailed Student’s t-test (GraphPad Prism, San Diego, CA).
Assessment of Phosphorylation of CREB and c-Jun in Cochlear Sections
Phosphorylation of the transcription factors CREB and c-Jun was determined by immunohistochemical detection, as described above. Cochlear sections from three normal hearing rats and four deafened rats (deafness duration, 6 weeks to 3 months) were used for analysis using the Alpha Imager. Immunofluorescence in the nuclei of SGNs was chosen for densitometric measurements. Nuclear localization was verified by DAPI nuclear staining. For pCREB analysis, we measured the average integrated density value (defined as the pixel value of the object divided by its area) of SGNs from normal hearing rats and determined the median pixel density value to be 88. Next, we measured the average integrated density value of SGNs from deafened rats, and then we determined the relative proportion of those neurons in both cohorts exceeding the median pixel density value of 88. For p c-Jun analysis, we measured the average integrated density value of SGNs from deafened animals and determined the median pixel density value to be 146. Then, we obtained measurements of the average integrated density value of SGNs from normal hearing animals and calculated the proportion of neurons in both cohorts exceeding the median pixel density value of 146. Statistical analyses between normal hearing and deafened groups were determined using the two-tailed Student’s t-test, as described previously. Graphs indicate means ± SEM. Statistical significance (P < 0.05) in deafened samples, relative to the control cohort, is indicated in asterisks.
Assessment of SGN Density in Cochlear Sections
Dual immunohistochemistry was performed using a monoclonal antibody against the 200-kd neurofilament to identify SGNs30 and one of the rabbit polyclonal antibodies against pan-TrkB, p75NTR, pCREB, and p c-Jun, as described above. SGN counts were obtained from the Rosenthal’s canal where the changes of these genes have been analyzed. The cross-sectional area of the Rosenthal’s canal was determined using a morphometric program (AxioVision LE, Hallbergmoos, Germany). Statistical analyses between normal hearing and deafened groups were evaluated using one-way analysis of variance Student-Newman-Keuls Method with pairwise comparison between control cohort and each of the deafened cohorts (SigmaStat). SGN density was normalized to the control cohort. Graph indicates means ± SEM. Statistical significance (P < 0.05) is indicated in asterisks.
Results
Aminoglycoside-Induced Deafness Causes Differential Regulation of TrkB and p75NTR Proteins
Because aminoglycoside antibiotics destroy hair and supporting cells of the organ of Corti, causing secondary degeneration of the peripheral processes of SGNs, we asked whether neurotrophin receptor expression in these fibers would be altered following deafness. In particular, we focused on p75NTR and TrkB because previous studies have mapped the mRNAs of these receptors in adult rodent SGNs by in situ hybridization.10,16 In our analysis, we used a commercially available TrkB antibody (pan-TrkB), raised against the extracellular domain, to detect both full-length (containing the catalytic domain) and truncated forms of the receptor. For p75NTR, we used an antibody that recognizes a neurotrophin-binding motif in its extracellular domain (Ab9650), and the expression of this receptor could not be detected in a p75NTR-deleted mouse.31,32
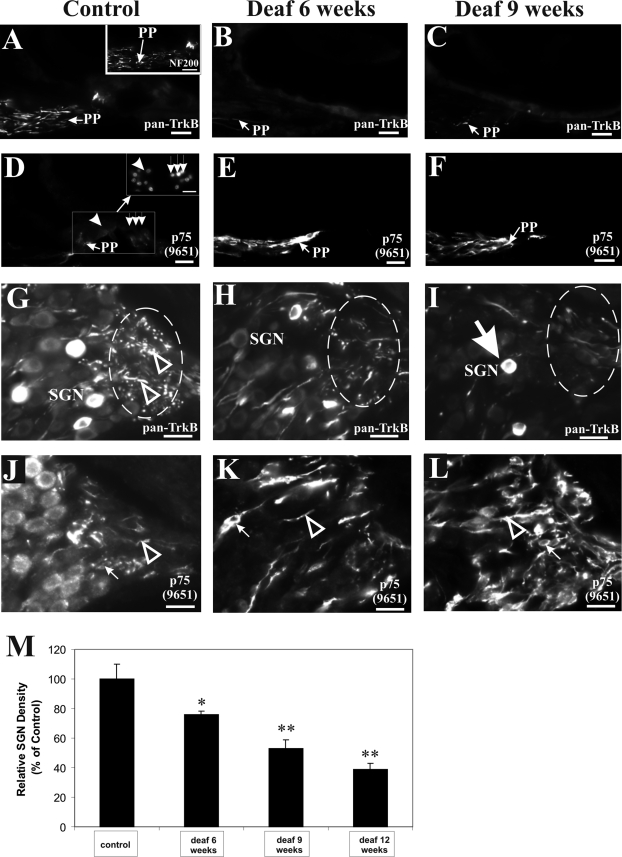
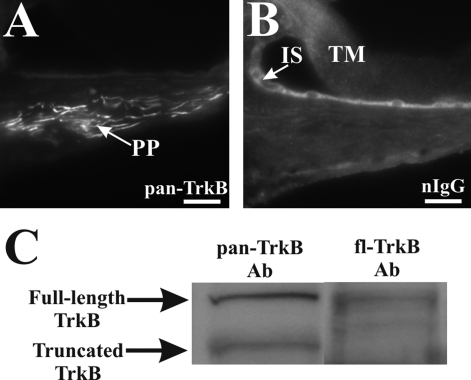
In the normal hearing cochlea, dense pan-TrkB immunolabeling could be observed in the peripheral processes within the osseous spiral lamina, projecting from the SGNs to the organ of Corti (Figures 1A and 2A, PP). We identified peripheral processes by using an antibody against the 200-kd neurofilament protein, which has been used as an afferent marker,5,33–35 and this is illustrated in Figure 1A, inset. No comparable staining pattern in the peripheral processes was obtained using naïve immunoglobulins supplied by the manufacturer (Figure 2B), but unspecific reaction could be observed in the tectorial membrane and cells lining the inner sulcus (Figure 2B). TrkB expression in the peripheral processes is unlikely to be attributed to truncated T1 TrkB isoform because we consistently failed to detect its expression in the peripheral processes but only in soma of SGNs (data not shown). To highlight the specificity of the pan-TrkB antibody used in this experiment, we performed a Western blot analysis of cochlear proteins in which we identified two fragments of ∼145 and ∼95 kd, corresponding, respectively, to the full-length and truncated TrkB receptors (Figure 2C) in agreement with a previous study.36 In contrast, the fl-TrkB antibody targeting the catalytic domain of TrkB identified the ∼145-kd fragment (Figure 2C) but not the ∼95-kd fragment, which lacks the catalytic domain and was recognized only by the t-TrkB antibody (data not shown). The expression of p75NTR, however, is weakly detected in peripheral processes of normal hearing animals (Figure 1D, PP) and in the nonmyelinated nerve endings beneath inner and outer hair cells (highlighted, respectively, as arrowhead and a row of three arrows in Figure 1D, inset).
Figure 1-6921.
SGN degeneration involves a reduction in TrkB expression, coincident with an aberrant elevation of p75NTR expression in the peripheral processes. Normal hearing animals express TrkB in the peripheral processes projecting from the SGNs to the organ of Corti, as shown in the osseous spiral lamina (A, left panel, PP). The identity of peripheral processes could be established by NF200 immunolabeling (A, inset). After aminoglycoside-induced deafening, TrkB immunoreactivity in the osseous spiral lamina was reduced as shown here after 6 weeks (B, middle panel, PP) and 9 weeks (C, right panel, PP) of deafness. D: In normal hearing cochleae, p75NTR is weakly expressed in the peripheral processes (PP) projecting to the inner (arrowhead) and outer hair cells (row of three downward arrows), identified with DAPI staining in the inset. After aminoglycoside-induced deafening, p75NTR immunoreactivity in the osseous spiral lamina was strongly up-regulated as illustrated here after 6 weeks (E, RF) and 9 weeks (F, RF) of deafness in adjacent sections to B and C, respectively. Most of the peripheral processes of SGNs are clustered in a region of the Rosenthal’s canal before projecting to the osseous spiral lamina (G, circle). In normal hearing animals, dense TrkB expression could be observed in this region as punctated or filamentous-like fluorescence (G, arrowhead). After 6 weeks of deafness, TrkB staining in this region was comparatively reduced (H, circle), and a further down-regulation of TrkB in this region was observed after 9 weeks of deafness (I, circle). Some surviving SGNs showed strong TrkB immunoreactivity (I, arrow), comparable with those seen in normal hearing animals. Using a polyclonal p75NTR antibody (Ab9651), a strong up-regulation of p75NTR could be observed in both fibers and putative Schwann cells after 6 weeks (K, arrowhead pointing to fiber and arrow to Schwann cell) and 9 weeks (L, arrowhead pointing to fiber and arrow to Schwann cell) of deafness, relative to similar structures in normal hearing cochleae (J). Cochlear sections shown here were taken from the middle turn. Scale bar = 20 μm. Original magnification, ×40. M: Quantification of SGN density in the middle turn at varying time points after aminoglycoside administration. Graphs indicate means ± SEM. A 24% reduction in SGN density relative to normal hearing cochleae (n = 4) was observed after 6 weeks of deafness (n = 3), progressing to 47 and 61% reduction after 9 weeks (n = 3) and 12 weeks (n = 2) of deafness, respectively. Statistical significance determined by Student-Newman-Keuls one-way analysis of variance between each deafness cohort and the control group is indicated by asterisks. There was a significant reduction in SGN density after 6 (*P < 0.05), 9 (**P < 0.01), and 12 (**P < 0.01) weeks of deafness.
Figure 2-6921.
Characterization of the pan-TrkB antibody. Peripheral processes from a normal hearing rat cochlea showed a prominent expression of TrkB using the pan-TrkB (sc-8316) antibody (A), and no expression could be detected in these fibers located within the osseous spiral lamina area when a naïve immunoglobulin was used (B). In contrast, nonspecific binding of this antibody was observed in both the tectorial membrane (B, TM) and cells lining the inner sulcus (B, IS). Immunoblotting of cochlear proteins with the pan-TrkB antibody revealed the presence of both the full-length and truncated isoforms at ∼145 and ∼95 kd, respectively (C, left lane, pan-TrkB Ab), whereas immunoblotting with fl-TrkB antibody showed only the presence of the full-length isoform (C, right lane, fl-TrkB Ab). Scale bars= 20 μm. Original magnification, ×40.
In at least three independent experiments, aminoglycoside-induced deafness caused a gradual loss of TrkB-positive fibers in the osseous spiral lamina, shown here 6 and 9 weeks after aminoglycoside administration (Figure 1, B and C, respectively). Interestingly, in the adjacent section of the same cochlea, we observed a dramatic increase in the intensity of p75NTR immunoreactivity in the peripheral processes of deafened cochleae at both 6 and 9 weeks after aminoglycoside administration (Figure 1, E and F, respectively). By performing a colocalization experiment with the 43-kd growth-associated protein (GAP43), a marker of neuronal processes, we confirmed that the elevated p75NTR expression (Figure 3A, PP) is attributed primarily to GAP43-positive processes in the osseous spiral lamina (Figure 3B, PP). The specificity of the antibody was illustrated using Western blot analysis of hippocampal proteins, which identified a ∼43-kd fragment that is abolished on pre-incubation with excess antigen peptide (Figure 3C). Together, the data show that the decrease of TrkB expression in the peripheral processes of deafened cochleae cannot be explained exclusively by a reduction of peripheral processes, because within surviving processes, we observed a coincident and dramatic increase of p75NTR expression.
Figure 3-6921.
Up-regulated p75NTR expression is attributed to GAP43-positive neural processes. In dual immunofluorescence experiments, p75NTR expression in the peripheral processes (A, PP) overlapped with the expression of a marker for neural processes, GAP43 (B, PP), as shown here after 9 weeks of deafness, indicating that the elevation of p75NTR in the osseous spiral lamina is predominantly restricted to peripheral processes. The GAP43 antibody identified a ∼43-kd fragment (C, left lane) in immunoblots of hippocampal proteins, which was abolished in neutralization experiment with the antigenic peptide (C, right lane). C: Coomassie-stained gel demonstrated analysis of comparable amounts of proteins. Scale bars= 20 μm. Original magnification, ×40.
In at least three independent experiments, we also examined the differential changes of TrkB and p75NTR in a region of the Rosenthal’s canal where the peripheral processes of SGNs are clustered before projecting as peripheral processes in the osseous spiral lamina to the organ of Corti. TrkB expression in this region of the Rosenthal’s canal (Figure 1G, circle) could be observed as a dense pattern of strong, punctated, and filamentous-like fluorescence (Figure 1G, arrowheads). After 6 and 9 weeks of deafness, this fluorescent pattern became more diffuse and weaker (Figure 1, H and I, respectively; circle), confirming our earlier analysis of reduced TrkB expression in peripheral processes of deafened cochleae (Figure 1, B and C). In contrast, p75NTR expression in the Rosenthal’s canal was strongly elevated after 6 and 9 weeks of deafness (Figure 1, K and L, respectively), relative to expression levels found in normal hearing cochlea (Figure 1J). Notably, elevated p75NTR expression was observed in neural processes (Figure 1, K and L, arrowheads) and putative Schwann cells (Figure 1, K and L, arrows). To illustrate the specificity of these changes, we also performed dual immunohistochemistry using the rabbit polyclonal pan-TrkB antibody and a monoclonal p75NTR antibody to observe specific changes within the same section, and similar results were obtained (data not shown).
Because previous reports on mouse mutants have shown that perturbations in BDNF-TrkB and NT-3-TrkC signaling resulted in reduced SGN survival,15,37,38 we sought to determine whether the differential changes of TrkB and p75NTR expression observed in deafened cochleae correlated with the SGN density in the Rosenthal’s canal where these receptors have been analyzed. With increasing duration of aminoglycoside-induced deafness (Figure 1M), we observed a progressive decline in SGN density (relative to normal hearing controls) by 24% after 6 weeks of deafness (P < 0.05), 47% after 9 weeks of deafness (P < 0.01), and 61% after 3 months of deafness (P < 0.01). Our data agree with previous findings reporting a temporal loss of SGN after aminoglycoside administration in rats and guinea pigs.7,39,40
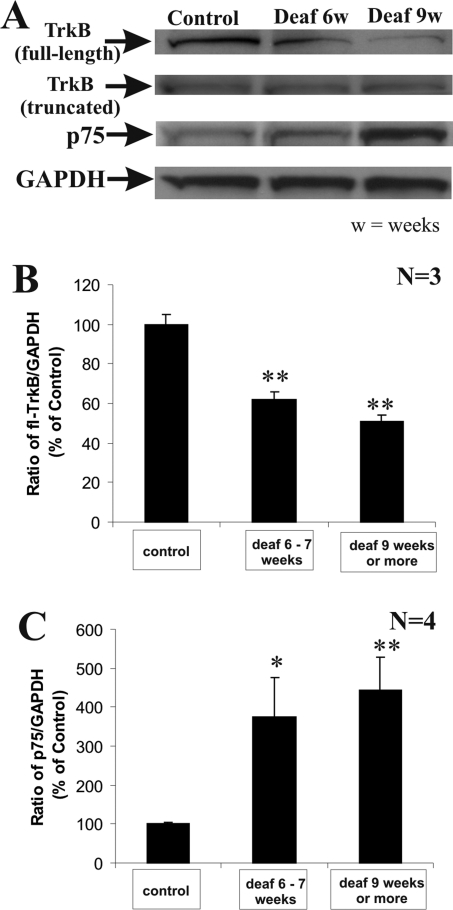
To quantify the contrasting changes of TrkB and p75NTR expression, we used another independent technique, semiquantitative Western blot analysis, to compare the levels of TrkB and p75NTR proteins in cochlear homogenates of control and deafened animals. At least three independent experiments comprising animals subjected to either 6 to 7 weeks or more than 9 weeks of deafness were performed. The commercial pan-TrkB polyclonal antibody was used to identify both the full-length and truncated TrkB isoforms, as described in Figure 2C. To identify the p75NTR neurotrophin receptor, we used the 9992 antibody, which has been tested in previous studies,30,41 to demonstrate specific recognition of the intracellular domain of p75NTR. We detected a fragment at ∼75 kd in our cochlear protein samples, and to verify the specificity of the antibodies in these Western blot experiments, negative control experiments were performed using normal affinity-purified type G immunoglobulins from naïve rabbit (data not shown).
As shown in a representative blot in Figure 4A, full-length TrkB immunoreactive bands (∼145 kd) decreased in intensity with progressive duration of deafness, in contrast to the up-regulation in intensity of the p75NTR immunoreactive bands, confirming the differential mod-ulation of these neurotrophin receptors in our immunohistochemical analysis on cochlear sections. In addition, Western blot experiments demonstrated that the decrease was in the full-length TrkB fragment but not in the truncated fragment, suggesting that truncated TrkB in the cochlea is unlikely to play a role in aminoglycoside-induced deafness. Thus it is more probable that the full-length isoform but not the truncated T2 isoform contributes to the reduced TrkB immunoreactivity in the peripheral processes after aminoglycoside-induced deafening (Figure 1, compare B and C with A). To ensure that equivalent amounts of proteins were analyzed in control and deafened samples, we used an antibody against a housekeeping gene, GAPDH, which detected an immunoreactive band at ∼37 kd and revealed approximately equal loading. By normalizing the intensity of the full-length TrkB fragment to the GAPDH fragment in three independent experiments, we observed a significant reduction of TrkB, relative to normal hearing animals, by 38% (P < 0.01) in animals experiencing aminoglycoside-induced deafening after 6 weeks and 49% (P < 0.01) in animals after more than 9 weeks of aminoglycoside-induced deafening (Figure 4B). On the other hand, in four independent experiments, p75NTR immunoreactive bands increased significantly in intensity, relative to normal hearing animals, from 370% (P < 0.05) in animals experiencing deafness after 6 weeks to 440% (P < 0.01) in animals subjected to a prolonged duration of deafness (9 weeks or longer), as shown in Figure 4C. In total, both immunohistochemical and Western blot analyses strongly suggest that aminoglycoside-induced degeneration of SGNs is closely correlated with a reduction of full-length TrkB expression, coincident with a dramatic, aberrant increase of p75NTR expression.
Figure 4-6921.
Western blot quantification of reduced TrkB but elevated p75NTR proteins in cochlear homogenates from deafened animals. Cochlear proteins from normal hearing and deafened animals experiencing two distinct durations of deafness (6 to 7 weeks versus 9 weeks or longer) were immunoblotted with pan-TrkB, Ab 9992 (p75NTR), and GAPDH antibodies. The intensity of the full-length TrkB immunoreactive band was progressively reduced after 6 and 9 weeks of deafness (A, TrkB, full-length), whereas the intensity of the truncated fragment did not vary considerably (A, TrkB, truncated). On the contrary, in the same blot, there was an up-regulation of p75NTR-immunoreactive band (A, p75), whereas GAPDH levels remained relatively constant between samples (A, GAPDH). The intensity of the full-length (fl)-TrkB band was normalized to the GAPDH band in three independent experiments, and the mean ratio for each group was then expressed as a relative percentage of the control group of normal hearing animals (B). Statistical significance between each deafness cohort and the control group is indicated by asterisks. There was a significant reduction in this ratio in the two deafness cohorts (B, **P < 0.01). Similarly, the intensity of the p75NTR-band was normalized to the GAPDH band in four independent experiments, and the mean ratio for each group was again expressed as a relative percentage of the control group of normal hearing animals (C). Comparison between the control group and each of the deafened cohorts showed a significant increase in this ratio in animals deafened for 6 to 7 weeks (C, *P < 0.05) and animals experiencing more than 9 weeks of deafness (C, **P < 0.01). Numerical data indicate means ± SEM.
Aminoglycoside-Induced Deafness Causes Differential Phosphorylation of CREB and c-Jun Nuclear Proteins
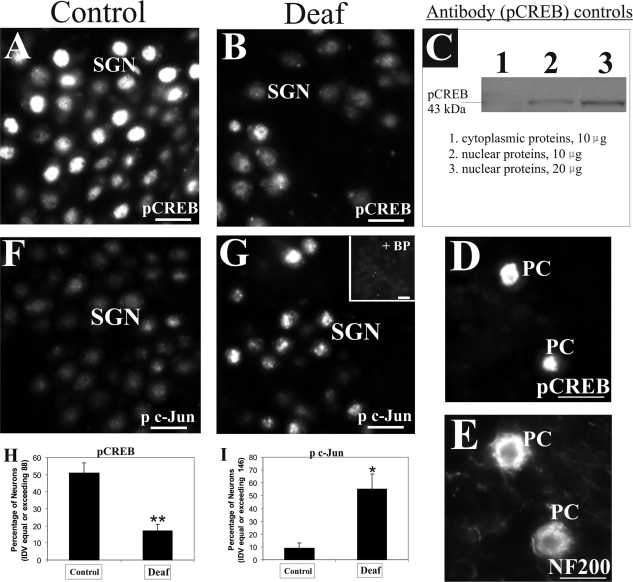
Activation of full-length TrkB receptors can trigger a downstream signaling cascade that eventually leads to the phosphorylation of CREB, a transcription factor critical for neuronal survival and function.42–44 We therefore considered the possibility that the reduction of TrkB expression in the peripheral processes of deafened cochleae may impinge on the phosphorylation of CREB in their SGNs. Immunostaining of cochlear sections from normal hearing animals with an antibody specific for pCREB demonstrated nuclear fluorescence in SGNs that varied in intensity, presumably reflecting the activity-dependent phosphorylation of CREB (Figure 5A). In cochleae from animals deafened from 6 weeks onward, we observed a loss of pCREB immunostaining in many of the remaining SGNs (Figure 5B). This antibody recognized a single band of 43 kd in nuclear proteins from hippocampus (Figure 5C, lanes 2 and 3) but not the cytoplasmic proteins (Figure 5C, lane 1). We also performed a positive control experiment showing nuclear localization of pCREB (Figure 5D) in Purkinje neurons as previously described45 but not in the cytoplasmic regions delineated by NF200 immunolabeling (Figure 5E). Whereas the overall decline in pCREB expression in SGNs of deafened animals could be easily explained by a loss in the number of neurons, we observed a reduction in the proportion of SGNs expressing strong pCREB immunoreactivity in deafened animals compared with normal hearing controls. Therefore, we used a densitometric approach to assess the proportion of neurons in the Rosenthal’s canal expressing an average integrated density value of 88 or above. This value represents the median of pCREB intensity in SGNs from representative sections of normal hearing animals. As shown in Figure 5H, 51% of SGNs in normal hearing cochlea meet this criterion, whereas in four deafened animals, we observed a significant decline to 17% (P < 0.01).
Figure 5-6921.
Aminoglycoside-induced sensorineural hearing loss induces differential phosphorylation of nuclear transcription factor in surviving SGNs. A: A large proportion of SGNs in normal hearing animals (left panel) expressed strong nuclear localization of pCREB protein in immunofluorescence studies. B: In deafened animals (middle panel), fewer neurons expressed such strong staining pattern of pCREB protein in their nuclei (as shown after 9 weeks of deafness). C: Characterization of pCREB antibody (right panel) revealed a single band of 43 kd in Western blots of nuclear proteins from hippocampal tissues (lane 2) but not in an equivalent amount of cytoplasmic proteins (lane 1). Furthermore, doubling the amount of hippocampal nuclear proteins led to a stronger band (compare lanes 2 and 3). To underline further the specificity of the antibody, immunolabeling of pCREB was observed in the nuclei of Purkinje neurons (D, PC) costained with NF200 to demarcate cytoplasmic regions (E, PC). F: In normal hearing animals, SGNs express low basal levels of p c-Jun protein in immunofluorescence analysis. G: However, deafened animals displayed elevation of p c-Jun fluorescence in their nuclei (shown here after 9 weeks of deafness), and most of this staining pattern was abolished by neutralization with the relevant control antigen (inset). Densitometric assessment of pCREB and p c-Jun fluorescence in the SGNs of four animals experiencing a deafness duration from 6 weeks to 3 months revealed a significant decrease in the proportion of SGNs demonstrating strong pCREB fluorescence (H, **P < 0.01), whereas in contrast, an increasing proportion of SGNs from these deafened animals demonstrated strong p c-Jun fluorescence (I, *P < 0.05). Numerical data indicate means ± SEM. Cochlear sections shown here were taken from the middle turn. Scale bars = 20 μm. Original magnification, ×40.
Activation of c-Jun kinase and the subsequent phosphorylation of the c-Jun transcription factor have been reported in stressed cells, and p75NTR activation has been considered as one of the upstream events triggering this signal cascade.27,46–48 In normal hearing cochlea, SGNs expressed low levels of phosphorylated c-Jun in their nuclei (Figure 5F, p c-Jun), but this expression was elevated in deafened cochleae (Figure 5G). The specificity of the antibody was evaluated in a neighboring section from the deafened cochlea by performing pre-incubation with the blocking peptide. As shown in Figure 5G, inset, most of the immunofluorescence was effectively blocked, except for weak spotted background fluorescence. To assess p c-Jun intensity in both normal hearing and deafened cohorts, we first measured the average integrated density value of p c-Jun immunostaining in SGNs from representative sections of four deafened animals and determined the median value to be 146. As shown in Figure 5I, only 9% of SGNs from normal hearing animals displayed an average integrated density value exceeding 146, whereas deafening induced a significant increase in this proportion to 55% (Figure 5I, P < 0.05). These findings suggest that degenerating SGNs not only show a differential modulation of TrkB and p75NTR, at the receptor level, but a comparable trend is also observed in the phosphorylation of transcription factors controlled by these neurotrophin receptors.
Adult Organ of Corti Expresses Brain-Derived Neurotrophic Factor
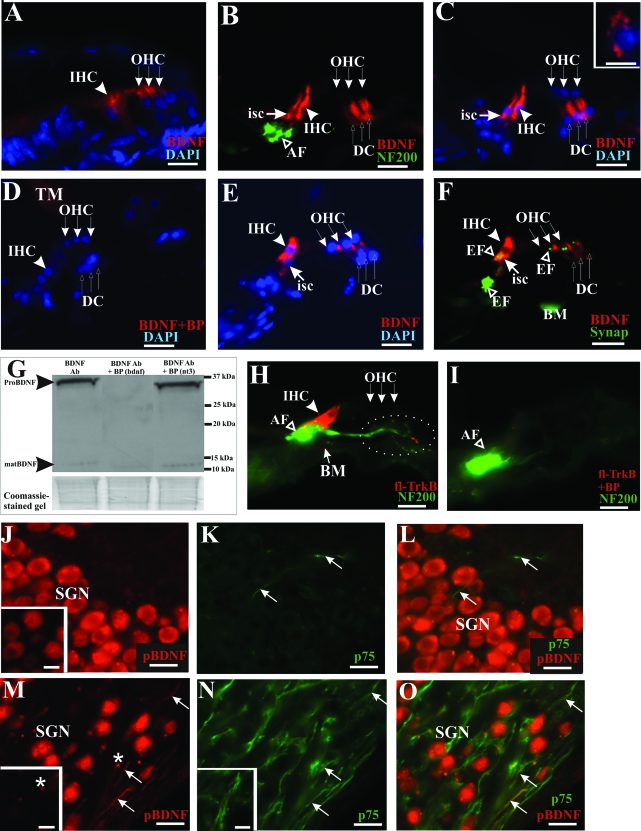
Aminoglycoside antibiotics destroy hair cells and supporting cells in the organ of Corti. Because we have observed strong immunostaining of TrkB in peripheral processes projecting to the organ of Corti, we asked if their cognate, high-affinity ligand, the brain-derived neurotrophic factor (BDNF), is expressed in the organ of Corti. In the postnatal cochlea, 6 days after birth, BDNF protein has been detected in both inner and outer hair cells.5 Using the same antibody, we confirmed this pattern of expression (Figure 6A, IHC and OHC), and at the same time, we detected the expression of BDNF protein in the inner hair cells, inner supporting cells, and Deiters’ cells of the adult rat organ of Corti (Figure 6, B and C), which was abolished on pre-incubation of the antibody with an excess of the control peptide (Figure 6D). As a positive control, we also detected punctated BDNF expression in the cytoplasm of cerebellar granule neuron (Figure 6C, inset, red fluorescence) as previously shown.49 To further seek evidence that BDNF protein is expressed in the organ of Corti, we also analyzed mouse cochlear sections and similarly observed BDNF protein expression in the inner hair and supporting cells and in Deiters’ cells (Figure 6, E and F). Notably, BDNF expression is found opposite type I afferent nerve endings, identified by NF200 immunolabeling (Figure 6B, AF) and efferent nerve endings around inner and outer hair cells, identified by synaptophysin immunostaining (Figure 6F, EF). The specificity of the BDNF antibody was tested, as shown in Figure 6G, by performing a Western blot of adult cochlear proteins (Figure 6G, left lane) which identified an ∼34-kd band (pro-neurotrophin) and a 13-kd band (mature neurotrophin), as previously reported in other studies.24,50 Both bands were also detected in postnatal cochlear proteins and hippocampal proteins (data not shown). A further control of the specificity of this antibody was demonstrated in other studies demonstrating the absence of BDNF immunoreactive bands in BDNF knockout mice.49,51 These bands were completely abolished by pre-incubation with the corresponding blocking peptide (Figure 6G, middle lane, BDNF Ab + BP (bdnf)), whereas a pre-incubation experiment with an irrelevant peptide derived from neurotrophin-3 could not abolish these two bands (Figure 6G, right lane, BDNF Ab + BP (nt3)). Interestingly, we observed the expression of full-length TrkB within and below the inner hair cells (Figure 6H) and in the nerve endings around the outer hair cells (Figure 6H, dotted circle). This pattern was completely abolished by pre-incubation with the blocking peptide (Figure 6I) in a neighboring section, without affecting NF200 immunoreactivity. Thus, our observation is consistent with the view that the organ of Corti acts as target-derived sources of neurotrophic factors for innervating SGNs possessing the corresponding neurotrophin receptors in their peripheral processes.
Figure 6-6921.
BDNF protein is expressed in both hair and supporting cells of the adult organ of Corti as well as spiral ganglion neurons. Both inner and outer hair cells from the postnatal rat cochlea expressed BDNF, as shown in A (arrowhead pointing to inner hair cell and row of three arrows to outer hair cells). In contrast, in the adult rat cochlea, BDNF expression was localized to inner supporting cells (B and C, isc), inner hair cells (B and C, bold arrowhead, IHC), and Deiters’ cells (B and C, row of three upward-pointing arrows, DC). Afferent innervation was identified using NF200 antibody (B, open arrowhead, AF), and specific cells of the organ of Corti were recognized using DAPI nuclear staining (C). D: The BDNF staining pattern was completely abolished on pre-incubation of the antibody with the recommended antigenic peptide. As a positive control, we also observed BDNF immunoreactivity in cerebellar granule neuron (C, inset). Expression pattern of BDNF in the adult organ of Corti was also verified in mice where immunolabeling was detected in inner hair cells (E and F, bold arrowhead, IHC), inner supporting cell (E and F, arrow, isc), Deiters’ cells (E and F, row of three upward-pointing arrows, DC). Consistently, no BDNF immunoreactivity could be found in the outer hair cells of adult rat (B and C, row of three downward-pointing arrows) or mouse (E and F, row of three downward-pointing arrows). BDNF immunoreactivity was also localized opposite synaptophysin-labeled efferent nerve endings (F, open arrowhead, EF), whereas labeling at the basilar membrane is nonspecific (F, BM). G: The specificity of the BDNF antibody was further investigated in Western blot analysis of cochlear proteins where both a ∼34-kd fragment corresponding to the pro-neurotrophin form and a ∼13-kd fragment representing the mature neurotrophin (left lane) were detected. These bands could not be detected when the antibody was pre-incubated with its antigenic peptide (middle lane). Pre-incubating the antibody with an irrelevant antigenic peptide derived from neurotrophin-3 did not result in a loss of these bands (right lane). A Coomassie-stained gel below indicated analysis of comparable amounts of proteins in this experiment. H: Full-length TrkB immunoreactivity was detected primarily at the baso-lateral region of inner hair cells (bold arrowhead, IHC) and partially colocalized with NF200 immunostaining at the afferent nerve endings (open arrowhead, AF). I: TrkB immunolabeling was abolished in neutralization experiment with the relevant antigenic peptide, without affecting NF200 immunostaining. J: SGNs from a normal hearing rat cochlea expressed BDNF protein in their soma, as shown using an antibody recognizing the uncleaved pro-neurotrophin form of BDNF (pBDNF) and another antibody targeting the mature BDNF isoform (inset). The p75NTR immunoreactivity could be weakly detected in projection fibers (K, arrows) in a normal hearing rat cochlea, and no colocalization could be observed between p75NTR and pBDNF in these fibers (L, arrows). However, in an aminoglycoside-deafened cochlea, pBDNF immunoreactivity appeared strongly in the neural fibers of the Rosenthal’s canal of deafened animals (M, arrows), shown here after 3 months of deafness. These pBDNF-positive fibers also expressed p75NTR (N, arrows) and showed colocalization with a subset of p75NTR-positive fibers (O, arrows). Pre-incubating the pBDNF antibody with its antigenic peptide abolished the filamentous fluorescence signal in these fibers (M, inset), whereas p75NTR immunoreactivity was not affected (N, inset). M: Nonspecific staining, presumably representing cellular debris, could be observed as fluorescent spots (asterisk) that could not be blocked (inset), suggesting that these fluorescent spots did not reflect pBDNF immunoreactivity. Cochlear sections shown here were taken from the middle turn. Scale bars: 20 μm; except inset in C (10 μm). Original magnification: ×40; except inset in C (×100).
Aminoglycoside-Induced Deafness Causes Differential Processing of Cochlear BDNF
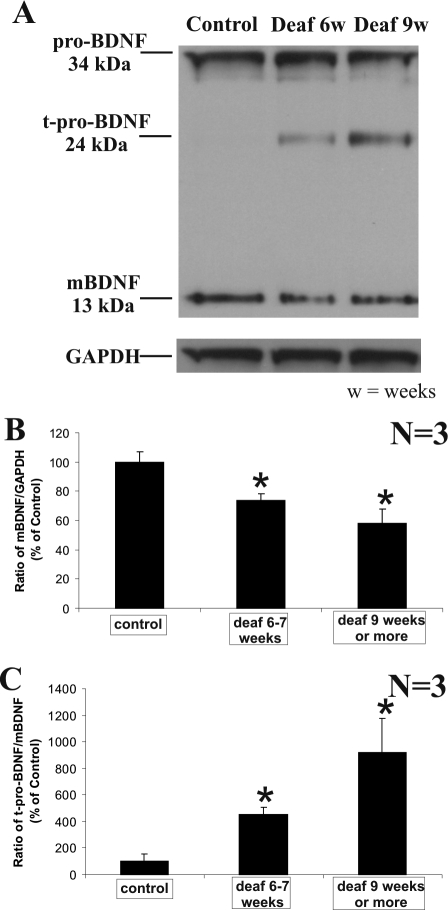
As a further indication of a loss of neurotrophic support by aminoglycoside-induced elimination of hair and supporting cells, we performed Western blot analysis of cochlear proteins isolated from normal hearing and deafened animals with two different durations of deafness. As shown in Figure 7A, after a progressive period of deafness, the mature form of BDNF (∼13 kd) showed a significant decrease in intensity, whereas no obvious fluctuation could be seen in the level of the pro-neurotrophin form (∼34 kd). In contrast, we observed a marked and statistically significant elevation of a truncated pro-neurotrophin form (∼24 kd; Figure 7A) after aminoglycoside-induced deafening, further indicating that the reduction of the mature BDNF form (∼13 kd) is specific. The reduction of the mature form of BDNF could not be attributed to unequal loading because an antibody against GAPDH revealed approximately equal amounts of proteins analyzed (Figure 7A). By normalizing the densitometric value of mature BDNF immunoreactive band to the GAPDH band, we demonstrated a significant decline of mature BDNF with deafness duration at 6 to 7 weeks (P < 0.05) and after 9 weeks (P < 0.05) in three separate experiments (Figure 7B). Furthermore, dividing the densitometric value of the truncated pro-BDNF immunoreactive band (∼24 kd) by the densitometric value of the mature BDNF immunoreactive band (∼13 kd) revealed a significant increase in the proportion of truncated pro-BDNF, relative to the mature BDNF isoform, for deafness durations of 6 to 7 weeks (P < 0.05) and >9 weeks (P < 0.05) in three separate experiments (Figure 7C).
Figure 7-6921.
BDNF protein is differentially processed in aminoglycoside-deafened cochleae. A: Western blot analysis of the cochleae from normal hearing animals and animals experiencing two distinct durations of deafness (shown here for 6 and 9 weeks) demonstrated a reduction in the mature form of BDNF (mBDNF) and the appearance of a truncated form of pro-BDNF (t-pro-BDNF) after 6 and 9 weeks of deafness. The intensity of the truncated pro-BDNF fragment increased from 6 to 9 weeks of deafness. In contrast, the expression of the unprocessed form of BDNF (proBDNF) showed relatively little fluctuation, in comparison with both the mature BDNF and truncated pro-BDNF species. As loading controls, the level of the housekeeping protein, glyceldehyde-3-phosphate dehydrogenase, remained constant in these samples (A, GAPDH). By quantifying the intensity of these bands, we normalized the intensity of the mature BDNF band to the GAPDH in three independent experiments and the mean ratio for each group was then expressed as a relative percentage of the control group of normal hearing animals (B). We observed a significant reduction in the ratio of mature BDNF to GAPDH in a cohort experiencing 6 to 7 weeks deafness (B, *P < 0.05), as well as another cohort with a deafness duration exceeding 9 weeks (B, *P < 0.05). C: To demonstrate the relative levels of truncated pro-BDNF versus mature BDNF in each group, we divided the intensity of truncated pro-BDNF band by that of mature BDNF band and expressed the mean ratio for each group as a relative percentage of the control group of normal hearing animals. Comparison between the control group and each of the deafened cohorts showed a significant increase in this ratio in animals deafened for 6 to 7 weeks (*P < 0.05) and animals experiencing more than 9 weeks of deafness (*P < 0.05). Numerical data indicate means ± SEM.
Because a recent study has implicated pro-BDNF as an apoptotic ligand for neurons by activating p75NTR,24 we sought to identify whether the p75NTR-immunopositive fibers observed in deafened cochleae (Figure 1) also expressed pro-BDNF. To this end, we used an antibody targeting the pro-domain of BDNF and performed neutralization experiments with the antigenic peptide to illustrate specificity. SGNs from normal hearing rats displayed pro-BDNF immunoreactivity in their soma (Figure 6J, SGN), and BDNF expression could be confirmed with another antibody targeting the mature domain of BDNF (sc-546; Figure 6J, inset), suggesting that both mature and pro-BDNF isoforms could be found in these neurons. However, in aminoglycoside-deafened cochleae, we found pro-BDNF immunoreactivity in fibers within Rosenthal’s canal (Figure 6M, arrows), which also expressed p75NTR (Figure 6N, arrows), and colocalization could be established between pro-BDNF and p75NTR immunoreactivities in a subset of these p75NTR-positive fibers (Figure 6O, arrows). In SGNs from normal hearing animals, p75NTR-expressing fibers (Figure 6K, arrows) did not show immunoreactivity for pro-BDNF (Figure 6L, arrows). Furthermore, pre-incubating the pro-BDNF antibody with its antigenic peptide abolished pro-BDNF immunoreactivity in the fibers (Figure 6M, inset) but not p75NTR immunoreactivity (Figure 6N, inset). Neutralization of pro-BDNF antibody with a peptide derived from the N terminus of mature BDNF (used to raise the antibody sc-546) did not eradicate pro-BDNF immunoreactivity in the fibers, as represented in Figure 6M.
Discussion
In this study, we present data that aminoglycoside-induced degeneration of cochlear hair cells, resulting in secondary loss of SGNs, is correlated with an augmentation of p75NTR expression in the peripheral processes of these degenerating neurons, coincident with a reduction of TrkB expression in these processes. These data agree with published observations of a pro-apoptotic role of enhanced p75NTR expression in injured nervous systems52 and a loss of pro-survival signaling cascades mediated by reduced TrkB expression.29 Consistent with these findings, there is a significant increase in the proportion of SGNs in Rosenthal’s canal showing a decline in the phosphorylation of CREB, a neuronal survival protein,43 following deafening. This pattern is contrasted by an increased proportion of SGNs showing strong phosphorylation of c-Jun, indicating the operation of a predominantly pro-apoptotic mechanism when TrkB receptors are down-regulated.53 Our investigation has identified the expression of BDNF in inner hair cells and supporting cells of the organ of Corti as a neurotrophic support in normal hearing rodents that is lost when these cells are destroyed by aminoglycoside antibiotics, thus explaining the gradual decline or retraction of TrkB-expressing fibers in the osseous spiral lamina of aminoglycoside-deafened animals. Our results also demonstrated a differential processing of BDNF in the cochleae of deafened animals with a predominant up-regulation of a truncated form of pro-BDNF against a decline in the mature form of BDNF, suggesting distinct physiological functions played by these BDNF isoforms.
Differential Modulation of Neurotrophin Receptors in the Injured Cochlea
The induction of p75NTR in the nervous system under pathological conditions has been documented.54–57 In recent years, the physiological relevance of these changes has identified a role of p75NTR in apoptosis during neuronal trauma.22,58–60 Sequence analysis of the p75NTR molecule identifies a death domain that bears homology to those found in the intracellular domain of the Fas and p55 tumor necrosis factor receptors,61,62 but the mechanism used by p75NTR to mediate cell death is different from apoptotic signaling by Fas and tumor necrosis factor receptors.63,64 Complete deletion of p75NTR in mouse mutants reveals significantly more cholinergic neurons in these mutants,65 reinforcing the role of p75NTR as an apoptotic inducer. In agreement with a death-inducing role of p75NTR under patho-physiological conditions, the present report shows that degenerating peripheral processes of SGNs dramatically increase their p75NTR expression (Figure 1), which could account for the significant and progressive death of SGNs after aminoglycoside-induced sensorineural hearing loss.
It has been proposed that the biological role of p75NTR is dependent on cellular Trk activation status, with its pro-apoptotic role enhanced when Trk-mediated pathways are reduced and vice versa.18 This hypothesis has been supported by in vitro studies in which TrkA activation rescued p75NTR-dependent apoptosis in oligodendrocyte cell cultures27 and by mouse mutant analysis in which TrkA supported neuronal survival by suppressing p75NTR-regulated death signal during developmental neuronal death.66 By examining simultaneously the relative expression of p75NTR and TrkB in this study, we demonstrated that the secondary degeneration of SGNs is further exacerbated by the reduction of TrkB expression in their peripheral processes (Figure 1). Thus, our findings favor a model in which an opposing relationship between p75NTR and TrkB activation determines neuronal death under pathological conditions (Figure 8). To our knowledge, this study provides the first in vivo evidence of an antagonistic interplay between p75NTR and TrkB receptors in a neurodegeneration model.
Figure 8-6921.
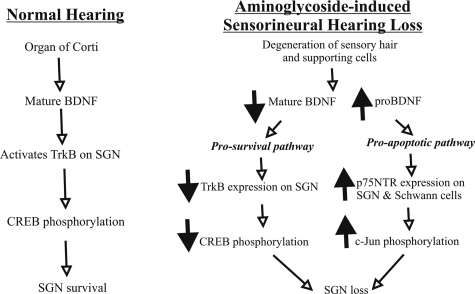
Schematic summary of the different patterns of gene regulation in the cochlea after aminoglycoside-induced sensorineural hearing loss. In a normal hearing cochlea (left panel), the hair and supporting cells of the organ of Corti produce target-derived mature BDNF to activate TrkB receptors on SGN triggering phosphorylation of CREB and consequently SGN survival. After aminoglycoside administration (right panel), sensory cells of the organ of Corti progressively degenerate, reducing the availability of mature BDNF and inducing a decline of TrkB expression and CREB phosphorylation in SGN. In contrast, p75NTR expression and c-Jun phosphorylation are up-regulated in SGN, suggesting an activation of apoptotic pathways when pro-survival neurotrophic support is diminished. An increase in the expression of a truncated pro-BDNF isoform in aminoglycoside-deafened cochleae also indicates defects in BDNF processing, which may contribute to SGN loss. Changes in gene expression (downward, down-regulation; upward, up-regulation) are indicated by the bold arrows.
Differential Phosphorylation of Nuclear Transcription Factors in the Injured Cochlea
One of the signaling pathways linked to neuronal death is the stress-activated protein kinase/c-Jun N-terminal kinase (JNK) signaling pathway, which has been implicated in injured nerves67 and in cells exposed to environmental stress.68 In unraveling the apoptotic mechanism mediated by p75NTR, an increase in JNK activity has been associated with p75NTR-dependent apoptosis.27,28 One of the substrates phosphorylated by activated JNK is c-Jun, a transcription factor.69 In the rodent cochlea, hair cells injured by aminoglycosides showed enhanced JNK activation and c-Jun phosphorylation.70,71 Therefore, we used increased c-Jun phosphorylation as an indicator of JNK activity/p75NTR-mediated signaling in degenerating SGNs. After a distinct period of deafness, we observed an increase in the proportion of the remaining SGNs demonstrating strong phosphorylated c-Jun immunoreactivity (Figure 5). This is in agreement with findings obtained from injured hair cells and the quantitative increase of phosphorylated c-Jun immunostaining in axotomized dopaminergic neurons.48
Whereas activation of the JNK pathway could account for the apoptotic loss of SGNs, our results demonstrated that there is a significant decrease in the proportion of neurons displaying intense CREB phosphorylation (Figure 5). A high proportion of SGNs from normal hearing animals display strong immunoreactivity to phosphorylated CREB, but this proportion is significantly reduced in deafened animals (Figure 5). Phosphorylated CREB protein is required for neuronal function and synaptic activity.43,44 Neurotrophins binding to Trk receptors can activate intracellular signaling cascades such as the mitogen-activated protein kinase pathway leading to the phosphorylation of CREB.29 Therefore, the diminishing levels of phosphorylated CREB in degenerating SGNs could be attributed to a reduction of Trk-activated intracellular signaling pathways, which is reflected here as a down-regulation of TrkB receptors in the peripheral processes of these neurons (Figure 1). Thus, our study supports a hypothesis in which an augmentation of death-inducing pathway mediated by p75NTR, together with an impairment of TrkB-activated downstream survival pathways, is involved in the degeneration of SGNs.
Inner Hair Cells and Supporting Cells of the Adult Organ of Corti Provide Target-Derived Neurotrophic Support to Innervating Spiral Ganglion Neurons
Apart from the down-regulation of TrkB receptors in the peripheral processes, immunoblot analysis demonstrated a reduced expression of the mature form of BDNF in cochlear samples from deafened animals, further implying a decline of TrkB activation in deafened cochleae. During cochlear development, BDNF and neurotrophin-3 (NT-3) function as target-derived neurotrophic factors to regulate the survival of SGNs and guide their innervation to the neuro-sensory epithelium in the organ of Corti.4,72 Deletion of these neurotrophic genes in mutant mice consequently resulted in a loss of cochlear neurons and in a retraction or retardation of cochlear innervation.5,16,37,38 The observed expression of BDNF in inner hair cells and supporting cells (including Deiters’ cells) of the organ of Corti (Figure 6), together with the expression of its cognate neurotrophin receptor, TrkB, in afferent nerve endings beneath hair cells (Figure 6), suggests that BDNF continues to play a role as a target-derived neurotrophic factor to maintain the innervation pattern in adult cochleae. As a target-derived growth factor, BDNF secreted from hair and supporting cells can bind to TrkB expressed in peripheral nerve fibers projecting from SGNs. Consequently, the retraction of TrkB-immunopositive projection fibers from the sensory epithelium (Figure 1) also argues for a loss of neurotrophic support from the organ of Corti, as shown by a significant decrease in the expression of the mature form of BDNF when these hair and supporting cells are destroyed by aminoglycosides (Figure 7). Similar fiber retraction has been reported coincident with a decline or absence of BDNF in target hair cells during cochlear development5 and in taste buds of the gustatory system.73 The lessened availability of target-derived BDNF would further abrogate TrkB-activated survival pathways in SGNs,18 resulting in decreased CREB phosphorylation (as shown in Figure 5) and neuronal death. Our observation that the adult cochlea expresses BDNF in inner hair and supporting cells is further strengthened by studies showing that exogenously applied BDNF can rescue SGNs from aminoglycoside-induced degeneration, and its withdrawal leads to accelerated degeneration.6,74,75
We cannot exclude that other neurotrophic factors may also contribute to the neuronal degeneration seen in this study. NT-3 is known to be expressed in adult inner hair cells,10,76 and recently it has been shown that inner supporting cells also express NT-3.3 In a mouse model of cochlear sensory neuron degeneration, a significant reduction of NT-3 expression, presumably in inner hair and supporting cells, has been identified as a likely factor contributing to the neuronal degeneration.3
The mature form of BDNF (∼13 kd) is considered to be the biologically active isoform mediating most of the pro-survival and synaptic functions of neurons through TrkB-dependent signaling.77 On the other hand, the immature form of BDNF, pro-BDNF, has recently been shown to be a pro-apoptotic ligand for a receptor complex consisting of p75NTR and the type 1 transmembrane sortilin and is cleaved by the extracellular proteinase plasmin to yield 24- and 13-kd products.24 The uncleaved form of nerve growth factor has similarly been shown to induce apoptosis through p75NTR in other in vivo studies.45,78,79 Our Western blot analysis of BDNF in deafened cochleae (Figure 7) showed a decline in the expression of the mature form of BDNF (∼13 kd) but an increase in the expression of a truncated pro-BDNF isoform (∼24 kd), suggesting that an imbalance of mature and pro-neurotrophin form of BDNF may determine neuronal survival or death. It has been estimated that pro-BDNF is at least 10 times more effective than mature BDNF in inducing apoptosis.24 Interestingly, our immunohistochemical analysis revealed colocalization of pro-BDNF and p75NTR in neuronal fibers within the Rosenthal’s canal (Figure 6) and osseous spiral lamina (data not shown) of deafened cochleae, indicating that pro-BDNF may act as an apoptotic ligand to induce secondary SGN degeneration. In another study, a truncated form of pro-BDNF (∼28 kd) has been reported, but its biological function could not be ascertained.80 Our data raise the possibility that the truncated pro-BDNF isoform (∼24 kd) may play a distinct physiological function in degenerating SGNs, but further investigation is required to determine whether the predominance of the truncated pro-BDNF isoform over the mature BDNF isoform is responsible for the secondary degeneration of SGNs in aminoglycoside-deafened cochleae.
In summary, using an in vivo model, we have mapped a molecular fingerprint of spiral ganglion degeneration, secondary to loss of hair and supporting cells (Figure 8). Our findings highlight an antagonistic relationship between p75NTR and TrkB receptor levels and their associated downstream signaling processes. Our data also indicate that the adult hair and supporting cells continue to provide neurotrophic support to SGNs beyond their essential role during cochlear development. Because surviving SGNs are essential for the success of rehabilitative interventions such as cochlear implants to ameliorate deafness, understanding these neurodegenerative events at the molecular level may identify the p75NTR and TrkB molecules as promising drug targets to alleviate secondary SGN degeneration in deafness.
Acknowledgments
We gratefully thank Elisa Borg, Maria Clarke, Anne Coco, Lauren Donley, Stephanie Epp, and Dr. Lisa Gillespie for assistance and Dr. Simon Murray for critically reading the manuscript.
Footnotes
Address reprint requests to Dr. Justin Tan, Department of Otolaryngology, 32 Gisborne St., East Melbourne, Victoria 3002, Australia. E-mail: jtan@bionicear.org.
Supported by the National Institute on Deafness and Other Communication Disorders (grant NO1-DC-3-1005), by Medical Research and Technology in Victoria (Australia), by Royal Victorian Eye and Ear Hospital, and by the Marion and E.H. Flack Trust.
References
- Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol. 2000;5:3–22. doi: 10.1159/000013861. [DOI] [PubMed] [Google Scholar]
- Matsui JI, Parker MA, Ryals BM, Cotanche DA. Regeneration and replacement in the vertebrate inner ear. Drug Discov Today. 2005;10:1307–1312. doi: 10.1016/S1359-6446(05)03577-4. [DOI] [PubMed] [Google Scholar]
- Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qun L, Pirvola U, Saarma M, Ylikoski J. Neurotrophic factors in the auditory periphery. Ann NY Acad Sci. 1999;884:292–304. doi: 10.1111/j.1749-6632.1999.tb08649.x. [DOI] [PubMed] [Google Scholar]
- Wiechers B, Gestwa G, Mack A, Carroll P, Zenner HP, Knipper M. A changing pattern of brain-derived neurotrophic factor expression correlates with the rearrangement of fibers during cochlear development of rats and mice. J Neurosci. 1999;19:3033–3042. doi: 10.1523/JNEUROSCI.19-08-03033.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson HC, Mohuiddin A. Response of spiral ganglion neurons to cochlear hair cell destruction in the guinea pig. J Neurocytol. 2000;29:525–537. doi: 10.1023/a:1007201913730. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Kiang NY. Acoustic trauma in cats: cochlear pathology and auditory-nerve activity. Acta Otolaryngol. 1978;358(Suppl):1–63. [PubMed] [Google Scholar]
- Gestwa G, Wiechers B, Zimmermann U, Praetorius M, Rohbock K, Koepschall I, Zenner HP, Knipper M. Differential expression of trkBT1 and trkBT2, truncated trkC and p75NGFR in the cochlea prior to hearing function. J Comp Neurol. 1999;414:33–49. doi: 10.1002/(sici)1096-9861(19991108)414:1<33::aid-cne3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumae U, Saarma M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- Lee FS, Kim AH, Khursigara G, Chao M. The uniqueness of being a neurotrophin receptor. Curr Opin Neurobiol. 2001;11:281–286. doi: 10.1016/s0959-4388(00)00209-9. [DOI] [PubMed] [Google Scholar]
- Bibel M, Hoppe E, Barde Y. Biochemical and functional interactions between the neurotrophin receptors Trk and p75NTR. EMBO J. 1999;18:616–622. doi: 10.1093/emboj/18.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti M, Levi A, Chao MV. Differential expression of nerve growth factor receptors leads to altered binding affinity and neurotrophin responsiveness. Proc Natl Acad Sci USA. 1993;90:7859–7863. doi: 10.1073/pnas.90.16.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Stewart RR, Gao WQ. Neurotrophin-4/5 enhances survival of cultured spiral ganglion neurons and protects them from cisplatin neurotoxicity. J Neurosci. 1995;15:5079–5087. doi: 10.1523/JNEUROSCI.15-07-05079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmang T, Minichiello L, Vazquez E, San Jose I, Giraldez F, Klein R, Represa J. Developing inner ear sensory neurons require TrkB and TrkC receptors for innervation of their peripheral targets. Development. 1995;121:3381–3391. doi: 10.1242/dev.121.10.3381. [DOI] [PubMed] [Google Scholar]
- Schimmang T, Tan J, Mueller M, Zimmermann U, Rohbock K, Koepschall I, Limberger A, Minichiello L, Knipper M. Lack of Bdnf and TrkB signalling in the postnatal cochlea leads to a spatial reshaping of innervation along the tonotopic axis and hearing loss. Development. 2003;130:4741–4750. doi: 10.1242/dev.00676. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Knipper M, Gestwa L, Ten Cate WJ, Lautermann J, Brugger H, Maier H, Zimmermann U, Rohbock K, Kopschall I, Wiechers B, Zenner HP. Distinct thyroid hormone-dependent expression of TrkB and p75NGFR in nonneuronal cells during the critical TH-dependent period of the cochlea. J Neurobiol. 1999;38:338–356. [PubMed] [Google Scholar]
- Lee FS, Kim AH, Khursigara G, Chao MV. The uniqueness of being a neurotrophin receptor. Curr Opin Neurobiol. 2001;11:281–286. doi: 10.1016/s0959-4388(00)00209-9. [DOI] [PubMed] [Google Scholar]
- Dowling P, Ming X, Raval S, Husar W, Casaccia-Bonnefil P, Chao M, Cook S, Blumberg B. Upregulated p75NTR neurotrophin receptor on glial cells in MS plaques. Neurology. 1999;53:1676–1682. doi: 10.1212/wnl.53.8.1676. [DOI] [PubMed] [Google Scholar]
- Roux PP, Colicos MA, Barker PA, Kennedy TE. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J Neurosci. 1999;19:6887–6896. doi: 10.1523/JNEUROSCI.19-16-06887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechant G, Barde YA. The neurotrophin receptor p75NTR: novel functions and implications for diseases of the nervous system. Nat Neurosci. 2002;5:1131–1136. doi: 10.1038/nn1102-1131. [DOI] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen Z, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Yoon SO, Casaccia-Bonnefil P, Carter B, Chao MV. Competitive signalling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakar AL, Howell JL, Paul CE, Salehi AH, Becker EB, Said F, Bonni A, Barker PA. Apoptosis induced by p75NTR overexpression requires Jun kinase-dependent phosphorylation of Bad. J Neurosci. 2003;23:11373–11381. doi: 10.1523/JNEUROSCI.23-36-11373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Adamson CL, Reid MA, Davis RL. Opposite actions of brain-derived neurotrophic factor and neurotrophin-3 on firing features and ion channel composition of murine spiral ganglion neurons. J Neurosci. 2002;22:1385–1396. doi: 10.1523/JNEUROSCI.22-04-01385.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SS, Bartlett PF, Lopes EC, Coulson EJ, Greferath U, Cheema SS. Low-affinity neurotrophin receptor with targeted mutation of exon 3 is capable of mediating the death of axotomized neurons. Clin Exp Pharmacol. 2003;30:217–222. doi: 10.1046/j.1440-1681.2003.03827.x. [DOI] [PubMed] [Google Scholar]
- Huber LJ, Chao MV. Mesenchymal and neuronal cell expression of the p75 neurotrophin receptor gene occur by different mechanisms. Dev Biol. 1995;167:227–238. doi: 10.1006/dbio.1995.1019. [DOI] [PubMed] [Google Scholar]
- Zheng L, Gao W. Differential damage to auditory neurons and hair cells by ototoxins and neuroprotection by specific neurotrophins in rat cochlear organotypic cultures. Eur J Neurosci. 1996;8:1897–1905. doi: 10.1111/j.1460-9568.1996.tb01333.x. [DOI] [PubMed] [Google Scholar]
- Wise AK, Richardson R, Hardman J, Clark G, O’Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487:147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- Coleman B, Hardman J, de Silva M, Epp S, Coco A, Crook J, Shepherd RK: Delivery strategies for cell-based therapy in the mammalian cochlea. Cell Transplant 2006, (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury TJ, Murray PD, Bambrick LL, Krueger BK. Ca2+-dependent regulation of TrkB expression in neurons. J Biol Chem. 2003;278:40744–40748. doi: 10.1074/jbc.M303082200. [DOI] [PubMed] [Google Scholar]
- Ernfors P, van de Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Farinas I, Reichardt LF. Lack of neurotrophin 3 causes loss of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion. J Neurosci. 1997;17:6213–6225. doi: 10.1523/JNEUROSCI.17-16-06213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness SL, Shepherd RK. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otol Neurotol. 2005;26:1064–1072. doi: 10.1097/01.mao.0000185063.20081.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson HC. Loss and survival of spiral ganglion neurons in the guinea pig after intracochlear perfusion with aminoglycosides. J Neurocytol. 1997;26:541–556. doi: 10.1023/a:1015434524040. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Zampieri N, Spencer T, Hilaire M, Mellado W, Chao MV, Filbin MT. MAG induces regulated intramembrane proteolysis of the p75 neurotrophin receptor to inhibit neurite outgrowth. Neuron. 2005;46:849–855. doi: 10.1016/j.neuron.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu Rev Neurosci. 2003;26:299–310. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, Netzeband JG, Quina LA, Blakely-Gonzalez PK. Contribution of L-type channels to Ca2+ regulation of neuronal properties in early developing Purkinje neurons. Cerebellum. 2005;4:128–139. doi: 10.1080/14734220510007969. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhang JS, Jakobsen J. Differential effect of p75 neurotrophin receptor on expression of pro-apoptotic proteins c-Jun, p38 and caspase-3 in dorsal root ganglion cells after axotomy in experimental diabetes. Neuroscience. 2005;132:1083–1092. doi: 10.1016/j.neuroscience.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Harrington AW, Kim JY, Yoon SO. Activation of Rac GTPase by p75 is necessary for c-Jun N-terminal kinase-mediated apoptosis. J Neurosci. 2002;22:156–166. doi: 10.1523/JNEUROSCI.22-01-00156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker SJ, Lamba WR, Smith PD, Callaghan SM, Slack RS, Anisman H, Park DS. c-Jun mediates axotomy-induced dopamine neuron death in vivo. Proc Natl Acad Sci USA. 2001;98:13385–13390. doi: 10.1073/pnas.231177098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz PM, Borghesani PR, Levy RL, Pomeroy SL, Segal RA. Abnormal cerebellar development and foliation in BDNF−/− mice reveals a role for neurotrophins in CNS patterning. Neuron. 1997;19:269–281. doi: 10.1016/s0896-6273(00)80938-1. [DOI] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongiorgi E, Armellin M, Guilianini PG, Bregola G, Zucchini S, Paradiso B, Steward O, Cattaneo A, Simonato M. Brain-derived neurotrophic factor mRNA and protein are targeted to discrete dendritic laminas by events that trigger epileptogenesis. J Neurosci. 2004;24:6842–6852. doi: 10.1523/JNEUROSCI.5471-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Willnow TE, Petersen CM. p75NTR: live or let die. Curr Opin Neurobiol. 2005;15:49–57. doi: 10.1016/j.conb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK1–p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Henschen A, Olson L, Persson H. Expression of nerve growth factor receptor mRNA is developmentally regulated and increased after axotomy in rat spinal cord motoneurons. Neuron. 1989;2:1605–1613. doi: 10.1016/0896-6273(89)90049-4. [DOI] [PubMed] [Google Scholar]
- Gage FH, Batchelor P, Chen KS, Chin D, Higgins GA, Koh S, Deputy S, Rosenberg MB, Fischer W, Bjorklund A. NGF receptor re-expression and NGF-mediated cholinergic neuronal hypertrophy in the damaged adult neostriatum. Neuron. 1989;2:1177–1184. doi: 10.1016/0896-6273(89)90184-0. [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, Crawford TO, Price DL. Axotomy induces nerve growth factor receptor immunoreactivity in spinal motor neurons. Brain Res. 1991;549:297–304. doi: 10.1016/0006-8993(91)90471-7. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Kordower JH. Cortical neurons express nerve growth factor receptors in advanced age and Alzheimer disease. Proc Natl Acad Sci USA. 1992;89:569–573. doi: 10.1073/pnas.89.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JD, Chartisathian K, Chase TN, Butcher LL. Overexpression of neurotrophin receptor p75 contributes to the excitotoxin-induced cholinergic neuronal death in rat basal forebrain. Brain Res. 2000;853:174–185. doi: 10.1016/s0006-8993(99)02054-5. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy CM, Friedman JE, Friedman WJ. Mechanisms of p75-mediated death of hippocampal neurons: role of caspases. J Biol Chem. 2002;277:34295–34302. doi: 10.1074/jbc.M205167200. [DOI] [PubMed] [Google Scholar]
- Chapman BS. A region of the 75 kDa neurotrophin receptor homologous to the death domains of TNFR-1 and Fas. FEBS Lett. 1995;374:216–220. doi: 10.1016/0014-5793(95)01113-s. [DOI] [PubMed] [Google Scholar]
- Liepinsh E, Ilag LL, Otting G, Ibanez CF. NMR structure of the death domain of the p75 neurotrophin receptor. EMBO J. 1997;16:4999–5005. doi: 10.1093/emboj/16.16.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson EJ, Reid K, Barrett GL, Bartlett PF. p75 neurotrophin receptor-mediated neuronal death is promoted by Bcl-2 and prevented by Bcl-xL. J Biol Chem. 1999;274:16387–16391. doi: 10.1074/jbc.274.23.16387. [DOI] [PubMed] [Google Scholar]
- Coulson EJ, Reid K, Baca M, Shipham KA, Hulett SM, Kilpatrick TJ, Bartlett PF. Chopper, a new death domain of the p75 neurotrophin receptor that mediates rapid neuronal cell death. J Biol Chem. 2000;275:30537–30545. doi: 10.1074/jbc.M005214200. [DOI] [PubMed] [Google Scholar]
- Naumann T, Casademunt E, Hollerbach E, Hofmann J, Dechant G, Frotscher M, Barde YA. Complete deletion of the neurotrophin receptor p75NTR leads to long-lasting increases in the number of basal forebrain cholinergic neurons. J Neurosci. 2002;22:2409–2418. doi: 10.1523/JNEUROSCI.22-07-02409.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdan M, Walsh GS, Aloyz R, Miller F. TrkA mediates developmental sympathetic neuron survival in vivo by silencing an ongoing p75NTR-mediated death signal. J Cell Biol. 2001;155:1275–1285. doi: 10.1083/jcb.200110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudano E, Rosenblad C, Bjorklund A. Injury induced c-Jun expression and phosphorylation in the dopaminergic nigral neurons of the rat: correlation with neuronal death and modulation by glial-cell-line-derived neurotrophic factor. Eur J Neurosci. 2001;13:1–14. [PubMed] [Google Scholar]
- Davies RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davies RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Qun LX, Virkkala J, Saarma M, Murakata C, Camoratto AM, Walton KM, Ylikoski J. Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibitor of c-Jun N-terminal kinase activation. J Neurosci. 2000;20:43–50. doi: 10.1523/JNEUROSCI.20-01-00043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Van De Water TR, Bonny C, de Ribaupierre F, Puel JL, Zine A. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003;23:8596–8607. doi: 10.1523/JNEUROSCI.23-24-08596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Blomlöf J, ElShamy WM, Ernfors P, Olson L. Lingual deficits in BDNF and NT3 mutant mice leading to gustatory and somatosensory disturbances, respectively. Development. 1997;124:1333–1342. doi: 10.1242/dev.124.7.1333. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Barlett PF, Marzella PL. BDNF-induced survival of auditory neurons in vivo: cessation of treatment leads to accelerated loss of survival effects. J Neurosci Res. 2003;71:785–790. doi: 10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Bredberg G, Ulfendahl M, Pyykkö I, Olivius NP, Kaksonen R, Lindström B, Altschuler R, Miller JM. Neurotrophic factor intervention restores auditory function in deafened animals. Proc Natl Acad Sci USA. 2002;99:1657–1660. doi: 10.1073/pnas.032677999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreicher E, Knipper M, Arnold A, Zenner HP, Felix D. Neurotrophin 3 potentiates glutamatergic responses of IHC afferents in the cochlea in vivo. Eur J Neurosci. 2000;12:1584–1590. doi: 10.1046/j.1460-9568.2000.00049.x. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza CE, Podlesniy P, Vidal N, Arevalo JC, Lee R, Hempstead B, Ferrer I, Iglesias M, Espinet C. Pro-NGF isolated from the human brain affected by Alzheimer’s disease induces neuronal apoptosis mediated by p75NTR. Am J Pathol. 2005;166:533–543. doi: 10.1016/S0002-9440(10)62275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EM, Stieglitz MG, Liezman C, Overall RW, Nakamura M, Hagen E, Klapp BF, Arck P, Paus R. p75 neurotrophin receptor-mediated signaling promotes human hair follicle regression (catagen) Am J Pathol. 2006;168:221–234. doi: 10.2353/ajpath.2006.050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem. 2001;276:12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]