Abstract
Antigen-presenting cells (APC) are specialized sentinel cells that sense pathogens within tissues and then activate appropriate immune effector cells in lymphoid organs. Recent evidence, however, suggests that APC can also induce effector cells in non-lymphoid organs. The purpose of this study was to determine the effect of intravaginal (IVAG) delivery of CpG-oligodeoxynucleotide (ODN) on expansion of resident genital APC. Our results show that delivery of CpG-ODN to the murine genital tract induced a rapid and significant, but transient expansion of genital APC in situ. As early as 12 hr post CpG-ODN delivery, we observed an enhanced level of F4/80+ major histocompatibility complex (MHC) class II-negative macrophages in the genital tissue. This was followed by increased levels of F4/80/MHC class II double-positive cells, as well as MHC class II, CD11c and CD86 triple-positive dendritic cells (DC) at 48 hr. Expanded APC levels at 48 hr post CpG-ODN resulted in increased ability of genital cells to induce an allogenic mixed leucocyte reaction. By 72 hr after CpG-ODN treatment, APC levels were not distinguishable from naïve levels. Therefore, these results clearly show that administration of CpG-ODN to the genital tract induced a marked but transient enhancement of APC within the genital tissue, and that these APC appear to possess functional capacity. Furthermore, these results indicate that IVAG–CpG-ODN may be an important factor for the enhancement of local antigen presentation in the genital tract through increased DC numbers.
Keywords: viral, antigen presentation, mucosa
Introduction
The major immune function for antigen-presenting cells (APC) lies in their ability to initiate antigen-specific adaptive immune responses in tissue-draining lymph nodes.1 The immune inductive capacity of APC outside lymphoid tissues, however, remains incompletely understood. Recent evidence suggests that APC do possess the capacity to activate effector cells in the tissues, prior to their migration to the lymphoid organs. Specifically, nasal immunization of lymphotoxin-α knockout mice results in APC-mediated T-cell activation within the lung2 while adoptive transfer of dendritic cells (DC) into a tumour site in vivo induces a natural killer (NK) cell-mediated innate immune antitumour response that inhibits tumour growth.3 Therefore, optimization of APC in tissues frequently exposed to pathogens may lead to increased immune mediated protection.
Because mucosal tissues are most frequently exposed to pathogens, it is not surprising that most of them have the capacity to induce local mucosal-associated lymphoid tissues (MALT) where antigen presentation occurs following pathogen invasion.2,4–7 Indeed, nasal and oral vaccination induce strong local and systemic antigen-specific antibody responses.8,9 In contrast to other mucosal sites, the vagina is considered to be a relatively poor immune inductive site. In particular, vaginal immunization with non-replicating antigens results in modest induction of specific antibodies in local secretions; specific antibodies are not found systemically.10 The poor immune inductive capacity of vaginal tissue may be explained by the absence of MALT. Administration of factors that stimulate bronchus-associated lymphoid tissue (BALT) results in faster antigen uptake in the lung.7 Moreover, surface immunoglobulin A (sIgA) found in bronchoalveolar lavage fluids of lungs is produced locally within the BALT.11,12 Therefore, in light of the lack of organized lymphoid tissue in the genital tissue, cellular interactions may be less organized and hence the ability of the immune inductive capacity at this mucosal site may be inherently limited. Alternatively, the tissue microenvironment of the vagina may interfere with APC activation. DC isolated from bronchial lymph nodes were recently shown to be distinct from those isolated from the mesenteric lymph nodes, showing that the microenvironment is crucial in shaping DC function.13 Therefore, immune stimulants that induce activation of APC within other mucosal tissues, may have limited effects within the genital tract. As such, local antigen presentation may be severely hampered in the genital tract.
CpG-oligodeoxynucleotide (ODN) is a potent immune stimulator capable of initiating both innate and adaptive immune responses.14,15 In vitro stimulation of monocytes with CpG-ODN leads to their differentiation into DC with strong antigen presenting capacity.16 While CpG-ODN have been shown to induce activation of APC in vivo, and to induce protective adaptive immune responses, the capacity of CpG-ODN to expand tissue APC in vivo remains incompletely understood. In a recent study, treatment of the lung with bacille Calmette–Guèrin, which contains CpG motifs, led to a marked expansion of DC within the lung.17 Furthermore, infection of murine genial tract with thymidine kinase (TK−) mutant herpes simplex virus-2 (HSV-2) also led to a marked recruitment of DC to the vaginal tissue.18 Because HSV-2 DNA has recently been shown to directly stimulate Toll-like receptor-9 (TLR9), it is possible that CpG-ODN stimulation may induce similar APC recruitment.19 Recently, we showed that intravaginal (IVAG) delivery of CpG-ODN led to transient innate immune-mediated protection against genital HSV-2 challenge.20,21 The CpG-ODN-induced protection was accompanied by rapid thickening of vaginal epithelium and significant influx of inflammatory cells to the genital tract.21 More recently, we showed that CpG-ODN can serve as an effective adjuvant following IVAG immunization with a non-replicating viral protein subunit-based vaccine to induce local and systemic immune responses and protection against genital challenge.22 However, the effect of CpG-ODN on expansion of genital APC remains unknown. Therefore, the purpose of this study was to determine the effect of intravaginal (IVAG) delivery of CpG-ODN on expansion of functional resident genital APC. To address this question, tissues were treated with CpG-ODN for varying amounts of time and assessed for the presence of various APC subsets. Our results show that intravaginal CpG-ODN delivery results in a transient but significant expansion of mature macrophages and functional dendritic cells to the vagina.
Materials and methods
Animals
Female C57BL/6 mice (Charles River Canada, St. Constant, Quebec, Canada), 6–8 weeks old, were used for these studies. All mice were maintained in Level B housing conditions in a 12-hr light–dark cycle. All experiments described here were approved by the Animal Research Ethics Board of McMaster University.
Reagents and primary antibodies
RPMI-1640 and fetal bovine serum (FBS) were purchased from Gibco Laboratories (Gibco, Burlington, Canada). Bovine serum albumin (BSA) was purchased from Sigma-Aldrich (Sigma, St. Louis, MO).
For immunohistochemical analysis all antibodies except F4/80 (Serotec, Oxford, UK) were purchased from BD PharMingen (Mississauga, Ontario, Canada). The following primary antibodies were used: purified Armenian hamster IgG1 anti-mouse CD11c, purified rat IgG2b anti-mouse CD11b, purified rat IgG2b anti-mouse F4/80 and biotinylated mouse IgG2a anti-mouse I-Ab, The antibodies were titrated to determine the optimal concentration.
For FACS analysis, all antibodies except F4/80 (Cedarlane Laboratories, Hornby, Ontario, Canada) were purchased from BD PharMingen. The following antibodies were used: phycoerythrin (PE)-conjugated Armenian hamster IgG anti-mouse CD11c, PE-conjugated rat IgG anti-mouse CD11b, Biotin-conjugated rat IgG anti-mouse CD80, Biotin-conjugated rat IgG anti-mouse CD86, Biotin-conjugated rat IgG anti-mouse F4/80, FITC-conjugated mouse IgG anti-mouse I-Ab; all appropriate isotype control antibodies. The antibodies were titrated to determine optimal concentration.
ODN delivery
The immunostimulatory CpG-ODN used in these studies was ODN 1826 which we have previously shown to induce innate immune protection from intravaginally (IVAG) HSV-2 challenge.20,21 Prior to CpG-ODN delivery, mice were subcutaneously inoculated with 2 mg of progesterone/mouse (Depo-Provera; Upjohn, Don Mills, Ontario, Canada) in the upper back using a 291/2 gauge needle. Four days later, mice were anaesthetized with ketamine (150 mg/kg) and xylazine (10 mg/kg) i.p and treated with 25 µg of CpG-ODN IVAG through a micropipette.
Tissue collection, preparation and immunohistochemistry
Groups of mice were killed by cervical dislocation at different time points following CpG-ODN delivery. Untreated mice were used as controls; mice treated with phosphate-buffered saline (PBS) for 24 hr did not differ from untreated mice in their responses (data not shown). Genital tissues (including cervix but not uterine tissue) were dissected, placed in Tissue-Tek (Sakura Finetek USA, Inc. Torrance CA) and immediately snap-frozen by placing the tissue on a culture plate floating on liquid nitrogen. Cryostat sections (8 µm) were air-dried for 24–48 hr, and fixed in cold acetone. Immunohistochemical analysis of vaginal tissue cryostat cross-sections was performed using the immunoperoxidase technique. In order to determine tissue expression of major histocompatibility complex (MHC) class II, CD11b, and F4/80, vaginal sections were incubated successively with: 2% BSA/PBS protein block for 20 min, 2% hydrogen peroxide/PBS for 10 min to block endogenous peroxidase, primary monoclonal antibodies, secondary multiple adsorbed biotinylated rabbit anti-rat IgG (Vector Laboratories, Inc. Burlingame, CA) for 1 hr (omitted for biotinylated Ms IgG2a anti-mouse I-Ab) and horseradish peroxidase-conjugated Avidin (Vector Laboratories) for 40 min in a humidified chamber at room temperature. In between each of the steps above, sections were washed three times with 0·1% BSA/PBS. Colour development of the antibody treated tissues was performed with 5% of a stock 3-amino-9-ethyl-carbazole solution (AEC, Sigma) and 0·07% hydrogen peroxide in AEC buffer (0·05 m sodium acetate, 0·02 m acetic acid; pH 5·0) for 15–30 min Sections were counterstained with aqueous haematoxylin (Biomeda corp.), and mounted with Crystal Mount (Sigma). In order to assess CD11c expression in the vaginal tissues, we needed to perform a signal amplification protocol, and thus we slightly modified the protocol mentioned above. First we increased the specificity of the anti-CD11c antibody by incubating it overnight with 10% mouse serum at 4°. Furthermore, the protein block in this case consisted of 2%BSA/15% goat serum. Following 1 hr incubation with CD11c, the tissues washed and treated with purified rabbit anti-hamster IgG antibody (Southern Biotechnology Associates, Birmingham, AL) for an additional hour. Finally, the tissues were treated with the anti rabbit envision system (DakoCytomation, Mississauga, Ontario, Canada) for 20 min The tissues were then developed with AEC and counterstained with aqueous haematoxylin as described above.
Isolation and fluorescence-activated cell sorting (FACS) analysis of genital cells
Groups of mice (5–10 mice/group) treated with IVAG–CpG-ODN for various lengths of time were killed and their tissues processed for FACS analysis. Untreated mice were used as controls. Specifically, genital tissues were dissected, placed in RPMI media on ice and cut into small (≈2 mm diameter) pieces and agitated at room temperature for 15 min in 15 ml of 0·05% ethylenediaminetetraacetic acid. Subsequently, the tissues were agitated at 37° for 1 hr in 15 ml collagenase A (Life Technologies, Rockville, MD) at a concentration of 150 U/ml in RMPI-1640 supplemented with 10% FBS, 1% penicillin-streptomycin, and l-glutamine (Gibco). The collagenase step was repeated, and the remaining genital pieces were triturated using the plunger from a 5-ml syringe through a 70-µm filter screen into RPMI. The resulting cell suspension was filtered through once more through a 40-µm filter screen. These cells were next washed twice with 0·4% BSA/PBS and then counted using a haemocytometer. The cells were re-suspended at a concentration of 0·5–1 × 106 cells/100 µl and treated with an anti-Fc Receptor antibody (BD PharMingen) for 15 min at 4°. In order to assay the expression of cell surface markers on the isolated genital cells, 0·5–1·0 × 106 cells were incubated with biotin or fluorochrome-conjugated monoclonal antibodies for 30 min at 4° in the dark. Samples treated with biotinylated antibodies were treated with Streptavidin Cy-Chrome (BD PharMingen) for an additional 30 min at 4° in the dark. Data were collected using a FACScan flow cytometer (Becton Dickinson, Sunnyvale, CA), and were analyzed using WinLIST software (Verity Software House, Topsham, ME).
Allogenic mixed lymphocyte reaction (MLR)
Genital tissues of either naïve mice or mice treated with IVAG CpG-ODN for 48 hr were isolated and irradiated (2000 rad) in Gammacell 1000 gamma-irradiator (MDS Nordion, Kanata, Ontario, Canada). Next, these genital cells were used to stimulate allogenic (BALB/c H-2d) splenocytes in U-bottomed 96-well plates (Invitrogen, Burlington, Ontario, Canada). Between 1500 and 300 000 irradiated stimulator cells in 100 µl were added per well to 800 000 splenocytes in 100 µl of complete medium. Control wells contained either splenocytes or genital cells alone. After 4 days of incubation at 37° and 5% CO2, 1 µCi of tritiated thymidine (PerkinElmer, Woodbridge, Ontario, Canada) was added to each well. After 26 hr, proliferation was determined by measuring radioactivity in a scintillation counter.
Statistical analysis
Data are expressed as mean ± SD for thymidine incorporation quantified proliferation and mean ± SEM for cell counts of differentially stimulated vaginal tissue. Statistical interpretation was performed using a two-sample t-test. Differences were considered statistically significant when P < 0·05.
Results
Vaginal CpG-ODN delivery induces increased inflammatory infiltrating cells in the genital tissue
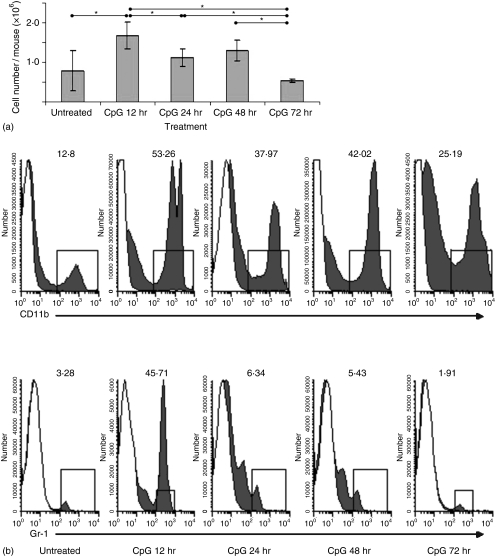
We previously showed that IVAG CpG-ODN delivery induced significant vaginal inflammation.21 To assess the time course of IVAG CpG-ODN-induced inflammation within the genital tissue, genital tissues of mice treated with 25 µg of CpG-ODN for 12, 24, 48 and 72 hr were removed, digested in collagenase A and counted. IVAG CpG-ODN resulted in a significantly higher number of cells in the genital tissue at 12 hr when compared to tissue from PBS-treated animal (P = 0·0075; Fig. 1a). Although significantly greater numbers when compared to PBS-treated animals were not observed thereafter, the trend for increased cellularity in the genital tract following CpG-ODN delivery was evident until the 48 hr time-point. Resolution of inflammation, with regards to total cellularity, was evident at 72 hr post IVAG CpG-ODN delivery since cell counts were indistinguishable from those observed in PBS-treated animals. To assess the kinetics of polymorphonuclear cell and neutrophil infiltration, we stained the single-cell suspensions of above described tissues with CD11b and Gr-1 antibodies (Fig. 1b). Our results show that the majority of the cells responsible for the increased cellularity following IVAG CpG-ODN are CD11b positive cells, which are considerably elevated throughout all time-points tested, with the peak observed between 12 and 48 hr. In contrast to other time-points, only the 12 hr time-point exhibited a marked elevation in neutrophil numbers, as assessed by high surface expression of Gr-1, indicating that while neutrophils were responsible for the majority of the inflammation at the 12 hr time-point, they were not the key inflammatory cells at other time-points tested.
Figure 1.
(a) Effects of vaginal CpG-ODN delivery on inflammatory cell numbers in the vagina. Groups of mice were treated with a single injection of Depo-Provera. On day 4 after Depo-Provera treatment, groups of mice were either killed immediately or killed at 12, 28, 48 or 72 hr following vaginal delivery of CpG-ODN. For each time point, vaginal tissues (including cervix but not the uterus) from at least five mice were pooled, digested and counted as described in Materials and methods). Cell counts are expressed as the number of cells/mouse. Error bars indicate the SEM of at least four separate experiments for all time-points except the 72 hr time-point, which was carried out twice. (b) Flow cytometric analysis was performed on differentially treated genital tissues as described in Materials and methods. White histogram plots indicate the staining obtained by an isotype-control antibody, while the dark histograms represent the staining of the specified antibody. The numbers indicated percentage of positive cells within the leukocyte subpopulation. Antibody-specific values are deemed positive if the expression levels are higher than 5% of the isotype control antibody.
Vaginal CpG-ODN delivery induces a rapid increase in APC within the genital tract
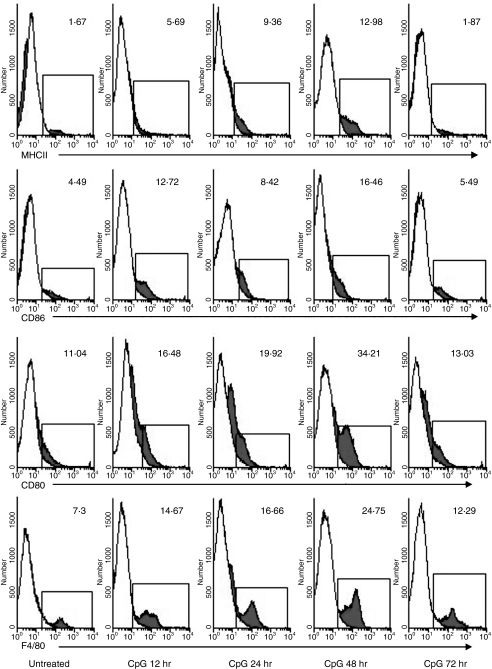
Next, we sought to determine the ability of vaginal CpG-ODN to expand the resident APC population. To this end, genital tissue of mice treated with IVAG CpG-ODN for various periods of time were isolated, digested and analysed by FACS for expression levels of several APC-related cell-surface markers. Maximal levels of cells expressing the typical APC markers, namely MHC class II as well as CD80 and CD86 costimulatory molecules were observed at 48 hr post IVAG CpG-ODN delivery (Fig. 2). Interestingly, the enhanced expression of these cell surface markers was transient, since the levels of the above-mentioned markers returned to levels that were not distinguishable from PBS-treated tissue as early as 72 hr post IVAG CpG-ODN delivery.
Figure 2.
Infiltration kinetics of APC into vaginal tissue following vaginal CpG-ODN delivery. Groups of mice were treated with a single injection of Depo-Provera. On day 4 after Depo-Provera treatment, groups of mice were either killed immediately or killed at 12, 28, 48 and 72 hr following vaginal delivery of CpG-ODN. Immediately thereafter, vaginal tissues (including cervix but not the uterus) from at least five mice were digested, stained for flow cytometry and analysed with WINLIST software as described in Materials and methods. White histogram plots indicate the staining obtained by an isotype-control antibody, while the dark histograms represent the staining of the specified antibody. The numbers indicated percent of positive cells within the leucocyte subpopulation. Antibody specific values are deemed positive if the expression levels are higher than 5% of the isotype-control antibody.
Vaginal CpG-ODN delivery induces a rapid increase in the number of CD11b and F4/80 APC in the genital tissue
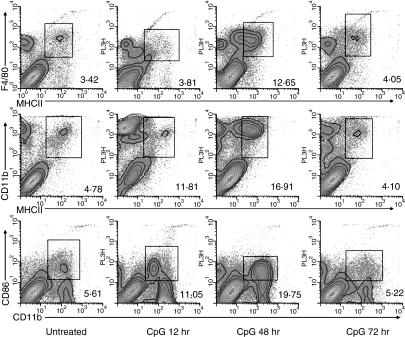
It is becoming increasingly clear that APC subsets differ in their ability to shape subsequent immune responses.23 Therefore, having established the rapid expansion of APC in the genital tissue following local CpG-ODN delivery, we next sought to determine the phenotype of these APC. Our results indicate that the first subset of APCs to be increased in the genital tissue following IVAG CpG-ODN delivery were the F4/80+ macrophages, which were up-regulated at 12 hr post IVAG CpG-ODN delivery (Fig. 2). Interestingly, the majority of the macrophages at this time-point were MHC class II negative (Fig. 3), although a marked increase in the MHC class II expression by F4/80-positive macrophages was observed at 48 hr post IVAG CpG-ODN delivery (Fig. 3). This initial enhancement of macrophages was followed by a marked increase in the level of MHC class II/CD11b double-positive cells into the genital tissue, with maximal levels observed 48 hr post CpG-ODN stimulation (Fig. 3). Coinciding with these results, the maximal level of CD11b-positive cells coexpressing a costimulatory molecule, CD86, were observed 48 hr post IVAG CpG-ODN delivery (Fig. 3). In contrast, we did not observe an increase in lymphoid tissue restricted MHC class II/CD8α double-positive cells at any of the time points tested (data not shown).
Figure 3.
Subset of APCs infiltrating the vaginal tissue following IVAG CpG-ODN delivery. Four days following Depo-Provera treatment, groups of mice were either killed immediately or killed at 12, 48 and 72 hr following vaginal delivery of CpG-ODN. Immediately thereafter, vaginal tissues (including cervix but not the uterus) from at least five mice were digested, stained for flow cytometry and analysed with WINLIST software as described in Materials and methods. The numbers shown are representative of cells that coexpress both of the markers in the dotplot and indicate the percent of positive cells within the leukocyte subpopulation.
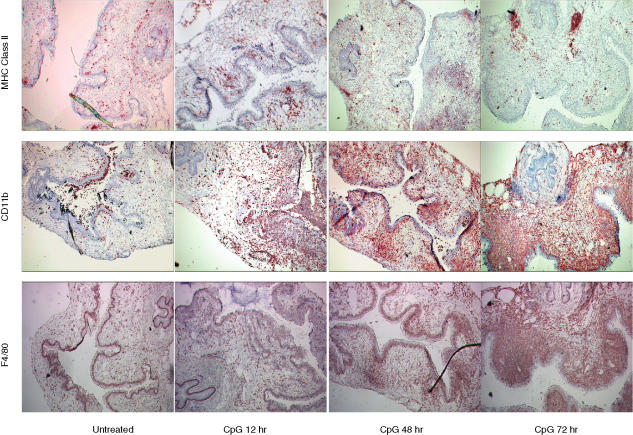
To assess the distribution and localization of APC subsets within the tissue, we performed immunohistochemical analysis on frozen vaginal tissue sections. At 12 hr post IVAG CpG-ODN delivery, MHC class II staining appeared to cluster beneath the epithelial surface, while at the 28 hr time point we observed marked focal staining of the epithelial layer. Interestingly, no epithelial MHC class II staining was observed at 48 hr post IVAG CpG-ODN (Fig. 4). Instead, we observed strong and focal MHC class II staining at the genital–urethral border. CD11b and F4/80 staining was observed within both the epidermal and dermal layers at all tested time-points, with the maximal levels of both markers observed 48 hr post IVAG CpG-ODN delivery.
Figure 4.
Distribution/localization of APCs within the vaginal tract following IVAG CpG-ODN delivery. Four days following Depo-Provera treatment, groups of mice were either killed immediately or killed at 12, 28 or 48 hr following vaginal delivery of CpG-ODN. Immediately thereafter, vaginal tissues were collected, placed in OCT and frozen in liquid nitrogen. Immunohistochemical analysis of MHC class II, CD11b and F4/80 was performed on 8 µm cryostat sections using the immunoperoxidase technique. Images were captured using a 4× objective lens. Average sections, representative of at least three independent experiments using at least three mice/group are shown.
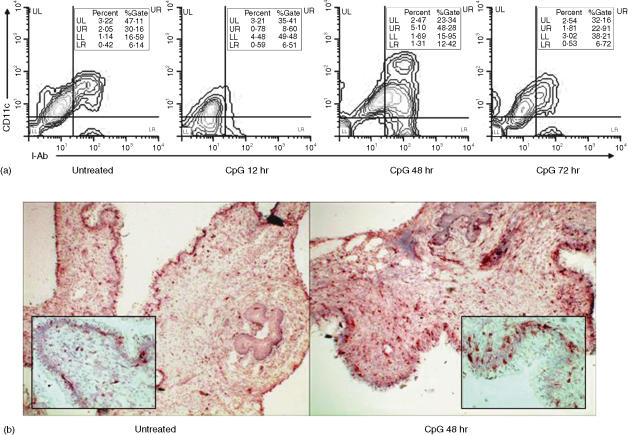
Delayed expansion of CD11c-positive cells in the genital tract following vaginal CpG-ODN stimulation
It is well known that monocytes can be induced to differentiate into DC following in vitro stimulation with IL-4 and GM-CSF.24 Recently, Gursel et al. showed that in vitro treatment of human blood monocytes with CpG-ODN resulted in the differentiation of these cells into DC.25 To assess the ability of CpG-ODN to expand DC in the genital tissue following local administration, we assessed the phenotype of cells expressing the CD11c marker by immunohistochemistry and multicolour FACS analysis. For FACS analysis, cells were considered to be DCs based on triple staining. Specifically, CD86 gated cells were assayed for the coexpression of both MHC class II and CD11c. Our results show that the highest levels of dendritic cells were observed at 48 hr post IVAG CpG-ODN delivery; although this up-regulation was preceded by decreased levels at 12 hr post CpG-ODN delivery (Fig. 5a).
Figure 5.
Infiltration kinetics of CD11c-positive cells into the vaginal tissue following vaginal CpG-ODN delivery. (a) Four days following Depo-Provera treatment, groups of mice were either killed immediately or killed at 12, 28 or 48 hr following vaginal delivery of CpG-ODN. Immediately thereafter, vaginal tissues (including cervix but not the uterus) from at least five mice were digested, stained for flow cytometry and analysed with WINMDI software as described in Materials and methods. During the analysis of the acquired data we first gated the leucocyte population on CD86+ cells, and then analysed for the expression of CD11c and MHC class II cell surface markers. Percent of gate values indicate the percentage of the cells positive for each marker among the CD86+ cells, while the percent values indicate the percentage of CD11c/MHC class II/CD86 triple-positive cells among the total cell population. (b) Four days following Depo-Provera treatment, groups of mice were either killed immediately or killed at 48 hr following vaginal delivery of CpG-ODN. Immediately thereafter, vaginal tissues were collected, placed in OCT and frozen in liquid nitrogen. Immunohistochemical analysis of CD11c was performed on 8 µm cryostat sections using the immunoperoxidase technique. Images were captured using either a 4× objective lens or a 20× objective lens (inset). Average sections, representative of at least three independent experiments using at least three mice/group are shown.
Coinciding with our FACS data, immunohistochemical analysis of frozen tissue sections for CD11c expression revealed a marked enhancement in the level of CD11c positive cells at 48 hr post IVAG CpG-ODN when compared to animals treated with PBS alone (Fig. 5b). These cells had an irregular appearance and morphology typically associated with dendritic cells with numerous cells clearly exhibiting dendritic processes (Fig. 5b, inset).
Increased functionality of genital APCs following IVAG CpG-ODN delivery
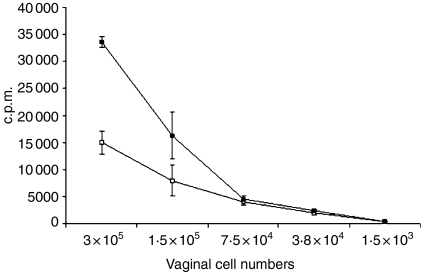
Finally, in order to show the functional capacity of CpG-ODN induced vaginal APC, we performed a MLR experiment. Results from the MLR experiment confirm the increased levels of APC at 48 hr post IVAG CpG-ODN delivery when compared to PBS-treated tissues. Following incubation of equal numbers of irradiated vaginal cells from either PBS-treated mice or mice treated with IVAG CpG-ODN for 48 hr, a significant increase (P = 0·0002) in the ability of vaginal cells from the CpG-ODN-treated group to induce cellular proliferation was observed (Fig. 6).
Figure 6.
Functional capacity of CpG-induced APCs in the genital tract. Four days following Depo-Provera treatment, groups of mice were either killed immediately (□) or killed 48 hr following vaginal delivery of CpG-ODN (▪). Immediately thereafter, vaginal tissues (including cervix but not the uterus) from at least five mice were digested and plated on 96-well plates in indicated numbers. Subsequently, 8 × 105 splenocytes from autologous BALB/c mice were administered to each well. Proliferation of T cells was measured by means of 3H thymidine incorporation after 5 days of coculture. Results are mean c.p.m. ± SD of triplicate cultures
Discussion
The purpose of the present study was to investigate the ability of intravaginal delivery of CpG-ODN to expand antigen presenting cells (APC) within the genital tissue. CpG-ODNs are synthetic oligodeoxynucleotides that express certain immunostimulatory motifs normally found within bacterial DNA; these motifs exert their immunostimulatory effects upon ligation of TLR-9.26 Previous reports have demonstrated that in vitro stimulation of APC by CpG-ODN leads to the up-regulation of costimulatory molecules and enhanced antigen presenting capacity. However, the ability of CpG-ODN to expand APC numbers in vivo, particularly in the genital tract, has not been well studied. Our laboratory and others have previously shown that administration of CpG-ODN alone to the vaginal mucosa is sufficient to induce protective innate immunity against HSV-2 challenge.20,27,28 Data presented here clearly show that IVAG CpG-ODN delivery results in a significant but transient expansion of antigen presenting cells within the vaginal tissue. In particular, a rapid, but transient up-regulation of cells expressing MHC class II as well as CD80 and CD86 costimulatory molecules was observed. Maximal levels of cells expressing these typical APC markers were observed at 48 hr post IVAG CpG-ODN delivery, rapidly subsiding thereafter (Figs 3 and 4). Our findings are consistent with previous reports showing rapid recruitment of DCs to mucosal sites after exposure to a variety of nonreplicating immune stimulants.29–33
Although these previous reports have described methods for increasing APC within mucosal sites, those results cannot be directly applied to the genital tissue. First, unlike other mucosal tissues, the level and function of APC in the genital tract is under hormonal regulation; high oestradiol levels have been show to inhibit antigen presenting functions, while the inhibitory effects of oestradiol are reversed with the administration of progesterone.34 Additionally, it is becoming increasingly clear that the tissue-specific microenvironment plays a pivotal role in shaping the nature of the subsequent immune response.35 Specifically, in vitro stimulation of APC obtained from different tissues with identical stimuli does not result in identical immune responses.13,36,37 As a corollary, in vivo enhancement of APC with one agent would not necessarily guarantee enhancement in all tissues.
Vaginal tissue has been shown to contain various APC subsets including epidermal DC, dermal DC, and dermal macrophages. In our model, the first subset of APC that were expanded within the vaginal tissue following IVAG CpG-ODN delivery were F4/80+/MHC class II− macrophages, which were markedly elevated as early as 12 hr post IVAG CpG-ODN delivery. In contrast, the level of DC in the vaginal tissue, typified by coexpression of CD86, MHC class II, and CD11c markers appeared to decrease at this time-point. This finding is consistent with previous reports illustrating the migration of tissue DC to regional lymphoid organs upon activation. Interestingly, the highest level of DCs following CpG-ODN delivery was observed at 48 hr post delivery, implying that a second wave of DC were recruited following the migration of the initial subset. Immunohistochemical studies with an anti-CD11c antibody confirmed these results and showed that cells with dendritic morphology were maximal in both the epidermal and dermal layers at 48 hr post IVAG CpG-ODN delivery. That the up-regulation of CD11c+ cells with dendritic morphology within the epidermal and dermal layers is preceded by an influx of monocyte/macrophages suggests that macrophages may play a critical role in the expansion of DC within the tissue by either differentiating into DC in situ, or through secretion of DC chemotactic factors. Although monocytes obtained from peripheral blood mononuclear cells differentiate into DC upon stimulation with granulocyte–macrophage colony-stimulating factor, interleukin-4 (IL-4) and transforming growth factor-α1) (TGFα1)38in vitro, less information is available under in vivo conditions. A study by Kradin et al. suggested that macrophages have the capacity to differentiate into DC within the lung, as a subpopulation of lung exudate macrophages expressed cell surface markers typically associated with DC, and was capable of stimulating T-cell responses.39 Whether and to what extent this process occurs following CpG-ODN administration in genital tissue is not presently known. Our finding that the majority of IVAG CpG-ODN expanded APC in the genital tissue are of the CD11b myeloid origin is consistent with a recent report by Zhao et al., whereby vaginal delivery of a rapidly resolving TK− mutant HSV-2 virus, which cannot cause neurological disease in mice, resulted in a significant expansion of CD11c+/CD11b+ DC within the vaginal tissue.18 The ability of the TK− mutant virus to induce protective immunity from subsequent wild-type HSV-2 infections in mice40–42 may be linked to its ability to expand APC numbers in the vaginal tissue. However, TK− mutant virus is pathogenic in humans, causing acute keratitis and establishing latency in trigeminal ganglion, and hence cannot be used clinically.43 Interestingly, HSV-2 DNA has recently been shown to directly stimulate TLR9, suggesting that both TK− HSV-2 and IVAG CpG-ODN alone may mediate the up-regulation of vaginal DC through a common TLR9-mediated pathway. To this end, CpG-ODN delivery may well be a viable means of expanding mucosal tissue APC in immuno-compromised individuals in a clinical setting. Further studies are required to dissect the exact mechanisms responsible for the up-regulation of DC within both models.
Recent studies from our lab have shown that CpG-ODN serves as an effective mucosal adjuvant with non-replicating subunit viral protein antigen when delivered intravaginally.22 Although the mechanism remains incompletely understood, the efficacy of CpG-ODN as an adjuvant within the vaginal tissue may lie in its ability to increase the level of resident APC. To assess the functional capacity of DC within the vaginal tissue at 48 hr post IVAG CpG-ODN we performed a MLR. Indeed, vaginal cells isolated 48 hr post IVAG CpG-ODN delivery were able to efficiently activate naïve allogenic splenic T cells in an MLR to a significantly higher extent than vaginal cells from PBS-treated animals. Therefore, our results indicate that these cells have enhanced capacity to induce cellular activation within the vaginal tissue.
Previous studies investigating the expansion of APCs within the vaginal tissues have utilized live replicating pathogens.18,46 To our knowledge, our study is the first to describe enhanced numbers and function of APC within genital tissue following administration of a TLR-activating ligand. Although it is clear that the major function of APC lies in their ability to initiate adaptive immune responses within the tissue draining lymph nodes, several lines of evidence suggest that the immune inductive capacity of APC is not limited to the lymphoid organs or adaptive immune cells.2,3 In the genital tissue, protection against Chlamydia trachomatis is associated with recruitment of MHC class II-positive cells to the uterine tissue.44 IVAG CpG-ODN has been shown by our lab and others to induce protection from IVAG HSV-2 challenge only when given within 2 days before HSV-2 challenge.20,42 Specifically, we have shown that IVAG CpG-ODN delivery 1 day prior to HSV-2 challenge is protective, while challenge at 3 days post CpG-ODN is not.20 Similarly, Harandi et al. have shown that CpG-ODN 2 days prior to IVAG HSV-2 challenge is protective27 a finding that we have confirmed in our lab (data not shown). Taken together with the results from our current study, where we observed the resolution of the IVAG CpG-ODN-mediated increase in vaginal APCs on day 3 post delivery, it appears that the kinetics of APC enhancement in the genital tissue correlates with IVAG CpG-ODN mediated protection from IVAG HSV-2 challenge. Thus, enhanced vaginal APC may play a role in the CpG-ODN-induced protection against IVAG HSV-2 challenge. As the prevalence of sexually transmitted diseases has not significantly declined despite improvements in detection methods and the effects of STD control programs45,46 strategies for optimizing immune responses in the genital tissue are of considerable interest. We believe that the IVAG CpG-ODN mediated increase in functional APC within the vaginal tissue may favour the induction of immune responses, both innate and adaptive, against sexually transmitted pathogens that infect the genital tract. A potential caveat to this approach is that the expansion of genital DC may enhance susceptibility to pathogens that exploit DC to increase their infectivity. Specifically, human and primate DC express DC-SIGN, a surface molecule to which human immunodeficiency virus (HIV) attaches, and thereby facilitates the trans infection of CD4+ T-cell targets following DC migration to the lymph node.47 However, several reports have shown that the expression of DC-SIGN can be regulated by the prevailing tissue microenvironment. Indeed, T helper 1 (Th1) cytokines such as interferon-α (IFN-α), tumour necrosis factor-α and IFN-γ, as well as TGF-β have been shown to down-regulate the expression of DC-SIGN, while Th2 cytokines IL-4 and IL-13 have been shown to induce it.48,49 Because CpG-ODN is a potent stimulant for Th1 cytokine production, the ability of CpG-ODN to down-regulate the expression of DC-SIGN and thus suppress the DC mediated transmission of HIV warrants further investigation. That another TLR ligand, LPS, has been recently shown to decrease the expression of DC-SIGN following in vitro stimulation of DC adds further support to this hypothesis.48 Future studies are needed to establish if the transient increase in APC numbers in the genital tissue following IVAG CpG-ODN is sufficient and necessary for induction of protective responses against sexually transmitted infections.
Acknowledgments
We are grateful to Mary Jo Smith for technical assistance with immunohistochemistry and Janina Q. Jiang for advice regarding isolation of mononuclear cells from the genital tract. We also thank Dr Ali A. Ashkar for critical reading of the manuscript. This work was supported by research grants from the Canadian Institutes for Health Research (CIHR) and the Canadian Network for Vaccines & Immunotherapeutics (CANVAC). D.S. is the recipient of the Ontario Graduate Scholarship Award (OGS). K.L.R. is supported by a Career Scientist Award from the Ontario HIV Treatment Network (OHTN).
Abbreviations
- ODN
oligodeoxynucleotide
- MALT
mucosal-associated lymphoid tissues
- IVAG
intravaginal
- TLR
Toll-like receptor
References
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271. doi: 10.1146/annurev.iy.09.040191.001415. 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Constant SL, Brogdon JL, Piggott DA, Herrick CA, Visintin I, Ruddle NH, Bottomly K. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J Clin Invest. 2002;110:1441. doi: 10.1172/JCI16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez NC, Lozier A, Flament C, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405. doi: 10.1038/7403. 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 4.Bienenstock J, Johnston N, Perey DY. Bronchial lymphoid tissue. I. Morphologic characteristics. Lab Invest. 1973;28:686. [PubMed] [Google Scholar]
- 5.Bienenstock J, Johnston N, Perey DY. Bronchial lymphoid tissue. II. Functional characterisitics. Lab Invest. 1973;28:693. [PubMed] [Google Scholar]
- 6.McSorley SJ, Asch S, Costalonga M, Reinhardt RL, Jenkins MK. Tracking salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365. doi: 10.1016/s1074-7613(02)00289-3. 10.1016/S1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 7.Toyoshima M, Chida K, Sato A. Antigen uptake and subsequent cell kinetics in bronchus-associated lymphoid tissue. Respirology. 2000;5:141. doi: 10.1046/j.1440-1843.2000.00241.x. 10.1046/j.1440-1843.2000.00241.x. [DOI] [PubMed] [Google Scholar]
- 8.Gallichan WS, Rosenthal KL. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J Exp Med. 1996:184–1879. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giuliani MM, Del Giudice G, Giannelli V, Dougan G, Douce G, Rappuoli R, Pizza M. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J Exp Med. 1998;187:1123. doi: 10.1084/jem.187.7.1123. 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell MW. Immunization for protection of the reproductive tract: a review. Am J Reprod Immunol. 2002;47:265. doi: 10.1034/j.1600-0897.2002.01099.x. 10.1034/j.1600-0897.2002.01099.x. [DOI] [PubMed] [Google Scholar]
- 11.Burnett D. Immunoglobulins in the lung. Thorax. 1986;41:337. doi: 10.1136/thx.41.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastian A, Tunkel C, Lins M, Bottcher H, Hirt SW, Cremer J, Bewig B. Immunoglobulin A and secretory immunoglobulin A in the bronchoalveolar lavage from patients after lung transplantation. Clin Transplant. 2000;14:580. doi: 10.1034/j.1399-0012.2000.140611.x. [DOI] [PubMed] [Google Scholar]
- 13.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725. doi: 10.1038/90667. 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 14.Ashkar AA, Rosenthal KL. Toll-like receptor 9, CpG DNA and innate immunity. Curr Mol Med. 2002;2:545. doi: 10.2174/1566524023362159. [DOI] [PubMed] [Google Scholar]
- 15.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709. doi: 10.1146/annurev.immunol.20.100301.064842. 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 16.Ichikawa HT, Williams LP, Segal BM. Activation of APCs through CD40 or Toll-like receptor 9 overcomes tolerance and precipitates autoimmune disease. J Immunol. 2002;169:2781. doi: 10.4049/jimmunol.169.5.2781. [DOI] [PubMed] [Google Scholar]
- 17.Lagranderie M, Nahori MA, Balazuc AM, Kiefer-Biasizzo H, Lapa e Silva JR, Milon G, Marchal G, Vargaftig BB. Dendritic cells recruited to the lung shortly after intranasal delivery of Mycobacterium bovis BCG drive the primary immune response towards a type 1 cytokine production. Immunology. 2003;108:352. doi: 10.1046/j.1365-2567.2003.01609.x. 10.1046/j.1365-2567.2003.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153. doi: 10.1084/jem.20021109. 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513. doi: 10.1084/jem.20030162. 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sajic D, Ashkar AA, Patrick AJ, McCluskie MJ, Davis HL, Levine KL, Holl R, Rosenthal KL. Parameters of CpG oligodeoxynucleotide-induced protection against intravaginal HSV-2 challenge. J Med Virol. 2003;71:561. doi: 10.1002/jmv.10518. 10.1002/jmv.10518. [DOI] [PubMed] [Google Scholar]
- 21.Ashkar AA, Bauer S, Mitchell WJ, Vieira J, Rosenthal KL. Local delivery of CpG oligodeoxynucleotides induces rapid changes in the genital mucosa and inhibits replication, but not entry, of herpes simplex virus type 2. J Virol. 2003;77:8948. doi: 10.1128/JVI.77.16.8948-8956.2003. 10.1128/JVI.77.16.8948-8956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwant AR, Rosenthal KL. Intravaginal immunization with viral subunit protein plus CpG oligodeoxynucleotides induces protective immunity against HSV-2. Vaccine. 2004;22:3108. doi: 10.1016/j.vaccine.2004.01.059. [DOI] [PubMed] [Google Scholar]
- 23.Palucka K, Banchereau J. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr Opin Immunol. 2002;14:420. doi: 10.1016/s0952-7915(02)00365-5. 10.1016/S0952-7915(02)00365-5. [DOI] [PubMed] [Google Scholar]
- 24.Jackson SH, Alicea C, Owens JW, Eigsti CL, Malech HL. Characterization of an early dendritic cell precursor derived from murine lineage-negative hematopoietic progenitor cells. Exp Hematol. 2002;30:430. doi: 10.1016/s0301-472x(02)00792-0. 10.1016/S0301-472X(02)00792-0. [DOI] [PubMed] [Google Scholar]
- 25.Gursel M, Verthelyi D, Klinman DM. CpG oligodeoxynucleotides induce human monocytes to mature into functional dendritic cells. Eur J Immunol. 2002;32:2617. doi: 10.1002/1521-4141(200209)32:9<2617::AID-IMMU2617>3.0.CO;2-F. 10.1002/1521-4141(200209)32:9<2617::AID-IMMU2617>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740. doi: 10.1038/35047123. 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 27.Harandi AM, Eriksson K, Holmgren J. A protective role of locally administered immunostimulatory CpG oligodeoxynucleotide in a mouse model of genital herpes infection. J Virol. 2003;77:953. doi: 10.1128/JVI.77.2.953-962.2003. 10.1128/JVI.77.2.953-962.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyles RB, Higgins D, Chalk C, Zalar A, Eiden J, Brown C, Van Nest G, Stanberry LR. Use of immunostimulatory sequence-containing oligonucleotides as topical therapy for genital herpes simplex virus type 2 infection. J Virol. 2002;76:11387. doi: 10.1128/JVI.76.22.11387-11396.2002. 10.1128/JVI.76.22.11387-11396.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahnsen FL, Lund-Johansen F, Dunne JF, Farkas L, Haye R, Brandtzaeg P. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J Immunol. 2000;165:4062. doi: 10.4049/jimmunol.165.7.4062. [DOI] [PubMed] [Google Scholar]
- 30.McWilliam AS, Nelson D, Thomas JA, Holt PG. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med. 1994;179:1331. doi: 10.1084/jem.179.4.1331. 10.1084/jem.179.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McWilliam AS, Napoli S, Marsh AM, et al. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med. 1996;184:2429. doi: 10.1084/jem.184.6.2429. 10.1084/jem.184.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jahnsen FL, Moloney ED, Hogan T, Upham JW, Burke CM, Holt PG. Rapid dendritic cell recruitment to the bronchial mucosa of patients with atopic asthma in response to local allergen challenge. Thorax. 2001;56:823. doi: 10.1136/thorax.56.11.823. 10.1136/thorax.56.11.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Etchart N, Desmoulins PO, Chemin K, Maliszewski C, Dubois B, Wild F, Kaiserlian D. Dendritic cells recruitment and in vivo priming of CD8+ CTL induced by a single topical or transepithelial immunization via the buccal mucosa with measles virus nucleoprotein. J Immunol. 2001;167:384. doi: 10.4049/jimmunol.167.1.384. [DOI] [PubMed] [Google Scholar]
- 34.Wira CR, Rossoll RM. Antigen-presenting cells in the female reproductive tract: influence of sex hormones on antigen presentation in the vagina. Immunology. 1995;84:505. [PMC free article] [PubMed] [Google Scholar]
- 35.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151. doi: 10.1146/annurev.iy.13.040195.001055. 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 36.Kelsall BL, Biron CA, Sharma O, Kaye PM. Dendritic cells at the host–pathogen interface. Nat Immunol. 2002;3:699. doi: 10.1038/ni0802-699. 10.1038/ni0802-699. [DOI] [PubMed] [Google Scholar]
- 37.Hasseus B, Jontell M, Bergenholtz G, Eklund C, Dahlgren UI. Langerhans cells from oral epithelium are more effective in stimulating allogeneic T-cells in vitro than Langerhans cells from skin epithelium. J Dent Res. 1999;78:751. doi: 10.1177/00220345990780030701. [DOI] [PubMed] [Google Scholar]
- 38.Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med. 1998;187:961. doi: 10.1084/jem.187.6.961. 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kradin RL, Sakamoto H, Preffer FI, Dombkowski D, Springer KM, Leary CP. Accumulation of macrophages with dendritic cell characteristics in the pulmonary response to Listeria. Am J Respir Crit Care Med. 2000;161:535. [PubMed] [Google Scholar]
- 40.McDermott MR, Smiley JR, Leslie P, Brais J, Rudzroga HE, Bienenstock J. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J Virol. 1984;51:747. doi: 10.1128/jvi.51.3.747-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parr MB, Parr EL. Protective immunity against HSV-2 in the mouse vagina. J Reprod Immunol. 1997;36:77. doi: 10.1016/s0165-0378(97)00055-7. 10.1016/S0165-0378(97)00055-7. [DOI] [PubMed] [Google Scholar]
- 42.Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Differential roles of B cells and IFN-gamma-secreting CD4 (+) T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J Gen Virol. 2001;82:845. doi: 10.1099/0022-1317-82-4-845. [DOI] [PubMed] [Google Scholar]
- 43.Stroop WG, Banks MC, Qavi H, Chodosh J, Brown SM. A thymidine kinase deficient HSV-2 strain causes acute keratitis and establishes trigeminal ganglionic latency, but poorly reactivates in vivo. J Med Virol. 1994;43:297. doi: 10.1002/jmv.1890430319. [DOI] [PubMed] [Google Scholar]
- 44.Stagg AJ, Tuffrey M, Woods C, Wunderink E, Knight SC. Protection against ascending infection of the genital tract by Chlamydia trachomatis is associated with recruitment of major histocompatibility complex class II antigen-presenting cells into uterine tissue. Infect Immun. 1998;66:3535. doi: 10.1128/iai.66.8.3535-3544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cates W., Jr Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. American Social Health Association Panel. Sex Transm Dis. 1999;26:S2. doi: 10.1097/00007435-199904001-00002. [DOI] [PubMed] [Google Scholar]
- 46.Leone P, Fleming DT, Gilsenan AW, Li L, Justus S. Seroprevalence of herpes simplex virus-2 in suburban primary care offices in the United States. Sex Transm Dis. 2004;31:311. doi: 10.1097/01.olq.0000123651.84697.d6. [DOI] [PubMed] [Google Scholar]
- 47.Curtis BM, Scharnowske S, Watson AJ. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc Natl Acad Sci USA. 1992;89:8356. doi: 10.1073/pnas.89.17.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puig-Kroger A, Serrano-Gomez D, Caparros E, Dominguez-Soto A, Relloso M, Colmenares M, Martinez-Munoz L, Longo N, Sanchez-Sanchez N, Rincon M, Rivas L, Sanchez-Mateos P, Fernandez-Ruiz E, Corbi AL. Regulated expression of the pathogen receptor dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin in THP-1 human leukemic cells, monocytes, and macrophages. J Biol Chem. 2004;279:25680. doi: 10.1074/jbc.M311516200. 10.1074/jbc.M311516200. [DOI] [PubMed] [Google Scholar]
- 49.Relloso M, Puig-Kroger A, Pello OM, Rodriguez-Fernandez JL, de la Rosa G, Longo N, Navarro J, Munoz-Fernandez MA, Sanchez-Mateos P, Corbi AL. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J Immunol. 2002;168:2634. doi: 10.4049/jimmunol.168.6.2634. [DOI] [PubMed] [Google Scholar]