Abstract
Autoimmune diabetes in the non-obese diabetic (NOD) mouse is associated with development of inflammation around the islets at around 4–5 weeks of age, which may be prolonged until frank diabetes begins to occur around 12 weeks of age. Although many interventions can halt disease progression if administration coincides with the beginning of the anti-β cell response, very few are able to prevent diabetes development once insulitis is established. Here we describe a strategy which blocks cellular infiltration of islets and prevents diabetes. Intranasal treatment with the B-subunit of Escherichia coli heat labile enterotoxin (EtxB), a protein that binds GM1 ganglioside (as well as GD1b, asialo-GM1 and lactosylceramide with lower affinities), protected NOD mice from developing diabetes in a receptor-binding dependent manner. Protection was associated with a significant reduction in the number of macrophages, CD4+ T cells, B cells, major histocompatibility complex class II+ cells infiltrating the islets. Despite this, treated mice showed increased number of interleukin-10+ cells in the pancreas, and a decrease in both T helper 1 (Th1) and Th2 cytokine production in the pancreatic lymph node. Disease protection was also transferred with CD4+ splenocytes from treated mice. Taken together, these results demonstrated that EtxB is a potent immune modulator capable of blocking diabetes.
Keywords: recombinant EtxB, enterotoxin, intranasal, receptor-binding, immunomodulation
Introduction
The non-obese diabetic (NOD) mouse spontaneously develops an autoimmune disease strongly resembling human type 1 diabetes (T1D).1,2 Disease in the NOD mouse is associated with development of inflammation around the islets at around 4–5 weeks of age. This state may be maintained for long periods; however, frank diabetes begins to occur from around 12 weeks of age and ultimately affects in excess of 80% of females. Although the processes leading to the inflammation in the pancreas and subsequent β cell destruction are not completely understood, activation of both CD8+ and CD4+ T cells in response to islet-associated antigens probably occurs in the pancreatic lymph node3 and progressive insulitis develops as these cells, together with macrophages, infiltrate the pancreas itself.4–6. By the time of diagnosis, over 90% of the β cells may have been destroyed.7
Current treatment of T1D involves subcutaneous administration of insulin. There are no available strategies either for preventing disease in susceptible individuals, or for treating the underlying inflammatory processes driving islet destruction during the early stages of the process. While a great deal of hope is being placed on the potential of islet cell transplantation as a means of restoring endogenous insulin production, this approach likely requires some additional strategy to protect the graft from attack by the same autoimmune process that rendered the patient diabetic in the first place.
Attack of the islets in diabetes is driven by T helper 1 (Th1)-like CD4+ and cytotoxic CD8+ T cells. There is therefore considerable interest in strategies that can control Th1 cell activity. The nontoxic B subunit of Escherichia coli heat-labile enterotoxin (EtxB) both promotes Th2-dominated immune responses to coadministered antigens8,9 and activates regulatory processes capable of suppressing Th1 responses when administered alone.10 A mixture of EtxB and herpes simplex virus-1 (HSV-1) glycoproteins elicits an antiviral response which is highly Th2 dominated following intranasal delivery.8 Importantly, vaccination of latently HSV-1 infected mice modulates the virally induced Th1-dominated response to produce a protective Th2 reaction9. In other experiments, EtxB has been shown to be able to prevent collagen-induced arthritis (CIA) when given alone.10 This disease protection was not associated with increased Th2 reactivity, but resulted from the activation of CD4+ T regulatory cells.
Immunomodulation by EtxB is linked to its capacity to bind cellular receptors. EtxB binds to GM1 and GD1b, as well as asialo-GM1, lactosylceramide, and certain glycoproteins, albeit at lower affinity.11 A close relative of EtxB, cholera toxin B-subunit (CtxB) has a lower inherent stability than EtxB and exhibits a more restricted binding pattern, interacting only with GM1 and GD1b. CtxB is a poor adjuvant following intranasal delivery8 and is unable to prevent CIA when used alone.10,12 Interestingly, CtxB can be used to prevent autoimmunity when it is directly conjugated to autoantigen. Thus, CtxB conjugated to type II collagen can prevent CIA,12 CtxB conjugated to MBP can prevent experimental autoimmune encephalomyelitis,13 and CtxB-insulin conjugates can block diabetes in the NOD mouse.14—16. In the NOD mouse some studies have suggested a small effect of using CtxB alone, while others have shown a lack of protection in the absence of conjugated insulin.14,17
Given the greater effectiveness of EtxB in CIA and the inherent difficulties in producing protein-B-subunit conjugates reliably and to the standards that are required for human use, we have investigated the potential use of EtxB either alone or admixed with insulin as a means of intervening in the diabetes process in the NOD mouse. We demonstrate that EtxB is a potent immune modulator capable of blocking diabetes. The data suggest that the mechanisms of protection differ when EtxB is given alone or mixed with insulin.
Materials and methods
Mice and diabetes monitoring
Female NOD mice were bred under specific pathogen-free conditions within the University of Bristol. Diabetes was diagnosed using Diastix (Bayer, UK) following two consecutive weekly indications of glycosuria (111 mmol/l). All work was carried out according to our institutional approval and according to the Home Office (UK) Animal Act.
Treatment of NOD mice
Recombinant EtxB and EtxB(G33D) (a non-receptor-binding mutant of EtxB) were synthesized and purified as reported previously.8 Preparations contained <30 endotoxin units/mg, as determined by using a Kinetic-QCL chromogenic limulus amoebocyte lysate assay (Biowhittaker, Walkersville, MD).
Female mice received intranasal treatment at different times on alternate days with EtxB or EtxB(G33D) in a total volume of 20 µl diluted in PBS. Age-matched mice were treated with phosphate-buffered saline (PBS) as controls. In some experiments, EtxB was admixed with 10 µg insulin purified from porcine pancreas (Sigma, Poole, UK) dissolved in phosphate-buffered saline (pH 7·4).
Histology
Histological analyses of islets of Langerhans were performed 4 weeks after completion of treatment. Pancreatic tissue were fixed and stained as reported18. Monoclonal antibodies (mAbs) against mouse CD8 (KT15) (Biosource, CA, USA),CD4 (RM4-5) and Gr-1 (RB6-8C5) antibodies (BD Biosciences, NJ, USA), CD11b (M1/70.15), F4/80 (CI:A3-1), major histocompatibility complex (MHC) II (Ye2/36HLK) antibodies (Serotec, Oxford, UK), B220 (clone RA3/3a1-6.1, culture supernatant), interleukin (IL)-10 (JES5-2A5), IL-12 (C15.6; Biosource) and interferon-gamma (XmG1.2, culture supernatant) were used.
Image analysis
The surface area of the islets and the infiltrate as well as the percentage of islet area positive for the markers were assessed with a Carl Zeiss Vision light microscope (Carl Zeiss Vision, Welwyn GC, UK) equipped with PC-based KS300 Version 3.0 image analysis programme (Zeiss) using a 40× magnification. In each case, a line was drawn around the islet, which the software uses to calculate the area. Then a further region was defined as the area in which positively stained cells were located, the computer uses this to calculate the percentage of the total islet in which staining is to be found. Finally, the density of the staining within the positive area was calculated. In order to do this, a threshold above which brown coloration was considered as positive staining was set. The brown diaminobenzidine-stained cells were then detected automatically by the computer, whereas cells stained with the counter-stain only were ignored. Data were collected from between 5 and 10 different islets in each of three pancreatic sections per mouse. An infiltration index was calculated for each mouse by multiplying the density of staining within the positive area by the percentage of the islet in which staining was present. This process allows the data to reflect situations where there is a dense infiltrate in a small area or where the area of infiltration is increased, but where an individual marker is poorly represented.
Cytokine secretion assessment
Purified single cell suspensions (2 × 106/ml/well) of pancreatic lymph node cells (PanLN) (six mice per group) or splenocytes were cultured with plate bound anti-CD3 monoclonal antibodies (10 µg/ml) for 48 hr. Cytokine measurement was determined by using a cellular sandwich enzyme-linked immunosorbent assay (ELISA) as previously described.10,19
Cell preparation and diabetes adoptive transfers
Donor mice were treated with EtxB or PBS alone. Two weeks after the last treatment, spleen cells were isolated and erythrocytes were removed by lysis with sterile distilled water, before purification of CD4+ T lymphocytes using CD4 (L3T4) Microbeads (Miltenyi Biotec, Bisley, UK). CD4+ T cells (5 × 106) were mixed with an equal number of spleen cells from diabetic NOD mice and injected intravenously to 10–12-week-old irradiated recipients. As a positive control, splenocytes from newly diabetic mice were injected into 10–12-week-old female NOD mice. Glycosuria was assessed every other day.
Statistical analysis
Statistical comparisons were made on normally distributed data using unpaired Student's t-test. For non-parametric data, Mann–Whitney test or Kruskal–Wallis anova was performed. Differences in disease incidence were tested by chi-squared analysis.
Results
Prevention of diabetes using EtxB
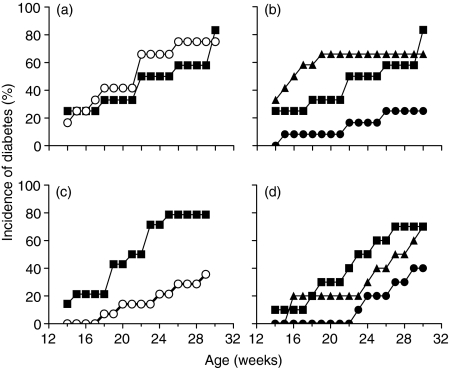
To determine whether EtxB could inhibit diabetes, groups of NOD mice were treated either at 4–6 weeks of age or at 10–12 weeks of age. These timepoints correspond to the period around which the first inflammatory infiltrates are seen in the pancreas, and the period during which destruction of the islets is first seen. Mice were given eight doses of PBS alone; EtxB, insulin or a combination of EtxB and insulin on alternate days intranasally and the development of diabetes was monitored. The results (Fig. 1) indicate that when treatment is given at 4–5 weeks of age, neither EtxB alone nor insulin alone delay diabetes development (P = 0·25, Fig. 1a). In contrast, coadministration of EtxB with insulin dramatically reduced diabetes incidence (P = 0·01 compared to PBS- or insulin-treated controls, Fig. 1b).
Figure 1.
EtxB prevents the development of diabetes Female NOD mice (12 per group) were treated with PBS (▪) or received intranasal delivery of 10 µg EtxB (○), 10 µg insulin (▴) or 10 µg EtxB + 10 µg insulin (•) on alternate days on eight occasions.In (a) and(b) mice were aged 4&1/3;6 weeks at the time of the first treatment, in (c) and (d) mice were 10&1/3;12 weeks old at the time of the first treatment.Diabetes was diagnosed on the basis of weekly urinary glucose testing
When treatment was delayed until 10–12 weeks of age, a different pattern of disease protection was observed. At this time point, treatment with EtxB alone was effective in delaying disease development (P = 0·05, Fig. 1c). The level of protection with EtxB alone was similar to that seen with EtxB + insulin (P = 0·047, Fig. 1d) indicating that, at this time point, there is no added benefit of including insulin.
Constraints of treatment with EtxB alone
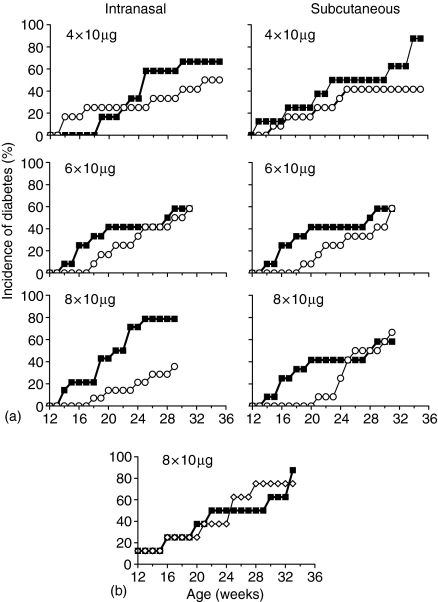
Studies have shown that EtxB can prevent CIA following either mucosal or parenteral administration, with four intranasal treatments using doses as low as 1 µg per dose being effective.10 CIA is, however, induced simultaneously in animals as a result of direct autoantigen challenge. In the NOD mouse, the inflammatory process arises spontaneously and the precise stage of progression will vary between similarly aged animals. We therefore investigated whether the constraints of successful EtxB treatment in the NOD mice were similar to CIA. Experiments compared the use of intranasally or subcutaneously administered EtxB given to 10–12-week-old NOD mice. As previously, eight doses of 10 µg EtxB dramatically reduced the incidence of diabetes following intranasal administration (P ≤ 0·05, Fig. 2a) up to week 30 (intranasal) and week 26 (subcutaneous). When the number of doses given was reduced to six or four the effect on diabetes was less marked. Similarly, reducing the dose level from 10 µg to 1 µg of EtxB led to a marked reduction in the level of disease protection (data not shown). When treatment with EtxB was given subcutaneously, a reduction in disease was observed up to 26 weeks following the administration of eight doses of 10 µg. The level of protection using this route of administration was not as high as that seen after intranasal delivery, and decreasing the number of occasions or the dose used led to a similar loss in protection (Fig. 2a, and data not shown).
Figure 2.
Constraints of treatment with EtxB 10–12-week old female NOD mice (12 per group) were treated either intranasally or subcutaneously with 10 µg EtxB(a; ○), with 10 10 µg EtxB(G33D)(b;•) or were given PBS alone (▪) on the number of occasions indicated. Diabetes was diagnosed on the basis of weekly urinary glucose testing
In other systems, the ability of EtxB to modulate the immune response has been inextricably linked to its receptor binding properties through the use of a non-receptor binding mutant, EtxB (G33D). Similarly, experiments in the NOD mouse showed that the ability to block diabetes resulted from receptor cross-linking by the B-subunit. Intranasal treatment with EtxB (G33D) failed to affect diabetes incidence (Fig. 2b). This observation also suggests that the very low levels of endotoxin present in our preparations of EtxB are not responsible for any of the disease modulating effects that we have observed using the wild type B-subunit. However, it remains a formal possibility that the very low amount of lipopolysaccharide in the EtxB preparation is a required cofactor in enabling the effects of receptor-binding by EtxB to alter the diabetic process.
Modulation of the T-cell response in diabetes following EtxB treatment
Islet-reactive T cells localize preferentially in the PanLN and, as a result, the nature of the autoimmune response can be investigated using cells from this tissue. We collected PanLN and splenocytes from NOD mice within 3 days after completion of treatment with PBS alone or EtxB either alone or admixed with insulin at 4–6 weeks of age, or at 10–12 weeks of age. Cells from these tissues were cultured with anti-CD3 antibodies, which when used alone stimulate previously activated T cells to respond in preference to resting naïve precursors, thus providing an indication of the nature of the ongoing anti-islet response.
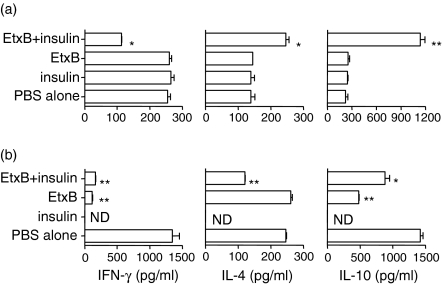
Those treatments, which were able to inhibit diabetes development, were also able to potently inhibit the production of interferon-γ (IFN-γ) by PanLN cells (Fig. 3) but not the splenocytes (not shown). Thus, when treatment was given at four to six weeks of age a combination of EtxB + insulin led to a dramatic reduction in IFN-γ production compared to PBS controls, while treatment with either EtxB or insulin alone failed to affect its release (Fig. 3a). When treatment was delayed until 10–12 weeks of age, then not only did EtxB + insulin suppress the IFN-γ response, but EtxB alone was similarly active. At this later time point, the overall level of IFN-γ production in control cultured PanLN cells was much higher than that in cells from younger mice (Fig. 3b), and once again the modulation of IFN-γ production was restricted to the PanLN response as no changes were observed in splenocyte cultures (data not shown).
Figure 3.
EtxB alters the production of cytokines by pancreatic lymph node T cellscells (a) 4&1/3;6-week-old or (b) 10&1/3;12-week-old female NOD mice (n = 6 per group) were given eight intranasal treatments on alternate days with PBS alone, 10µg EtxB, 10µg insulin or 10µg insulin mixed with 10µg EtxB. Pancreatic lymph node cells were prepared two days after the final treatment and were cultured with soluble anti-CD3 antibodies for 48 hr. The level of cytokines in the supernatants of such cultures was assessed by CelELISA. Data are the mean and SEM value of triplicates of treated versus untreated controls. *P > 0.01, **P > 0.001, ND=not determined
Importantly, there was clear evidence that the nature of the immune modulation was different under differing circumstances. Inhibition of IFN-γ production by treatment with EtxB + insulin at four to 6 weeks of age was associated with a concomitant increase in the amounts of secreted IL-4 and IL-10. When treatments were delayed until 10–12 weeks of age, the administration of EtxB + insulin or EtxB alone was not associated with an increase in these cytokines. The levels of IL-4 and IL-10 were either unchanged or were lowered following treatment.
Protection from diabetes can be transferred from EtxB-treated mice
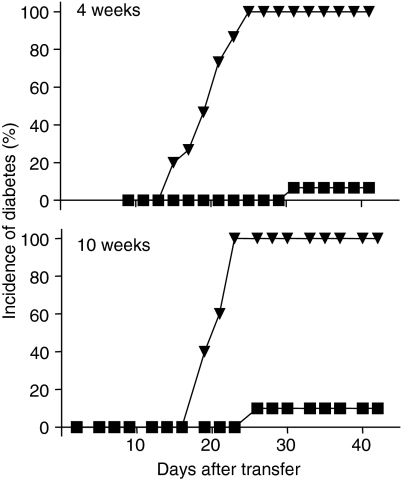
To determine the mechanism by which EtxB administration inhibited the development of diabetes, experiments were carried out in which CD4+ T cells from the spleens of treated animals were cotransferred with diabetogenic spleen cells. Similar experiments were performed in which cells were purified and transferred from the spleens of mice treated with EtxB either at 4–6 weeks of age or at 10–12 weeks of age. As expected, the transfer of spleen cells from diabetic mice caused rapid onset diabetes in recipient NOD mice. In both of the experiments shown (Fig. 4) diabetes in these control groups reached 100% incidence by around day 20–25. In marked contrast, the cotransfer of CD4+ T cells from the spleens of treated animals dramatically suppressed this response. Thus, the incidence of diabetes in animals that received cells from animals treated with EtxB + insulin at 4–6 weeks of age, and in those that received cells from animals treated with EtxB alone at 10–12 weeks of age, was dramatically reduced. Similar disease protection was not seen either when animals received the CD4– fraction, or received CD4+ cells from the spleens of untreated mice (data not shown), indicating the critical role of a population of CD4+ cells in controlling diabetes after EtxB treatments.
Figure 4.
Protection from diabetes resulting from EtxB treatment is mediated by regulatory CD4+ T cells 4–6-week-old NOD mice (top panel; six per group) were given 8 intranasal treatments with 10 µg EtxB mixed with 10 µg insulin. Ten−12-week-old female NOD mice (lower panel; six per group) were given 10 µg EtxB alone on eight occasions by intranasal administration. Two weeks after the final treatment, 5 × 106 CD4+ spleen cells from treated mice were mixed with 5×106 spleen cells from diabetic female NOD mice and were transferred i.v. into 10–12-week-old female NOD recipients (▪). Control recipients received spleen cells from diabetic mice alone (▿). Diabetes was diagnosed in groups of recipients (10 per group) on the basis of urinary glucose testing on alternate days.
Intranasal administration of EtxB suppresses inflammatory cell infiltration and islet tissue damage
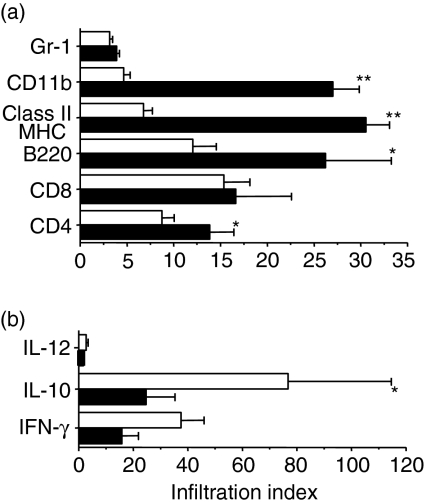
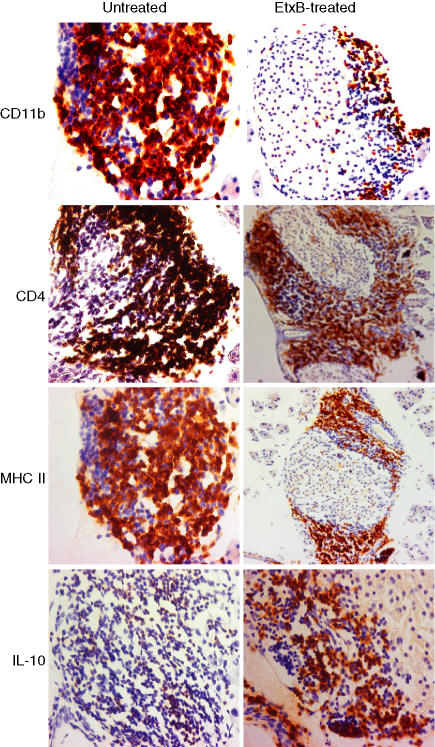
While protection from diabetes following early treatment with EtxB + insulin may result from a Th1 to a Th2-dominated anti-insulin response20,21 the mechanisms by which late treatment with EtxB alone prevent diabetes are less clear. To investigate the effect of EtxB treatment at 10 weeks of age on islet inflammation, we carried out a detailed immunohistological analysis. Pancreata were harvested from mice 4 weeks after the completion of treatment with PBS or EtxB alone. Intranasal administration of EtxB dramatically affected the infiltration of the islets with inflammatory cells (Figs 5 and 6). The amount of CD4+ T-cell infiltration along with the numbers of B220+ cells (primarily B cells) and macrophages (CD11b+) were significantly reduced in treated animals (P < 0·05 for CD4+ and B220+P < 0·0001 for CD11b+). In addition, there was a marked reduction in the levels of MHC class II staining within the islets of treated animals (P < 0·0001). Interestingly, the only cell population that was present in the pancreas in significant numbers that was not affected by EtxB treatment was the CD8+ T-cell population.
Figure 5.
Protection of NOD mice by EtxB is associated with altered islet infiltration Pancreatic islet tissue sections from 10 to 12 week old female NOD mice treated with 10 µg EtxB on eight occasions and control groups were stained for different cell surface markers (a) and cytokines (b). Cellular infiltration of islets from mice treated with EtxB (□) in comparison with the control groups (▪) was assessed by standard image analysis. The proportion of islets with infiltrating cells was multiplied by the number of cells present in infiltrated tissues to generate an ‘infiltration index’. *P = 0·04, **P = 0·0001.
Figure 6.
Immunohistochemistry for CD11b, CD4, MHC II and IL-10+ in response to EtxB treatment Protection of NOD mice from developing diabetes is associated with a reduction in cellular infiltration. Degree of different cellular infiltration in untreated control mice and EtxB-treated NOD mice at 14 weeks of age (n = 6/group). Similar morphology was observed throughout the same study group; representative slides are shown here. Pancreatic sections were stained with antibodies against the markers indicated using immunohistochemical staining methods (see Materials and methods).
Further, immunohistochemical analysis studied the expression of key cytokines in the islets of treated and untreated mice (Figs 5 and 6). Importantly, protection from disease was associated with a significant increase in the numbers of IL-10+ cells within the islets of treated animals (P < 0·05). This increase is particularly interesting given that the primary cell types that are known producers of IL-10 were all present in much lower numbers after treatment (macrophages, B cells and CD4+ T cells). Although there was also an increase in the numbers of IFN-γ+ cells this change did not reach significance. There were very few IL-12+ cells in the islets of either control or treated animals.
Discussion
Various immunotherapies have been shown to be able to ameliorate progression of NOD mice into diabetes if they are given early in the process of sensitization and islet infiltration (classically before 10 weeks of age). However, later intervention beyond 10 weeks of age, a time when there is established T-cell infiltration around islets has been shown to be ineffective in most cases and sometimes damaging.22,23 Exceptions to this include approaches that are not viable therapies in man; for example, active infection of mice with Salmonella typhimurium24 or lymphocyte choriomeningitis virus (LCMV)25 have been demonstrated to be able to prevent progression of established islet infiltrates. We report that EtxB is a potent immunomodulator capable of blocking the progression of insulitis to diabetes in the NOD mouse. The timing of EtxB administration affected its ability to modulate disease. When used alone, EtxB was unable to protect mice from developing diabetes if it was given around the time that insulitis develops (4–6 weeks of age). However, delaying treatment until animals have an established insulitis (10–12 weeks of age) provided conditions where EtxB alone was highly effective. The inability of EtxB to block the disease process when given to young NOD mice could be overcome if it was admixed with a low dose of insulin.
It is interesting to view the findings of this study in the context of other investigations using a close homologue of EtxB, CtxB. The ability of CtxB to inhibit diabetes when conjugated to insulin has been attributed to it acting as a delivery vehicle for insulin into normal tolerance pathways of the gut4,16. However, other studies indicate that conjugation is not a prerequisite to disease amelioration and suggest immune modulation by CtxB plays a more active role.17 In many systems, EtxB has been shown to exert stronger immunomodulatory effects than CtxB. Intranasal treatment with CtxB alone cannot prevent CIA, while the same dose of EtxB is a potent disease suppressor.10 Similarly, EtxB is a strong intranasal adjuvant8,26 whereas CtxB is not. Taken together with the findings reported here, this suggests that the immunomodulatory effects of EtxB are more marked than those of CtxB. Further, when EtxB is coadministered with antigen, the nature of the immune modulation leads to the generation of a non-inflammatory response characterized by production of non-complement fixing antibodies, IL-4 without immunoglobulin E (IgE), and down-regulation of IFN-γ production. When used in this way, EtxB is an adjuvant promoting an immune response with a particular bias. This bias is useful in supporting the production of antibodies that can protect against HSV-1, and is useful in biasing the response to a self-antigen away from a destructive pro-inflammatory Th1 response. Accordingly, protection from diabetes following the administration of EtxB + insulin to the 4–6-week-old mouse was associated with increased IL-4 and IL-10 production and suppression of IFN-γ secretion by PanLN cells. The establishment of a Th2-like response to insulin is consistent with the ability of CD4+ T cells taken from the spleens of treated animals to transfer disease protection; the mechanism of transferred diabetes modulation presumably relating to counter-regulation of the pro-inflammatory Th1 response.
The altered nature of the PanLN response following administration of EtxB + insulin to 10–12-week-old animals was not the same as that seen when the same treatment was given to 4–6-week-old mice. In older animals, dramatic suppression of IFN-γ production was not associated with a concomitant increase in IL-4 or IL-10. The likely reason for this difference lies in the fact that the 10–12-week-old mice have an established anti-pancreatic antigen immune response. In the 4–6-week-old animal this response is not fully developed. Therefore, the profile observed when EtxB + insulin is given to 10–12-week-old mice probably reflects the effect of modulating an established Th1 reaction, rather than generating a response de novo. The ability of EtxB + insulin to potently inhibit the IFN-γ response even when treatment is delayed is therefore particularly interesting.
A critical finding of these studies was the observation that EtxB was ineffective at preventing diabetes when administered alone preinsulitis, however, it was a potent suppressor of disease when treatment was delayed untilinsulitis was established. When given to 4–6-week-old animals, EtxB did not alter the PanLN T-cell response, whereas, when given to 10–12-week-old animals, EtxB suppressed PanLN IFN-γ production. This change was not associated with an increase in IL-10 or IL-4 levels and was therefore reminiscent of alterations to the anti-collagen response reported following the administration of EtxB in CIA.10 In CIA, the ability of EtxB to modulate disease is associated with activation of a population of CD4+ T regulatory cells, which could transfer disease protection. Similar results were reported here. Taken together with the observed cytokine modulation, these data indicate that EtxB was activating a population of T cells that are capable of suppressing the pathogenic Th1 response, but that did not produce enhanced levels of Th2 cytokines. The precise nature of this T regulatory cell population activated by EtxB has yet to be defined. Nevertheless, the association between disease modulation and the activation of T regulatory cells does provide an explanation for the failure of EtxB to prevent diabetes when given to 4–6-week-old animals. At this age insulitis is not yet established. As with any cellular response within the immune system, T regulatory cells activated following treatment with EtxB would remain in the circulation until either returning to a resting state or dying. It is only when there is an inflammatory site into which the cells can migrate that they would have been able to exert their effector function, and may have received the necessary environmental signals to maintain their activity.
The inflammatory site in which T regulatory cells activated following exposure to EtxB would exert their effects may be the pancreas itself, or may be the PanLN. Modulation of the T-cell response in the PanLN was clearly a feature in our experiments and this alone could account for the lack of disease progression. However, it is also possible that T regulatory cells enter the pancreas itself and modulate the inflammatory response there. In this regard, T regulatory cells have been reported to be able to suppress both T-cell mediated responses as well as inflammation that occurs as a result of innate mechanisms.27 Our analysis of the cellular infiltration and cytokine production in the islets following EtxB treatment revealed a significant reduction in numbers of CD4+ T cells, B cells and macrophages. Numbers of MHC class II+ cells were also reduced by treatment, presumably reflecting the lowered numbers of B cells and macrophages. Despite the lowered numbers of these cell types, there were significantly more cells staining for IL-10 than in controls. This indicates that EtxB treatment was not only preventing the Th1 response from driving infiltration and damage in the islets, but was also altering the environment in the pancreas to favour regulatory cytokine production. The observation that EtxB treated animals also had a slightly, although not significantly, increased amount of IFN-γ staining in the islets could be an indication that the T regulatory cells that modulate the effects of EtxB are Tr1-like.28 Co-staining experiments would be required to confirm if this is the case.
The mechanisms by which EtxB either delivered alone or admixed with antigen can modulate T-cell activity remain unclear. It is clear that EtxB exerts numerous effects on leucocyte populations as a result of its ability to bind glycolipid receptors. In vitro EtxB causes B-cell activation,29 triggers apoptosis of CD8+ T cells,30 and stimulates monocytes to produce IL-10 while suppressing their ability to release IL-12.31 It is likely that the effects of EtxB on the inflammatory processes in the NOD mouse result from the alteration of cellular activation in the tissues local to the site of delivery. We propose that, when given in the absence of antigen, the changes to the environment lead to the activation of a population of T regulatory cells. This could be the result of elevated levels of IL-10 secretion by antigen-presenting cells, or could result from the presentation of antigens from apoptosing CD8+ T cells.32,33 When antigen is admixed with EtxB, it is likely that B-cell activation following receptor binding would enhance T : B cell interactions and favour the differentiation of T cells into the Th2 pathway. The activation of T regulatory cells within a Th2 environment could be the reason why the adjuvant effects of EtxB are not associated with IgE production, but instead favour IgG1 and IgA.9 Although this is an attractive model for the mechanisms of action of EtxB, which explains both its effects when used alone or in combination with antigen, it is important to note that the precise effects of EtxB on cells in vivo have proven difficult to reveal. Until this has been done, the relative contributions of the effects observed in vitro can not be determined.
EtxB may represent a useful approach to the prevention of diabetes. Its use in combination with insulin could be viewed as a prophylactic therapy where the nature of the response to islet antigens is set in an anti-inflammatory profile. Alternatively, its use either alone or with antigen may be used in individuals with signs of ongoing anti-islet responses (such as anti-islet antigen antibodies) or early diabetes in order to preserve and even possibly reverse β-cell depletion. Clearly, it will be important to establish the safety profile of EtxB in advance of attempts to use it in diabetes patients. In addition, further studies are required in order to determine the optimal protocols and to define the most appropriate patient groups for which such a treatment could be a benefit.
Acknowledgments
This research was supported in part by Hunter Fleming Ltd. We would like to thank Rachel Case for help with the histology, and Rachel Williams, Tim Hirst and Keisha Methuin for providing EtxB for use in our experiments.
References
- 1.Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets? Nat Med. 1999;5:601–4. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- 2.Bach JF. Insulin-dependent diabetes mellitus as a beta cell targeted disease of immunoregulation. J Autoimmun. 1995;8:439–63. doi: 10.1016/0896-8411(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 3.Hoglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med. 1999;189:331–9. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christianson SWLD, Shultz EH. Leiter: Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. relative contributions of CD4+ and CD8+ T lymphocytes from diabetic versus prediabetic NOD. NON-Thy 1a donors. Diabetes. 1993;42:44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- 5.Graser RT, DiLorenzo TP, Wang F, Christianson GJ, Chapman HD, Roopenian DC, Nathenson SG, Serreze DV. Identification of a CD8 T cell that can independently mediate autoimmune diabetes development in the complete absence of CD4 T cell helper functions. J Immunol. 2000;164:3913–8. doi: 10.4049/jimmunol.164.7.3913. [DOI] [PubMed] [Google Scholar]
- 6.Wong FS, Janeway CA., Jr Insulin-dependent diabetes mellitus and its animal models. Curr Opin Immunol. 1999;11:643–7. doi: 10.1016/s0952-7915(99)00031-x. [DOI] [PubMed] [Google Scholar]
- 7.Colman PG, McNair P, Margetts H, et al. The Melbourne Pre-Diabetes Study: prediction of type 1 diabetes mellitus using antibody and metabolic testing. Med J Aust. 1998;169:81–4. doi: 10.5694/j.1326-5377.1998.tb140188.x. [DOI] [PubMed] [Google Scholar]
- 8.Richards CM, Aman AT, Hirst TR, Hill TJ, Williams NA. Protective mucosal immunity to ocular herpes simplex virus type 1 infection in mice by using Escherichia coli heat-labile enterotoxin B subunit as an adjuvant. J Virol. 2001;75:1664–71. doi: 10.1128/JVI.75.4.1664-1671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards CM, Case R, Hirst TR, Hill TJ, Williams NA. Protection against recurrent ocular herpes simplex virus type 1 disease after therapeutic vaccination of latently infected mice. J Virol. 2003;77:6692–9. doi: 10.1128/JVI.77.12.6692-6699.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luross JA, Heaton T, Hirst TR, Williams NA. Escherichia coli heat-labile enterotoxin B subunit prevents autoimmune arthritis through induction of regulatory CD4+ T cells. Arthritis Rheum. 2002;46:1671–82. doi: 10.1002/art.10328. [DOI] [PubMed] [Google Scholar]
- 11.Williams NA, Hirst TR, Nashar TO. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol Today. 1999;20:95–101. doi: 10.1016/s0167-5699(98)01397-8. [DOI] [PubMed] [Google Scholar]
- 12.Tarkowski A, Sun JB, Holmdahl R, Holmgren J, Czerkinsky C. Treatment of experimental autoimmune arthritis by nasal administration of a type II collagen-cholera toxoid conjugate vaccine. Arthritis Rheum. 1999;42:1628–34. doi: 10.1002/1529-0131(199908)42:8<1628::AID-ANR10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 13.Sun JB, Rask C, Olsson T, Holmgren J, Czerkinsky C. Treatment of experimental autoimmune encephalomyelitis by feeding myelin basic protein conjugated to cholera toxin B subunit. Proc Natl Acad Sci USA. 1996;93:7196–201. doi: 10.1073/pnas.93.14.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergerot I, Ploix C, Petersen J, et al. A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc Natl Acad Sci USA. 1997;94:4610–4. doi: 10.1073/pnas.94.9.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ploix C, Bergerot I, Durand A, Czerkinsky C, Holmgren J, Thivolet C. Oral administration of cholera toxin B-insulin conjugates protects NOD mice from autoimmune diabetes by inducing CD4+ regulatory T-cells. Diabetes. 1999;48:2150–6. doi: 10.2337/diabetes.48.11.2150. [DOI] [PubMed] [Google Scholar]
- 16.Aspord C, Thiovet C. Nasal administration of CTB-insulin induces active tolerance against autoimmune diabetes in non-obese diabetic (NOD) mice. Clin Exp Immunol. 2002;130:204–11. doi: 10.1046/j.1365-2249.2002.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sobel DO, Yankelevich B, Goyal D, Nelson D, Mazumder A. The B-subunit of cholera toxin induces immunoregulatory cells and prevents diabetes in the NOD mouse. Diabetes. 1998;47:186–91. doi: 10.2337/diab.47.2.186. [DOI] [PubMed] [Google Scholar]
- 18.Whiteland JL, Nicholls SM, Shimeld C, Easty DL, Williams NA, Hill TJ. Immunohistochemical detection of T-cell subsets and other leukocytes in paraffin-embedded rat and mouse tissues with monoclonal antibodies. J Histochem Cytochem. 1995;43:313–20. doi: 10.1177/43.3.7868861. [DOI] [PubMed] [Google Scholar]
- 19.Beech JT, Bainbridge T, Thompson SJ. Incorporation of cells into an ELISA system enhances antigen-driven lymphokine detection. J Immunol Methods. 1997;205:163–8. doi: 10.1016/s0022-1759(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 20.Calcinaro F, Gambelunghe G, Lafferty KJ. Protection from autoimmune diabetes by adjuvant therapy in the non-obese diabetic mouse. the role of interleukin-4 and interleukin-10. Immunol Cell Biol. 1997;75:467–71. doi: 10.1038/icb.1997.72. [DOI] [PubMed] [Google Scholar]
- 21.Maron R, Melican NS, Weiner HL. Regulatory Th2-type T cell lines against insulin and GAD peptides derived from orally- and nasally-treated NOD mice suppress diabetes. J Autoimmun. 1999;12:251–8. doi: 10.1006/jaut.1999.0278. [DOI] [PubMed] [Google Scholar]
- 22.Tisch R, Wang B, Serreze DV. Induction of glutamic acid decarboxylase 65-specific Th2 cells and suppression of autoimmune diabetes at late stages of disease is epitope dependent. J Immunol. 1999;163:1178–87. [PubMed] [Google Scholar]
- 23.Winer S, Gunaratnam L, Astsatourov I, et al. Peptide dose, MHC affinity, and target self-antigen expression are critical for effective immunotherapy of nonobese diabetic mouse prediabetes. J Immunol. 2000;165:4086–94. doi: 10.4049/jimmunol.165.7.4086. [DOI] [PubMed] [Google Scholar]
- 24.Zaccone P, Raine T, Sidobre S, Kronenberg M, Mastroeni P, Cooke A. Salmonella typhimurium infection halts development of type 1 diabetes in NOD mice. Eur J Immunol. 2004;34:3246–56. doi: 10.1002/eji.200425285. [DOI] [PubMed] [Google Scholar]
- 25.Urs C, Benke D, Wolfe T, Rodrigo E, Rhode A, Hughes AC, Oldstone MBA, von Herrath MG. Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient. J Clin Invest. 2004;113:74–84. doi: 10.1172/JCI200417005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millar DG, Hirst TR, Snider DP. Escherichia coli heat-labile enterotoxin B subunit is a more potent mucosal adjuvant than its closely related homologue, the B subunit of cholera toxin. Infect Immun. 2001;69:3476–82. doi: 10.1128/IAI.69.5.3476-3482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+ CD25+ T (R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–9. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nashar TO, Hirst TR, Williams NA. Modulation of B-cell activation by the B subunit of Escherichia coli enterotoxin: receptor interaction up-regulates MHC class II, B7, CD40, CD25 and ICAM-1. Immunology. 1997;91:572–8. doi: 10.1046/j.1365-2567.1997.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nashar TO, Williams NA, Hirst TR, Nahar TO. Cross-linking of cell surface ganglioside GM1 induces the selective apoptosis of matured CD8+ T lymphocytes. Int Immunol. 1996;8:731–6. doi: 10.1093/intimm/8.5.731. [DOI] [PubMed] [Google Scholar]
- 31.Turcanu V, Hirst TR, Williams NA. Modulation of human monocytes by Escherichia coli heat-labile enterotoxin B-subunit; altered cytokine production and its functional consequences. Immunology. 2002;106:316–25. doi: 10.1046/j.1365-2567.2002.01429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 33.Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–44. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]