Abstract
Recently there has been increasing evidence to suggest that membrane translocating peptides enter cells by a receptor-dependent pathway. There have been some studies on the mechanism of major histocompatibility complex (MHC) class I presentation of membrane translocating peptides incorporating cytotoxic T lymphocyte epitopes. However, these have been on different cell lines and only a limited number of inhibitors of the antigen presentation pathway were used. Herein, we demonstrate a comprehensive study utilizing a full spectrum of inhibitors to various pathways of MHC class I to elucidate the mechanism of the membrane translocating peptide, penetratin from Antennapedia (Int). It is clear that Int, RQIKIWFQNRRMKWKK when tandemly linked to a cytotoxic T lymphocyte peptide of ovalbumin, SIINFEKL (IntSIIN) is endocytosed via phagocytosis or macropinocytosis by dendritic cells in an ATP-dependent manner and is processed by a proteasome- and tapasin-independent pathway for presentation and loading to MHC class I molecules. In addition, the majority of antigen is taken up by negatively charged receptors. IntSIIN activates T cells in vitro and in vivo and protects mice against challenge with an ovalbumin-expressing tumour.
Keywords: Antennapedia, Int, mechanism, membrane translocating, penetratin, Trojan
Introduction
Vaccination of mice or humans with synthetic peptides based on sequences derived from over-expressed proteins in tumour cells, such as MUC1, MAGE or Mart-1 in the presence of adjuvant, can elicit tumour-specific CD8+ responses in vivo.1 Although immunization with peptides has yielded encouraging results there is still a need for improvements such as more efficient and safer adjuvants. Proteins or synthetic peptides containing specific cytotoxic T lymphocyte (CTL) epitopes need to enter the cytoplasmic compartment for processing and loading onto major histocompatibility complex (MHC) class I molecules. In general, antigen-presenting cells (APC) are not efficient in uptake and processing of exogenous antigens through the MHC class I pathway.2 In addition to the use of conventional adjuvants such as Quil A, liposomes or virus-like particles to improve the intracellular access of proteins and peptides into the cell, some delivery systems utilize targeting to receptors on APC such as mannose or DEC-205 receptors.3–15
In recent years there has been much interest in membrane translocating peptides such as Tat and penetratin that are able to facilitate cytoplasmic delivery proteins or CTL epitopes and induce efficient CTL responses in vivo. Many membrane translocating peptides are currently being investigated including a 16-amino-acid peptide called penetratin (Int) from the DNA binding domain of the Drosophila transcription factor (Antennapedia). Antennapedia protein and peptide have been used to deliver CTL epitopes into the cytoplasm of cells and induce T-cell responses. A recombinant fusion protein consisting of the 60-amino-acid Antennapedia homeodomain and an influenza-derived HLA-Cw3-restricted CTL epitope showed cytoplasmic internalization and subsequent stimulation of CTL responses by MHC class I in the presence of sodium dodecyl sulphate (SDS).16 Another study investigated the same recombinant protein formulated in liposomes that facilitated internalization into APC, such as macrophages and dendritic cells (DC), and induced killing by influenza-specific CTL clones in vitro without the use of SDS as an adjuvant as was necessary in the previous study. 17–19 Our laboratory has previously demonstrated that the 16-amino-acid peptide, Int, can facilitate the cytoplasmic uptake of Int linked in tandem to the ovalbumin (OVA) CD8 epitope, SIINFEKL (IntSIIN) into mouse peritoneal macrophages and EL-4 cells.20 Immunization with IntSIIN induced CTL in mice and protected against growth of an OVA-expressing tumour cell line, E.G7-OVA without the use of adjuvant.20 A more recent study showed that the topical application of recombinant IntSIIN applied to mice induced potent CTL responses, however, the induction of tumour protection required IntSIIN mixed with adjuvant CpG oligodinucleotides.21 These studies emphasize the efficient delivery of CTL epitopes linked to the Antennapedia peptide into the cytoplasm and presentation by MHC class I molecules and its potential in peptide-based cancer immunotherapy.22
The underlying intracellular processing pathway of Int and Int linked in tandem to CTL epitopes is yet to be fully understood. There have been no studies that have completely elucidated the processing pathway of penetratin alone or conjugated to cargo (peptide or protein). In addition, previous studies assessing the uptake and MHC class I processing pathway of penetratin cargo used primary cell lines and not professional APC, which would be the cells capable of stimulating CD8 T-cell responses in vivo. The mechanism of uptake will largely depend on the particular membrane translocating peptide and the cell type being used. Early mechanistic studies showed that Int alone is translocated across biological membranes in a receptor-independent manner.23 Later evaluation of these uptake studies showed that cell fixation leads to artifactual uptake of Int and trypsin digest of the cell membrane-adsorbed peptide was required to avoid this artifact.24 More recent studies have shown that either depletion of cellular energy with sodium azide and 2-deoxyglucose or incubation of cells at 4° resulted in a decreased internalization of Int, suggesting an endocytic mechanism.17,18,25,26 A few studies have described the antigen presentation pathways of either Int or Tat peptide tandemly linked to CTL epitopes; however, these studies have used different cell lines and the mechanism of uptake was assumed to be direct translocation into the cytoplasm of cells.
In the current study, we have used for the first time a range of biochemical inhibitors to investigate the uptake, processing and presentation of Int tandemly linked to the OVA CD8 CTL epitope, SIINFEKL (IntSIIN) in bone marrow-derived DC. IntSIIN enters the DC and is processed and presented by MHC class I molecules by a cross-presentation pathway. IntSIIN uptake is found to be ATP-dependent, indicating the involvement of endocytosis consistent with recent studies of other membrane translocating peptides.24 A panel of specific chemical inhibitors of MHC class I processing compartments showed IntSIIN is processed via a proteasome and tapasin (TAP) independent pathway and further trimming is not required by aminopeptidases in the endoplasmic reticulum (ER) or by furin endopeptidases in the trans-Golgi, as reported for HIV-Tat-SIINFEKL.27 In addition, the majority of IntSIIN uptake involves negatively charged receptors, shown with blocking by dextran sulphate. However, the role of other receptors in the cell membrane cannot be ignored and may also be important in the cellular uptake of IntSIIN.
Materials and methods
Peptides
Peptides were synthesized by Mimotopes, Clayton, Victoria, Australia; the purity of the peptides (> 95%) was determined by mass spectrometry. SIINFEKL (SIIN) is the ovalbumin H-2Kb CTL epitope eight-mer peptide.28 IntSIIN is the peptide incorporating the 16-amino-acid Antennapedia peptide and SIIN (RQIKIWFQNRRMKWKKSIINFEKL). OVA323–339 is the ovalbumin IAb CD4 epitope 16-mer peptide (ISQAVHAAHAEINEAGR) (CD4).
DC cultures
Bone marrow cells from C57BL/6 or TAP−/− female mice were cultured at 106 cells/ml. Petri dishes containing 10 ml of complete RPMI-1640 medium (CSL, Parkville, Australia) with 10 ng/ml each of recombinant mouse granulocyte–macrophage colony-stimulating factor and interleukin-4.29,30 Complete RPMI-1640 media is RPMI-1640 supplemented with 10% (v/v) heat inactivated fetal calf serum, 4 mm l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin sulphate and 100 μm β-mercaptoethanol). At day 6, cells expressed medium levels of CD40, CD80, CD86 and are characteristic of semi-mature DC and are 80% CD11c+, MHC class II (not shown). Cells were flushed from each dish, washed and counted.
Stimulation of lacZ-inducible ovalbumin-specific T-cell hybrid
The B3Z mouse T-cell hybridoma line contains a gene construct of Escherichia coli lacZ reporter gene linked to the nuclear factor of activated T cells. Recognition of the SIINFEKL peptide in the context of class I by the T-cell receptor (TCR) results in activation of the enzyme and conversion of the chromogenic substrate that can be measured by absorbance spectrophotometry.31 DC, EL-4 and RMA-S cells, (106 cells) were pulsed with different doses of IntSIIN and SIIN (1 or 10 μg/ml) for 24 hr; 2 × 105 DC were added to 105 B3Z cells (a 2 : 1 ratio) in 96-well microtitre plates. After overnight incubation at 37°, cells were washed with sterile phosphate-buffered saline (PBS) and incubated with chlorophenol red-β-galactoside (CPRG, Calbiochem, San Diego, CA). After a 4-hr incubation at 37°, the absorbance of the wells was read at 560 nm using a microplate reader.
Antigen-specific OT-I T-cell responses in vitro
TAP−/− and C57BL/6 DC were pulsed with IntSIIN (1 μg/ml) and SIIN (1 μg/ml) for 3 hr and added to purified OT-I T cells. Purified T cells were obtained from the spleens of OT-I mice using a T-cell antibody cocktail (kindly provided by Dr Mark Wright, Austin Research Institute). T cells were separated using anti-rat immunoglobulin magnetic beads (Qiagen, CA, USA) and the purity of the population was > 85% as identified by CD3 staining by flow cytometry – remaining cells were composed of predominantly B cells (∼7%), natural killer cells (∼2·2%), monocytes/macrophages (∼1·2%), granulocytes (∼1%) and DC (∼0·8%). T cells were cocultured in 96-well plates in the presence or absence of pulsed DC. DC were pulsed with media (control), IntSIIN (1 or 10 μg/ml) or SIIN (1 or 10 μg/ml) for 24 hr. The cocultures comprised 2 × 105 T cells and 2 × 104 DC in a total volume of 200 μl. Proliferative responses were assessed from day 1 to day 6 of culture at 37°. Cultures were pulsed with 1 μCi [3H]thymidine for 18 hr, and incorporation of the radionucleotide was measured using a β-scintillation counter (TopCount Gamma Counter; Packard, MA, USA). A comparative study of the efficiency of presentation of IntSIIN with a similar sized peptide without the Int sequence using the B3Z T-cell hybridoma has been reported by us earlier.20
Antigen-specific IFN-γ responses in vivo
Spleen cells from C57BL/6 mice immunized intradermally (base of tail) on days 0 and 14 with IntSIIN were isolated 16 days after the last injection and assessed by ELISpot for interferon-γ (IFN-γ) secretion. Mixed acetate plates (MAIP, Millipore, North Ryde, Australia) were coated overnight with anti-mouse IFN-γ (AN18, 5 μg/ml; Mabtech, Hamburg, Germany). Then, 5 × 105 spleen cells/well were added and incubated in 10% (v/v) complete RPMI-1640 medium in the presence of either SIINFEKL at 5 μg/ml, CD4 epitope of ovalbumin (OVA323–339) at 20 μg/ml or OVA at 20 μg/ml for 18 hr. Concanavalin A (1 μg/ml) or cells alone were used as positive and negative controls, respectively. Cells were washed (0·05% Tween-20/PBS) and anti-mouse IFN-γ antibody–biotin (R4-6A2; Mabtech) was added for 2 hr followed by extravidin–alkaline phosphatase at 0·1 µg/ml (Sigma, St Louis, USA) for 2 hr at room temperature. Spots of activity were detected using a colorimetric AP kit (Biorad, Hercules, CA, USA). Cytokine spots were counted with an AID ELISpot Reader system (Autoimmun Diagnostika GmbH, Strassberg, Germany). Data are presented as mean spot-forming units (SFU) per 0·5 × 106 cells ± standard deviation of the mean (SD).
Tumour protection
Groups of C57BL/6 mice (n = 7) were immunized intradermally with PBS or IntSIIN (25 μg per mouse) on days 0 and 14. Ten days later, mice were challenged with a subcutaneous dose of 107 E.G7-OVA cells (obtained from Dr Frank Carbone, University of Melbourne). E.G7-OVA cells are the EG7 tumour cell line (C57BL/6-derived, H-2b) transfected with OVA cDNA and were cultured in 10% (v/v) complete RPMI-1640 media. The expression of OVA in E.G7-OVA tumour cells was confirmed by flow cytometry (data not shown). The subcutaneous growth of the tumour was monitored by measuring the two perpendicular diameters using callipers and the results were expressed as the product of the two perpendicular diameters.
MHC class I mechanism studies
DC, EL-4 (TAP-competent) and RMA-S (TAP-deficient) cells were cultured in 10% (v/v) complete RPMI-1640 media. Cells were added to 24-well plates (Falcon, BD Biosciences, North Ryde, Australia) at 6 × 105/300 µl and preincubated for 45 min with various inhibitors: NaN3 (0·01–10 mm) (Sigma, St Louis, MO) and 2-deoxyglucose (0·01–10 mm) (Sigma), amiloride (0·006–6 mm) (Sigma), cytochalasin D (0·01–10 μg/ml) (Sigma), dextran sulphate (Progen Industries Ltd, Darra, Australia) (0·01–10 μg/ml), Nystatin (10 or 50 μg/ml), filipin III (1 or 10 μg/ml), chloroquine (0·2–200 μm) (Sigma), ammonium chloride (0·2–200 μm) (Ajax Chemicals, Sydney, Australia), monensin (1–1000 μm) (Sigma), lactacystin (0·01–10 μm) (Calbiochem) and brefeldin A (0·01–10 μg/ml) (Sigma), furin inhibitor decRVKR-CMK (0·01–10 μm) (Calbiochem), bestatin (0·01–10 μm) (Sigma). After the preincubation period, IntSIIN was added to the cell culture at 1 μg/ml at 37° and added to B3Z T cells as described above. Inhibitors were not removed from the T-cell cultures. In all experimental cultures, DC pulsed with SIINFEKL (SIIN(+)) or without (SIIN(−)), the maximum concentration of inhibitor was also added to B3Z T cells in parallel experiments. SIINFEKL in these experiments is used as a positive control and is not for comparison purposes because SIINFEKL will be surface-loaded whilst IntSIIN will be internalized and processed before presentation. All inhibitors were active as they have been used in similar studies (where the inhibitors were active) to determine the mechanism of Int covalently linked to ovalbumin protein (manuscript in preparation) and mannan-MUC1 protein.9
Statistical analysis
Assays were set up in triplicate. Mean values were compared using the two-tailed unpaired t-test. Two P-value thresholds were used for protection and immunogenicity assays: P < 0·001 to indicate a highly significant difference, and P < 0·05 to indicate a significant difference.
Results
Presentation of IntSIIN by MHC class I and stimulation of T cells
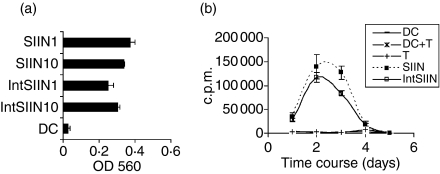
To assess whether IntSIIN is processed and presented by DC to the TCR of B3Z T cells, DC were cultured and pulsed with 1 and 10 μg/ml of IntSIIN. DC were incubated with B3Z T cells for 24 hr and the recognition of the SIIN epitope on the MHC class I molecule by its specific TCR was assessed via a colorimetric assay. DC alone were used as negative controls. DC pulsed with IntSIIN at both 1 and 10 μg/ml induced presentation to B3Z T cells that was significantly above background (Fig. 1a). The level of presentation of IntSIIN was directly compared to SIIN and results showed that the abilities to stimulate B3Z T cells were similar (Fig. 1a). DC pulsed with SIIN is surface-loaded and was used as a positive control. IntSIIN was also shown to induce significant presentation after pulsing DC for 1 hr (data not shown). Previously we demonstrated that IntSIIN is presented more efficiently to B3Z T cells than a non-internalizing 24-mer peptide.20 These results clearly show that DC pulsed with IntSIIN can present SIIN on MHC class I molecules to T cells.
Figure 1.
IntSIIN stimulates T cells in vitro. (a) C57BL/6 DC were stimulated with IntSIIN and SIIN at 10 and 1 μg/ml for 1 hr and added to B3Z T cells for 24 hr. LacZ activity in B3Z T cells was assayed by total culture lysates with LacZ substrate CPRG. The absorbance (560 nm) of culture wells was read after 4 hr incubation at 37°. (b) C57BL/6 DC were pulsed with SIIN and IntSIIN at 1 μg/ml for 1 hr, washed, and added to purified OT-I T cells and cultured for 6 days. Cells were harvested at each time-point and [3H]thymidine uptake was measured to assess T-cell proliferation. DC alone, OT-I T cells and DC added to T cells were used in experiments as negative controls and are shown in both (a) and (b). Data are presented as counts per minute (c.p.m.) of [3H]thymidine uptake from quadruple wells (± SD).
To assess the cellular uptake and subsequent presentation of IntSIIN by DC to OT-I T cells, in vitro proliferation assays were performed. DC pulsed with SIIN or IntSIIN at 1 μg/ml for 3 hr were added to OT-I T cells at a ratio of 1 : 10. Co-cultured cells were incubated for up to 6 days and each day, proliferation activity was assessed by [3H]thymidine uptake. DC pulsed with SIIN induced significant proliferation of OT-I T cells and peaked at day 2–3 and responses had returned back to background responses by day 4 (Fig. 1b). DC pulsed with IntSIIN induced similar proliferation to DC pulsed with SIIN (Fig. 1b). These results show that IntSIIN induces proliferation of antigen-specific purified OT-I T cells in vitro.
IntSIIN induces potent CD8 T-cell-specific IFN-γ responses in vivo
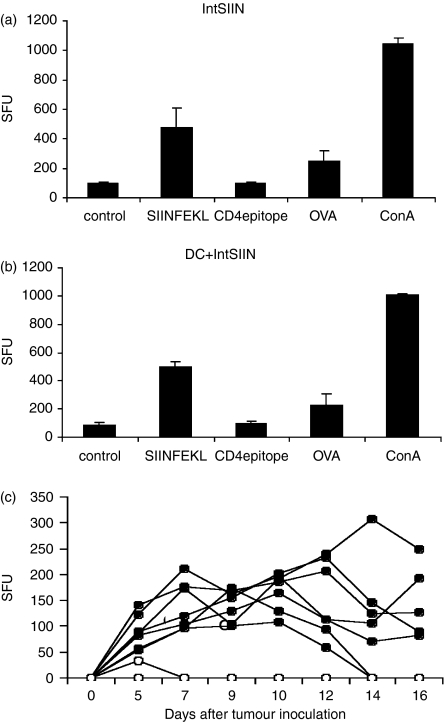
The potential of IntSIIN to induce CD8+ T-cell responses in vivo after two immunizations was measured using IFN-γ by ELISpot analysis. Splenocytes from mice immunized with IntSIIN alone and IntSIIN-pulsed DC were used in the assays. C57BL/6 mice immunized with 25 μg IntSIIN alone generated IFN-γ-secreting cells which recognized SIIN (OVA to a lesser extent), but not OVA323−339 (CD4) (Fig. 2a). Comparatively, mice immunized with DC pulsed with 20 μg/ml IntSIIN also induced IFN-γ-secreting cells which recognized SIIN and OVA but not CD4, as expected (Fig. 2b). The CD4 epitope of OVA, OVA323–339 was used as an internal negative control for antigen-specific IFN-γ responses. Experiments were also set up with one immunization using the same protocol and similar IFN-γ responses in ELISpot assays were observed (data not shown).
Figure 2.
IFN-γ responses and tumour protection in C57BL/6 mice immunized with IntSIIN. C57BL/6 mice were immunized twice at day 0 and 14 with (a)IntSIIN and (b) DC pulsed with IntSIIN. Immunizations were given intradermally and 16 days post-immunization, spleens were removed for ELISpot analysis. IFN-γ responses are given as spot-forming units (SFU) per 0·5 million cells. Representative results from one of three experiments are shown. One or two immunizations did not show any difference in responses; presented here after one injection. (c) Groups of C57BL/6 mice received one injection intradermally with (closed circles) PBS alone and (open circles) IntSIIN at 25 μg per mouse. After 14 days mice received a subcutaneous challenge of 107 E.G7.OVA tumour cells. Individual mouse curves are shown (n = 7).
IntSIIN induces tumour protection against E.G7.OVA
The ability of mice immunized with IntSIIN to be protected from challenge with OVA-expressing E.G7.OVA tumour cells was investigated. Groups of mice (n = 7) were injected intradermally with 25 μg IntSIIN per mouse on days 0 and 14, compared to mice injected with PBS alone. Fourteen days after immunization mice were challenged subcutaneously with 1 × 107 E.G7.-OVA tumour cells. Mice injected with PBS all grew tumours (7/7, Fig. 2c); however, in mice immunized with IntSIIN none of the tumours grew (0/7), except for one mouse that grew a very small tumour (< 50 mm2) by day 5 and was cleared by day 7 (Fig. 2c). These results show that immunization with IntSIIN intradermally can induce potent tumour protection in mice challenged with E.G7-OVA without the need for adjuvant and confirmed our previous findings using intraperitoneally administered IntSIIN.20
IntSIIN is processed via a TAP-independent MHC class I pathway
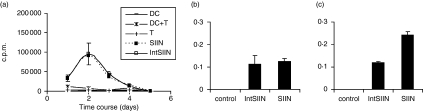
We investigated the TAP dependence of MHC class I presentation using DC from TAP−/− mice pulsed with IntSIIN at 1 μg/ml for 1 hr and added to OT-I T cells in culture. TAP−/− DC pulsed with SIIN at 1 μg/ml were used as a positive control for the assay, because SIIN is surface loaded and processing through TAP is not required. The stimulation of OT-I T cells by TAP−/− DC pulsed with IntSIIN was comparable to the responses induced by DC pulsed with SIIN (Fig. 3a). OT-I stimulation was similar to responses induced by C57BL/6 pulsed DC (Fig. 1B), the responses peaked at day 2 and decreased thereafter. Flow cytometry analysis on the residual cells in the purified OT-I T cells showed predominantly B cells (∼7%), natural killer cells (∼2·2%) and negligible amounts of monocytes/macrophages (∼1·2%), granulocytes (∼1%) and most importantly DC (CD11c+ ∼0·75%), which were the smallest population in the residual cells. B cells, natural killer cells, monocytes/macrophages and granulocytes present in the residual population are not able to cross-present exogenous antigen for stimulation of naïve T cells, therefore our hypothesis that the TAP-deficient DC are able to take up IntSIINFEKL and present via the cross-presentation pathway for stimulation of naïve antigen-specific T cells is supported by this analysis. These results show that IntSIIN is processed via a TAP-independent MHC class I processing pathway.
Figure 3.
TAP-independent stimulation of CD8 T-cell responses by IntSIIN. (a) In vitro grown TAP−/− DC were pulsed with SIIN and IntSIIN at 1 μg/ml for 1 hr then added to purified OT-I T cells and cultured for 6 days. Cells were harvested at each time-point and uptake of [3H]thymidine was measured to assess T-cell proliferation. DC pulsed with nothing, OT-I T cells and DC added to T cells were used in experiments as negative controls. Data are presented as counts per minute (c. p.m.) from quadruple wells (± SD). (b) EL-4 and (c) RMA-S were stimulated with IntSIIN and SIIN at 1 μg/ml for 1 hr and added to B3Z T cells for 24 hr. LacZ activity in B3Z T cells was assayed by total culture lysates with LacZ substrate CPRG. EL-4 and RMA-S added to B3Z T cells without peptides were used in experiments as negative controls. The absorbance (560 nm) of culture wells was read after 4 hr incubation at 37°.
To confirm the results from the in vitro proliferation studies, RMA-S cells, deficient in TAP, were pulsed with IntSIIN or SIIN at 1 μg/ml for 1 hr and added to B3Z T cells. EL-4 cells are TAP competent and were used as a positive control for the experiment (Fig. 3b). RMA-S pulsed with IntSIIN showed significant presentation to B3Z T cells above background responses (Fig. 3c). These responses were comparable to DC pulsed with SIIN at 1 μg/ml. These results in combination with proliferation assays confirm that IntSIIN is processed and presented by MHC class I molecules by a TAP-independent mechanism.
Uptake of IntSIIN by DC
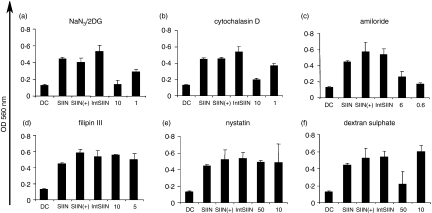
It has been reported that internalization of free Tat and Int peptides may be by endocytosis instead of a receptor-independent mechanism.24 To investigate if IntSIIN is taken up by an energy-dependent mechanism, DC were preincubated with sodium azide and 2-deoxyglucose to deplete ATP, then pulsed with IntSIIN and added to B3Z T cells.32 After 45 min, DC were pulsed for 1 hr with IntSIIN and added to B3Z T cells for 24 hr. ATP depletion by sodium azide and 2-deoxyglucose completely inhibited IntSIIN presentation at 10 mm and partially inhibited it at 1 mm (Fig. 4a). These results suggest that IntSIIN is taken up by energy-dependent endocytosis.
Figure 4.
Uptake of IntSIIN. DC were incubated for 45 min with inhibitors (a) sodium azide/2-deoxyglucose (10, 1 mm), (b) cytochalasin D (10, 1 μg/ml), (c) amiloride (6, 0·6 μm), (d) filipin III (10, 1 μg/ml), (e) nystatin (50, 10 μg/ml) and (f) dextran sulphate (50, 10 μg/ml), followed by incubation with IntSIIN at 1 μg/ml and added to B3Z T cells for 24 hr. DC were pulsed with SIINFEKL in the presence of the biochemical inhibitor at the maximum dose and were added to B3Z T cells in parallel experiments to ensure no inhibitory effects on the presentation of SIINFEKL CTL epitope. LacZ activity in B3Z T cells was assayed by total culture lysates with LacZ substrate CPRG. The absorbance (560 nm) of culture wells was read after 4 hr incubation at 37°.
Endocytosis may occur via a number of distinct mechanisms which encompass clathrin-mediated endocytosis, macropinocytosis, caveolae-mediated endocytosis, clathrin-caveolae-independent endocytosis pathways. To ascertain if the uptake of IntSIIN by DC is via macropinocytosis or phagocytosis, cytochalasin D and amiloride were used in antigen presentation assays. As a result of the requirement for actin in phagocytosis and macropinocytosis the inhibitor of F-actin elongation inhibits these mechanistic pathways.33,34 Preincubation of cytochalasin D with DC before the addition of IntSIIN inhibited IntSIIN presentation by 90–100% in two independent experiments by B3Z T cells at 10 μg/ml and partially inhibited at 1 μg/ml (Fig. 4b). Similarly, amiloride, an inhibitor of the Na+/H+ exchange inhibited presentation of IntSIIN by up to 90% (Fig. 4c).35 Depletion of ATP by incubation of DC with sodium azide and 2-deoxyglucose would inhibit all endocytic pathways including clathrin-dependent endocytosis. The inhibition by cytochalasin D and amiloride demonstrates the major uptake mechanism (> 90%) of IntSIIN is by a clathrin-independent endocytic process involving phagocytosis and/or macropinocytosis.
Caveolae are enriched in cholesterol and express caveolin. To study the possible role of caveolae in cellular uptake and subsequent processing of IntSIIN, filipin III and nystatin, both inhibitors of caveolae formation were used.36–38 Filipin III (1 or 10 μg/ml) and nystatin (10 or 50 μg/ml) had no effect on the presentation of IntSIIN to B3Z T cells (Fig. 4d,e). Therefore, the mechanism of uptake and presentation of IntSIIN does not appear to be mediated via caveolae-dependent uptake, consistent with the proposed mechanism of cellular uptake of conjugated Tat.39
The results of IntSIIN uptake demonstrate a caveolae-independent, phagocytosis- and/or macropinocytosis-mediated uptake. Dextran sulphate, which blocks negatively charged receptors on the surface of cells, was incubated with DC before the addition of IntSIIN and B3Z T cells.40,41 Dextran sulphate at 50 μg/ml partially inhibited the presentation of IntSIIN to B3Z T cells and at 10 μg/ml had no effect (Fig. 4f). These results suggest that IntSIIN gains access to the endosomes and subsequently enters the antigen presentation pathway after binding to negatively charged receptors on the cell membrane. In all experiments, DC pulsed with SIINFEKL in the presence of the maximum dose of biochemical inhibitor (SIIN +) or the absence of inhibitor (SIIN) demonstrated that the inhibitor did not have any effects on the T cells (Fig. 4).
The role of endosomes and lysosomes in the uptake and presentation of IntSIIN
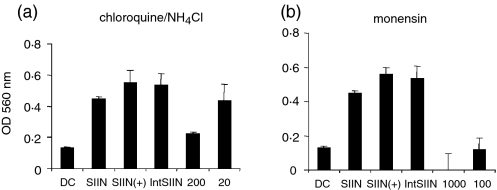
The inhibition of IntSIIN presentation by sodium azide, 2-deoxyglucose, cytochalasin D and dextran sulphate strongly suggests uptake via an endocytic process including phagocytosis and/or macropinocytosis. The possible role for endosomal processing was investigated using the inhibitors chloroquine and ammonium chloride, which prevent acidification of endosomes.42–44 After addition of chloroquine and ammonium chloride to pulsed DC, presentation to B3Z T cells was inhibited by 75% in two independent experiments at 200 μm and not inhibited at 20 μm (Fig. 5a). This suggests that the majority of IntSIIN is taken up and processed via the endosomal compartments.
Figure 5.
IntSIIN is endocytosed and processed in endosomes. DC were incubated for 45 min with inhibitors (a) chloroquine/NH4CL (200, 20 μm), and (b) monensin (1000, 100 μm) followed by incubation with IntSIIN at 1 μg/ml and added to B3Z T cells for 24 hr. DC were pulsed with SIINFEKL in the presence of the biochemical inhibitor at the maximum dose and added to B3Z T cells in parallel experiments to ensure no inhibitory effects on the presentation of SIINFEKL CTL epitope. LacZ activity in B3Z T cells was assayed by total culture lysates with LacZ substrate CPRG. The absorbance (560 nm) of culture wells was read after 4 hr incubation at 37°.
Monensin, a sodium/potassium proton ionophore has multiple roles and interferes with Golgi transport, acidification of intracellular compartments and can block protein transfer from endosomes to lysosomes.45,46 Monensin was able to completely inhibit the presentation of IntSIIN by DC at both 100 and 1000 μm (Fig. 5b) consistent with the results observed with chloroquine and ammonium chloride and supporting the hypothesis that IntSIIN is processed by the endosomes and lysosome for MHC class I presentation. In all experiments, DC pulsed with SIINFEKL in the presence of inhibitor (SIIN+) or the absence of inhibitor (SIIN) demonstrated that the inhibitor did not have any effects on the T cells (Fig. 5).
Proteolysis and peptide loading of IntSIIN
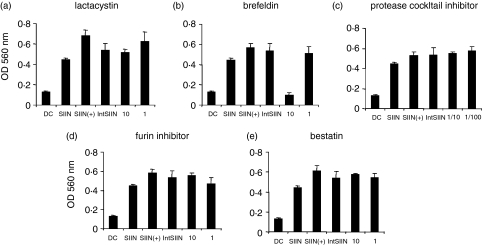
Peptides can be further degraded into smaller peptide fragments in the proteasomes for loading onto MHC class I molecules. To investigate the need for IntSIIN to be processed in the proteasomes for presentation on MHC, lactacystin was preincubated with DC before being pulsed with IntSIIN and presented to B3Z T cells.47 Lactacystin had no effect on the presentation of IntSIIN to B3Z T cells, at either 1 or 10 μm (Fig. 6a). Therefore, IntSIIN can be processed and presented in DC without the requirement for proteasomes. To further evaluate how CTL peptides derived from IntSIIN are loaded onto MHC class I molecules, we incubated DC with brefeldin A.48,49 Brefeldin A inhibits vesicle transport of newly synthesized MHC class I and II molecules between ER and Golgi complex.42 Pre-incubation with brefeldin A resulted in complete inhibition of IntSIIN to B3Z T cells at 10 μm and no inhibition was observed at 1 μm (Fig. 6b). These results certainly suggest that although IntSIIN is not further degraded in the proteasome and is TAP-independent, brefeldin-induced inhibition indicates that IntSIIN is taken up by endocytosis and lysosomes with possible processing to generate the CTL epitopes occurring in these compartments. These fragments might then escape the endosomes and bypass the proteasome and TAP compartments and be loaded onto MHC class I molecules.
Figure 6.
Proteolysis and peptide loading of IntSIIN. DC were incubated for 45 min with inhibitors, (a) lactacystin (10, 1 μm), (b) brefeldin (10, 1 μg/ml), (c) protease inhibitor (1/100, 1/10 dilution), (d) furin inhibitor (10, 1 μm) and (e) bestatin (10, 1 μm) followed by incubation with IntSIIN at 1 μg/ml and added to B3Z T-cell hybridoma for 24 hr. DC were pulsed with SIINFEKL in the presence of the biochemical inhibitor at the maximum dose and added to B3Z T cells in parallel experiments to ensure no inhibitory effects on the presentation of SIINFEKL CTL epitope. LacZ activity in B3Z T cells was assayed by total culture lysates with LacZ substrate CPRG. The absorbance (560 nm) of culture wells was read after 4 hr incubation at 37°.
To confirm that IntSIIN was not degraded extracellularly by proteases and surface-loaded on the DC, we incubated pulsed DC with protease inhibitor cocktail. Pre-incubation with protease inhibitor cocktail did not have any effect on IntSIIN presentation to B3Z T cells and confirmed that IntSIIN was degraded intracellularly (Fig. 6c).
To further investigate possible compartments involved in the degradation of IntSIIN into CTL epitopes in intracellular compartments, inhibitors of aminopeptidases in the ER and furin endopeptidases in the trans-Golgi were investigated.27,50,51 It is known that unconjugated Tat peptide can be trimmed in the trans-Golgi compartment, with the participation of the endopeptidase furin and possibly with the additional participation of a carboxypeptidase and aminopeptidases in the ER.27 Aminopeptidase and furin inhibitors were preincubated with DC pulsed with IntSIIN.52–54 The endopeptidase furin inhibitor decRVKR-CMK at 1 and 10 μm had no effect on the presentation of IntSIIN to B3Z T cells (Fig. 6d). In addition, the use of an inhibitor of aminopeptidase which is located in the ER, bestatin, induced no inhibition of IntSIIN presentation to B3Z T cells at 1 and 10 μm (Fig. 6e). The roles of furin endopeptidase in the trans-Golgi and aminopeptidases in the ER do not contribute to further trimming of IntSIIN into smaller peptides, contrary to what has been observed with conjugated Tat peptide.27,55 Trimming in the endosomes and lysosomes appears to be sufficient to generate CTL epitopes capable of loading MHC class I molecules. In all experiments, DC pulsed with SIINFEKL in the presence of inhibitor (SIIN+) or the absence of inhibitor (SIIN) demonstrated that the inhibitor did not have any effects on the T cells (Fig. 6).
Discussion
Exogenous antigens are usually processed in late endosomes/lysosomes and presented by MHC class II molecules to CD4 cells. DC in particular are capable of processing exogenous antigens and peptides for presentation by class I molecules via the cross-presentation pathway. A wide range of biomolecules such as antigenic peptides,20,55 peptide nucleic acids,24,56 oligonucleotides,57 full-length proteins,58 nanoparticles and liposomes18 have been shown to be delivered into cells by membrane translocating peptides. Membrane translocating peptides from the Drosophila Antennapedia protein, human immunodeficiency virus Tat protein and measles virus fusion protein have also been used for peptide delivery. Microbial toxins such as shiga toxin, anthrax toxin, diphtheria toxin, bordetella pertussis toxin and ricin have been used because of their ability to translocate from the endosome into the cytoplasm. The 16-mer peptide responsible for the membrane translocating activity of Antennapedia can be used to internalize peptides or proteins directly into the cytoplasm.23 We had previously demonstrated that IntSIIN was rapidly internalized into the cytoplasm of macrophages, induced peptide-specific T cells and protected mice against a tumour challenge.20 This was independently confirmed recently when it was demonstrated that this fusion peptide when injected epicutaneously in mice generated T-cell responses and protected mice against a tumour challenge.21
The present study demonstrates that the 16-amino-acid peptide from Antennapedia (Int) tandemly linked to the ovalbumin SIINFEKL CTL peptide was efficiently taken up by in vitro grown DC and the CTL epitope was displayed on the cell surface bound to MHC class I as demonstrated by stimulation of the B3Z T cell hybridoma and OVA antigen-specific OT-I T cells in vitro (Fig. 1). In addition, mice immunized with IntSIIN induced potent CD8+-specific IFN-γ responses shown by ELISpot assay (Fig. 2a). These responses were induced in the presence or absence of DC, emphasizing that penetratin may be used directly for peptide-based immunization without the need for DC or other adjuvants. Mice immunized with 25 μg IntSIIN per mouse did not grow OVA-expressing tumours compared to control mice (Fig. 2c).
At present, little is known about the mechanisms of antigen presentation of Int linked to CTL peptide. We assessed the ability of IntSIIN to be taken up by DC in vitro and present peptides on the cell surface in complex with MHC class I using several biochemical inhibitors of various stages of the antigen presentation pathways. The interaction between Int and the cellular membrane seems to be an essential step for its uptake into cells. It has been suggested that Int translocates through the lipid bilayer of the cell membrane by transiently forming inverted micelles.23 Heparin sulphate proteoglycans were recently shown to be responsible for the uptake of the Tat protein in a large number of mammalian cell lines.59 The Tat peptide, however, is taken up by a route that does not involve the heparin sulphate proteoglycans.60 More interestingly, uptake of Tat-streptavidin complexes by CHO cells was inhibited by heparin and dextran sulphate; however, Int-streptavidin complexes were inhibited by only heparin and not dextran sulphate.61 Comparatively, when Tat and Antennapedia liposomes were assessed for uptake into B16 cells, heparin and dextran sulphate inhibited uptake of both complexes.61
In our studies, it is clear that internalized IntSIIN is processed and presented by MHC class I molecules by cross-presentation. The majority (> 90%) uptake of IntSIIN is via an endocytic process involving phagocytosis or macropinocytosis, as demonstrated by the inhibition of antigen presentation when DC were treated with cytochalasin D, NaN3/2DG and amiloride (Fig. 4). Since the major uptake of IntSIIN is via a clathrin-independent pathway the interaction of the cationic Int peptide with the negatively charged receptors promotes the concentration of peptide on the cell surface for phagocytic or pinocytic uptake rather than for uptake by specific receptor-mediated endocytosis. The lack of a specific receptor-mediated endocytic uptake was demonstrated previously by identical uptake of peptides synthesized entirely with d-amino acids rather than natural l-amino acids.23 The inhibitory effect on presentation by chloroquine/NH4Cl and monensin indicated cleavage in the endosomal compartment (Fig. 5). The subsequent steps to access the ER for loading into class I molecules are proteasome- and TAP-independent with no further trimming required by aminopeptidases in the ER or by furin endopeptidases in the trans-Golgi (Fig. 6).
We have characterized the antigen uptake, processing and presentation pathway, utilized by DC pulsed with an Antennapedia peptide, Int, tandemly linked to a CTL epitope for the first time. It is possible that the mode of internalization and presentation pathways could be different depending on the sequence of the peptide and also cell types. Whatever the mode of mechanism, it is clear that the Antennapedia peptide-based delivery of antigen containing CTL epitopes is very effective in eliciting cellular responses in vivo.
Acknowledgments
This work was supported by the National Breast Cancer Foundation Kathleen Cunningham project grant (V.A., G.P.), an NH & MRC project grant 223310 (V.A.) and the Susan G Komen Breast Cancer Foundation grant (G.P.). V.A. is an NH & MRC R. Douglas Wright Fellow 223316.
Abbreviations
- AP
alkaline phosphatase
- APC
antigen-presenting cells
- CPRG
chlorophenol red-β-galactoside
- CTL
cytotoxic T lymphocytes
- DC
dendritic cells
- Int
penetratin 16mer peptide – RQIKIWFQNRRMKWKK
- IntSIIN
Int tandemly linked to SIINFEKL peptide
- OVA
ovalbumin
- SDS
sodium dodecyl sulphate
- SIIN
SIINFEKL peptide
- SFU
spot-forming units
- TAP
tapasin
- TCR
T-cell receptor
References
- 1.Pietersz GA, Apostolopoulos V, McKenzie IF. Generation of cellular immune responses to antigenic tumor peptides. Cell Mol Life Sci. 2000;57:290–310. doi: 10.1007/PL00000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 3.Apostolopoulos V, Barnes N, Pietersz GA, McKenzie IF. Ex vivo targeting of the macrophage mannose receptor generates anti-tumor CTL responses. Vaccine. 2000;18:3174–84. doi: 10.1016/s0264-410x(00)00090-6. [DOI] [PubMed] [Google Scholar]
- 4.Apostolopoulos V, Lofthouse SA, Popovski V, Chelvanayagam G, Sandrin MS, McKenzie IF. Peptide mimics of a tumor antigen induce functional cytotoxic T cells. Nat Biotechnol. 1998;16:276–80. doi: 10.1038/nbt0398-276. [DOI] [PubMed] [Google Scholar]
- 5.Apostolopoulos V, McKenzie IF. Cellular mucins: targets for immunotherapy. Crit Rev Immunol. 1994;14:293–309. doi: 10.1615/critrevimmunol.v14.i3-4.40. [DOI] [PubMed] [Google Scholar]
- 6.Apostolopoulos V, McKenzie IF. Role of the mannose receptor in the immune response. Curr Mol Med. 2001;1:469–74. doi: 10.2174/1566524013363645. [DOI] [PubMed] [Google Scholar]
- 7.Apostolopoulos V, McKenzie IF, Pietersz GA. Breast cancer immunotherapy: current status and future prospects. Immunol Cell Biol. 1996;74:457–64. doi: 10.1038/icb.1996.76. [DOI] [PubMed] [Google Scholar]
- 8.Apostolopoulos V, Osinski C, McKenzie IF. MUC1 cross-reactive Gal alpha (1,3) Gal antibodies in humans switch immune responses from cellular to humoral. Nat Med. 1998;4:315–20. doi: 10.1038/nm0398-315. [DOI] [PubMed] [Google Scholar]
- 9.Apostolopoulos V, Pietersz GA, Gordon S, Martinez-Pomares L, McKenzie IF. Aldehyde–mannan antigen complexes target the MHC class I antigen-presentation pathway. Eur J Immunol. 2000;30:1714–23. doi: 10.1002/1521-4141(200006)30:6<1714::AID-IMMU1714>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 10.Apostolopoulos V, Pietersz GA, Loveland BE, Sandrin MS, McKenzie IF. Oxidative/reductive conjugation of mannan to antigen selects for T1 or T2 immune responses. Proc Natl Acad Sci U S A. 1995;92:10128–32. doi: 10.1073/pnas.92.22.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apostolopoulos V, Pietersz GA, McKenzie IF. Cell-mediated immune responses to MUC1 fusion protein coupled to mannan. Vaccine. 1996;14:930–8. doi: 10.1016/0264-410x(95)00258-3. [DOI] [PubMed] [Google Scholar]
- 12.Apostolopoulos V, Pietersz GA, McKenzie IF. MUC1 and breast cancer. Curr Opin Mol Ther. 1999;1:98–103. [PubMed] [Google Scholar]
- 13.Apostolopoulos V, Sandrin MS, McKenzie IF. Mimics and cross reactions of relevance to tumour immunotherapy. Vaccine. 1999;18:268–75. doi: 10.1016/s0264-410x(99)00197-8. [DOI] [PubMed] [Google Scholar]
- 14.Apostolopoulos V, Sandrin MS, McKenzie IF. Carbohydrate/peptide mimics. Effect on MUC1 cancer immunotherapy. J Mol Med. 1999;77:427–36. doi: 10.1007/s001090050373. [DOI] [PubMed] [Google Scholar]
- 15.McKenzie IF, Apostolopoulos V, Lees C, et al. Oxidised mannan antigen conjugates preferentially stimulate T1 type immune responses. Vet Immunol Immunopathol. 1998;63:185–90. doi: 10.1016/s0165-2427(98)00094-4. [DOI] [PubMed] [Google Scholar]
- 16.Schutze-Redelmeier MP, Gournier H, Garcia-Pons F, Moussa M, Joliot AH, Volovitch M, Prochiantz A, Lemonnier FA. Introduction of exogenous antigens into the MHC class I processing and presentation pathway by Drosophila antennapedia homeodomain primes cytotoxic T cells in vivo. J Immunol. 1996;157:650–5. [PubMed] [Google Scholar]
- 17.Chikh G, Bally M, Schutze-Redelmeier MP. Characterization of hybrid CTL epitope delivery systems consisting of the Antennapedia homeodomain peptide vector formulated in liposomes. J Immunol Meth. 2001;254:119–35. doi: 10.1016/s0022-1759(01)00411-2. [DOI] [PubMed] [Google Scholar]
- 18.Chikh GG, Kong S, Bally MB, Meunier JC, Schutze-Redelmeier MP. Efficient delivery of Antennapedia homeodomain fused to CTL epitope with liposomes into dendritic cells results in the activation of CD8+ T cells. J Immunol. 2001;167:6462–70. doi: 10.4049/jimmunol.167.11.6462. [DOI] [PubMed] [Google Scholar]
- 19.Karttunen J, Shastri N. Measurement of ligand-induced activation in single viable T cells using the lacZ reporter gene. Proc Natl Acad Sci USA. 1991;88:3972–6. doi: 10.1073/pnas.88.9.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietersz GA, Li W, Apostolopoulos V. A 16-mer peptide (RQIKIWFQNRRMKWKK) from antennapedia preferentially targets the Class I pathway. Vaccine. 2001;19:1397–405. doi: 10.1016/s0264-410x(00)00373-x. [DOI] [PubMed] [Google Scholar]
- 21.Schutze-Redelmeier MP, Kong S, Bally MB, Dutz JP. Antennapedia transduction sequence promotes anti-tumour immunity to epicutaneously administered CTL epitopes. Vaccine. 2004;22:1985–91. doi: 10.1016/j.vaccine.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Chikh G, Schutze-Redelmeier MP. Liposomal delivery of CTL epitopes to dendritic cells. Biosci Rep. 2002;22:339–53. doi: 10.1023/a:1020151025412. [DOI] [PubMed] [Google Scholar]
- 23.Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J Biol Chem. 1996;271:18188–93. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- 24.Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–90. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 25.Drin G, Cottin S, Blanc E, Rees AR, Temsamani J. Studies on the internalization mechanism of cationic cell-penetrating peptides. J Biol Chem. 2003;278:31192–201. doi: 10.1074/jbc.M303938200. [DOI] [PubMed] [Google Scholar]
- 26.Saalik P, Elmquist A, Hansen M, Padari K, Saar K, Viht K, Langel U, Pooga M. Protein cargo delivery properties of cell-penetrating peptides. A comparative study. Bioconjug Chem. 2004;15:1246–53. doi: 10.1021/bc049938y. [DOI] [PubMed] [Google Scholar]
- 27.Lu J, Wettstein PJ, Higashimoto Y, Appella E, Celis E. TAP-independent presentation of CTL epitopes by Trojan antigens. J Immunol. 2001;166:7063–71. doi: 10.4049/jimmunol.166.12.7063. [DOI] [PubMed] [Google Scholar]
- 28.Lipford GB, Hoffman M, Wagner H, Heeg K. Primary in vivo responses to ovalbumin. Probing the predictive value of the Kb binding motif. J Immunol. 1993;150:1212–22. [PubMed] [Google Scholar]
- 29.Apostolopoulos V, Yuriev E, Ramsland PA, et al. A glycopeptide in complex with MHC class I uses the GalNAc residue as an anchor. Proc Natl Acad Sci USA. 2003;100:15029–34. doi: 10.1073/pnas.2432220100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pouniotis DS, Proudfoot O, Bogdanoska V, Apostolopoulos V, Fifis T, Plebanski M. Dendritic cells induce immunity and long-lasting protection against blood-stage malaria despite an in vitro parasite-induced maturation defect. Infect Immun. 2004;72:5331–9. doi: 10.1128/IAI.72.9.5331-5339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanderson S, Shastri N. LacZ inducible, antigen/MHC-specific T cell hybrids. Int Immunol. 1994;6:369–76. doi: 10.1093/intimm/6.3.369. [DOI] [PubMed] [Google Scholar]
- 32.Potocky TB, Menon AK, Gellman SH. Cytoplasmic and nuclear delivery of a TAT-derived peptide and a beta-peptide after endocytic uptake into HeLa cells. J Biol Chem. 2003;278:50188–94. doi: 10.1074/jbc.M308719200. [DOI] [PubMed] [Google Scholar]
- 33.Lo WF, Dunn CD, Ong H, Metcalf ES, Soloski MJ. Bacterial and host factors involved in the major histocompatibility complex class Ib-restricted presentation of Salmonella Hsp 60: novel pathway. Infect Immun. 2004;72:2843–9. doi: 10.1128/IAI.72.5.2843-2849.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sampath P, Pollard TD. Effects of cytochalasin, phalloidin, and pH on the elongation of actin filaments. Biochemistry. 1991;30:1973–80. doi: 10.1021/bi00221a034. [DOI] [PubMed] [Google Scholar]
- 35.West MA, Bretscher MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol. 1989;109:2731–9. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905–15. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pohl J, Ring A, Stremmel W. Uptake of long-chain fatty acids in HepG2 cells involves caveolae: analysis of a novel pathway. J Lipid Res. 2002;43:1390–9. doi: 10.1194/jlr.m100404-jlr200. [DOI] [PubMed] [Google Scholar]
- 38.Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–32. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richard JP, Melikov K, Brooks H, Prevot P, Lebleu B, Chernomordik LV. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J Biol Chem. 2005;280:15300–6. doi: 10.1074/jbc.M401604200. [DOI] [PubMed] [Google Scholar]
- 40.Fretz MM, Koning GA, Mastrobattista E, Jiskoot W, Storm G. OVCAR-3 cells internalize TAT-peptide modified liposomes by endocytosis. Biochim Biophys Acta. 2004;1665:48–56. doi: 10.1016/j.bbamem.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 41.Mai JC, Shen H, Watkins SC, Cheng T, Robbins PD. Efficiency of protein transduction is cell type-dependent and is enhanced by dextran sulfate. J Biol Chem. 2002;277:30208–18. doi: 10.1074/jbc.M204202200. [DOI] [PubMed] [Google Scholar]
- 42.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–6. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 43.Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 44.Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–91. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 45.Midoux P, Roche AC, Monsigny M. Quantitation of the binding, uptake, and degradation of fluoresceinylated neoglycoproteins by flow cytometry. Cytometry. 1987;8:327–34. doi: 10.1002/cyto.990080314. [DOI] [PubMed] [Google Scholar]
- 46.Wileman T, Boshans RL, Schlesinger P, Stahl P. Monensin inhibits recycling of macrophage mannose-glycoprotein receptors and ligand delivery to lysosomes. Biochem J. 1984;220:665–75. doi: 10.1042/bj2200665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science. 1995;268:726–31. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 48.Pond L, Watts C. Characterization of transport of newly assembled, T cell-stimulatory MHC class II–peptide complexes from MHC class II compartments to the cell surface. J Immunol. 1997;159:543–53. [PubMed] [Google Scholar]
- 49.Yewdell JW, Bennink JR. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science. 1989;244:1072–5. doi: 10.1126/science.2471266. [DOI] [PubMed] [Google Scholar]
- 50.Saric T, Beninga J, Graef CI, Akopian TN, Rock KL, Goldberg AL. Major histocompatibility complex class I-presented antigenic peptides are degraded in cytosolic extracts primarily by thimet oligopeptidase. J Biol Chem. 2001;276:36474–81. doi: 10.1074/jbc.M105517200. [DOI] [PubMed] [Google Scholar]
- 51.Saric T, Graef CI, Goldberg AL. Pathway for degradation of peptides generated by proteasomes: a key role for thimet oligopeptidase and other metallopeptidases. J Biol Chem. 2004;279:46723–32. doi: 10.1074/jbc.M406537200. [DOI] [PubMed] [Google Scholar]
- 52.Gil-Torregrosa BC, Raul Castano A, Del Val M. Major histocompatibility complex class I viral antigen processing in the secretory pathway defined by the trans-Golgi network protease furin. J Exp Med. 1998;188:1105–16. doi: 10.1084/jem.188.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kozlowski S, Corr M, Shirai M, Boyd LF, Pendleton CD, Berzofsky JA, Margulies DH. Multiple pathways are involved in the extracellular processing of MHC class I-restricted peptides. J Immunol. 1993;151:4033–44. [PubMed] [Google Scholar]
- 54.Umezawa H. Low-molecular-weight enzyme inhibitors of microbial origin. Annu Rev Microbiol. 1982;36:75–99. doi: 10.1146/annurev.mi.36.100182.000451. [DOI] [PubMed] [Google Scholar]
- 55.Lu J, Higashimoto Y, Appella E, Celis E. Multiepitope Trojan antigen peptide vaccines for the induction of antitumor CTL and Th immune responses. J Immunol. 2004;172:4575–82. doi: 10.4049/jimmunol.172.7.4575. [DOI] [PubMed] [Google Scholar]
- 56.Pooga M, Soomets U, Hallbrink M, et al. Cell penetrating PNA constructs regulate galanin receptor levels and modify pain transmission in vivo. Nat Biotechnol. 1998;16:857–61. doi: 10.1038/nbt0998-857. [DOI] [PubMed] [Google Scholar]
- 57.Astriab-Fisher A, Sergueev DS, Fisher M, Shaw BR, Juliano RL. Antisense inhibition of P-glycoprotein expression using peptide–oligonucleotide conjugates. Biochem Pharmacol. 2000;60:83–90. doi: 10.1016/s0006-2952(00)00310-5. [DOI] [PubMed] [Google Scholar]
- 58.Shibagaki N, Udey MC. Dendritic cells transduced with protein antigens induce cytotoxic lymphocytes and elicit antitumor immunity. J Immunol. 2002;168:2393–401. doi: 10.4049/jimmunol.168.5.2393. [DOI] [PubMed] [Google Scholar]
- 59.Tyagi M, Rusnati M, Presta M, Giacca M. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J Biol Chem. 2001;276:3254–61. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- 60.Silhol M, Tyagi M, Giacca M, Lebleu B, Vives E. Different mechanisms for cellular internalization of the HIV-1 Tat-derived cell penetrating peptide and recombinant proteins fused to Tat. Eur J Biochem. 2002;269:494–501. doi: 10.1046/j.0014-2956.2001.02671.x. [DOI] [PubMed] [Google Scholar]
- 61.Console S, Marty C, Garcia-Echeverria C, Schwendener R, Ballmer-Hofer K. Antennapedia and HIV transactivator of transcription (TAT) ‘protein transduction domains’ promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J Biol Chem. 2003;278:35109–14. doi: 10.1074/jbc.M301726200. [DOI] [PubMed] [Google Scholar]