Abstract
Expression of interleukin (IL)-16 is increased in bronchial mucosal biopsies of atopic asthmatics compared to normal controls. The functional significance of increased expression of IL-16 at sites of allergic inflammation is not yet clear. We have previously shown that IL-16 inhibits IL-5 secretion by allergen-stimulated peripheral blood mononuclear cells (PBMC). We investigated whether IL-16 inhibits the production of other T helper 2 cytokines, namely IL-13 and IL-4, by allergen-specific T cells. PBMC from ragweed-sensitive atopic subjects were stimulated with allergen extract for cytokine production in the presence or absence of rhIL-16. Production of cytokines was assessed by enzyme-linked immunosorbent assay and reverse transcription–polymerase chain reaction. To evaluate whether the modulatory effect of IL-16 on cytokine synthesis was mediated by interferon-γ (IFN-γ), IL-10, IL-12 or IL-18, allergen-stimulated PBMC were cultured in presence of IL-16 and neutralizing concentrations of relevant antibodies. Allergen-stimulated PBMC produced significantly elevated levels of IL-13 (90–740 pg/ml) as compared to unstimulated PBMC (0–375 pg/ml, P < 0·01). Addition of rhIL-16 resulted in down-regulation of IL-13 mRNA expression as well as significantly reduced amounts of IL-13 released by allergen-stimulated PBMC (0–457 pg/ml, P < 0·001), as observed for IL-5. No effect of IL-16 was observed on IL-4 mRNA expression. Treatment with IL-16 resulted in increased levels of IL-10 and IL-18 in allergen-stimulated cell culture. Neutralization of IFN-γ, IL-12, IL-10 or IL-18 did not alter the inhibitory effects of IL-16 on IL-13 and IL-5 secretion by allergen-stimulated PBMC. IL-16 did not modify IL-13 synthesis by anti-CD3-stimulated CD4+ T cells, but it significantly reduced the production of IL-5. These data suggest that IL-16 may play an important immunoregulatory role in allergic states in response to allergen.
Keywords: lymphocyte, IL-16, allergy, human
Introduction
Airway inflammation is a hallmark of asthma. The development of allergic airway inflammation is dependent on CD4+ T cells partly through the release of T helper 2 (Th2)-type cytokines. Interleukin (IL)-16 has specific effects on CD4-bearing cells (i.e. T cells, monocytes, eosinophils).1 Besides its chemotactic activity on human T cells, IL-16 promotes cell cycle progression and induces IL-2 receptor and human leucocyte antigen-DR surface expression in CD4+ target cells. We have previously shown increased expression of IL-16 in bronchial biopsy specimens from atopic asthmatics compared to normal subjects.2 Endobronchial segmental provocation with allergen induced further up-regulation of airway expression of IL-16 which correlated with the numbers of infiltrating CD4+ cells and CD25+ cells in asthmatics.3 Similarly, IL-16 expression was up-regulated in late nasal responses of allergen-induced rhinitis.4 The functional significance of elevated production of IL-16 at sites of allergic inflammation is presently unclear. Because IL-16 interacts with CD4+ T cells, IL-16 may exert important immunoregulatory effects on these cells such as modulation of cytokine synthesis. Earlier, we investigated the effects of exogenous IL-16 on allergen-induced cytokine production by peripheral blood T cells. Our data showed that IL-16 modulates the pattern of cytokines produced by allergen-specific T cells in atopic subjects, abolishing the secretion of IL-5 and promoting the release of interferon-γ (IFN-γ).5 In the present study, we determined whether IL-16 also inhibits IL-13 and IL-4 production, as observed with IL-5, in response to allergenic stimulation. IL-13 is a pleiotropic cytokine produced in large quantities by stimulated CD4+ Th2 cells. IL-13 and IL-4 have many overlapping biological activities pertinent to the development of the allergic response in asthmatic airways, such as the regulation of isotype switching to immunoglobulin E synthesis in human B cells, induction of major histocompatibility complex (MHC) II and CD23 on monocytes, induction of endothelial cell vascular cell adhesion molecule-1 expression and production of eotaxin.6,7 IL-13 is highly expressed in airways of subjects with atopic and non atopic asthma and is possibly involved in the airway remodelling response and the development of airway hyperresponsiveness. 8–11 However, unlike IL-4, IL-13 does not have the ability to promote the differentiation of T cells towards a Th2 phenotype, but it may affect T-cell functions and differentiation indirectly through its ability to down-regulate the production of IL-12 from antigen-presenting cells.7 We therefore investigated whether the modulatory effects of IL-16 on IL-13 and IL-5 production are related to its known enhancing effect on IFN-γ secretion or may be related to synthesis of other soluble factors known to down-regulate the production of so-called Th2 cytokines. Here we show that IL-16, in addition to inhibiting IL-5, down-regulates IL-13 production by peripheral blood mononuclear cells at both transcription and protein secretion levels. The inhibitory effects of IL-16 on IL-13 and IL-5 production were not found to be mediated by the release of IFN-γ, IL-12, IL-10 or IL-18.
Materials and methods
Synthesis of rhIL-16
Recombinant human IL-16 (rhIL-16) was produced in Escherichia coli as a polyhistidine fusion protein containing the 121 C-terminal biologically active residues using the expression vector pET-30 LIC (Novagen, Madison, WI). rhIL-16 was purified by metal chelation chromatography and its bioactivity was determined by chemotaxis of human T cells as previously reported.12 It was thus established that the preparation used showed a maximal chemotactic response at concentrations of 10–200 ng/ml, corresponding to ∼0·8–16·7 nM. The rhIL-16 preparation used contained less than 1·0 unit of endotoxin per millilitre, as determined using a Limulus amoebocyte lysate assay (BioWhittaker QCL 1000, Walkerville, MD).
Subject population and preparation of cells
Venous blood was drawn from atopic subjects with a history of symptomatic seasonal allergic rhinitis and/or atopic asthma for at least 2 years' duration (n = 12). The atopic subjects showed strong positive skin prick test responses to ragweed pollen extract (a mix of Ambrosia trifida and Ambrosia artemisiifolia; Hollister Stier) in the presence of negative diluent and positive histamine controls. These subjects were not taking any systemic or inhaled glucocorticoids, or anti-inflammatory drugs (nedocromil sodium, ketotifen, sodium cromoglycate, leukotriene modifiers) for the previous 6 months. None of the subjects had a history of immunotherapy during the previous 5 years, nor a history of immunization or viral infection within the previous 4 weeks. The study was approved by the institutional Ethics Committee and all the participants gave prior written informed consent to participation.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood samples by density gradient centrifugation over Ficoll-Paque (Amersham Pharmacia Biotech, Quebec, Canada), washed twice and resuspended in RPMI-1640 culture medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin. In depletion experiments, PBMC were depleted of CD4+ cells by negative selection using magnetic-activated cell sorting (MACS) CD4 microbeads (Miltenyi Biotec, Auburn, CA). Under these conditions, greater than 98·5% depletion was achieved as assessed by flow cytometry.
Cell stimulation for cytokine production
PBMC or CD4-depleted cell preparations were cultured at 1·5 × 106 cells/ml in round-bottomed 96-well plates (200 µl/well) at 37° in a humidified 5% CO2 atmosphere in medium alone or in the presence of ragweed allergen extract (mix of A. trifida and A. artemisiifolia, 50 000 PNU/ml; Hollister Stier) or tuberculin (10 µg/ml; Staten Serum Institute, Copenhagen, Denmark) used as an irrelevant antigen. To determine the effects of IL-16 on allergen-induced cytokine production, PBMC were incubated with ragweed allergen extract in the presence of rhIL-16 (100 ng/ml) added 30 min before allergen extract. Supernatants were harvested at different time points between 2 and 6 days and stored at −20° pending cytokine measurements. To determine whether the observed effects on allergen-induced cytokine production were specific to IL-16, similar experiments have been conducted using β-galactosidase, an irrelevant recombinant protein produced in the same system as rhIL-16. Cell viability was assessed following staining with propidium iodide and the number of propidium iodide-positive cells were determined by flow cytometry and fluorescence-activated cell sorting (FACS) analysis.
To determine whether the effects of IL-16 on cytokine production were mediated by IFN-γ, PBMC were incubated in 96-well plates at 1·5 × 106/ml and stimulated with ragweed allergen extract in the presence of rhIL-16 (200 ng/ml) and neutralizing concentrations of either anti-IFN-γ monoclonal antibody (mAb; R & D Systems, Minneapolis, MN)) or appropriate isotype control. Optimal concentration of anti-IFN-γ mAb was determined by titration of the concentration of monoclonal antibody required to reverse the inhibition of allergen-driven IL-5 and IL-13 production by PBMC induced by rhIFN-γ (10 ng/ml). rhIL-16 was added 30 min before allergen stimulation and 1 hr after the addition of neutralizing mAb. After 6 days incubation, supernatants were assayed for cytokine production. We also tested the effects of neutralizing concentrations of anti-IL-10 mAb (R & D Systems), anti-IL-12 mAb (Endogen, Woburn, MA) and anti-IL-18 mAb (MBL International, Woburn, MA) on IL-16-induced responses. Optimal concentrations of antibodies were determined as for anti-IFN-γ mAb. In other experiments, purified CD4+ T cells were cultured in round bottomed 96-well culture plates which have been coated with anti-CD3 mAb at 1 µg/ml (clone UCHT1, BD Pharmingen, San Diego, CA) and incubated overnight at 4° in phosphate-buffered saline (PBS, pH 7·4). After three washes with cold PBS, CD4+ T cells were cultured at a concentration of 1·5 × 106 cells/ml and rhIL-16 was added at a concentration of 100 ng/ml 30 min prior to costimulation with 1 µg/ml anti-CD28 mAb (clone CD28.2, BD Pharmingen). After 72 hr of culture, supernatants were harvested and cytokine production was assayed by enzyme-linked immunosorbent assay (ELISA).
Cytokine protein measurement
ELISA kits for IL-5, IL-13, IL-10 and IL-12 (p70) were purchased from Pharmingen (Mississauga, ON, Canada) and the IL-4 ELISA kit was purchased from R & D Systems. These assays were sensitive above 5 pg/ml for IL-5, 3 pg/ml for IL-13, 10 pg/ml for IL-4, 2 pg/ml for IL-10 and 4 pg/ml for IL-12 (p70).
Reverse transcription–polymerase chain reaction (RT–PCR) detection of cytokine mRNA
Total cellular RNA was extracted using total RNA extraction kit (Sigma Aldrich, Oakville, ON, Canada). The RT–PCR was run using the OneStep RT–PCR kit (Qiagen, Mississauga, ON, Canada) according to manufacturer's recommendations. Four hundred ng of total RNA from each sample was used for the one step RT–PCR in 50 µl total volume reaction. Target-specific primer pairs of IL-4, IL-13 and β-actin were purchased from Biosource International (Medicorp Inc., Montreal, QC, Canada) and target-specific primer pairs of IL-5 were purchased from R & D Systems. PCR conditions were 94° for 4 min followed by 35 cycles of 45 s at 94°, 45 s at 55°, and 45 s at 72° with a final 10 min extension step at 72°. Normalization of cytokine RNA was achieved with amplification reactions of the marker gene β-actin at subsaturating cycle number. The PCR products for IL-4, IL-5, IL-13 and β-actin were 387, 271, 336 and 417 bp, respectively. Amplified products were separated using 2% agarose DNA gel electrophoresis, stained with ethidium bromide and were visualized by ultraviolet illumination using Multimage light cabinet (Alpha Innotech Corp., San Leandro, CA). Negative controls were carried out in the absence of RNA templates or by omitting the RT from the RT–PCR reaction.
Statistical analysis
All results are reported as means and standard error values. Statistical analyses were performed by using the one-way repeated analysis of variance (anova) and the Bonferonni's multiple comparison tests. Statistical significance was accepted as the 5% level of confidence.
Results
Effect of rhIL-16 on allergen-induced IL-13 secretion: comparison with IL-5
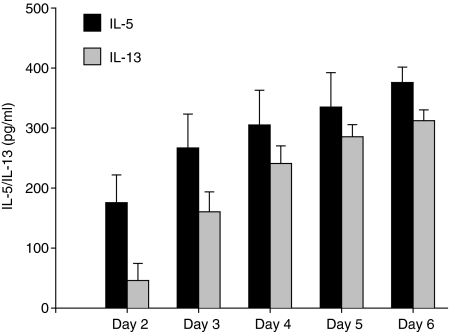
We previously reported that IL-16 inhibits IL-5 production by allergen-stimulated PBMC. We continued this investigation by determining whether IL-16 could also inhibit other Th2-type cytokines involved in the pathogenesis of asthma, IL-13 and IL-4. PBMC from atopic subjects were stimulated with rhIL-16 in the presence or absence of ragweed allergen extract and incubated for 6 days. We first examined and compared the time course of IL-13 and IL-5 production by PBMC in response to allergen stimulation. Cytokine production was measured in culture supernatants harvested at day 2, 3, 4, 5 and 6. During this time interval, cytokine secretion in supernatants reached its highest level on day 6 for both IL-5 and IL-13 (Fig. 1). Levels of allergen-induced IL-13 measured were similar in cell cultures derived from allergic asthmatic subjects and patients with allergic rhinitis. In none of the CD4-depleted PBMC cultures was measurable IL-13 detected in cell culture supernatants, demonstrating that CD4+ cells were the main source of secreted cytokines under these conditions (data not shown) as we previously observed for IL-5.5 In none of the atopic subjects was consistent IL-4 detected in cell culture supernatants between days 2 and 6 after allergen stimulation precluding any determination of the effects of IL-16 on the secretion of IL-4. In non-atopic subjects, levels of secreted IL-5 and IL-13 measured in allergen-stimulated PBMC culture supernatants did not differ from those measured in unstimulated PBMC culture supernatants. In addition, no significant cytokine production over basal levels was observed when PBMC from ragweed-sensitive subjects were stimulated with tuberculin used as an irrelevant antigen (data not shown). These data suggest that the secretion of IL-5 and IL-13 following stimulation with ragweed allergen extract was allergen specific.
Figure 1.
Kinetics of cytokine production by allergen-stimulated PBMC. PBMC were incubated with ragweed allergen extract (50 000 PNU/ml). Cytokine levels were assessed by ELISA in cell culture supernatants harvested at day 2, 3, 4, 5 and 6. Results are expressed as the mean ± SEM.
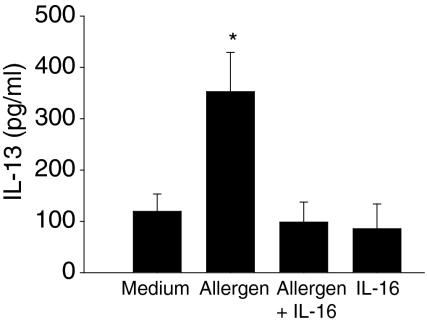
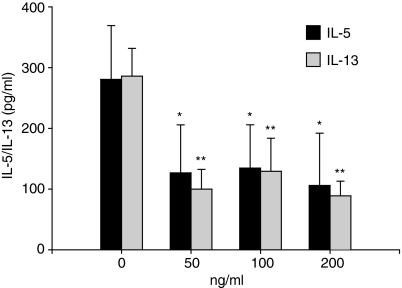
As shown in Fig. 2, a significant increase in IL-13 levels was measured on day 6 following allergen stimulation of PBMC from atopic donors (90–740 pg/ml) as compared to control medium (0–375 pg/ml, P < 0·001). The addition of exogenous rhIL-16 (100 ng/ml) significantly reduced allergen-induced IL-13 secretion (0–457 pg/ml, P < 0·001). Similarly, IL-16 inhibited the secretion of IL-5 by allergen-stimulated PBMC (medium, 49·4 pg/ml [0–292·1 pg/ml]; allergen, 253·6 pg/ml [147·2–370·8 pg/ml]; allergen plus rhIL-16, 18·7 pg/ml [0–74·6 pg/ml], P < 0·001), in line with our previous work.5 Maximal biological effects of IL-16 on target cells have been observed at concentrations ranging from 10−9 to 10−10 mol/l.13–15 Accordingly, in the present study, we tested the effect of rhIL-16 on allergen-induced IL-13 and IL-5 production at doses ranging from 50 to 200 ng/ml, which fall into that range. Figure 3 shows that IL-16 used at concentrations of 50, 100 and 200 ng/ml significantly inhibits allergen-induced cytokine production. Recombinant human IL-16 alone in cell cultures, in the absence of allergen, did not significantly modify the release of IL-13 and IL-5 (P > 0·05). As control, stimulated PBMC were cultured with an equivalent concentration of β-galactosidase, an irrelevant protein produced in a similar fashion in E. coli. The production of IL-13 and IL-5 was not altered in the presence of β-galactosidase(Table 1). Furthermore, the effects of IL-16 on allergen-induced IL-13 and IL-5 production were not modified in presence of polymyxin B, suggesting that the inhibitory effects of IL-16 on cytokine production were not related to residual lipopolysaccharide contamination of the rhIL-16 preparation. Cell viability was unaffected by allergen stimulation or the treatment with rhIL-16, as assessed by cytometric analysis of propidium iodide-stained cells (less than 1% mortality in all conditions) suggesting that the reduction of allergen-induced cytokine secretion was not caused by IL-16-induced cell death. Treatment with IL-16 did not modify IL-13Rα1 or IL-13Rα2 cell surface expression, suggesting that the reduction in allergen-induced IL-13 secretion following IL-16 treatment was not related to any regulatory effect of IL-16 on IL-13 receptor expression (data not shown). These data indicate that IL-16, in addition to inhibiting IL-5, reduces the secretion of another Th2-type cytokine, IL-13.
Figure 2.
Effect of IL-16 on allergen-induced IL-13 production. PBMC were isolated from atopic donors (n = 11) and incubated for 6 days with culture medium, ragweed allergen extract, ragweed allergen extract plus rhIL-16 (100 ng/ml) or rhIL-16 alone. IL-13 release in cell culture supernatants was quantitated by ELISA. Results are expressed as the mean ± SEM. *P < 0·001 compared to cell cultures with culture medium only and cell cultures with allergen plus rhIL-16 (anova).
Figure 3.
Effects of IL-16 on cytokine secretion by allergen-stimulated PBMC. PBMC were incubated for 6 days with ragweed allergen extract in presence of rhIL-16 (0–200 ng/ml). Results are expressed as the mean ± SEM (n = 5). *P < 0·05 and **P < 0·01 compared to allergen-stimulated cells (anova).
Table 1.
Effects of rIL-16 on allergen-induced cytokine production by PBMC
| Experimental conditions (n = 8) | IL-13 pg/ml (mean ± SEM) | IL-5 pg/ml (mean ± SEM) |
|---|---|---|
| Allergen | 284·5 ± 31·18 | 261·6 ± 33·84 |
| Allergen + IL-16 | 167·7 ± 54·40* | 55·6 ± 36·192 |
| Allergen + β-galactosidase | 279·7 ± 32·64 | 201·8 ± 32·92 |
| Allergen + IL-16 + polymyxin B | 169·9 ± 54·301 | 80·9 ± 30·892 |
P < 0·05 compared to allergen-stimulated cells;
P < 0·001 compared to allergen-stimulated cells.
Effects of IL-16 on steady-state levels of IL-4, IL-13 and IL-5 mRNA in allergen-stimulated PBMC
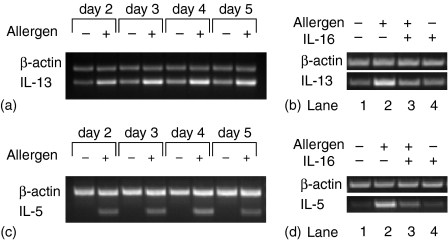
Having determined that IL-16 inhibits IL-13 secretion, we next investigated whether the inhibitory effects of IL-16 on cytokine release reflect a decrease in mRNA expression. RNA was isolated from PBMC cells stimulated with ragweed allergen extract in the presence or absence of rhIL-16 and analysed by RT–PCR. The kinetics of allergen-induced cytokine mRNA expression was first examined by incubating PBMC in presence of ragweed allergen extract for 2–5 days. During this time interval, IL-13 mRNA expression was up-regulated upon allergen stimulation in comparison to unstimulated controls and was highest on day 4 (Fig. 4a). Figure 4(b) shows the effects of IL-16 on IL-13 mRNA expression on day 4. Treatment with rhIL-16 clearly down-regulated the expression of IL-13 mRNA. The kinetics of allergen-driven IL-5 mRNA accumulation was similar to that observed for IL-13 (Fig. 4c) as well as the effect of IL-16 on IL-5 mRNA expression (Fig. 4d). Effect of IL-16 on the expression of IL-4 mRNA in ragweed-stimulated cells was inconsistent, showing no effect in most subjects (data not shown). Amplification products specific for β-actin at subsaturating cycle number were of similar intensity in all samples which suggests the comparable RNA content between the samples. No PCR products were detected in control assays carried out in the absence of RNA or in the absence of reverse transcriptase. These data demonstrate that, in addition to its effects on IL-13 and IL-5 secretion, IL-16 also down-regulates their gene transcription.
Figure 4.
Modulation of cytokine mRNA expression by rhIL-16. (a, c) Kinetics of IL-13 and IL-5 mRNA expression in PBMC incubated in the presence or absence of allergen extract for the indicated times. (b, d) IL-16 down-regulates IL-13 and IL-5 mRNA expression in allergen-stimulated PBMC harvested on day 4. Representative results of RT–PCR products for IL-13 and IL-5 mRNA are shown for allergen-stimulated PBMC under the conditions indicated. Normalization by equivalent β-actin gene expression at subsaturating cycle number is depicted for each condition. The amplified cDNA fragments have the expected lengths of 336 bp for IL-13, 271 bp for IL-5, and 417 bp for β-actin. One representative of four independent experiments is shown.
Role of IFN-γ, IL-10, IL-12 and IL-18 in mediating the inhibitory effects of IL-16 on allergen-induced cytokine production
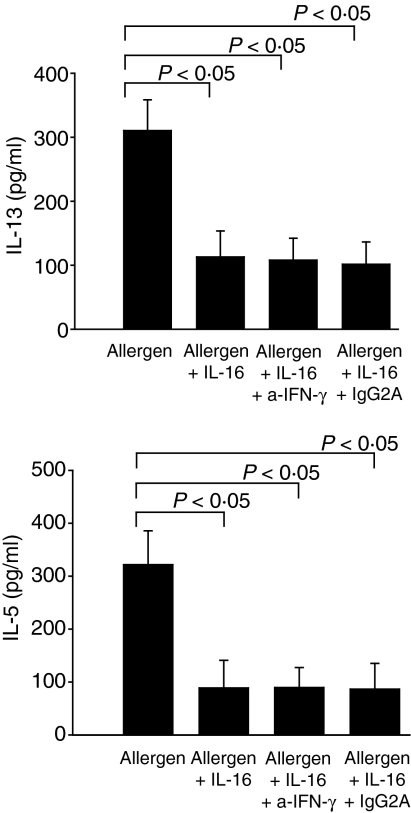
We next investigated whether the inhibitory effects of IL-16 on allergen-induced cytokine secretion may be related to the release of some soluble factors and focused our investigation on IFN-γ, IL-10, IL-12 and IL-18. We reported previously that incubation of allergen-stimulated PBMC with IL-16 resulted in increased production of IFN-γ by allergen-stimulated PBMC.5 Levels of IL-10, IL-12 and IL-18 were also measured in supernatants from allergen-stimulated PBMC collected between day 1 and 6 after treatment with IL-16. During this time interval, no measurable IL-12 was detected in supernatants whereas significant levels of IL-10 and IL-18 were measured with highest levels on day 2. Treatment with IL-16 resulted in increased production of both IL-10 (allergen: 55·4 pg/ml [16·2–129·3 pg/ml], allergen + IL-16: 167·4 pg/ml [61·3–210·9 pg/ml], P < 0·05) and IL-18 (allergen: 50·1 pg/ml [29·8–185·6 pg/ml], allergen + IL-16: 141·2 pg/ml [74·6–257·7 pg/ml], P < 0·05) by allergen-stimulated cells Therefore, to investigate whether the effects of IL-16 on IL-13 and IL-5 production were mediated by these soluble factors, allergen-stimulated PBMC were incubated in the presence of rhIL-16 and neutralizing concentrations of either anti-IFN-γ mAb, anti-IL-10 mAb, anti-IL-12 mAb or anti-IL-18 mAb. Neutralizing concentrations of anti-IFN-γ mAb did not modify the effects of IL-16 on allergen-induced IL-13 and IL-5 release (Fig. 5) nor did neutralizing concentrations of anti-IL-10, anti-IL-12 and anti-IL-18 used alone or in combination (data not shown).
Figure 5.
Role of IFN-γ in mediating the effects of IL-16 on allergen-induced IL-13 and IL-5 production. PBMC were incubated for 6 days with ragweed allergen extract and treated with rhIL-16 in the presence or absence of anti-IFN-γ mAb (10 µg/ml) and appropriate isotype controls. Cytokine production was assayed by ELISA. Results are expressed as the mean ± SEM (n = 12).
Modulation by IL-16 of cytokine synthesis by purified CD4+ T cells
To determine whether IL-16 modulates cytokine production by T cells in an APC-independent system, purified CD4+ T cells were stimulated with anti-CD3 and anti-CD28 mAbs in the presence or the absence of rhIL-16. IL-16 did not modify the production of IL-13 by stimulated CD4+ T cells. However, IL-16 significantly reduced the levels of IL-5 produced by stimulated CD4+ T cells (37 ± 8% inhibition) (Table 2). These results may indicate that while IL-16-induced inhibition of IL-13 production may operate through a direct effect on antigen-presenting cells, the modulation of IL-5 production by IL-16 may also involve a direct effect on CD4+ T cells.
Table 2.
Effects of IL-16 on IL-13 and of IL-5 production by purified CD4+ T cells stimulated with anti-CD3 and anti-CD28 mAbs
| IL-13 pg/ml (mean ± SEM) | IL-5 pg/ml (mean ± SEM) | |
|---|---|---|
| Medium | 8·3 ± 8·3 | 0 |
| Anti-CD3/anti-CD28 | 367·75 ± 41·71 | 215·25 ± 19·31 |
| Anti-CD3/anti-CD28 + rhIL-16 | 318·8 ± 22·11 | 131·9 ± 14·31,2 |
| rhIL-16 | 0 ± 0 | 0 ± 0 |
P < 0·001 compared to cells cultured with medium alone and with rhIL-16 alone;
P < 0·001 compared to cells cultured with anti-CD3/anti-CD28 mAbs.
Discussion
Enhanced production of IL-13 in atopic and nonatopic asthma and in allergic rhinitis is well documented.10,16 Although other cell types produce IL-13, CD4+ T cells are the major source of IL-13 in inflamed airways.11 Additional support for the predominant role of IL-13 in the pathogenesis of asthma comes from experimental murine models of allergic disease implicating IL-13 in the development of eosinophilic airway inflammation, mucous cell hyperplasia and airway hyperresponsiveness.17,18 It is believed that IL-13 is a key mediator of the effector phase of the allergic response, independently of IL-4. In this study, we demonstrated that IL-16, a cytokine highly expressed at sites of allergic inflammation, inhibits IL-13 mRNA expression and protein secretion in allergen-stimulated PBMC from atopic subjects. The effects of IL-16 on the production of IL-13 by allergen-stimulated PBMC mirrored those observed with IL-5; this is consistent with the close association between IL-13 and IL-5 expression in response to allergen stimulation in atopic subjects.19
Despite its association with various forms of allergic inflammation, the relationship between IL-16 and allergic immune responses is unclear and may be complex. For instance, IL-16 is a chemoattractant for CD4+ T cells and eosinophils,20 promotes cytokine and chemokine release from eosinophils,15 can participate in dendritic–T-cell interactions,21 inhibits CD3-activated lymphocyte responses22 and can modulate the pattern of cytokines produced by allergen-stimulated T cells in vitro.5 There exist sparse data from animal studies to elucidate the potential role of IL-16 in mediating allergic airway responses in vivo. Previous studies have shown that administration of anti-human IL-16 antibody attenuated allergen-induced airway hyperresponsiveness without affecting the pattern of the airway inflammatory infiltrate in mice, suggesting that IL-16 may participate to the development of some specific features associated with allergic airway responses in vivo.23 These latter data may appear at variance with the results of our current and previous findings showing an inhibition of IL-5 and IL-13 production and an induction of IFN-γ in allergen-stimulated PBMC by IL-16. It is noteworthy that IFN-γ is required for the development of airway hyperresponsiveness in this latter murine model since treatment with anti-IFN-γ antibodies completely abolished the development of airway hyperresponsiveness in allergen-challenged mice.24 More recently, inhibition of allergen-induced hyperresponsiveness, airway eosinophilia and Th2-type cytokine production in sensitized mice was observed following the administration of exogenous IL-16 suggesting that IL-16 may also have immunosuppressive effects on Th2 dominated allergic airway responses in vivo.25 These latter data are in line with a recent study showing that IL-16 preferentially induced Th1 cell migration in vitro and may therefore play a role in the regulation of Th1 cell recruitment and activation at sites of inflammation.26 Whether endogenous levels of IL-16 reach concentrations required for inhibition of Th2 responses is unknown.
In this investigation, we chose to analyse cytokine expression using a simple primary cell culture model involving a single allergenic stimulation of freshly isolated PBMC ex vivo. In this model, fully differentiated T cells from sensitized atopic subjects, particularly allergen-specific CD4+ T cells, are characterized by elevated secretion of IL-5 and IL-13 upon allergen-mediated activation as compared to cells from non-atopic subjects. This is a sensitive model system for detecting changes in IL-13 and IL-5 but not IL-4 production as shown by others27,28 The clear association between allergen-induced IL-13 and IL-5 production and allergic diseases also suggest that this method is physiologically relevant. Depletion experiments confirmed that CD4+ T cells were the source of IL-13 and IL-5 under these conditions. Although use of allergen stimulation and short-term cultures minimize the impact of selective influences on which T-cell populations survive and which are activated it should be recognized that the possibility of selective T-cell differentiation or activation exists for any technique utilizing in vitro culture. However, Parada et al. has previously shown that IL-16/IL-2 cotreatment of resting CD4+ T cells expands the CD4+ T cell population in vitro but does not induce selective proliferation of any Th subset.29 For these reasons, it is more likely that the IL-16-induced inhibition of IL-13 and IL-5 production in our model reflects an inhibition of allergen-driven Th2 recall responses in differentiated T cells rather than an induction of a shift from Th2 to Th1 differentiation. An important caveat to this study is the possibility that the regulation of cytokine synthesis by circulating PBMC may differ from that of T cells at sites of allergen exposure. Further studies are required to determine to which extent the inhibition of IL-13 and IL-5 production by IL-16 applies to airway T cells and is thus relevant to in vivo situation.
Although the mechanism by which IL-16 inhibits allergen-driven Th2-type cytokine synthesis is presently unknown, some hypotheses can be put forth. Previous studies showed that IL-16 enhances allergen-induced IFN-γ production by PBMC. Therefore, we investigated whether the inhibitory effects of IL-16 on IL-13 and IL-5 production were related to induction of IFN-γ. Inhibition of allergen-induced cytokine release by IL-16 was not modified in presence of neutralizing concentrations of anti-IFN-γ suggesting that the effects of IL-16 are not likely to be attributed to its enhancing effect on IFN-γ production. We also investigated whether the inhibitory effects of IL-16 on IL-13 and IL-5 synthesis were related to induction of other cytokines, such as IL-10, IL-12 and IL-18, known to down-regulate the production of so-called Th2 cytokines. No significant IL-12 production was induced following treatment with IL-16. While a significant induction of IL-10 and IL-18 secretion occurs in presence of IL-16, neutralizing IL-10 and IL-18, either individually or in combination, failed to restore allergen-induced cytokine production. Potential target cells for IL-16 include T cells, in which CD4 is the putative receptor, and monocytes which function as antigen-presenting cells under these experimental conditions. The inability of IL-16 to reduce the production of IL-13 by anti-CD3-stimulated CD4+ T cells may suggest that IL-16 does not act directly on T cells but rather operates through an effect on antigen presenting cells. On the other hand, IL-16 significantly reduced the production of IL-5 by anti-CD3-stimulated CD4+ T cells which suggests that the inhibition of IL-5 production by IL-16 is partly related to functional activity of IL-16 on target T cells. However, this does not exclude the possibility that IL-16 may also modulate the synthesis of IL-5 by regulating the activity of antigen presenting cells. In this regard, the magnitude of the effect of IL-16 on IL-5 synthesis in CD3-stimulated CD4+ T cells (≈37% reduction) was much less to that observed in allergen-stimulated PBMC cultures in which a nearly complete abrogation was obtained. Although not conclusive, this difference points to a predominant effect of IL-16 on accessory cells. Consistent with this, we have previously shown that IL-16 does not affect cytokine synthesis by PBMC stimulated with PHA used at a concentration that bypasses antigen-presenting cell-dependant activation pathways.5 The biological functions of IL-16 on accessory cells are largely unknown. Interestingly, IL-16 was found to modulate receptor expression and cytokine release in monocyte and monocyte-derived dendritic cells suggesting that IL-16 may regulate T-cell responses that are dependent on antigen processing.30,31 Experiments to elucidate whether IL-16 regulates the biology of antigen presenting cells in the context of allergen stimulation are currently in progress.
In conclusion, our data show that IL-16 abrogates allergen-driven IL-13 and IL-5 production suggesting that IL-16 may provide a protective mechanism in the development of allergic inflammation. Modulation of IL-16 in asthma and allergic disorders could lead to new therapeutic approaches in dealing with these disorders.
Acknowledgments
This work was supported by a CIHR grant no. MOP43904 and by the Association Pulmonaire du Québec. Mrs El Bassam is the recipient of a studentship award from the Fonds de la recherche en santé du Québec. Dr Laberge is the recipient of a scholarship award from the Fonds de la recherche en santé du Québec. Drs Kornfeld and Ren are supported by NIH grant HL72647. We are grateful to Raffaela Ballarano for her excellent secretarial assistance.
References
- 1.Center DM, Kornfeld H, Ryan TC, Cruikshank WW. Interleukin 16. implications for CD4 functions and HIV-1 progression. Immunol Today. 2000;21:273–80. doi: 10.1016/s0167-5699(00)01629-7. [DOI] [PubMed] [Google Scholar]
- 2.Laberge S, Ernst P, Ghaffar O, Cruikshank WW, Kornfeld H, Center DM, Hamid Q. Increased expression of interleukin-16 in bronchial mucosa of subjects with atopic asthma. Am J Respir Cell Mol Biol. 1997;17:193–202. doi: 10.1165/ajrcmb.17.2.2750. [DOI] [PubMed] [Google Scholar]
- 3.Laberge S, Pinsonneault S, Varga EM, et al. Increased expression of IL-16 immunoreactivity in bronchial mucosa after segmental allergen challenge in patients with asthma. J Allergy Clin Immunol. 2000;106:293–301. doi: 10.1067/mai.2000.108112. [DOI] [PubMed] [Google Scholar]
- 4.Laberge S, Durham SR, Ghaffar O, Rak S, Center DM, Jacobson M, Hamid Q. Expression of IL-16 in allergen-induced late-phase nasal responses and relation to topical glucocorticosteroid treatment. J Allergy Clin Immunol. 1997;100:569–74. doi: 10.1016/s0091-6749(97)70152-0. [DOI] [PubMed] [Google Scholar]
- 5.Pinsonneault S, El Bassam S, Mazer B, Cruikshank WW, Laberge S. IL-16 inhibits IL-5 production by antigen-stimulated T cells in atopic subjects. J Allergy Clin Immunol. 2001;107:477–82. doi: 10.1067/mai.2001.112373. [DOI] [PubMed] [Google Scholar]
- 6.Wills-Karp M. IL-12/IL-13 axis in allergic asthma. J Allergy Clin Immunol. 2001;107:9–18. doi: 10.1067/mai.2001.112265. [DOI] [PubMed] [Google Scholar]
- 7.Punnonen J, Aversa G, Cocks BG, McKenzie AN, Menon S, Zurawski G, de Waal MR, de Vries JE. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–4. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraft M, Lewis C, Pham D, Chu HW. IL-4, IL-13, and dexamethasone augment fibroblast proliferation in asthma. J Allergy Clin Immunol. 2001;107:602–6. doi: 10.1067/mai.2001.113760. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto S, Gon Y, Takeshita I, Maruoka S, Horie T. IL-4 and IL-13 induce myofibroblastic phenotype of human lung fibroblasts through c-Jun NH2-terminal kinase-dependent pathway. J Allergy Clin Immunol. 2001;107:1001–8. doi: 10.1067/mai.2001.114702. [DOI] [PubMed] [Google Scholar]
- 10.Humbert M, Durham SR, Kimmitt P, et al. Elevated expression of messenger ribonucleic acid encoding IL-13 in the bronchial mucosa of atopic and nonatopic subjects with asthma. J Allergy Clin Immunol. 1997;99:657–65. doi: 10.1016/s0091-6749(97)70028-9. [DOI] [PubMed] [Google Scholar]
- 11.Naseer T, Minshall EM, Leung DY, Laberge S, Ernst P, Martin RJ, Hamid Q. Expression of IL-12 and IL-13 mRNA in asthma and their modulation in response to steroid therapy. Am J Resp Crit Care Med. 1997;155:845–51. doi: 10.1164/ajrccm.155.3.9117015. [DOI] [PubMed] [Google Scholar]
- 12.Cruikshank WW, Center DM, Nisar N, Wu M, Natke B, Theodore AC, Kornfeld H. Molecular and functional analysis of a lymphocyte chemoattractant factor: association of biologic function with CD4 expression. Proc Natl Acad Sci USA. 1994;91:5109–13. doi: 10.1073/pnas.91.11.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan TC, Cruikshank WW, Kornfeld H, Collins TL, Center DM. The CD4-associated tyrosine kinase p56lck is required for lymphocyte chemoattractant factor-induced T lymphocyte migration. J Biol Chem. 1995;270:17081–6. doi: 10.1074/jbc.270.29.17081. [DOI] [PubMed] [Google Scholar]
- 14.Theodore AC, Center DM, Nicoll J, Fine G, Kornfeld H, Cruikshank WW. CD4 ligand IL-16 inhibits the mixed lymphocyte reaction. J Immunol. 1996;157:1958–64. [PubMed] [Google Scholar]
- 15.Bandeira-Melo C, Sugiyama K, Woods LJ, Phoofolo M, Center DM, Cruikshank WW, Weller PF. IL-16 promotes leukotriene C (4) and IL-4 release from human eosinophils via CD4- and autocrine CCR3-chemokine-mediated signaling. J Immunol. 2002;168:4756–63. doi: 10.4049/jimmunol.168.9.4756. [DOI] [PubMed] [Google Scholar]
- 16.Ghaffar O, Laberge S, Jacobson MR, Lowhagen O, Rak S, Durham SR, Hamid Q. IL-13 mRNA and immunoreactivity in allergen-induced rhinitis. comparison with IL-4 expression and modulation by topical glucocorticoid therapy. Am J Respir Cell Mol Biol. 1997;17:17–24. doi: 10.1165/ajrcmb.17.1.2696. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–88. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grunig G, Warnock M, Wakil AE, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Till S, Durham S, Dickason R, et al. IL-13 production by allergen-stimulated T cells is increased in allergic disease and associated with IL-5 but not IFN-gamma expression. Immunology. 1997;91:53–7. doi: 10.1046/j.1365-2567.1997.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rand TH, Cruikshank WW, Center DM, Weller PF. CD4-mediated stimulation of human eosinophils: lymphocyte chemoattractant factor and other CD4-binding ligands elicit eosinophil migration. J Exp Med. 1991;173:1521–8. doi: 10.1084/jem.173.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaser A, Dunzendorfer S, Offner FA, et al. A role for IL-16 in the cross-talk between dendritic cells and T cells. J Immunol. 1999;163:3232–8. [PubMed] [Google Scholar]
- 22.Cruikshank WW, Lim K, Theodore AC, Cook J, Fine G, Weller PF, Center DM. IL-16 inhibition of CD3-dependent lymphocyte activation and proliferation. J Immunol. 1996;157:5240–8. [PubMed] [Google Scholar]
- 23.Hessel EM, Cruikshank WW, Van Ark I, et al. Involvement of IL-16 in the induction of airway hyper-responsiveness and up-regulation of IgE in a murine model of allergic asthma. J Immunol. 1998;160:2998–3005. [PubMed] [Google Scholar]
- 24.Hessel EM, Van Oosterhout AJAI, Van Esch B, Hofman G, Van Loveren H, Savelkoul HF, Nijkamp FP. Development of airway hyperresponsiveness is dependent on interferon-gamma and independent of eosinophil infiltration. Am J Respir Cell Mol Biol. 1997;16:325–34. doi: 10.1165/ajrcmb.16.3.9070618. [DOI] [PubMed] [Google Scholar]
- 25.de Bie JJ, Henricks PA, Cruikshank WW, Hofman G, van Nijkamp FP, Oosterhout AJ. Effect of interleukin-16-blocking peptide on parameters of allergic asthma in a murine model. Eur J Pharmacol. 1999;383:189–96. doi: 10.1016/s0014-2999(99)00547-6. [DOI] [PubMed] [Google Scholar]
- 26.Lynch EA, Heijens CA, Horst NF, Center DM, Cruikshank WW. Cutting edge: IL-16/CD4 preferentially induces Th1 cell migration. requirement of CCR5. J Immunol. 2003;171:4965–8. doi: 10.4049/jimmunol.171.10.4965. [DOI] [PubMed] [Google Scholar]
- 27.Till S, Dickason R, Huston D, et al. IL-5 secretion by allergen-stimulated CD4+ T cells in primary culture: relationship to expression of allergic disease. J Allergy Clin Immunol. 1997;99:563–9. doi: 10.1016/s0091-6749(97)70085-x. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Simons FE, Hayglass KT. Environmental antigen-induced IL-13 responses are elevated among subjects with allergic rhinitis, are independent of IL-4, and are inhibited by endogenous IFN-gamma synthesis. J Immunol. 1998;161:7007–14. [PubMed] [Google Scholar]
- 29.Parada NA, Center DM, Kornfeld H, Rodriguez WL, Cook J, Vallen M, Cruikshank WW. Synergistic activation of CD4+ T cells by IL-16 and IL-2. J Immunol. 1998;160:2115–20. [PubMed] [Google Scholar]
- 30.Hermann E, Darcissac E, Idziorek T, Capron A, Bahr GM. Recombinant interleukin-16 selectively modulates surface receptor expression and cytokine release in macrophages and dendritic cells. Immunology. 1999;97:241–8. doi: 10.1046/j.1365-2567.1999.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathy NL, Scheuer W, Lanzendorfer M, Honold K, Ambrosius D, Norley S, Kurth R. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology. 2000;100:63–9. doi: 10.1046/j.1365-2567.2000.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]