Abstract
The germinal centre is a dynamic microenvironment where antigen-activated B cells rapidly expand and differentiate, generating plasma cells and memory B cells. These cellular events are accompanied by dramatic changes in the antibody molecules that undergo somatic hypermutation and isotype switching. Follicular dendritic cells (FDCs) are the stromal cells located in the germinal centre. Although the capacity of FDCs to present antigen to B cells through antigen–antibody complexes has been recognized for many years, additional critical functions of FDCs have only recently been recognized. FDCs prevent apoptosis of germinal centre B cells and stimulate cellular interaction and proliferation. Here, we review the FDC signalling molecules that have recently been identified, some of which offer potential therapeutic targets for autoimmune diseases and B-cell lymphomas.
Keywords: B cells, follicular dendritic cells, germinal centre, lymphoma
Introduction
The peripheral lymphoid organs where acquired immune responses occur are well-organized microanatomical structures that are composed of T-cell and B-cell zones.1–3 These two lymphoid cell zones are anatomically separated in lymphoid tissues such as spleen, lymph nodes and tonsils. B-cell areas are found in the form of either primary follicles of naive B cells or secondary follicles of activated B cells forming the germinal centre (GC). The T-cell zone is outside the follicle. Follicular dendritic cells (FDCs) are the stromal cells located in the GC.
The primary immune response, occurring within 2–3 days following antigen (Ag) stimulation, produces immunoglobulin M (IgM).4,5 In 5–10 days, selected B cells migrate from the primary follicles to the GC where they accumulate within the extensive processes of FDCs. The immigrant lymphocytes rapidly proliferate and differentiate into memory B cells and plasma cells, accompanied by phenotypic changes. Simultaneously, there are dramatic changes in the immunoglobulin molecules. Somatic hypermutation of IgVH and isotype switching from IgM to other isotypes take place during the proliferation of GC B cells.6–8
The GC is a dynamic microenvironment where specific antibodies (Abs) against invading organisms are generated to protect the host. The production of Ag-specific Abs is precisely regulated by the cellular interactions among T cells, B cells and FDCs. Although the cellular and molecular mechanisms for T- and B-cell differentiation have been studied extensively, the function of FDCs is not well defined, but has recently been recognized as a critical component in the regulation of humoral immune responses.9–11
FDCs have been known for many years as auxiliary cells that hold Ag for a long time, but remain relatively inert.12 As a number of excellent reviews have been published in the past,13,14 this review will be focused on the recently challenging questions of FDC functions and FDC signalling molecules in a more dynamic and interactive perspective.
Development of functional FDCs
Development of mature progenies from precursors requires intimate interactions with their microenvironments. It has been shown that the generation of a proper microenvironment relies on reciprocal interactions with the developing cells it contains.15,16 As GC B cells need a proper microenvironment for GC reactions, FDCs also need microenvironmental factors to develop and differentiate.
In the mouse, B and T cells appear to be essential for development of the FDC network in the GC.17,18 No FDC develop in the severe combined immunodeficiency (SCID) mouse, in which B and T cells are absent;19,20 however, the FDC network does develop in SCID mice following reconstitution with B and T cells. In particular, activation of B cells by T cells is critical for the development of FDC networks, as demonstrated by defective GC formation in CD40 deficient or CD40-ligand deficient mice, and in nude mice.21–23 A number of investigations with gene-targeted mice have demonstrated that members of tumour necrosis factor (TNF) and TNF receptor families are crucial for the communication between lymphoid cells and FDCs, for example TNF-α, lymphotoxin (LT)α, and LTβ on B cells and their receptors on FDCs.24–28 Moreover, mice with impaired B-cell-activating factor of the TNF family (BAFF/BLys) are capable of forming GCs after immunization, but they are smaller than in wild-type controls and dissipate more rapidly.29,30 The mature FDC network in the BAFF/Blys-deficient mouse is not organized, despite intact CD35-positive primary reticulum, suggesting the requirement of B-cell interactions for the maintenance and further maturation of FDCs.
In humans, the requirement of microenvironmental factors in developing and maintaining the FDC phenotype has also been suggested. Isolated human tonsillar FDCs lose expression of important surface molecules, such as CD21, CD23, DRC-1 Ag and surface immunoglobulin, after in vitro culture in the absence of exogenous factors.31 Conversely, a cultured FDC cell line showed enhanced proliferation and up-regulation of surface interleukin (IL)-15 induced by activated T cells and B cells, respectively.32 These results are supported by in vivo observations of inflammatory lesions in patients. One of the characteristic features of the inflammatory infiltrate is the accumulation of leucocytes in the microanatomical structure that resembles the GC.33 In the ectopic GC, leucocytes are recruited and interact with stromal cells to form a GC-like microenvironment. At the end of an inflammatory response, leucocytes are cleared by apoptosis owing to loss of survival signals from stromal cells, and the infiltrates undergo a healing process akin to shrinking lymph nodes. This ectopic GC formation outside lymphoid tissues, such as in the synovial membrane, salivary glands and skin, implies de novo development of FDCs in response to the microenvironmental inflammatory signals.34,35 It is quite likely that these FDCs have arisen from the phenotypic changes of local fibroblasts (or differentiation from stromal cell precursors) from the inflammatory tissues, as FDCs are derived from non-bone marrow origins and share fibroblast surface markers such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), 1B10 and 3C8.31,36,37
FDCs consist of at least two heterogeneous subpopulations.38–40 In order to develop into mature subpopulations, FDCs need to obtain signals from the microenvironment, including signals from developing GC B cells. This mutual dependence could have important implications in clinical situations. Targeting of either B cells or FDCs would result in the loss of both populations and the microenvironment. This has been seen with rituxan treatment of autoimmune diseases,41,42 where depletion of B cells with an anti-CD20 immunoglobulin also interrupted the FDC development of the GC.
Function of FDCs
FDCs are recognized for their ability to retain Ag for a long period of time. In contrast to Ag-presenting cells that present Ag to T cells, FDCs do not internalize, process and present Ag in the context of major histocompatibility complex class II (MHC II) but present intact Ag–Ab complexes on their cell surface. Immune complexes are held by Fc receptors, such as CD32 and CD23, or by complement receptors, such as CD21 and CD35.43 It has recently been shown that immune complexes on FDCs were markedly more effective at stimulating B cells than soluble antigens in an in vitro Ag-presentation assay.44,45 Only the GC B cells that bind to immune complexes can survive and differentiate into memory B cells or plasma cells. However, this well-recognized dogma is challenged by recent observations made with mice that lack secreted immunoglobulin or Fc receptor.46,47 In such mice, the GC formation and antibody responses are not affected in the absence of Ag–Ab complexes. This observation can be explained by the interactions between CD21 and its ligand.48 Ags on FDCs engage immunoglobulin receptors in association with activated complement components that interact with CD21 in B-cell receptor (BCR) complexes, providing potent stimulatory signals to B cells.49,50 Although the controversy surrounding the role of immune complexes on FDCs has not been resolved, the role of FDCs in supporting GC B-cell survival and proliferation, via a non-Ag-specific mechanism, has been well established.14,51–53
How do FDCs support the proliferation of GC B cells in addition to Ag–Ab complexes?
Although in vivo experiments with genetically modified mice have revealed the essential role of many genes required for GC formation and FDC development, it is difficult to analyse stromal cell function by using this method. Hence, the majority of experiments performed to date have used primary FDCs isolated from murine lymph nodes and from human tonsils.54,55 Using these FDCs, it has been shown that FDCs bind GC B cells, preventing apoptosis.
FDCs comprise ≈ 1% of all GC cells.54 FDCs are isolated by digestion with DNAse and collagenase to release them from the lymphoid tissue.53 However, the number of primary FDCs isolated by using this method is inadequate for performing a detailed analysis of FDC functions. Furthermore, it is practically very difficult to dissociate the in vivo-bound B cells from isolated FDCs. To overcome this technical obstacle, several laboratories have succeeded in establishing FDC-like cell lines from human tonsils.31,56–58 These cell lines are negative for DRC-1, but weakly positive for CD14, CD11b, CD21, CD54 and CD40. Functionally, these cell lines preferentially bind GC B cells and stimulate GC B-cell growth in the presence of anti-CD40. However, these lines do not resemble typical dendritic cells in that they do not stimulate allogeneic T cells. Our laboratory has established an FDC cell line, termed HK, from human tonsil.31 HK cells are HJ2+ GP93+ 3C8+ DRC-1− KIM4−, suggesting that they may represent one of the FDC subpopulations. As HK cells share surface markers (e.g. 3C8, 8D6, DRC1, 4G10) with FDCs, they probably originate from FDCs.59,60
Functionally, HK cells preferentially bind GC B cells, providing them with survival signals, while the majority of unbound B cells undergo apoptosis within 24 hr.61 HK cells provide important costimulatory signals for GC B-cell proliferation. When cultured in the presence of HK cells, GC B cells proliferate and differentiate into plasma cells and memory B cells if CD40 ligand and cytokines are present.59,62,63
The availability of the HK cell line has provided a unique in vitro experimental model that mimics the GC reaction in vivo. FDCs do not induce differentiation of centroblasts, but provide anti-apoptotic and growth factors for rapid proliferation of GC B cells in the dark zone of the GC, where Ag-activated T cells are rare.
Activated T cells play distinct roles in the differentiation of GC B cells. In addition to CD40 ligand, which induces differentiation of centroblasts to centrocytes, cytokines secreted by activated T cells determine the pathway of GC B-cell differentiation.62,63 IL-4 directs GC B cells to differentiate into memory B cells, whereas IL-10 steers them towards plasma cells. IL-2 does not determine the differentiation pathway, but synergizes with IL-4 or IL-10 to augment cellular expansion of memory and plasma cells. The source of IL-2, IL-4 and IL-10 is T cells, as B cells and FDCs do not produce them. Therefore, FDCs collaborate with T cells in the protection and expansion of the Ag-specific GC B cells.
More than 80% of GC B cells isolated from human tonsils are CD38+ CD44− CD77+ centroblasts, while 10–20% are CD38+ CD44+ CD77− centrocytes. Centroblasts proliferate rapidly with a dividing time of 6–7 hr.64 Centrocytes in the light zone of the GC are derived from centroblasts in the dark zone.65 Both subsets of GC B cells undergo apoptosis when isolated from FDCs. Over 90% of cells die in culture within 24 hr, suggesting that FDCs provide the signalling molecules (FDC-SMs) to prevent apoptosis.61,66–68 For centrocytes, FDCs also provide Ag signals through Ag–Ab complexes, while centroblasts may receive only FDC-SMs because they do not express surface immunoglobulin molecules to bind Ag.69
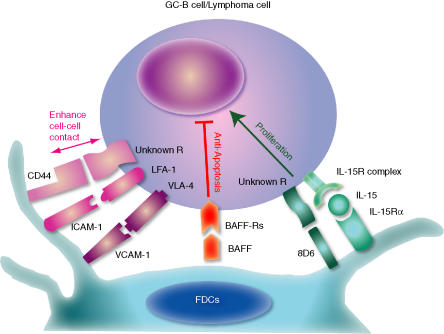
The cellular interactions between GC B cells and FDCs are carried out in co-operation with a variety of molecules (Fig. 1). The first group of molecules comprise adhesion molecules such as lymphocyte function-associated antigen-1 (LFA-1) with ICAM-1 and very-late activation antigen 4 (VLA-4) with VCAM-1.70 These molecules do not provide anti-apoptotic signals, but facilitate the anti-apoptotic functions of FDCs by enhancing cell–cell contacts.
Figure 1.
Follicular dendritic cell (FDC) signalling molecules. Vigorous proliferation and apoptosis of germinal centre (GC) B cells within the microenvironment created by FDCs is a hallmark of the GC reaction. In addition to the signal delivered through the B-cell receptor, cofactors provided by the FDCs have become increasingly recognized as critical factors that can alter the strength or the threshold of responses, possibly resulting in alteration of the specificity and intensity of immune responses. CD44, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) enhance cell–cell contact (pink bidirectional arrow). B-cell-activating factor of the tumour necrosis factor family (BAFF/BLys) prevents apoptosis of GC B cells (red blocking bar), while 8D6 and interleukin-15 (IL-15) co-stimulate GC B-cell proliferation (green arrow).
The second group comprises anti-apoptotic molecules. Recently, it was discovered that BAFF/BLys was produced by FDCs.71 BAFF/BLys rescues GC B cells from apoptosis in vitro. Furthermore, it has been reported that the radio-resistant stromal cells are the major source of BAFF/BLys, which is required to maintain normal B-cell homeostasis in vivo.72 T cells do not produce BAFF/BLys, while dendritic cells and macrophages that produce BAFF/BLys are rare in the GC. BAFF/BLys binds three receptors: BAFF receptor (BAFF-R/BR3);73,74 transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI);75 and B-cell maturation antigen (BCMA).76 BAFF-R is the predominant receptor on naive B cells outside the GC. Naive B cells do not express BCMA or TACI. However, the expression of three receptors is modulated in the course of GC B-cell differentiation (X. Zhang & C.-S. Park, manuscript in preparation). When GC B cells differentiate, BAFF-R is down-regulated while the expression of BCMA and TACI increases, indicating a fine-tuning mechanism by FDCs for the generation of plasma cell precursors. Indeed, there are no long-life plasma cells in BCMA knockout mice, suggesting an important role of FDC-BAFF/BLys in humoral immune responses.77 Apoptosis is a critical mechanism for regulating specific Ab production (Fig. 2). In the absence of apoptosis, abnormal autoimmune B cells are produced, as demonstrated in the BAFF/BLys-transgenic mouse. In contrast, normal B-cell homeostasis is completely disrupted in BAFF/BLys or BAFF-R-deficient mice.78,79 The latter mice are not capable of mounting an Ab response or GC reactions.
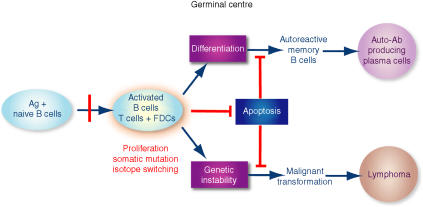
Figure 2.
Fine tuning of B-cell apoptosis by follicular dendritic cells (FDC). In order to produce high-affinity protective antibody (Ab), antigen (Ag)-activated B cells proliferate vigorously and undergo somatic hypermutation and isotype switching in the presence of FDC and T cells in the germinal centre (GC). These GC reactions are prone to produce genetic instability, such as mutation and translocation of critical genes. Failure to eliminate these aberrant B cells by apoptosis in the GC would be a critical component of autoimmune diseases and lymphomagenesis. The apoptotic mechanism in the GC needs to be precisely regulated to maintain normal B-cell homeostasis in the peripheral lymphoid tissues. In the absence of regulation of B-cell apoptosis, elimination of self-reactive B cells is impaired, resulting in autoimmune diseases. FDCs are the main source of anti-apoptotic factors such as BAFF/BLys and IL-15 in the GC. The physiological interaction between FDCs and B cells may regulate the appropriate concentrations of these cytokines in the GC microenvironment. We suggest that blockade of the survival signals provided by FDCs is one way to treat autoimmune diseases and B-cell lymphomas, in addition to the elimination of pathological B cells.
The third group of molecules comprises those related to the proliferation of GC B cells. Vigorous proliferation of centroblasts within the GC microenvironment created by FDCs is a hallmark of the GC reaction. However, factors that are responsible for this rapid cell division have not been identified. Many cytokines have been reported to augment human GC B-cell proliferation in vitro, one of the most potent being IL-2.59,80 In the absence of IL-2, GC B-cell recovery has been shown to be decreased by at least threefold in cultures.59 However, activated T cells that produce IL-2 are not common in the dark zone of the GC,81 and centroblasts do not express IL-2 receptor α, which is essential for binding IL-2 with high affinity.82 In addition to anti-apoptosis factors, FDCs produce a number of growth factors, such as 8D6, IL-15 and IL-6.60,83 These factors collaborate with BAFF/BLys in enhancing GC B-cell proliferation. A novel cytokine, 8D6, promotes GC B-cell growth and supports proliferations of plasma cell precursors, enhancing antibody secretion.60,63 Interestingly, 8D6 protein synergizes with CD44 in stimulating GC B cells or lymphoma cell lines.84 FDCs produce IL-15, and membrane-bound IL-15 stimulates GC B-cell proliferation by transducing signals through IL-2/IL-15Rβ.32 GC B cells proliferate in the presence of IL-15, dividing faster than cells cultured without IL-15. These results imply that IL-15 and 8D6 stimulate the rapid proliferation of centroblasts in the GC in vivo. Although IL-6 does not stimulate cellular proliferation of GC B cells in vitro, eliminating FDC-derived IL-6 alters the formation of light and dark zones of the GC.85,86
FDCs in B-cell lymphoma and other diseases
The majority of B-cell lymphomas originate from the GC.87,88 The preferential localization of lymphoma cells in the GC suggests a unique relationship between tumour cells and their microenvironment. The generation and blast transformation of lymphomas occurs in close association with FDCs.89 As shown in Fig. 2, FDCs contribute to lymphoma generation by preventing apoptosis as well as by promoting the proliferation of transformed B cells. The tumorigenic cells can emerge by selection for additional genetic changes or through adaptation to the protumorigenic environment provided by FDCs. Because various cancer cells metastasize to lymphoid follicles, initiating a niche, it is probable that FDCs also provide the growth factors for these metastasized cancer cells.
Our hypothesis is that there are three stages of lymphoma development. The first stage is the survival stage for a small number of lymphoma cells generated by the genetic instability that occurs during the rapid cellular proliferation and somatic mutation of B cells in the GC. The second stage is cellular proliferation (i.e. growth) of the surviving lymphoma cells. The third stage is tumour formation by angiogenesis (i.e. lymphomagenesis). These three stages of tumour development in vivo (survival→growth→lymphomagenesis) are constantly in progress during malignant transformation. FDCs play a pivotal role in driving this process by providing the necessary signalling molecules. During this development, lymphoma cells that require FDCs for their survival and growth become FDC-independent in the late stage. This hypothesis is consistent with the clinical progress of B-cell lymphomas.90,91 B-cell lymphomas are usually indolent in the early stage. However, they become malignant after blast transformation. The molecular basis for such blast transformation is not known.
The Burkitt lymphoma cell line, L3055 cells and the follicular lymphoma (FL) cell line, FLK-1, require FDCs for their growth in vitro.92,93 Furthermore, L3055 cells form tumours when inoculated with HK cells into nude mice.84 In the absence of HK cells, L3055 cells undergo apoptosis in vitro and do not form tumours in vivo. Recently, it was shown that the combination of neutralizing monoclonal antibodies (mAb) specific to FDC-SMs (CD44 and 8D6) completely prevented tumour formation, suggesting an important role of FDC-SMs in lymphomagenesis.60,84
The development of an inducible lymphoma model in vivo offers unprecedented opportunities in cancer research, such as investigation of the genetic changes that occur in lymphoma cells during escape using the DNA microarray method.94,95 Identification of FDC-SMs and the characterization of their functions that promote survival, growth and angiogenesis of lymphomas, could well lead to the discovery of the therapeutic agents to intervene in the pathway to malignant transformation.
In addition to their role in lymphomagenesis, FDCs also play an important role in other intractable diseases, such as acquired immune-deficiency syndrome (AIDS), transmissible sponsiform encephalitis (TSE) and autoimmune diseases.36,96–98 FDCs trap large quantities of human immunodeficiency virus (HIV) or prions and thus serve as a major reservoir for each disease.99–102 Furthermore, there have been reports suggesting that FDCs play an active role in pathogenesis that involves more than simply holding pathogens.96,103,104 FDCs provide the necessary microenvironment for HIV to enhance infection to CD4 T cells and/or replication of virus. HIV on FDCs has been shown to be resistant to neutralizing Ab. It has been reported that the distance between FDCs and the peripheral nerve is the critical factor for TSE.105 Prion neuroinvasion is highly dependent on FDCs, while tetanus and other bacterial toxins have also been shown to accumulate on FDCs.106
As described previously, the recent successes of rituxan treatment in patients with rheumatoid arthritis re-emphasizes the significant involvement of B cells and autoantibodies in the pathogenesis of autoimmune diseases. Germinal centres containing FDC networks are frequently found in the lesions and are suggested as locations where autoreactive B cells proliferate and differentiate to PCs producing autoantibodies.107–109 Establishing lymphoid architecture in the close vicinity of autoantigen-production sites would certainly contribute to disease progression.35 Furthermore, synoviocytes of patients with rheumatoid arthritis are reported to share some of the intrinsic properties of FDCs, such as the binding of GC B cells and the prevention of apoptosis.36 Rheumatoid synoviocytes express a number of adhesion molecules, such as VCAM-1 and ICAM-1, and produce cytokines such as granulocyte–macrophage colony-stimulating factor (GM-CSF), stromal-derived factor-1 (SDF-1), IL-15 and IL-6.98 Suppressing such ectopic FDC development and/or function might permit the treatment of the most severe type of autoimmune diseases. Therefore, further research on FDCs could provide a more efficacious therapeutic target for attenuating these diseases.
Applications of FDC cell lines in lymphoma research
For the past 30 years, all B-lymphoma cell lines have been obtained by growing lymphoma cells without FDC cells. For example, several FL lines have been established that grow in the absence of FDCs or other feeder cells. However, as FL cells from patients frequently fail to grow in vitro, it is probable that these previously established cell lines have become FDC independent by selection and mutation in vitro and therefore may have lost their in vivo characteristics. We have recently established an HK cell-dependent FL cell line (FLK) from a patient.92 This FLK cell line requires HK cells for its propagation and, like the L3055 cell subclone, undergoes apoptosis in the absence of HK cells. Such a cell line could not have been obtained until FDC cell lines, such as HK, became available. Immortalized FDC lines will provide invaluable tools for studying the biology of lymphoma cells in vitro and in vivo.
FDCs are not homogenous but are composed of different subpopulations, as defined by their surface markers.40 However, the functional distinction between these subpopulations is not known. For survival, different types of lymphomas may require unique FDC subsets providing different SMs. Alternatively, distinct types of lymphomas may influence the differentiation of FDCs into different subpopulations. The interplay between tumour and stromal cells has been shown in a study of transgenic mice expressing green fluorescent protein under the control of the human vascular endothelial growth factor (VEGF) promoter.110 Implanted tumours were able to induce the transgene, indicating the potential for the tumour environment to induce protumorigenic factors (e.g. VEGF) in stromal cells of host origin.
In order to characterize the FDC subpopulations, we have recently immortalized freshly isolated FDCs by constitutively expressing telomerase (S.-Y. Yoon et al., manuscript in preparation). Compared to HK, which is a primary cell line, immortalized FDCs are more stable and provide more consistent results. It is now possible to compare the capacity of the FDC clones to stimulate lymphoma cell growth and to produce FDC-SMs. The changes in the gene expression of FDC subpopulations, following interaction with various B-cell lymphoma cells, can be studied by microarray technology and compared to the functional capacity of FDC subpopulations to promote lymphoma cell growth in vitro and in vivo.
Malignant progression of tumour cells is driven by intrinsic events, such as activation of oncogenes, loss of tumour suppressor genes, and maintenance of telomere length and function. Although the intrinsic cellular defects causing malignant transformation have been investigated extensively, the protumorigenic factors from the microenvironment have not been clearly delineated. However, it has become increasingly recognized that these are critical factors for the tumorigenic process. The stromal cells in the adjacent tissue must provide a supporting microenvironment for tumour growth in the early stage of tumorigenesis. Direct cell–cell contact and soluble factors may be important for the in vivo survival and growth of tumour cells.
It has now become feasible to investigate the dynamic interactions between tumour cells and stromal cells owing to the recent availability of FDC lines.
Acknowledgments
We acknowledge the contributions made by the former and current postdoctoral fellows: Drs Han-Soo Kim, Jongseon Choe, Xin Zhang, Sun-Ok Yoon, and Li Li. We also appreciate Dr Richard Armitage for critical reading of the manuscript. We are indebted to many colleagues in the field whose work we may have not cited because of reference limits. This work was supported by National Institute of Health Grant of United States of America (CA092126).
References
- 1.MacLennan ICM. Germinal centers. Annu Rev Immunol. 1994;12:117–39. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y-J, Arpin C. Germinal center development. Immunol Rev. 1997;156:111–26. doi: 10.1111/j.1600-065x.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 3.Kelsoe G. The germinal center: a crucible for lymphocyte selection. Semin Immunol. 1996;8:179–84. doi: 10.1006/smim.1996.0022. [DOI] [PubMed] [Google Scholar]
- 4.Kelsoe G. Life and death in germinal centers (Redux) Immunity. 1996;4:107–11. doi: 10.1016/s1074-7613(00)80675-5. [DOI] [PubMed] [Google Scholar]
- 5.Toellner K-M, Gulbranson-Judge A, Taylor DR, Sze DM-Y, MacLennan ICM. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. J Exp Med. 1996;183:2303–12. doi: 10.1084/jem.183.5.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pascual V, Liu Y-J, Magalski A, de Bouteiller O, Banchereau J, Capra JD. Analysis of somatic mutation in five B cell subsets of human tonsil. J Exp Med. 1994;180:329–39. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y-J, Arpin C, deBouteiller O, Guret C, Banchereau J, Martinez-Valdez H, Lebecque S. Sequential triggering of apoptosis, somatic mutation and isotype switch during germinal center development. Semin Immunol. 1996;8:169–77. doi: 10.1006/smim.1996.0021. [DOI] [PubMed] [Google Scholar]
- 8.Kimoti H, Nagaoka H, Adachi Y, et al. Accumulation of somatic hypermutation and antigen-driven selection in rapidly cycling surface Ig+ germinal center (GC) B cells which occupy GC at a high frequency during the primary anti-hapten response in mice. Eur J Immunol. 1997;27:268–79. doi: 10.1002/eji.1830270140. [DOI] [PubMed] [Google Scholar]
- 9.Kosco-Vilbois MH, Scheidegger D. Follicular dendritic cells: antigen retention, B cell activation, and cytokine production. Curr Top Microbiol Immunol. 1995;201:69–82. doi: 10.1007/978-3-642-79603-6_4. [DOI] [PubMed] [Google Scholar]
- 10.Tew JG, Wu J, Qin D, Helm S, Burton GF, Szakal AK. Follicular dendritic cells and presentation of antigen and costimulatory signals to B cells. Immunol Rev. 1997;156:39–52. doi: 10.1111/j.1600-065x.1997.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 11.Choi YS. Differentiation and apoptosis of human germinal center B-lymphocytes. Immunol Res. 1997;16:161–74. doi: 10.1007/BF02786360. [DOI] [PubMed] [Google Scholar]
- 12.Nossal GJV, Ada GL, Austin CM. Antigens in immunity. IV. Cellular localization of 125I-and 131I-labelled flagella in lymph nodes. Aust J Exp Biol. 1964;42:311–30. [PubMed] [Google Scholar]
- 13.Tew JG, Kosco MH, Burton GF, Szakal AK. Follicular dendritic cells as accessory cells. Immunol Rev. 1990;117:185–211. doi: 10.1111/j.1600-065x.1990.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 14.Kosco-Vilbois MH. Are follicular dendritic cells really good for nothing? Nat Rev Immunol. 2003;3:764–9. doi: 10.1038/nri1179. [DOI] [PubMed] [Google Scholar]
- 15.Ritter MA, Boyd RL. Development in the thymus: it takes two to tango. Immunol Today. 1993;14:462–9. doi: 10.1016/0167-5699(93)90250-O. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 17.Cerny A, Zinkernagel RM, Groscurth P. Development of follicular dendritic cells in lymph nodes of B-cell-depleted mice. Cell Tissue Res. 1988;254:449–54. doi: 10.1007/BF00225818. [DOI] [PubMed] [Google Scholar]
- 18.MacLennan ICM, Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 19.Kapasi ZF, Burton GF, Shultz LD, Tew JG, Szakal AK. Induction of functional follicular dendritic cell development in severe combined immunodeficiency mice. Influence of B and T cells. J Immunol. 1993;150:2648–58. [PubMed] [Google Scholar]
- 20.Yoshida K, Kaji M, Takahashi T, van den Berg TK, Dijkstra CD. Host origin of follicular dendritic cells induced in the spleen of SCID mice after transfer of allogeneic lymphocytes. Immunology. 1995;84:117–26. [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson EB, Caporale LH, Thorbecke GJ. Effect of thymus cell injections on germinal center formation in lymphoid tissues of nude (thymusless) mice. Cell Immunol. 1974;13:416–30. doi: 10.1016/0008-8749(74)90261-5. [DOI] [PubMed] [Google Scholar]
- 22.Kawabe T, Naka T, Yoshida K, et al. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–78. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 23.Renshaw BR, Fanslow WC, III, Armitage RJ, Campbell KA, Liggett D, Wright B, Davison BL, Maliszewski CR. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaplin DD, Fu Y. Cytokine regulation of secondary lymphoid organ development. Curr Opin Immunol. 1998;10:289–97. doi: 10.1016/s0952-7915(98)80167-2. [DOI] [PubMed] [Google Scholar]
- 25.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNFα-deficient mice: a critical requirement for TNFα in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins α and β revealed in lymphotoxin-β deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto M, Fu Y-X, Molina H, Huang G, Kim J, Thomas DA, Nahm MH, Chaplin DD. Distinct roles of lymphotoxin α and the Type I tumor necrosis factor (TNF) receptor in the establishment of follicular dendritic cells from non-bone marrow-derived cells. J Exp Med. 1997;186:1997–2004. doi: 10.1084/jem.186.12.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu Y-X, Huang G, Wang Y, Chaplin DD. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin α-dependent fashion. J Exp Med. 1998;187:1009–18. doi: 10.1084/jem.187.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vora KA, Wang LC, Rao SP, et al. Germinal centers formed in the absence of B cell-activating factor belonging to the TNF family exhibit impaired maturation and function. J Immunol. 2003;171:547–51. doi: 10.4049/jimmunol.171.2.547. [DOI] [PubMed] [Google Scholar]
- 30.Rahman ZS, Rao SP, Kalled SL, Manser T. Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice. J Exp Med. 2003;198:1157–69. doi: 10.1084/jem.20030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim H-S, Zhang X, Choi YS. Activation and proliferation of follicular dendritic cell-like cells by activated T lymphocytes. J Immunol. 1994;153:2951–61. [PubMed] [Google Scholar]
- 32.Park C-S, Yoon S-O, Armitage RJ, Choi YS. Folllicular dendritic cell produce IL-15 that enhances germinal center B cell proliferation in membrane bound form. J Immunol. 2004;173:6676–83. doi: 10.4049/jimmunol.173.11.6676. [DOI] [PubMed] [Google Scholar]
- 33.Buckley CD. Michael Mason prize essay 2003. Why do leucocytes accumulate within chronically inflamed joints? Rheumatology (Oxford) 2003;42:1433–44. doi: 10.1093/rheumatology/keg413. [DOI] [PubMed] [Google Scholar]
- 34.Berek C, Kim HJ. B-cell activation and development within chronically inflamed synovium in rheumatoid and reactive arthritis. Semin Immunol. 1997;9:261–8. doi: 10.1006/smim.1997.0076. [DOI] [PubMed] [Google Scholar]
- 35.Weyand CM, Kurtin PJ, Goronzy JJ. Ectopic lymphoid organogenesis: a fast track for autoimmunity. Am J Pathol. 2001;159:787–93. doi: 10.1016/S0002-9440(10)61751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindhout E, van Eijk M, van Pel M, Lindeman J, Dinant HJ, de Groot C. Fibroblast-like synoviocytes from rheumatoid arthritis patients have intrinsic properties of follicular dendritic cells. J Immunol. 1999;162:5949–56. [PubMed] [Google Scholar]
- 37.Lee IY, Choe J. Human follicular dendritic cells and fibroblasts share the 3C8 antigen. Biochem Biophys Res Commun. 2003;304:701–7. doi: 10.1016/s0006-291x(03)00649-1. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida K, van den Berg TK, Dijkstra CD. Two functionally different follicular dendritic cells in secondary lymphoid follicles of mouse spleen, as revealed by CR1/2 and FcR gamma II-mediated immune-complex trapping. Immunology. 1993;80:34–9. [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson GD, Hardie DL, Ling NR, Maclennan IC. Human follicular dendritic cells (FDC): a study with monoclonal antibodies (MoAb) Clin Exp Immunol. 1986;64:205–13. [PMC free article] [PubMed] [Google Scholar]
- 40.Imai Y, Maeda K, Yamakawa M, Karube Y, Matsuda M, Dobashi M, Sato H, Terashima K. Heterogeneity and cellular origin of follicular dendritic cells. Adv Exp Med Biol. 1993;329:339–44. doi: 10.1007/978-1-4615-2930-9_57. [DOI] [PubMed] [Google Scholar]
- 41.Shaw T, Quan J, Totoritis MC. B cell therapy for rheumatoid arthritis: the rituximab (anti-CD20) experience. Ann Rheum Dis. 2003;62(Suppl. 2):55–9. doi: 10.1136/ard.62.suppl_2.ii55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–81. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 43.Tew JG, Mandel TE, Burgess AW. Retention of intact HSA for prolonged periods in the popliteal lymph nodes of specifically immunized mice. Cell Immunol. 1979;45:207–12. doi: 10.1016/0008-8749(79)90378-2. [DOI] [PubMed] [Google Scholar]
- 44.Kosco MH, Szakal AK, Tew JG. In vivo obtained antigen presented by germinal center B cells to T cells in vitro. J Immunol. 1988;140:354–60. [PubMed] [Google Scholar]
- 45.Wu J, Qin D, Burton GF, Szakal AK, Tew JG. Follicular dendritic cell-derived antigen and accessory activity in initiation of memory IgG responses in vitro. J Immunol. 1996;157:3404–11. [PubMed] [Google Scholar]
- 46.Vora KA, Ravetch JV, Manser T. Amplified follicular immune complex deposition in mice lacking the Fc receptor γ-chain does not alter maturation of the B cell response. J Immunol. 1997;159:2116–24. [PubMed] [Google Scholar]
- 47.Hannum LG, Haberman AM, Anderson SM, Shlomchik MJ. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J Exp Med. 2000;192:931–42. doi: 10.1084/jem.192.7.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Croix DA, Ahearn JM, Rosengard AM, Han S, Kelsoe G, Ma M, Carroll MC. Antibody response to a T-dependent antigen requires B cell expression of complement receptors. J Exp Med. 1996;183:1857–64. doi: 10.1084/jem.183.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fearon DT, Carroll MC. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu Rev Immunol. 2000;18:393–422. doi: 10.1146/annurev.immunol.18.1.393. [DOI] [PubMed] [Google Scholar]
- 50.Qin D, Wu J, Carroll MC, Burton GF, Szakal AK, Tew JG. Evidence for an important interaction between a complement-derived CD21 ligand on follicular dendritic cells and CD21 on B cells in the initiation of IgG responses. J Immunol. 1998;161:4549–54. [PubMed] [Google Scholar]
- 51.Burton GF, Conrad DH, Szakal AK, Tew JG. Follicular dendritic cells and B cell costimulation. J Immunol. 1993;150:31–8. [PubMed] [Google Scholar]
- 52.Kosco MH, Pflugfelder E, Gray D. Follicular dendritic cell-dependent adhesion and proliferation of B cells in vitro. J Immunol. 1992;148:2331–9. [PubMed] [Google Scholar]
- 53.Grouard G, de Bouteiller O, Banchereau J, Liu YJ. Human follicular dendritic cells enhance cytokine-dependent growth and differentiation of CD40-activated B cells. J Immunol. 1995;155:3345–52. [PubMed] [Google Scholar]
- 54.Schnizlein CT, Kosco MH, Szakal AK, Tew JG. Follicular dendritic cells in suspension. identification, enrichment, and initial characterization indicating immune complex trapping and lack of adherence and phagocytic activity. J Immunol. 1985;134:1360. [PubMed] [Google Scholar]
- 55.Tsunoda R, Nakayama M, Onozaki K, Heinen E, Cormann N, Kinet-Denoel C, Kojima M. Isolation and long-term cultivation of human tonsil follicular dendritic cells. Virchows Arch B Cell Pathol. 1990;59:95–105. doi: 10.1007/BF02899393. [DOI] [PubMed] [Google Scholar]
- 56.Clark EA, Grabstein KH, Shu GL. Cultured human follicular dendritic cells. Growth characteristics and interactions with B lymphocytes. J Immunol. 1992;148:3327–35. [PubMed] [Google Scholar]
- 57.Lindhout E, Lakeman A, Mevissen MLCM, de Groot C. Functionally active Epstein–Barr virus-transformed follicular dendritic cell-like cell lines. J Exp Med. 1994;179:1173–84. doi: 10.1084/jem.179.4.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsunoda R, Bosseloir A, Onozaki K, et al. Human follicular dendritic cells in vitro and follicular dendritic cell-like cells. Cell Tissue Res. 1997;288:381–9. doi: 10.1007/s004410050824. [DOI] [PubMed] [Google Scholar]
- 59.Choe J, Kim H-S, Zhang X, Armitage RJ, Choi YS. Cellular and molecular factors that regulate the differentiation and apoptosis of germinal center B cells. J Immunol. 1996;157:1006–16. [PubMed] [Google Scholar]
- 60.Li L, Zhang X, Kovacic S, Long AJ, Bourque K, Wood CR, Choi YS. Identification of a human follicular dendritic cell molecule that stimulates germinal center B cell growth. J Exp Med. 2000;191:1077–84. doi: 10.1084/jem.191.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim H-S, Zhang X, Klyushnenkova E, Choi YS. Stimulation of germinal center B lymphocyte proliferation by an FDC-like cell line, HK. J Immunol. 1995;155:1101–9. [PubMed] [Google Scholar]
- 62.Choe J, Choi YS. Interleukin-10 interrupts memory B cell expansion in the germinal center by inducing differentiation into plasma. Eur J Immunol. 1998;28:508–15. doi: 10.1002/(SICI)1521-4141(199802)28:02<508::AID-IMMU508>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, Li L, Jung J, Xiang S, Hollmann C, Choi YS. The distinct roles of T cell derived cytokines and a novel follicular dendritic cell-signaling molecule 8D6 in germinal center-B cell differentiation. J Immunol. 2001;167:49–56. doi: 10.4049/jimmunol.167.1.49. [DOI] [PubMed] [Google Scholar]
- 64.Zhang J, MacLennan IC, Liu YJ, Lane PJ. Is rapid proliferation in B centroblasts linked to somatic mutation in memory B cell clones? Immunol Lett. 1988;18:297–9. doi: 10.1016/0165-2478(88)90178-2. [DOI] [PubMed] [Google Scholar]
- 65.Feuillard J, Taylor D, Cassamayor-Palleja M, Johnson GD, MacLennan ICM. Isolation and characteristics of tonsil centroblasts with reference to Ig class switching. Int Immunol. 1995;7:121–30. doi: 10.1093/intimm/7.1.121. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y-J, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan ICM. Mechanism of antigen-driven selection in germinal centers. Nature. 1989;342:929–31. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- 67.Lindhout E, Lakeman A, de Groot C. Follicular dendritic cells inhibit apoptosis in human B lymphocytes by a rapid and irreversible blockade of preexisting endonuclease. J Exp Med. 1995;181:1985–95. doi: 10.1084/jem.181.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Eijk M, Medema JP, de Groot C. Cutting edge: cellular Fas-associated death domain-like IL-1-converting enzyme-inhibitory protein protects germinal center B cells from apoptosis during germinal center reactions. J Immunol. 2001;166:6473–6. doi: 10.4049/jimmunol.166.11.6473. [DOI] [PubMed] [Google Scholar]
- 69.Galibert L, Burdin N, de Saint-Vis B, Garrone P, Van Kooten C, Banchereau J, Rousset F. CD40 and B cell antigen receptor dual triggering of resting B lymphocytes turns on a partial germinal center phenotype. J Exp Med. 1996;183:77–85. doi: 10.1084/jem.183.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koopman G, Keehnen RMJ, Lindhout E, Newman W, Shimizu Y, van Seventer GA, de Groot C, Pals ST. Adhesion through the LFA-1 (CD11a/CD18) -ICAM-1 (CD54) and the VLA-4 (CD49d) -VCAM-1 (CD106) pathways prevents apoptosis of germinal center B cells. J Immunol. 1994;152:3760–7. [PubMed] [Google Scholar]
- 71.Hase H, Kanno Y, Kojima M, et al. BAFF/BLyS can potentiate B-cell selection with the B-cell coreceptor complex. Blood. 2004;103:2257–65. doi: 10.1182/blood-2003-08-2694. [DOI] [PubMed] [Google Scholar]
- 72.Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med. 2003;198:937–45. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thompson JS, Bixler SA, Qian F, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–11. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 74.Yan M, Brady JR, Chan B, et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11:1547–52. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]
- 75.Xia XZ, Treanor J, Senaldi G, et al. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J Exp Med. 2000;192:137–43. doi: 10.1084/jem.192.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thompson JS, Schneider P, Kalled SL, et al. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J Exp Med. 2000;192:129–35. doi: 10.1084/jem.192.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O'Connor BP, Raman VS, Erickson LD, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–8. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–4. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 80.Arpin C, Dechanet J, Van Kooten C, Merville P, Frouard G, Briere F, Banchereau J, Liu Y-J. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720–2. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- 81.Liu Y-J, Johnson GD, Gordon J, MacLennan ICM. Germinal centres in T-cell-dependent antibody responses. Immunol Today. 1992;13:17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- 82.Jego G, Bataille R, Pellat-Deceunynck C. Interleukin-6 is a growth factor for nonmalignant human plasmablasts. Blood. 2001;97:1817–22. doi: 10.1182/blood.v97.6.1817. [DOI] [PubMed] [Google Scholar]
- 83.Husson H, Lugli SM, Ghia P, et al. Functional effects of TNF and lymphotoxin alpha1beta2 on FDC-like cells. Cell Immunol. 2000;203:134–43. doi: 10.1006/cimm.2000.1688. [DOI] [PubMed] [Google Scholar]
- 84.Li L, Yoon SO, Fu DD, Zhang X, Choi YS. Novel follicular dendritic cell molecule, 8D6, collaborates with CD44 in supporting lymphomagenesis by a Burkitt lymphoma cell line, L3055. Blood. 2004;104:815–21. doi: 10.1182/blood-2004-01-0292. [DOI] [PubMed] [Google Scholar]
- 85.Kopf M, Herren Z, Wiles MV, Pepys MB, Kosco-Vilbois MH. Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J Exp Med. 1998;188:1895–906. doi: 10.1084/jem.188.10.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Butch AW, Chung G-H, Hoffmann JW, Nahm MH. Cytokine expression by germinal center cells. J Immunol. 1993;150:39–47. [PubMed] [Google Scholar]
- 87.Stevenson F, Sahota S, Zhu D, Ottensmeier C, Oscier D, Hamblin T. Insight into the origin and clonal history of B-cell tumors as revealed by analysis of immunoglobulin variable region genes. Immunol Rev. 1998;162:247–59. doi: 10.1111/j.1600-065x.1998.tb01446.x. [DOI] [PubMed] [Google Scholar]
- 88.Kuppers R, Klein U, Hansmann ML, Rajewsky K. Cellular origin of human B-cell lymphomas. N Engl J Med. 1999;341:1520–9. doi: 10.1056/NEJM199911113412007. [DOI] [PubMed] [Google Scholar]
- 89.Petrasch S, Kosco M, Perez-Alvarez C, Schmitz J, Brittinger G. Proliferation of non-Hodgkin lymphocytes in vitro is dependent upon follicular dendritic cell interactions. Br J Haematol. 1992;80:21–6. doi: 10.1111/j.1365-2141.1992.tb06395.x. [DOI] [PubMed] [Google Scholar]
- 90.Horning SJ, Rosenberg SA. The natural history of initially untreated low-grade non-Hodgkin's lymphoma. N Engl J Med. 1984;311:1471–5. doi: 10.1056/NEJM198412063112303. [DOI] [PubMed] [Google Scholar]
- 91.Lossos IS, Levy R. Higher-grade transformation of follicle center lymphoma is associated with somatic mutation of the 5′ noncoding regulatory region of the BCL-6 gene. Blood. 2000;96:635–9. [PubMed] [Google Scholar]
- 92.Kagami Y, Jung J, Choi YS, Osumi K, Nakamura S, Morishima Y, Seto M. Establishment of a follicular lymphoma cell line (FLK-1) dependent on follicular dendritic cell-like cell line HK. Leukemia. 2001;15:148–56. doi: 10.1038/sj.leu.2402002. [DOI] [PubMed] [Google Scholar]
- 93.Choe J, Li L, Zhang X, Gregory CD, Choi YS. Distinct role of follicular dendritic cells and T cells in the proliferation, differentiation, and apoptosis of a centroblast cell line, L3055. J Immunol. 2000;164:56–63. doi: 10.4049/jimmunol.164.1.56. [DOI] [PubMed] [Google Scholar]
- 94.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–11. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 95.Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 96.Burton GF, Keele BF, Estes JD, Thacker TC, Gartner S. Follicular dendritic cell contributions to HIV pathogenesis. Semin Immunol. 2002;14:275–84. doi: 10.1016/s1044-5323(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 97.Mabbott NA, Bruce ME. Follicular dendritic cells as targets for intervention in transmissible spongiform encephalopathies. Semin Immunol. 2002;14:285–93. doi: 10.1016/s1044-5323(02)00061-1. [DOI] [PubMed] [Google Scholar]
- 98.Miranda-Carus ME, Balsa A, Benito-Miguel M, Perez de Ayala C, Martin-Mola E. IL-15 and the initiation of cell contact-dependent synovial fibroblast-T lymphocyte cross-talk in rheumatoid arthritis: effect of methotrexate. J Immunol. 2004;173:1463–76. doi: 10.4049/jimmunol.173.2.1463. [DOI] [PubMed] [Google Scholar]
- 99.Haase AT. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu Rev Immunol. 1999;17:625–56. doi: 10.1146/annurev.immunol.17.1.625. [DOI] [PubMed] [Google Scholar]
- 100.Pantaleo G, Graziosi C, Butini L, Pizzo PA, Schnittman SM, Kotler DP, Fauci AS. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci USA. 1991;88:9838–42. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kitamoto T, Muramoto T, Mohri S, Doh-Ura K, Tateishi J. Abnormal isoform of prion protein accumulates in follicular dendritic cells in mice with Creutzfeldt–Jakob disease. J Virol. 1991;65:6292–5. doi: 10.1128/jvi.65.11.6292-6295.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hill AF, Butterworth RJ, Joiner S, et al. Investigation of variant Creutzfeldt–Jakob disease and other human prion diseases with tonsil biopsy samples. Lancet. 1999;353:183–9. doi: 10.1016/s0140-6736(98)12075-5. [DOI] [PubMed] [Google Scholar]
- 103.Heath S, Tew JG, Tew JG, Szakal AK, Burton GF. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature. 1995;377:740–4. doi: 10.1038/377740a0. [DOI] [PubMed] [Google Scholar]
- 104.Brown KL, Stewart K, Ritchie DL, Mabbott NA, Williams A, Fraser H, Morrison WI, Bruce ME. Scrapie replication in lymphoid tissues depends on prion protein-expressing follicular dendritic cells. Nat Med. 1999;5:1308–12. doi: 10.1038/15264. [DOI] [PubMed] [Google Scholar]
- 105.Prinz M, Heikenwalder M, Junt T, et al. Positioning of follicular dendritic cells within the spleen controls prion neuroinvasion. Nature. 2003;425:957–62. doi: 10.1038/nature02072. [DOI] [PubMed] [Google Scholar]
- 106.Pozdnyakova O, Guttormsen HK, Lalani FN, Carroll MC, Kasper DL. Impaired antibody response to group B streptococcal type III capsular polysaccharide in C3- and complement receptor 2-deficient mice. J Immunol. 2003;170:84–90. doi: 10.4049/jimmunol.170.1.84. [DOI] [PubMed] [Google Scholar]
- 107.Armengol MP, Juan M, Lucas-Martin A, Fernandez-Figueras MT, Jaraquemada D, Gallart T, Pujol-Borrell R. Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active intrathyroidal germinal centers. Am J Pathol. 2001;159:861–73. doi: 10.1016/S0002-9440(10)61762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Randen I, Mellbye OJ, Forre O, Natvig JB. The identification of germinal centres and follicular dendritic cell networks in rheumatoid synovial tissue. Scand J Immunol. 1995;41:481–6. doi: 10.1111/j.1365-3083.1995.tb03596.x. [DOI] [PubMed] [Google Scholar]
- 109.Tarlinton DM, Hodgkin PD. Targeting plasma cells in autoimmune diseases. J Exp Med. 2004;199:1451–4. doi: 10.1084/jem.20040719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fukumura D, Xavier R, Sugiura T, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–25. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]