Abstract
Vibrio vulnificus, a Gram-negative estuarine bacterium, is a causative agent of food-borne diseases, such as life-threatening septicaemia and wound infection disease. V. vulnificus penetrating into the epithelial barrier stimulates an inflammatory response in the adjacent mucosa. Therefore, interaction between V. vulnificus and epithelial cells is important for understanding of both the immunology of mucosal surfaces and V. vulnificus. In this study, we investigated the effect and action mechanism of V. vulnificus infection on production of interleukin (IL)-8, a proinflammatory cytokine, in human intestinal epithelial INT-407 cells. V. vulnificus infection significantly induced IL-8 production in a time- and multiplicity of infection (MOI)-dependent manner, as determined by human IL-8 enzyme-linked immunosorbent assay (ELISA). In addition, V. vulnificus infection significantly increased IL-8 mRNA levels in INT-407 cells, indicating that the increased IL-8 production by V. vulnificus occurred at the transcriptional level. V. vulnificus infection also enhanced IL-8 gene promoter activity in INT-407 cells transiently transfected with IL-8 promoter constructs, but this effect was impaired in INT-407 cells transfected with IL-8 promoter constructs deleted or mutated of a κB site. V. vulnificus infection increased the nuclear factor-kappaB (NF-κB) binding activity to a κB site and the degradation of ΙκB-α protein in a time- and a MOI-dependent manner. Furthermore, BAY11-7082, an inhibitor of NF-κB activation, significantly reduced the IL-8 production, NF-κB binding activity and ΙκB-α degradation induced by V. vulnificus infection. Taken together, these results indicate clearly that V. vulnificus infection significantly induces IL-8 production in human intestinal epithelial cells via NF-κB activation.
Keywords: epithelial cell, interleukin-8, nuclear factor-κB, Vibrio vulnificus
Introduction
Vibrio vulnificus is a Gram-negative estuarine bacterium, known as a significant human pathogen. Vibrio vulnificus infection, acquired via direct contact or the gastrointestinal route, is characterized by food-borne septicaemia and skin infections with ulcer and oedema in many clinical cases.1,2 The fatality rate for V. vulnificus commonly ranges from 30% to 50%. It increases to about 70% in the case of people who have chronic diseases that affect either the liver function or the immune system, such as cirrhosis, alcoholism, hepatitis and immunosuppressive disease.3 Most of the fatal cases are caused by a septic shock,4 which results from various virulence factors of V. vulnificus, including capsular polysaccharide, lipopolysaccharide and haemolysin.5 These virulence factors may persistently activate the production of proinflammatory mediators such as tumour necrosis factor-α, interleukin (IL)-1β, IL-6, IL-8 and nitric oxide from the affected host.6,7 Several reports have demonstrated that the mucosal immune system responds to pathogenic and non-pathogenic bacteria. The epithelial cells infected with bacteria including Helicobacter pylori, Staphylococcus typhimurium and Bacillus subtilis produce inflammatory cytokines such as IL-1β, IL-6 and IL-8.8–11
IL-8 is expressed in many different cell types, including monocytes and macrophages, dermal fibroblasts, endothelial cells, keratinocytes, mesangial cells and several human tumour cell lines. IL-8 is a potent neutrophil-activating chemotactic cytokine.12,13 Thus, IL-8 release by infected intestinal epithelial cells may be instrumental in regulating neutrophil infiltration of the epithelial mucosa in V. vulnificus infection. The expression of IL-8 gene is regulated at both transcriptional and post-transcriptional levels. The former is mediated primarily by multiple cis elements, including a CCAAT box, a steroid-responsive element, and HNF-1 element, two IRF-1 elements, an activating protein 1 (AP-1) sequence, an AP-3 site, a C/EBP sequence and a nuclear factor-κB (NF-κB)-NF-IL-6 overlapping sequence.14,15 Activation of NF-κB is the most crucial step for IL-8 gene transcription in most cells, but NF-IL-6 and AP-1 binding sites are also required for IL-8 transcriptional activation by IL-1 or tumour necrosis factor (TNF)-α.16 Synergistic interaction between NF-κB and NF-IL-6 may play an important role in the transcription of the IL-8 gene.17 Depending on the cell lines, co-operation between NF-κB and either NF-IL-6 or AP-1 is sufficient for IL-8 gene activation.18
The transcription factor NF-κB is important for the inducible expression of a wide variety of cellular and viral genes.19 In the majority of cells, NF-κB exists in an inactive form in the cytoplasm, bound to the inhibitory ΙκB proteins.20 Treatment of cells with various inducers activates a signalling cascade that culminates in the phosphorylation of ΙκBs, resulting in the degradation of ΙκB proteins.21 The bound NF-κB is released and translocates to the nucleus, where it activates appropriate target genes.
In this study, we investigated the effect and action mechanism of V. vulnificus infection on production of IL-8, a proinflammatory cytokine, in human intestinal epithelial INT-407 cells. We have demonstrated that V. vulnificus infection significantly induces IL-8 production in human intestinal epithelial cells via NF-κB activation.
Materials and methods
Cell cultures
Human intestinal epithelial cell-lines, INT-407 and Caco-2 cells, were purchased from American Type Culture Collection (ATCC, Manassas, VA) and maintained at 37° in 5% CO2 in minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) (Gibco BRL, Grand Island, NY) and antibiotics (10 unit/ml penicillin G and 10 µg/ml streptomycin) (growth medium).
Bacterial strain and growth conditions
Vibrio vulnificus strain ATCC 29307 used in this study was purchased from the ATCC, and cultured as described previously.22 For infection experiments, bacteria were grown overnight at 30°C in Luria–Bertani medium (LBS) supplemented with 2·0% NaCl, and diluted to 0·8 of OD600[approximately 6 × 108 colony-forming units (CFU)/ml] in LBS, and then centrifuged and resuspended in antibiotics-free growth medium prior to the infection into epithelial cells. Bacterial concentrations were confirmed by viable cell count on LBS agar.
Infection protocol
Human intestinal epithelial cells were infected with V. vulnificus as described previously.23 In brief, intestinal epithelial cells were grown in culture flasks at 37° in 5% CO2 incubator. The cells (8 × 105 cells/dish) were seeded onto 6-cm culture dishes and cultured for 24 hr in the growth medium without antibiotics. Prior to infection, bacteria were centrifuged at 2500 g for 3 min, resuspended, and adjusted to approximately 6 × 108 CFU/ml in MEM without antibiotics. The bacterial suspensions were added to epithelial cells at a multiplicity of infection (MOI, ratio of bacteria no. to epithelial cell no.) from 0·1 to 100, and then the infected cells were incubated in a 5% CO2 incubator for various times at 37° in antibiotics-free growth medium.
IL-8 gene promoter constructs, transient transfection and luciferase reporter assay
The −144/+44 full fragments of the human IL-8 promoter were generated by polymerase chain reaction (PCR) from genomic DNA of the human intestinal epithelial INT-407 cells. Afterwards, the −120/+44, −89/+44 and −60/+44 fragments were generated by PCR from the −144/+44 full fragment.24 The generated fragments by PCR were cloned into the KpnI/BglII sites of the pGL3-basic luciferase vector (Promega, Madison, WI), followed by sequencing of both strands to verify sequence fidelity. A linker-scanning mutant was generated by a two-step PCR procedure with overlapping internal primers that contained mutated sequences for the NF-κB site (−89 to −71, GGAATTTCCT→TAACTTTCCT). INT-407 cells were plated at a density of 2 × 104 cells/well in 24-well culture plate. After 24 hr-culture, INT-407 cells were transfected with each of the IL-8 gene promoter constructs using the Superfect method (Qiagen, Valencia, CA), according to the manufacturer's instructions. The transfected cells were incubated for 12 hr at 37° in 5% CO2 incubator, followed by infection with V. vulnificus in antibiotics-free MEM medium at 1, 10 and 50 MOI for 1 hr. The cells were washed with phosphate buffered saline (PBS) and the cells were then incubated for 20 hr in the presence of gentamycin (100 µg/ml). Afterwards, the cells were harvested and suspended in 50 µl of lysis buffer for 20 min. The supernatant fluid was harvested and assayed for luciferase activity with luciferase reagent (Promega) in a luminometer. The results were normalized to LacZ expression and are expressed as relative fold induction.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was prepared from the cells and reverse-transcribed into cDNA, and then PCR amplification of the cDNA was performed. Total cellular RNA was isolated by the single-step method using the Trizol reagent (Sigma). Primers used were as follows; human IL-8 [302 base pairs (bp)], 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ (sense) and 5′-TTATGAATTCTCAGCCCTCTTCAAAAACTTCTC-3′ (anti-sense); β-actin (373 bp), 5′-TTCTACAATGAGCTGCGTGTGGCT-3′ (sense) and 5′-GCTTCTCCTTAATGTCACGCACGA-3′ (anti-sense). Reactions were carried out in a MJ Thermal Cycler (Watertowm, MA) for 36 cycles, each including denaturing at 94°C for 30 seconds, annealing at 60°C for 30 seconds and extending to 72° for 30 seconds. After the amplification, the RT-PCR products were separated in 2% (w/v) agarose gels and stained with ethidium bromide.
IL-8 enzyme-linked immunosorbent assay (ELISA)
INT-407 cells were cultured for 24 hr in a 60-mm culture dish prior to infection with V. vulnificus. The cells were infected with V. vulnificus at 10 MOI for 60 min or at various MOIs for 60 min. Afterwards, the cells were washed with PBS and postcultured for 2–24 hr in MEM medium containing gentamycin (100 µg/ml). The culture supernatants were analysed for the levels of IL-8 protein by using the OptEIA™ human IL-8 ELISA kit 2 (BD Biosciences Pharmingen, San Diego, CA), according to the manufacturer's instruction. Briefly, the culture supernatants were added to anti-human IL-8 monoclonal antibody (mAb)-coated ELISA plates and incubated for 2 hr at room temperature. Afterwards, the plates were washed with washing buffer, and incubated with a biotinylated antihuman IL-8 mAb for 1 hr. After washing, the plates were incubated with horseradish peroxidase-streptavidin for 1 hr at room temperature. The assay was developed with tetramethyl benzidine as substrate reagent. After incubating for approximately 30 min at room temperature, the absorbance at 490 nm of each well in 96-well plates was determined in an ELISA reader.
Inhibitor treatment
The ΙκB-α phosphorylation inhibitor BAY11-7082 was stored in dimethyl sulphoxide at −20°C. The INT-407 cells were incubated with BAY11-7082 (5–30 µm) for 1 hr. Afterwards, the cells were washed with prewarmed antibiotics-free growth medium and then infected with V. vulnificus for 1 hr at 10 MOI. The cells were washed with PBS and then total RNA, nuclear extract and cell lysates were prepared for RT-PCR, electrophoretic mobility shift assay (EMSA) and Western blot analysis, respectively.
Preparation of nuclear extracts and EMSA
INT-407 cells (8 × 105 cells/dish) were seeded onto a 6-cm culture dish for 24 hr in antibiotics-free growth medium, and then infected with V. vulnificus at various MOIs from 0·1 to 100. The infected cells were incubated for 5–60 min at 37° in antibiotics-free growth medium in a 5% CO2 incubator. Nuclear extracts from INT-407 cells were prepared as described previously.25 In brief, the infected cells were washed twice with PBS and resuspended in hypotonic buffer [10 mm HEPES buffer, pH 7·9, containing 0·5 mm KCl, 1·5 mm MgCl2, 0·5 mm DTT and 0·2 mm phenylmethylsulphonyl fluoride (PMSF)]. After the cells were allowed to swell on ice for 10 min, 10% solution of Nonidet P-40 was added and the cells were left on ice for 15 min. The homogenate was centrifuged at 1500 g for 15 min, and the resulting nuclear pellet was resuspended in 50 μl of low salt buffer (20 mm HEPES buffer, pH 7·9, containing 25% glycerol, 1·5 mm MgCl2, 0·2 mm PMSF) and then 50 μl of high salt buffer [20 mm HEPES buffer, pH 7·9, containing 25% glycerol, 1·5 mm MgCl2, 0·8 m KCl, 0·2 mm ethylenediaminetetraacetic acid (EDTA), 0·5 mm dithiothreitol (DTT), 0·2 mm PMSF] in a dropwise fashion. The nuclear extract was centrifuged for 30 min at 20 000 g at 4°C. Ten µg of each nuclear extract was incubated with labelled oligonucleotides for 30 min in 20°C of binding buffer (10 mm Tris-HCl, pH 7·6, 500 mm KCl, 10 mm EDTA, 50% glycerol, 250 ng of poly(dI-dC), and 1 mm dithiothreitol). The reaction mixture was analysed by electrophoresis on a 4% polyacrylamide gel in 0·5× Tris borate buffer. An oligonucleotide containing an NF-κB binding site within the immunoglobulin (Ig)-chain (5′-CCGGTTAACAGAGGGGGCTTTCCGAG-3′) was used as a probe. Specific binding was confirmed by competition experiments with a 50-fold excess of unlabelled, identical oligonucleotides of cAMP response element (CRE)-containing oligonucleotides. For the supershift assay, the nuclear extract was incubated with rabbit anti-p50, anti-p52, anti-p65 or anti-p65 (Rel B) IgG antibodies (Santa Cruz Biotechnology, Inc.) in the binding buffer and poly dI-dC for 45 min, followed by the addition of the radiolabelled probe.
Northern blot analysis
INT-407 cells were treated with actinomycin D (10 μg/ml) for 1 hr, 1·5 hr or 2 hr, followed by V. vulnificus infection for 1 hr. Total RNA was isolated from the treated cells and separated by electrophoresis on a denaturing 1% agarose gel containing formaldehyde in 4-morpholinopropanesulphonic acid buffer, and blotted overnight onto nitrocellulose membranes (Bio-Rad, CA). The membrane was prehybridized for 12 hr at 42° in a solution containing 50% formamide. IL-8 cDNA fragment was labelled with [α-32P]dCTP by random hexanucleotide priming for 2 hr at 37°. The membranes were hybridized for 48 hr at 42° and washed at high stringency and exposed to X-ray film and stripped for 30 min at 95°C in a solution containing sodium dodecyl sulphate (SDS).
Preparation of cell lysates and Western blot analysis
Cell lysates from INT-407 cells were prepared as described previously.26 The cells were lysed in lysis buffer (50 mm Tris buffer, pH 7·5, containing 100 mm NaCl, 1% Nonidet P-40, 10% glycerol, 1 mm EDTA, 1 mm NaF, 1 mm sodium orthovanadate, 50 µg/ml each of leupeptin, aprotinin and PMSF) by incubation on ice for 30 min. Lysates were then centrifuged at 13 000 g at 4° for 10 min, and the supernatants were transferred to fresh tubes and stored at − 70°C until required. Protein concentrations of the lysates were determined using the BCA™ Protein Assay Reagent A (Pierce, IL, USA). Equal amounts (10 µg/ml) of whole cell lysates were subjected to sodium dodecyl sulphate-12% polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred onto a polyvinylidene fluoride membrane using using a Semi-Phor (Hoefer Scientific Instruments, San Francisco, CA). The membrane was then incubated with washing buffer (PBS solution containing 0·1% Tween 20) containing 2% bovine serum albumin (BSA)for at least 1 hr to block non-specific protein binding. Afterwards, the membrane was, respectively, treated with rabbit anti-ΙκΒα, anti-pΙκΒα, anti-p52, anti-p68 (Rel B), anti-pp38, and anti-β-actin antibodies. After incubation with horseradish peroxidase (HRP)-conjugated anti-rabbit antibody, immunoreactive proteins were detected with the ECL system (Amersham Biosciences, UK).
Statistical analyses
Student's t-test and one-way analysis of variance (anova) followed by the Bonferroni method were used to determine statistical differences between values of the various experimental and control groups. P-values < 0·05 were considered statistically significant.
Results
Vibrio vulnificus infection increased the production of IL-8 by human intestinal epithelial cells
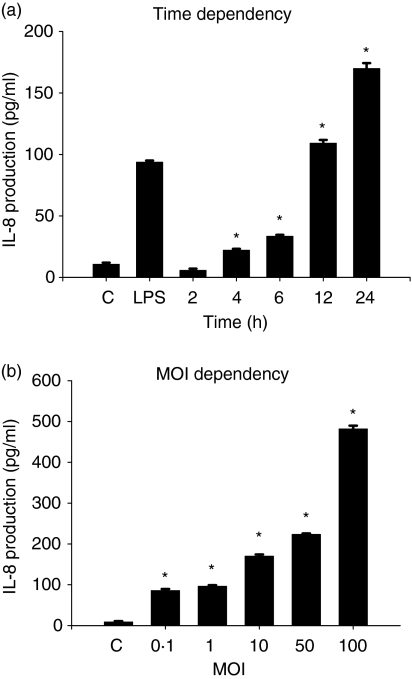
We examined the effect of V. vulnificus infection on the production of IL-8 by human intestinal epithelial cells. As shown in Fig. 1a, incubation with V. vulnifcus significantly increased IL-8 production by human intestinal epithelial INT-407 cells. Exposure of the epithelial cells to V. vulnificus for 1 hr at 10 MOI (multiplicity of infection, ratio of bacteria no. to epithelial cell no.) secreted approximately 169·8 ± 4·4 pg/ml of IL-8 for 24 hr, significantly higher than the IL-8 levels (93·6 ± 1·4 pg/ml) secreted by lipopolysaccharide (LPS)-stimulated INT-407 cells. The production of IL-8 in INT-407 cells was significantly increased by V. vulnificus in a MOI-dependent manner (Fig. 1b).
Figure 1.
Induction of IL-8 production in human intestinal epithelial INT-407 cells by V. vulnificus infection. INT-407 cells were infected with V. vulnificus at 10 MOI for 60 min, followed by washing with PBS and postculturing for 2–24 hr (a), or the cells were infected with V. vulnificus at various MOIs for 60 min, followed by washing and postculturing for 24 hr (b). Some cells were stimulated with LPS or medium alone as controls. The culture supernatants were analysed for IL-8 protein levels by ELISA. The data represent the mean ± SE (n = 3). *P < 0·02, relative to groups uninfected with V. vulnificus at each time or MOI, respectively.
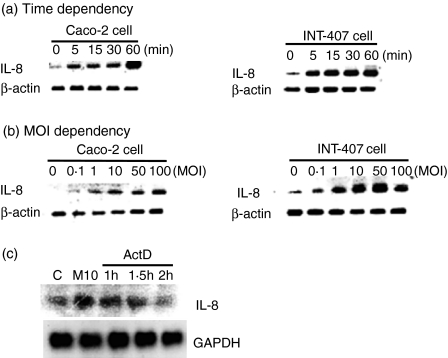
Furthermore, to determine whether the induction of IL-8 production is the result of increased mRNA expression, the effect of V. vulnificus infection on the expression of IL-8 mRNA was analysed in human intestinal epithelial cells, INT-407 and Caco-2 cells. As shown in Fig. 2, incubation of the epithelial cells with V. vulnificus significantly increased the levels of IL-8 mRNA in a time- and MOI-dependent manner, indicating that the induction of IL-8 production by V. vulnificus occurred at the mRNA level. In contrast, incubation with V. vulnificus did not influence β-actin mRNA level in V. vulnificus-infected epithelial cells, suggesting that the stimulatory effect of IL-8 production by V. vulnificus was not the result of the general phenomenon of cellular activation.
Figure 2.
Increased IL-8 mRNA expression in INT-407 and Caco-2 cells by V. vulnificus infection. Human intestinal epithelial INT-407 cells and Caco-2 cells were, respectively, infected with V. vulnificus at 10 MOI for 5–60 min (a) or various MOIs for 60 min (b). Cellular RNA at each point was extracted and IL-8 mRNA expression was determined by RT-PCR. The RT-PCR products were analysed in 2·0% agarose gels. (c) INT-407 cells were treated with 10 μg/ml actinomycin D for 1 hr, 1·5 hr and 2 hr, followed by V. vulnificus infection for 1 hr at 10 MOI. The mRNA levels of IL-8 and GAPDH as a control were detected by Northern blot analysis. The data are representative of two independent experiments.
Next, to determine whether the increased level of IL-8 transcription in V. vulnificus-infected INT-407 cells was due to increased IL-8 mRNA stability, INT-407 cells were pretreated with actinomycin D, a transcription inhibitor, for 1 hr, 1·5 hr or 2 hr before V. vulnificus infection. As shown in Fig. 2c, infection of INT-407 cells with V. vulnificus resulted in the increased IL-8 transcript levels that were significantly inhibited by actinomycin D in a time-dependent manner. The data indicate that the increased IL-8 transcription in V. vulnificus-infected INT-407 cells may not be related to IL-8 mRNA stability. Furthermore, IL-8 induction by V. vulnificus did not result from a general stimulatory effect on cell proliferation as cells number and viability in all cultures remained approximately constant throughout the incubation period in the presence of V. vulnificus, as demonstrated by trypan blue exclusion test (data not shown).
NF-κB-mediated activation of IL-8 gene promoter by V. vulnificus infection
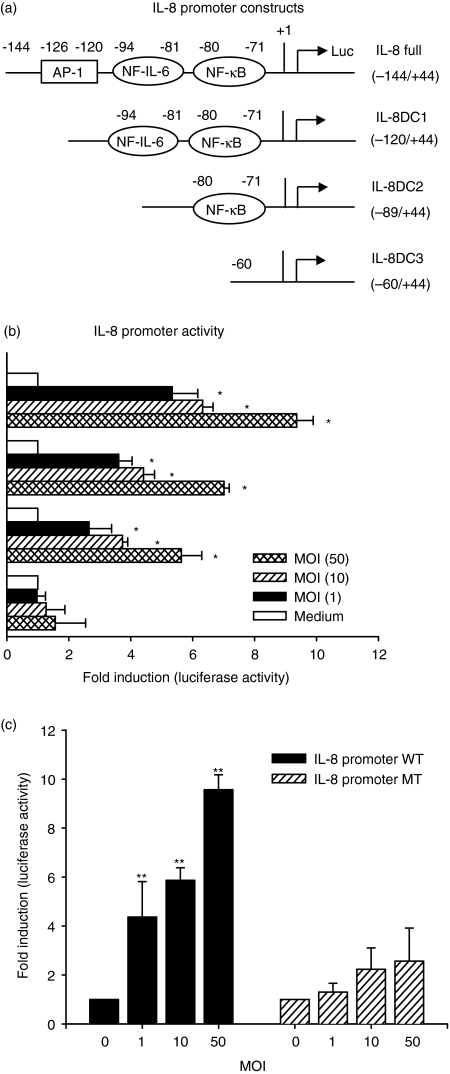
NF-κB, NF-IL-6 and AP-1 are three crucial transcriptional factors for regulation of IL-8 gene expression.24,27 To identify the region involved in the IL-8 production induced by V. vulnificus infection, we generated a series of luciferase reporter constructs containing the IL-8 promoter sequences from positions −144 to +44 (−144/+44), −120 to +44 (−120/+44), −89 to +44 (−89/+44) and −60 to +44 (−60/+44) relative to the transcription initiation site, as shown in Fig. 3a. Human epithelial INT-407 cells were transfected with each of these constructs, followed by V. vulnificus infection, and the luciferase activity was determined. As shown in Fig. 3b, the −144/+44 full construct showed strong stimulation with V. vulnificus in a MOI-dependent manner. In particular, deleting sequences to −89 (−89/+44) still showed significant stimulation with V. vulnificus. However, the stimulatory effect of V. vulnificus infection on IL-8 promoter activity was not observed in INT-407 cells transfected with a promoter construct deleting to a NF-κB site (−60/+44). To directly test the role of a κB site found between −71 and −81 of the IL-8 promoter in the V. vulnificus-mediated stimulatory action, we introduced a linker scanning mutation into the κB site within the context of the −144/+44 construct (IL-8 promoter MT). As shown in Fig. 3c, the V. vulnificus-dependent promoter activation was significantly decreased with IL-8 promoter MT, confirming the importance of the NF-κB in the IL-8 up-regulation by V. vulnificus infection in human intestinal epithelial INT-407 cells. These data indicate that the stimulatory effect of V. vulnificus infection on IL-8 production was mediated through the κB site.
Figure 3.
Analysis of V. vulnificus-mediated transcriptional activation of IL-8 promoter constructs in INT-407 cells. (a) Schematic representation of the human IL-8 promoter constructs is as shown, along with AP-1, NF-IL-6 and NF-κB binding sites. The nucleotide sequence numbers for each construct are shown. (b) Transient transfection of INT-407 cells with the IL-8 promoter constructs, followed by infection with V. vulnificus at 1, 10 and 50 MOI for 1 hr. Afterwards the cells were postincubated for 20 hr in the presence of gentamycin (100 µg/ml) and the supernatants were assayed for luciferase activity. The results are expressed as induction fold over the value obtained with the uninfected INT-407 cells transfected with the −144/+44 construct, which was given as an arbitray value of 1. (c) INT-407 cells were transfected with either IL-8 full promoter WT or linker scanning IL-8 promoter mutated at a NF-κB site (IL-8 promoter MT), followed by infection with V. vulnificus at 10 MOI for 1 hr. The data represent the mean ± SE (n = 3). *P < 0·05, relative to an uninfected group incubated with medium alone. **P < 0·05, relative to groups transfected with IL-8 promoter WT at each MOI.
NF-κB binding to the κB site stimulated by V. vulnificus infection
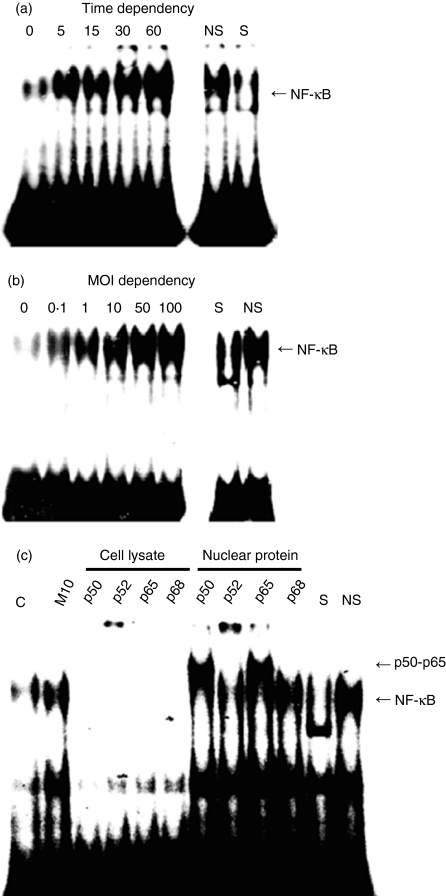
Infection of some microorganisms to epithelial cells has been shown to stimulate NF-κB binding to κB sites.28 To examine whether V. vulnificus-mediated stimulation of the NF-κB transactivation also exploits similar mechanisms, we analysed the NF-κB binding activity present in nuclear extract of V. vulnificus-infected INT-407 cells. As shown in Fig. 4, nuclear extracts from V. vulnificus-infected epithelial cells exhibited strong NF-κB binding activity in a time- and MOI-dependent manner, as demonstrated by the electrophoretic mobility shift assays using a labelled oligonucleotide containing a consensus NF-κB site. The binding was specific as it was competed with an unlabelled, identical oligonucleotide but not with unrelated, non-specific oligonucleotide, and was absent with nuclear extracts from non-stimulated cells. These studies indicate that direct infection of V. vulificus into human intestinal cells increased the binding activity of transcription factor NF-κB to the κB sites in a MOI- and time-dependent manner.
Figure 4.
Vibrio vulnificus-mediated enhancement of κB binding by NF-κB. INT-407 cells were infected with V. vulnificus at 10 MOI for 5–60 min (a) or at various MOIs for 60 min (b). The nuclear extracts were tested for NF-κB DNA binding activity in the electrophoretic mobility shift assay. S and NS indicate the presence of a 50-fold excess of specific oligonucleotide (NF-κB) and non-specific oligonucleotide (CRE), respectively. (c) For the supershift assay, the cells were infected with V. vulnificus at 10 MOI for 60 min and then cell lysates or nuclear extracts were incubated with anti-p50, anti-p52, anti-p65 and anti-p68 (Rel B) antibodies.
To characterize further the κB complex induced by V. vulnificus infection, INT-407 cells were cultured with V. vulnificus for 1 hr at 10 MOI. Afterwards, the cell lysates and nuclear proteins bound to NF-κB oligonucleotide were incubated with anti-p50, anti-p52, anti-p65 or anti-Rel B antibodies. As shown in Fig. 3c, antibodies against NF-κB p50 or p65 specifically shifted, while antibodies against p52 or Rel-B had no effect. The data suggest that the NF-κB complex represents a p50-p65 heterodimer.
V. vulnificus induced the degradation of ΙκB-α protein in INT-407 cells
In the majority of cells, NF-κB exists in an inactive form in the cytoplasm, bound to the inhibitory ΙκB proteins.20 Treatment of cells with various inducers results in the degradation of ΙκB proteins. The bound NF-κB is released and translocates to the nucleus, where it activates appropriate target genes.
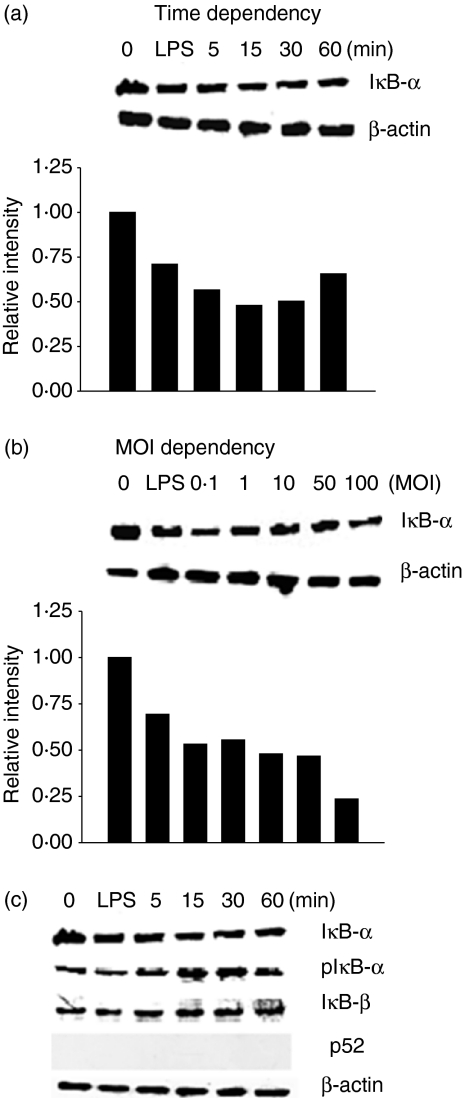
To examine an involvement of ΙκB-α degradation in the increased NF-κB binding activity by V. vulnificus, we determined the levels of ΙκB-α protein in V. vulnificus-infected INT-407 cells by Western blot analysis using anti-ΙκB-α mAb. As controls, the cells were not infected with V. vulnificus or stimulated with LPS. As shown in Fig. 5, infection with V. vulnificus significantly decreased the levels of ΙκB-α protein in INT-407 cells, compared with those in uninfected cells.
Figure 5.
Increased degradation of ΙκB-α in INT-407 cells by V. vulnificus infection. INT-407 cells were infected with V. vulnificus at 10 MOI for 5–60 min (a) or at various MOIs for 60 min (b). Some cells were treated with LPS as a control. The cell lysates were analysed by Western blot analysis using an anti-ΙκB-α antibody. (c) The cell lysates were analysed by using anti-ΙκB-α, anti-pΙκΒ-α, anti-IkB-a, anti-ΙκB-β and anti-p52. Intensity of each band was determined densitometrically and expressed as relative intensity to the corresponding control treated with medium alone. The cell lysates were also probed with an anti-β-actin antibody to confirm equal loading of cell proteins.
To determine further the involvement of ΙκΒ-α degradation in the V. vulnificus–mediatory stimulatory action, the ability of V. vulnificus to induce ΙκΒ-α phosphorylation was determined by Western blot analysis. In addition, the expression levels of ΙκB-α and p52 were investigated in INT-407 cells infected with V. vulnificus. As shown in Fig. 5c, the phosphorylation of ΙκΒ-α protein was increased within 5 min and sustained for at least 60 min. V. vulnificus infection did not affect the expression of ΙκB-β protein. The p52 protein was not detected. This result suggests that V. vulnificus infection might increase NF-κB binding activity in INT-407 cells by up-regulating the degradation of ΙκB-α protein.
An inhibitor of NF-κB activation, BAY11-7082, suppressed IL-8 production and ΙκB-α degradation induced by V. vulnificus infection
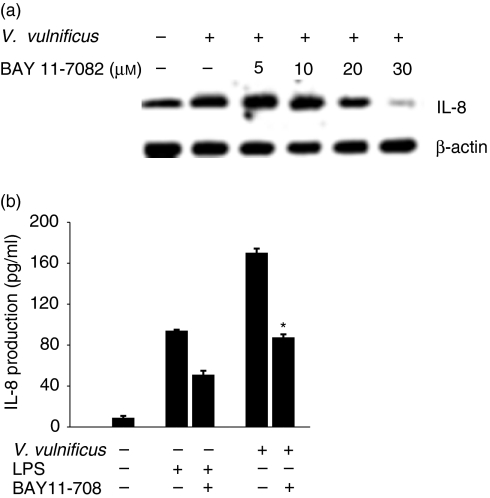
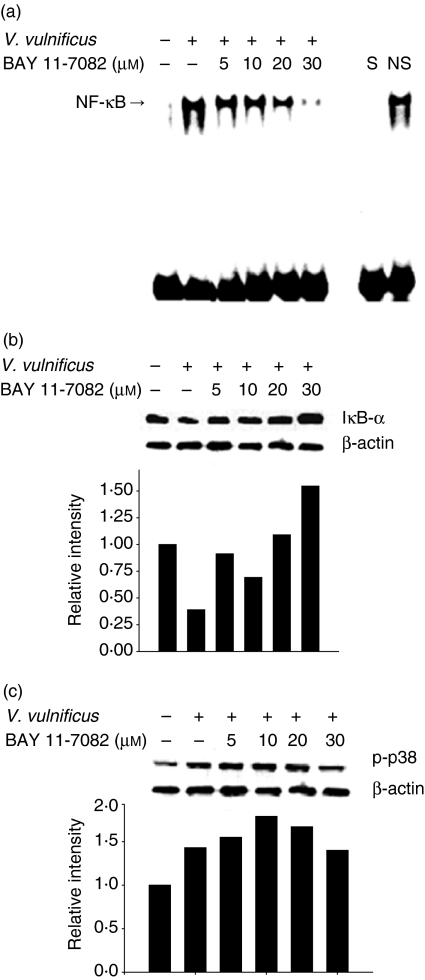
To determine further an involvement of NF-κB activation in the IL-8 production induced by V. vulnificus infection, INT-407 cells were pretreated for 1 hr with a specific inhibitor for NF-κB activation (BAY11-7082, 5–30 µm), followed by V. vulnificus infection. As shown in Fig. 6, treatment with BAY11-7082 resulted in a significant diminishment of both IL-8 mRNA and IL-8 protein levels increased by V. vulnificus infection. Furthermore, BAY11-7082 inhibited the NF-κB DNA binding activity and ΙκB-α degradation induced by V. vulnificus infection in a dose-dependent manner, as determined by the EMSA and Western blot analyses (Fig. 7). The concentrations of BAY11-7082 used in the experiment were not high enough to block ΙκΒ-α degradation non-specifically because the increased phosphorylation of p38 by V. vulnificus infection in INT-407 cells was not affected by treatment with BAY11-7082 (Fig. 7c). The results indicate clearly that infection with V. vulnificus induces the degradation of ΙκB-α and the subsequent activation of NF-κB, leading to the up-regulation of IL-8 production in human intestinal epithelial cells.
Figure 6.
Inhibition of V. vulnificus-mediated IL-8 production in INT-407 cells by a specific inhibitor for NF-κB activation, BAY11-7082. (a) INT-407 cells were pretreated with the NF-κB inhibitor BAY11-7082 (5–30 µm) for 1 hr, followed by infection with V. vulnificus. The cellular RNA was prepared and subjected to RT-PCR for evaluation of IL-8 mRNA levels. (b) INT-407 cells were infected with V. vulnificus at 10 MOI for 60 min. Afterwards the cells were washed with PBS and postcultured for 24 hr. The culture supernatants were analysed for IL-8 protein by ELISA. As a control, the cells were pretreated with BAY11-7082, followed by stimulation with LPS. The data represent the mean ± SE (n = 3). *P < 0·002, relative to a group infected with V. vulnificus without BAY11-7082 treatment.
Figure 7.
Effect of an inhibitor for NF-κB activation, BAY11-7082, on NF-κB binding activity and ΙκB-α degradation in INT-407 cells infected with V. vulnificus. INT-407 cells were pretreated with BAY11-7082 (5–30 µm) for 1 hr, followed by infection with V. vulnificus. The nuclear extracts from each treatment were prepared and subjected to the EMSA for evaluation of NF-κB DNA binding activity (a). The cell lysates were prepared and analysed by Western blot analysis using anti-ΙκΒ-α (b), anti-p38 (c), and anti-β-actin antibody as a control.
Discussion
Chemokines belonging to the CXC intercrine family of cytokines, such as IL-8, play a major role in mobilizing cellular defence mechanisms to eliminate pathogens by recruiting and activating neutrophils and T cells.29 In addition, IL-8 has been thought to signal the onset of an acute inflammatory response.30 Several recent studies have reported on the responses of the mucosal immune system to pathogenic bacteria. Infection of epithelial cells with pathogenic bacteria such as H. pylori, S. typhimurium, EPEC or Y. enterocolitica results in the increased production of IL-8.11,31 In addition, the responses of epithelial cells to non-pathogenic bacteria, such as Bacillus subtilis, B. vulgatus and Escherichia coli, have been examined recently.8–10 Therefore, IL-8 may be an important mediator of the inflammatory response that contributes to epithelium injury in V. vulnificus infection. In this report we investigated the molecular mechanisms by which IL-8 gene expression is regulated upon exposure of intestinal epithelial INT-407 cells to V. vulnificus. Here we have demonstrated for the first time that V. vulnificus infection increases IL-8 gene expression via NF-κB activation in human intestinal epithelial cells. Vibrio vulnificus infection also induced the expression of other proinflammatory cytokines such as IL-1, IL-6, IL-12 and IL-13 (data not shown).
The mechanism by which V. vulnificus induces IL-8 production in human epithelial cells seems to be through the enhancement of NF-κB-mediated activation and binding to the κB site. This point was supported by several lines of evidence. First, the stimulatory effect of V. vulnificus on a series of 5′ deletions of the IL-8 promoter was retained within −89 bp upstream of the transcription initiation site (Fig. 3b). Further deletions up to −60 bp completely abolished the response of the promoter to V. vulnificus, suggesting that the NF-κB site at the −80/−71 position is essential for V. vulnificus-mediated IL-8 production. A sequence spanning nucleotides −133 to −1 within the 5′ flanking region of the IL-8 gene is known to be sufficient and essential for transcriptional regulation of IL-8.12 This sequence contains AP-1, NF-IL-6 and NF-κB binding sites.32,33 The NF-κB element is crucial for transcriptional activation, whereas AP-1 and NF-IL-6 sites may not be relevant in various cell types.17 Furthermore, V. vulnificus significantly increased the NF-κB binding to the κB site in human epithelial cells in a time- and MOI-dependent manner, as demonstrated by the electrophoretic mobility assays (Fig. 4). The NFκB-mediated induction of IL-8 production is in accord with previous observations that infection of epithelial cells with pathogenic bacteria such as H. pylori, S. typhimurium or Y. enterocolitica resulted in up-regulation of NF-κB and secretion of IL-8.20,27,28,34
The activation of NF-κB in the cytoplasm involves the inducible phosphorylation of ΙκB, which then undergoes ubiquitin-mediated proteolysis, thereby releasing NF-κB dimers that translocate to the nucleus.19 In this study we found that the exposure of human epithelial cells to V. vulnificus significantly decreased the level of ΙκB-α protein (Fig. 5). Furthermore, the enhanced NF-κB DNA binding activity (Fig. 7a), ΙκB-α degradation (Fig. 7b) and IL-8 production (Fig. 6) by V. vulnificus infection were significantly suppressed by BAY11-7082, an inhibitor of ΙκB-α phosphorylation, indicating that V. vulnificus induced IL-8 production in human epithelial cells by enhancing the NF-κB DNA binding activity via increased ΙκB-α degradation.
The signalling pathways leading to IL-8 production triggered by the proinflammatory cytokines IL-1 and TNF-α are well characterized. It has been shown recently that IL-1-induced IL-8 production is regulated by a complex interplay of different mitogen-activated protein kinase cascades at the transcriptional level as well as at the post-transcriptional level regulating mRNA stability.15 Recent reports also demonstrated the involvement of bacterial products in IL-8 production of epithelial cells. LPS is known to induce IL-8 production via Toll-like receptor-4 (TLR-4).35 Uropathogenic E. coli activates IL-8 via P-fimbriae,36 and flagellin of different enteropathogenic bacteria activates IL-8 via TLR5.37,38 Therefore, further work will be required to elucidate any involvements of surface molecules on either epithelial cells or V. vulnificus, involved in the IL-8 production induced by V. vulnificus.
In conclusion, we have shown that V. vulnificus significantly induces IL-8 production by human intestinal epithelial cells via an NF-κB dependent transcriptional process. Vibrio vulnificus-mediated induction of proinflammatory cytokines such as IL-8 in human intestinal epithelia cells may explain some of its pathogenic reactions, including inflammatory activity.
Acknowledgments
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (03-PJ1-PG10-22000-0002).
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- EMSA
electrophoretic mobility shift assay
- IL
interleukin
- MOI
multiplication of infection
- NF-κB
nuclear factor-kappaB
- PCR
polymerase chain reaction
References
- 1.Kumamoto KS, Vukich DJ. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J Emerg Med. 1993;16:61–6. doi: 10.1016/s0736-4679(97)00230-8. 10.1016/S0736-4679(97)00230-8. [DOI] [PubMed] [Google Scholar]
- 2.Jeong HS, Lee MH, Lee KH, Park SJ, Choi SH. SmcR and cyclic AMP receptor protein coactivate Vibrio vulnificus vvpE encoding elastase through the RpoS-dependent promoter in a synergistic manner. J Biol Chem. 2003;278:45072–81. doi: 10.1074/jbc.M308184200. 10.1074/jbc.M308184200. [DOI] [PubMed] [Google Scholar]
- 3.Chan TY, Chow DP, Ng KC, Pan KW, McBride GA. Vibrio vulnificus septicemia in a patient with liver cirrhosis. Southeast Asian J Trop Med Public Health. 1994;25:215–6. [PubMed] [Google Scholar]
- 4.Strom MS, Paranjpye RN. Epidemiology and pathogenesis of Vibrio vulnificus. Microb Infect. 2000;2:177–88. doi: 10.1016/s1286-4579(00)00270-7. 10.1016/S1286-4579(00)00270-7. [DOI] [PubMed] [Google Scholar]
- 5.Wright AC, Powell JL, Kaper JB, Morris JG. Identification of a group 1-like capsular polysaccharide operon for Vibrio vulnificus. Infect Immun. 2001;69:6893–901. doi: 10.1128/IAI.69.11.6893-6901.2001. 10.1128/IAI.69.11.6893-6901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espat NJ, Auffenberg T, Abouhamze A, Baumhofer J, Moldawer LL, Howard RJ. A role for tumour necrosis factor-α in the increased mortality associated with Vibrio vulnificus infection in the presence of hepatic dysfunction. Ann Surg. 1996;223:428–33. doi: 10.1097/00000658-199604000-00012. 10.1097/00000658-199604000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin SH, Shin DH, Ryu PY, Chung SS, Rhee JH. Proinflammatory cytokine profile in Vibrio vulnificus septicemic patients' sera. FEMS Immunol Med Microbiol. 2002;33:133–8. doi: 10.1111/j.1574-695X.2002.tb00582.x. 10.1016/S0928-8244(02)00300-0. [DOI] [PubMed] [Google Scholar]
- 8.Haller D, Bode C, Hammes WP, Pfeifer AM, Schiffrin EJ, Blum S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut. 2000;47:79–87. doi: 10.1136/gut.47.1.79. 10.1136/gut.47.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haller D, Holt L, Parlesak A, Zanga J, Bauerlein A, Sartor RB, Jobin C. Differential effect of immune cells on non-pathogenic Gram-negative bacteria induced nuclear factor-κB activation and proinflammatory gene expression in intestinal epithelial cells. Immunology. 2004;112:310–20. doi: 10.1111/j.1365-2567.2004.01874.x. 10.1111/j.1365-2567.2004.01874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosoi T, Hirose R, Saegusa S, Ametani A, Kiuchi K, Kaminogawa S. Cytokine responses of human intestinal epithelial-like Caco-2 cells to the nonpathogenic bacterium Bacillus subtilis (natto) Int J Food Microbiol. 2003;15:255–64. doi: 10.1016/s0168-1605(02)00311-2. 10.1016/S0168-1605(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 11.Eaves-Pyles T, Szabo C, Salzman AL. Bacterial invasion is not required for activation of NF-κB in enterocytes. Infect Immun. 1999;67:800–4. doi: 10.1128/iai.67.2.800-804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell J, Wright AC, Wasserman SS, Hone DM, Morris JG., Jr Release of tumour necrosis factor alpha in response to Vibrio vulnificus capsular polysaccharide in vivo and in vitro models. Infect Immun. 1997;65:3713–18. doi: 10.1128/iai.65.9.3713-3718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung YD, Fan F, McConkey DJ, et al. Role of p38 MAPK, AP-1, and NF-κB in interleukin-1β-induced IL-8 expression in human vascular smooth muscle cells. Cytokine. 2002;18:206–13. doi: 10.1006/cyto.2002.1034. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann E, Dittric-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–55. [PubMed] [Google Scholar]
- 15.Roebuck KA. Regulation of interleukin-8 gene expression. J Interferon Cytokine Res. 1999;19:429–38. doi: 10.1089/107999099313866. 10.1089/107999099313866. [DOI] [PubMed] [Google Scholar]
- 16.Mori N, Oishi K, Sar B, Mukaida N, Nagatake T, Matsushima K, Yamamoto N. Essential role of transcription factor nuclear factor-κB in regulation of interleukin-8 gene expression by nitrite reductase from Pseudomonas aeruginosa in respiratory epithelial cells. Infect Immun. 1999;67:3872–8. doi: 10.1128/iai.67.8.3872-3878.1999. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Kunsch C, Lang RK, Rosen CA, Shannon MF. Synergistic transcriptional activation of the IL-8 gene by NF-κB p65 (RelA) and NF-IL-6. J Immunol. 1994;153:153–64. [PubMed] [Google Scholar]
- 18.Murayama T, Mukaida N, Sadanari H, et al. The immediate early gene 1 product of human cytomegalovirus is sufficient for up-regulation of interleukin-8 gene expression. Biochem Biophys Res Commun. 2000;279:298–304. doi: 10.1006/bbrc.2000.3923. 10.1006/bbrc.2000.3923. [DOI] [PubMed] [Google Scholar]
- 19.Christian J, Sartor RB. The ΙκB/NF-κB system: a key determinant of mucosal inflammation and protection. Am J Physiol Cell Physiol. 2000;278:451–62. doi: 10.1152/ajpcell.2000.278.3.C451. [DOI] [PubMed] [Google Scholar]
- 20.Kim JM, Kim JS, Jung HC, Oh YK, Kim N, Song IS. Inhibition of Helicobacter pylori-induced nuclear factor-κB activation and interleukin-8 gene expression by ecabet sodium in gastric epithelial cells. Helicobacter. 2003;8:542–53. doi: 10.1046/j.1523-5378.2003.00175.x. 10.1046/j.1523-5378.2003.00175.x. [DOI] [PubMed] [Google Scholar]
- 21.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive ΙκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–54. doi: 10.1038/41493. 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 22.Lee JJ, Park NY, Park SJ, Choi SH. Identification and characterization of the Vibrio vulnificus phosphomannomutase gene. J Microbiol Biotehnol. 2003;13:149–54. [Google Scholar]
- 23.Lee BC, Choi SH, Kim TS. Sulforhodamine B assay to determine cytotoxicity of Vibrio vulnificus against human intestinal cells. J Microbiol Biotechnol. 2004;14:355–40. [Google Scholar]
- 24.Chang YH, Hsieh SL, Chen MC, Lin WW. Lymphotoxin β receptor induces interleukin 8 gene expression via NF-κB and AP-1 activation. Exp Cell Res. 2002;278:166–74. doi: 10.1006/excr.2002.5573. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez BL, Rojas A, Campos J, Ledo T, Valle E, Toledo W, Fando R. Differential interleukin-8 response of intestinal epithelial cell line to reactogenic and nonreactogenic candidate vaccine strains of Vibrio cholerae. Infect Immun. 2001;69:613–6. doi: 10.1128/IAI.69.1.613-616.2001. 10.1128/IAI.69.1.613-616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang BY, Chung SW, Cho D, Kim TS. Involvement of p38 mitogen-activated protein kinase in the induction of interleukin-12 p40 production in mouse macrophages by berberine, a benzodioxoloquinolizine alkaloid. Biochem Pharmacol. 2002;63:1901–10. doi: 10.1016/s0006-2952(02)00982-6. 10.1016/S0006-2952(02)00982-6. [DOI] [PubMed] [Google Scholar]
- 27.Chu SH, Kim H, Seo JY, Lim JW, Mukaida N, Kim KH. Role of NF-κB and AP-1 on Helicobater pylori-induced IL-8 expression in AGS cells. Dig Dis Sci. 2003;48:257–65. doi: 10.1023/a:1021963007225. [DOI] [PubMed] [Google Scholar]
- 28.Schulte R, Grassl GA, Preger S, Fessele S, Jacobi CA, Schaller M, Nelson PJ, Autenrieth IB. Yersinia enterocolitica invasion protein triggers IL-8 production in epithelial cells via activation of Rel p65-p65 homodimers. FASEB J. 2000;14:1471–84. doi: 10.1096/fj.14.11.1471. 10.1096/fj.14.11.1471. [DOI] [PubMed] [Google Scholar]
- 29.Kim JK, Oh YK, Kim YJ, Oh HB, Cho YJ. Polarized secretion of CXC chemokines by human intestinal epithelial cells in response to Bacteroides fragilis enterotoxin: NF-κB plays a major role in the regulation of IL-8 expression. Clin Exp Immunol. 2001;123:421–7. doi: 10.1046/j.1365-2249.2001.01462.x. 10.1046/j.1365-2249.2001.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nibbering PH, Pos O, Stevenhagen A, Van Furth R. Interleukin-8 enhances nonoxidative intracellular killing of Mycobacterium fortuitum by human granulocytes. Infect Immun. 1993;61:3111–16. doi: 10.1128/iai.61.8.3111-3116.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer W, Puls J, Buhrdorf R, Gebert B, Odenbreil S, Haas R. Systematic mutagenesis of the Helicobacter pylori Cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol Microbiol. 2001;42:1337–48. doi: 10.1046/j.1365-2958.2001.02714.x. 10.1046/j.1365-2958.2001.02714.x. [DOI] [PubMed] [Google Scholar]
- 32.Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S. Transcription factors NF-IL6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin-6 and interleukin-8. Proc Natl Acad Sci USA. 1993;90:10193–7. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neff L, Zeisel M, Sibilia J, Scholler-Guinard M, Klein JP, Wachsmann D. NF-κB and the MAP kinases/AP-1 pathways are both involved in interleukin-6 and interleukin-8 expression in fibroblast-like synoviocytes stimulated by protein I/II, a modulin from oral streptococci. Cell Microbiol. 2001;3:7336–43. doi: 10.1046/j.1462-5822.2001.00148.x. [DOI] [PubMed] [Google Scholar]
- 34.Huang FC, Werne A, Li Q, Galyov EE, Walker WA, Cherayil BJ. Cooperative interactions between flagellin and SopE2 in the epithelial interleukin-8 response to Salmonella enterica serovar typhimurium infection. Infect Immun. 2004;72:5052–62. doi: 10.1128/IAI.72.9.5052-5062.2004. 10.1128/IAI.72.9.5052-5062.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 36.Wullt B, Bergsten G, Connell H, Rollano P, Gebratsedik N, Hang L, Svanborg C. P-fimbriae trigger mucosal responses to Escherichia coli in the human urinary tract. Cell Microbiol. 2001;3:255–64. doi: 10.1046/j.1462-5822.2001.00111.x. 10.1046/j.1462-5822.2001.00111.x. [DOI] [PubMed] [Google Scholar]
- 37.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–5. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 38.Donally MA, Steiner TS. Two nonadjacent regions in enteroaggregative Escherichia coli flagellin are required for activation of toll-like receptor 5. J Biol Chem. 2002;277:40456–61. doi: 10.1074/jbc.M206851200. 10.1074/jbc.M206851200. [DOI] [PubMed] [Google Scholar]