Abstract
Oral tolerance is a T-cell mediated phenomenon defined by inhibition of immune responsiveness to a protein previously contacted by the oral route. Oral tolerance may prevent autoimmune and allergic diseases that involve the recruitment and/or activation of different cell types including mast cells, neutrophils, eosinophils, monocytes and lymphocytes. The mechanisms by which oral tolerance avoids these immunological disorders are still controversial. Herein we used a murine model of ovalbumin (OVA)-induced peritonitis to investigate the effect of oral tolerance on allergic inflammation. Frequency of leucocyte subpopulations was evaluated by global and differential cell counts in peritoneal lavage fluid, peripheral blood, and bone marrow. Changes on lymphocyte subsets and adhesion molecules expression by these cells were analysed by flow cytometry. As compared with OVA-immune mice, intraperitoneal challenge of tolerant animals with OVA resulted in a significantly milder peritonitis, mostly affecting neutrophils and eosinophils; a concomitant reduction in total white blood cell counts was also observed, mainly because of lower neutrophil and eosinophil counts. Eosinophils, but not neutrophils, were also reduced in the bone-marrow of OVA-challenged tolerant mice. No changes occurred in total peritoneal lymphocyte counts in OVA-tolerant mice, however, there was a significant decrease in CD3+ CD8+ T cells and an increase in B cells (CD45R+) in these animals as compared to immune OVA-challenged animals. Altered expression of CD18 and CD54, respectively, in blood and peritoneal lymphocytes was also noted. These results suggest that, in addition to local specific effects, oral tolerance has systemic effects on the mobilization of leucocytes and bone-marrow eosinopoiesis.
Keywords: oral tolerance, inflammation, allergy, eosinophil, ova
Introduction
Mucosal lymphoid tissues are the largest component of the immune system spread over an area of hundreds of square metres, which is in constant contact with an immense variety of antigenic materials. Most of the body's lymphocytes are found in the mucosal associated lymphoid tissues. In fact, 80–90% of all immunoglobulin-producing B lymphocytes in the mouse are located in the gut epithelial lining.1 Small amounts of partially degraded or intact proteins regularly penetrate the circulation and antibodies to dietary antigen are a common finding in normal individuals.2 However, the progressive quality of clonal expansion from antigen-specific lymphocytes is absent in animals that have been previously exposed to antigen by mucosal routes and then immunized by parenteral routes. This is the relevant point: whereas animals primed by parenteral routes increase specific antibody titres at each additional injection of antigen, the titres of antibodies formed after ingestion of proteins remain remarkably stable in spite of repeated injections of antigen.3 Significantly, the most frequent consequence of the ingestion of proteins is the development of oral tolerance.
Oral tolerance is a T-cell mediated phenomenon defined by refractoriness to subsequent parenteral immunization with proteins first contacted by the oral route. Several factors affect the induction and maintenance of oral tolerance, including age, animal strain and species, immunological status of the animal, as well as adjuvants, dose and nature of the antigen used.4 Oral tolerant animals form less specific antibodies, and display weak delayed-type hypersensitivity (DTH) reactions in vivo and lymphocyte proliferation in the presence of the cognate antigen in vitro.5 Oral tolerance prevents the development of some experimental autoimmune diseases,6,7 allergies8,9 and experimental asthma.10 However, few advances have been achieved in treating diseases already ongoing by oral administration of antigens and a search for adjuvants that potentiate tolerance is required.11 Autoimmune and allergic diseases have become increasingly common and we are far from understanding the mechanisms that trigger and maintain them. Dietary changes, probiotics and modifications of the intestinal flora may be correlated with the incidence of allergies.12
Allergic inflammation involves the recruitment and/or activation of different cell types including mast cells, neutrophils, eosinophils, monocytes and lymphocytes.13 The cytological patterns of inflammatory infiltrates depend on the profiles of secretion of different inflammatory mediators and growth factors. Eosinophils are thought to play a central role in asthma where they contribute to tissue damage by secretion of their products on bronchial epithelium which include a wide array of cytokines.14 Eosinophils in asthmatic airways and other localized tissue are derived from circulating progenitors recruited by local inflammatory signals or from bone-marrow cells released after allergen exposure. Oral tolerance was able to prevent airway infiltration by eosinophils15–18 and bone-marrow eosinophilia10 by mechanisms that are still controversial, at times associated15,18 or not17 with increased transforming growth factor-β and interleukin (IL)-10.
Although atopic diseases such as asthma, allergic rhinitis and dermatitis have been conceived as occurring in specific local tissues, the systemic nature of allergy has also been considered.19 Oral tolerance may possibly have systemic effects upon the differentiation and/or migration of non-adaptive inflammatory cells triggered by antigen exposure. Herein, we used a model of ovalbumin (OVA)-induced peritonitis20 to evaluate whether oral tolerance interferes with leucocyte production, mobilization and/or migration to inflammatory sites. Global and differential cell counts in peritoneal lavage fluid (PLF), peripheral blood, and bone marrow (BM) were made. We also checked for differences in lymphocyte subtypes and expression of adhesion molecules.
Our results indicate that oral tolerance, besides its immunological specific and local effects, also blocks the global increase in leucocyte numbers triggered by immunization and inhibits the mobilization of some cell types from the bone marrow and their migration to inflammatory sites. Changes in the expression of molecules engaged on cell migration (CD18 and CD54) were observed on lymphocytes from OVA-tolerant versus OVA-immunized mice.
Materials and methods
Experimental animals
Female 8-week-old (C57BL/6 × DBA/2 J)F1, or B6D2F1 mice, bred and maintained in our animal breeding unit according to the rules established by the ethical committee, were used. Experimental groups contained six mice. Three groups were compared, as described below: (1) immune saline-challenged; (2) immune OVA-challenged; and (3) tolerant OVA-challenged.
Antigen and antibodies used
The antigen used was OVA (grade V, Sigma, St. Louis, MO). The antibody for enzyme-linked immunosorbent assay (ELISA) was goat anti-mouse immunoglobulin (IgM + IgG + IgA, H + L) horseradish peroxidase (HRP)-labelled (Southern Biotechnology Associates, Birmingham, AL). Antibodies for flow cytometry were: rat IgG2b anti-mouse CD3 R-phycoerythrin (PE; Sigma); rat IgG2a anti-mouse CD4 fluoroscein isothiocyanate (FITC; Sigma); rat IgG2a anti-mouse CD8 FITC (Sigma); rat IgG2a anti-mouse CD45R R-PE (Sigma); hamster IgG anti-mouse CD54 FITC (Pharmingen, San Diego, CA); rat IgG2a anti-mouse CD18 FITC (Caltag, Burlingame CA) or corresponding isotype-matched controls.
Feeding regimens for oral tolerance induction
Oral tolerance to OVA was induced by enforcing mice to drink, ad libitum, a 1/5 solution of hen egg white in drinking water for 3 consecutive days. The egg white solution was prepared in our laboratory from commercially available eggs. Daily estimated average consumption was 20 mg OVA/mouse and this resulted in significant levels of tolerance. Bottles were changed every day to avoid contamination. Control groups received filtered tap water.
Parenteral immunizations and antigen-induced peritonitis
Mice were actively sensitized by two subcutaneous (s.c.) injections of 0·2 ml of a suspension containing 100 µg of OVA and 1·6 mg of Al(OH)3 given 7 days apart. One week thereafter peritonitis was induced by the intraperitineal (i.p.) injection of 1 µg of OVA (0·2 ml of a sterile solution containing 5 µg/ml OVA in saline). Control animals sensitized s.c. with OVA received i.p. the same volume of sterile saline.
Peripheral blood counts
At 6, 24 and 48 hr after antigen challenge, mice were bled under ether anaesthesia from the axillary plexus and blood was collected without anticoagulant for antibody assays and smears, or with ethylenediaminetetra-acetic acid for global leucocyte counts. Total white blood cell counts were obtained in an automatic counter (Beckman Instruments, Inc.). peripheral blood smears were stained with May–Grünwald–Giemsa and differential white blood cell count determined under oil immersion (×1000). At least 200 cells were counted and results were expressed as the number of leucocytes, neutrophils or eosinophils per millilitre of blood. For cytometry analysis blood cells were collected in heparin 24 hr after OVA challenge, and samples of 25 µl were plated and incubated with the antibody or isotype control at appropriated dilution for 1 hr. Then, red blood cells were lysed using FACSTM lysing solution (Becton Dickinson, San Jose, CA). After lysis cells were washed twice in phosphate-buffered saline (PBS) containing 3% fetal calf serum (FCS) and 0·1% sodium azide. Stained cells were resuspended in PBS containing 3% FCS and 0·1% sodium azide. Cell samples were analysed on a FACScan Flow Cytometer (Becton Dickinson, Mountain View, CA). For each sample 10 000 events were collected and analysis were performed using CellQuest. FACS analysis was performed by gating the lymphocyte population on the basis of relative size (forward light scatter) and granularity (side angle scatter). Results were expressed as the percentage of each lymphocyte population.
Peritoneal lavage fluid (PLF)
After bleeding, animals were killed by cervical displacement, the peritoneal cavity was opened and washed with 3 ml of Hanks' balanced salt solution (HBSS) containing heparin (10 U/ml); approximately 90% of the initial volume was recovered and in cases when haemorrhages were detected in the peritoneal cavity, the animals were discarded. Total cell count present in the PLF was determined in a haemocytometer. Differential cell counts were performed after cytocentrifugation and staining with May–Grünwald–Giemsa under oil immersion (×1000). At least 400 cells were counted and results were expressed as the number of cells per millilitre of PLF. For cytometry analysis cells were collected 24 hr after OVA challenge, washed twice in PBS containing 3% FCS and 0·1% sodium azide and subsequently stained, according to the standard method. Stained cells were resuspended in PBS containing 3% FCS and 0·1% sodium azide. Cell samples were analysed on a FACScan Flow Cytometer in the same way as those from peripheral blood.
Bone marrow (BM)
Bone marrow cells were obtained by flushing the femur with 5 ml of HBSS containing heparin (50 U/ml). The cell suspensions were gently homogenized to break large clumps, centrifuged and resuspended in HBSS for final volume of 1 ml. Total cell count present in the BM cell suspensions was evaluated microscopically by haemocytometer. Differential cell counts were performed after cytocentrifugation and staining with May–Grünwald–Giemsa under oil immersion (×1000). At least 400 cells were counted and results were expressed as the number of cells per millilitre of BM cell suspension.
Antibody assay
Anti-OVA antibody titres were determined by standard ELISA using an automatic ELISA reader (Bio-Rad, Hercules, CA). In short, plates (Nunc, Roskilde, Denmark) were coated overnight with 2 µg of OVA in 100 µl/well of sodium carbonate buffer, pH 9·6, at 4°. Plates were washed with PBS containing 0·05% Tween-20 and blocked for 1 hr at room temperature (RT) with PBS containing 0·25% casein. The plates were incubated for 1 hr at RT with six dilutions of mouse serum samples starting at 1/100 in PBS-casein. Plates were washed again, six times, and incubated with goat anti-mouse immunoglobulin–HRP for 1 hr at 37°. Then the plates were washed six times, and incubated in the dark with H2O2 in the presence of orthophenylenediamine (OPD, Sigma) in sodium citrate buffer, pH 5·0 for 20 min. The reaction was stopped by the addition of 20 µl of 2 N H2SO4. Colour development was measured at OD 492 nm. ELISA scores were computed by running sums of optical densities between 1 : 100 and 1 : 3200 of serum dilutions in individual mice, as indicated in the figure legends. Each score is shown as mean ± SEM of groups of six animals.
Statistical analysis
Statistical analyses were performed using StatView or Sigma Statistical software and the statistical significance was determined using Umpaired t-test (for cytometry data) and Student–Newman–Keuls comparison test. P-values of 0·05 or less were considered significant. The results are expressed as the mean ± SEM.
Results
Oral tolerance blocks granulocyte accumulation in the peritoneal cavity
To induce oral tolerance to OVA, B6D2F1 mice were enforced to drink an egg white solution for 3 days; control mice drank tap water. Seven and 14 days after the oral treatment all mice received primary and secondary s.c. immunizations with 100 µg of OVA in Al(OH)3 and 7 days thereafter were i.p. challenged with a small dose (1 µg) of soluble sterile OVA. An additional control group not tolerant to OVA was s.c. immunized as the others, but i.p. challenged with sterile saline. All mice were tested for anti-OVA antibodies in the serum. In parallel experiments PLF were collected for immunophenotypic analysis by flow cytometry 24 hr after i.p. challenge and differential cell counts after cytocentrifugation and staining with May–Grünwald–Giemsa at 6, 24 and 48 hr after i.p. challenge.
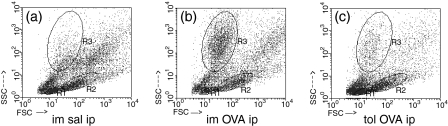
Mice that drank OVA became tolerant and formed less anti-OVA antibodies; the immune and immune-control groups had similar anti-OVA titres 24 hr after i.p. injection of, respectively, OVA or saline (Fig. 2a). Representative dot-plot distribution of leucocytes by size (forward light scatter) versus granularity (side angle scatter) are illustrated on Fig. 1(a–c). OVA-challenge in immune mice led to peritonitis, characterized by increased frequency of granulocytes (Fig. 1b). This inflammatory reaction was blocked in tolerant OVA-challenged mice (Fig. 1c), in which the reaction resembled that of the injection of i.p. saline in OVA-immune mice (Fig. 1a).
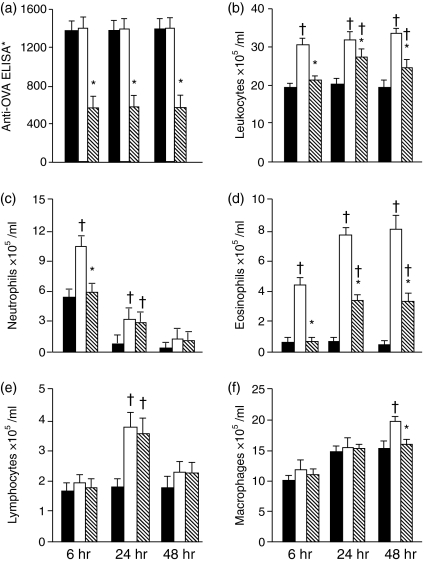
Figure 2.
Oral tolerance blocks neutrophil, eosinophil and macrophage's accumulation in the peritoneal cavity. Serum Anti-OVA antibodies (a), and total(b) or differential (c-f) leucocyte counts (×105/ml) in the peritoneal lavage fluid from sensitized mice killed 6, 24 or 48 hr after i.p. challenge. Solid bars: s.c. immunized and i.p. saline challenged; open bars: s.c. immunized and i.p. OVA challenged; hatched bars: previously tolerized to OVA, s.c. immunized and i.p. OVA challenged. Mean ± SEM. †P < 0·05 and *P < 0·05 compared, respectively, with saline- or OVA-challenged mice.
Figure 1.
Oral tolerance blocks granulocyte accumulation in the peritoneal cavity. Flow cytometry of peritoneal lavage fluid collected 24 hr after i.p. challenge. Shown are representative dot plot distribution of lymphocytes (R1), monocytes (R2) and granulocytes (R3) by size (forward light scatter) versus granularity (side angle scatter) of one mice of each group: (a)s.c. immunized and saline (im sal) i.p. injected mice; (b) s.c. immunized (im) and OVA i.p. challenged mice; (c) previously tolerized (tol) to OVA, s.c. immunized and OVA i.p. challenged.
In other experiments, using the same experimental protocol, we made global and differential cell counts at 6, 24 and 48 hr after i.p. OVA challenge. Again the anti-OVA antibodies were blocked in mice that previously drank OVA, characterizing the state of oral tolerance induction (Fig. 2a). There was a marked global increase in leucocyte counts in the PLF of immune mice, already installed 6 hr after OVA challenge, which persisted unchanged after 24 and 48 hr (Fig. 2b). Differential cell counts showed significant increases in neutrophils (Fig. 2c) and eosinophils (Fig. 2d) 6 hr after i.p. challenge with OVA, which were both blocked in oral-tolerant mice. At 24 hr after i.p. OVA challenge the numbers of neutrophils had dropped in immune mice but, conversely, the number eosinophils significantly increased and persisted high up to 48 hr. The frequency of neutrophils and eosinophils was significantly reduced in OVA-tolerant mice. There was also an increase in lymphocyte counts in PLF at 24 hr after i.p. OVA challenge, which was not blocked by oral tolerance (Fig. 2e). Macrophages were slightly higher in immune than in tolerant OVA-challenged mice at 48 hr (Fig. 2f). No differences were found in mast cell numbers between the three groups (results not shown).
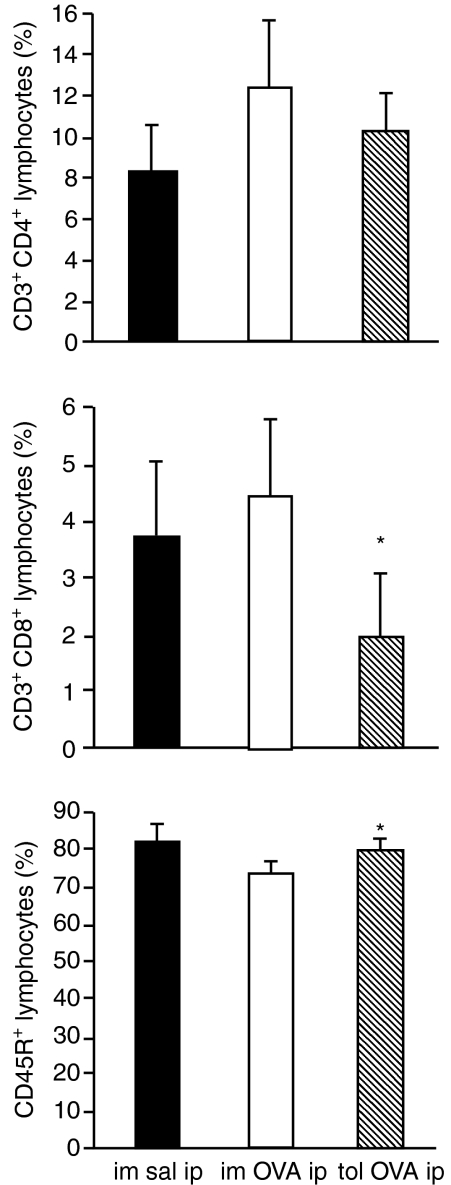
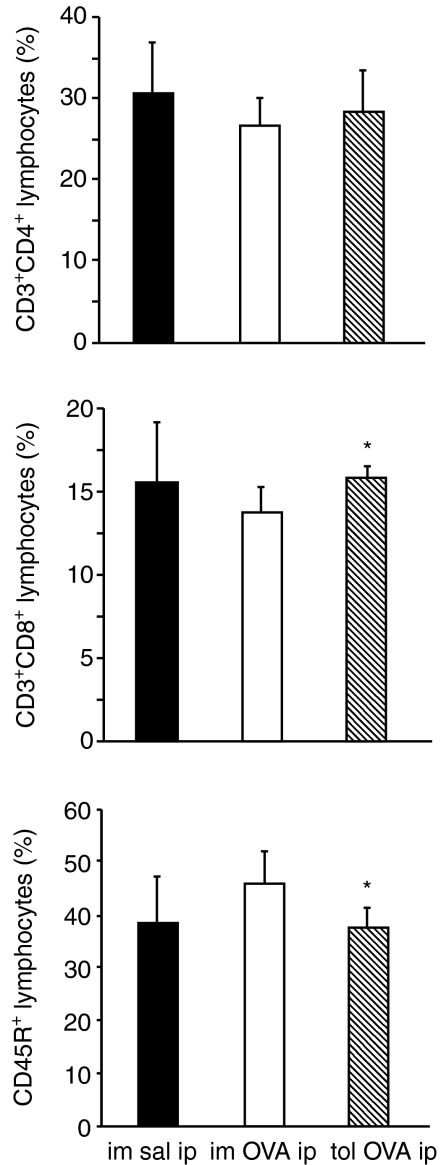
Although no differences were apparent in total lymphocyte numbers in the PLF from immune and tolerant mice 24 hr after OVA-challenge (Fig. 2e), we analysed if there were any difference in CD3+ CD4+ cells, CD3+ CD8+ cells and CD45R+ cells, which encompass the majority of lymphocyte subtypes. Phenotypic analysis by flow cytometry showed a significant reduction in the percentage of CD3+ CD8+ and an increase in CD45R+ lymphocytes in oral tolerant mice (Fig. 3).
Figure 3.
Reduction in the percentage of CD3+ CD8+ and increase in CD45R+ lymphocytes from peritoneal cavity of orally tolerant mice. Flow cytometry of peritoneal lymphocytes 24 hr after i.p challenge. Peritoneal cells gated on lymphocytes were analysed for the expression of CD3/CD4, CD3/CD8 or CD45R. Solid, open and hatched bars, and statistical significance*, as in Fig. 2.
Oral tolerance blocks neutrophilia and eosinophilia
To evaluate whether oral tolerance affects the production and/or mobilization of leucocytes we enumerated cells in peripheral blood and BM from OVA-immunized and OVA-tolerant mice at 6, 24 and 48 hr after i.p. OVA-challenge (Fig. 4).
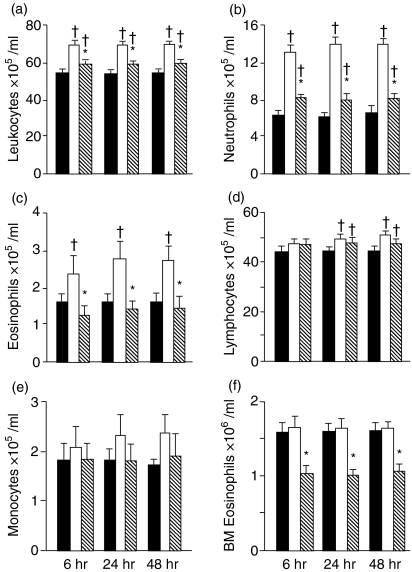
Figure 4.
Oral tolerance blocks neutrophilia and eosinophilia. Total (a) and differential (b–e) leucocyte counts (×105/ml) in the blood and (f) bone marrow (×106/ml) at 6, 24 and 48 hr after i.p. challenge. Solid, open and hatched bars, and statistical significance † and *, as in Fig. 2
After i.p. OVA-challenge, OVA-immunized mice showed a global increase in leucocyte numbers in the peripheral blood (Fig. 4a), which was also evident by differential cell count analysis (Fig. 4b–e). OVA oral tolerance blocked the increase of neutrophils, eosinophils and monocytes, but did not affect the lymphocyte counts.
Differential counts of BM cells allowed the analysis of mature neutrophils and eosinophils, based on major morphological features, such as cytoplasmatic and nuclear characteristics. On both groups of immune mice, challenged either with OVA or saline, the eosinophil numbers (Fig. 4f) were similar, and higher than mean values found in normal mice (Table 1). Thus, after s.c. immunization, independently of the i.p. challenge with OVA, there was an increase in BM eosinophil numbers. Marrow eosinophilia was blocked in orally tolerant mice, as revealed by the significantly lower counts in tolerant, OVA-challenged mice (Fig. 4f). No difference was detected either on total nucleated cells or on neutrophils counts in BM from all groups (results not shown).
Table 1.
Total and differential cell counts (mean ± SE) from naïve B6D2F1 female mice, at the same age of those used in the experiments
| Cell type | Cell count | |
|---|---|---|
| PLF1 | Total leucocyte | 20·77 ± 2·42 |
| Neutrophils | ND4 | |
| Eosinophils | 0·17 ± 0·07 | |
| Lymphocytes | 1·65 ± 0·08 | |
| Macrophages | 16·71 ± 1·02 | |
| Mast cells | 1·73 ± 0·16 | |
| PB2 | Total leucocyte | 46·08 ± 1·08 |
| Neutrophils | 3·99 ± 0·36 | |
| Eosinophils | 0·96 ± 0·09 | |
| Lymphocytes | 40·25 ± 1·22 | |
| Monocytes | 0·92 ± 0·21 | |
| BM3 | Total nucleated cells | 10·33 ± 0·23 |
| Neutrophils | 5·29 ± 0·11 | |
| Eosinophils | 0·53 ± 0·02 |
Peritoneal lavage fluid (×105/ml).
Peripheral blood (×105/ml).
Bone marrow (×106/ml).
Not detected.
Altered distribution of lymphocytes in tolerant mice
Although total lymphocyte numbers did not vary in PLF (Fig. 2) and in peripheral blood (Fig. 4) of tolerant mice we noted a lower proportion of CD3+ CD8+ lymphocytes and a higher proportion of CD45R+ lymphocytes in the PLF from tolerant OVA-challenged mice (Fig. 3). We further analysed the lymphocyte phenotypes in the peripheral blood 24 hr after OVA challenge and found a higher proportion of CD3+ CD8+ lymphocytes and a lower proportion of CD45R+ lymphocytes in tolerant OVA-challenged mice compared with immune OVA-challenged mice (Fig. 5).
Figure 5.
Increase in the percentage of CD3+ CD8+ and reduction in CD45R+ lymphocytes from peripheral blood of orally tolerant mice.Flow cytometry of blood lymphocytes 24 hr after i.p. challenge. Blood cells gated on lymphocytes were analysed for the expression of CD3/CD4, CD3/CD8 or CD45R. Solid, open and hatched bars, and statistical significance *, as in Fig. 2.
Next, we analysed the expression of CD54 and CD18 molecules in lymphocytes because altered expression of these adhesion molecules may differentially contribute to migration of lymphocytes to inflammatory sites. We compared the mean fluorescence intensity of CD18 and CD54 in B lymphocytes (CD45R+) and non-B lymphocytes (CD45R–) in the PLF and in peripheral blood of tolerant and not-tolerant mice (Table 2). After challenge with OVA the expression of CD54 on both CD45R+ and CD45R– lymphocytes is significantly lower in the PLF from immune mice but not significantly altered in tolerant mice. On the other hand the expression of CD18 in CD45R+ lymphocytes is significantly lower in the peripheral blood from tolerant mice.
Table 2.
Adhesion molecules (CD18 and CD54) expressed by lymphocytes from peritoneal lavage fluid (PLF) and peripheral blood 24 hr after i.p. challenge
| CD18(MFI1) | CD54(MFI1) | ||||
|---|---|---|---|---|---|
| CD45R+ | CD45R– | CD45R+ | CD45R– | ||
| PLF | Im sal i.p. | 10·10 ± 0·51 | 24·54 ± 0·82 | 8·01 ± 0·37 | 11·30 ± 0·19 |
| Im OVA i.p. | 8·91 ± 0·30 | 23·52 ± 1·54 | 6·80 ± 0·27† | 9·95 ± 0·27† | |
| Tol OVA i.p. | 10·24 ± 1·07 | 22·89 ± 2·26 | 7·98 ± 0·46 | 10·13 ± 0·31 | |
| Peripheral | Im sal i.p. | 7·69 ± 0·16 | 15·53 ± 0·28 | 2·34 ± 0·05 | 7·01 ± 0·18 |
| blood | Im OVA i.p. | 7·86 ± 0·18 | 15·30 ± 0·26 | 2·36 ± 0·14 | 6·72 ± 0·40 |
| Tol OVA i.p. | 7·27 ± 0·10* | 14·62 ± 0·29 | 2·20 ± 0·05 | 6·45 ± 0·16 |
Mean fluorescence intensity (mean ± SE);
P < 0·05 compared with im sal i.p. group,
P < 0·05 compared with im OVA i.p. group.
Im sal i.p.: s.c. immunized and i.p. saline challenged; Im OVA ip: s.c. immunized and i.p. OVA challenged; Tol OVA ip: previously tolerized to OVA, s.c. immunized and i.p. OVA challenged.
Discussion
Oral tolerance is quite efficient in blocking DTH reactions, IgG1 and IgE antibody formation and in preventing inflammatory immune reactions in allergy and in other models of autoimmune diseases (reviewed in 4, 21 and 22). In the present study, as predicted, it prevents OVA-induced peritonitis. Although very effective in blocking the initiation of immunological events in naïve animals, feeding with the antigen tends to enhance, rather than inhibit, immune reactions in previously immunized mice.23 In a murine model of asthma, oral antigen administration after antigen priming has limited therapeutic effects.16,17 The hope is that understanding the mechanisms that underlie oral tolerance, may improve its use in immunotherapy.11
Proposed mechanisms to explain oral tolerance include dominant, active suppression by regulatory T cells, clonal anergy/deletion of lymphocytes, immune deviation (T helper 1 (Th1), Th2 and Th3) and inhibition by anti-idiotypic antibodies.22 Which of these mechanisms inhibit inflammatory reactions in tolerant organisms, is not well understood.
It is usually not considered that parenteral re-exposure to a tolerated antigen triggers inhibitory phenomena that may, for example, block the initiation of immune responses to a second unrelated antigen. This phenomenon is known as bystander suppression,24 but we prefer to call it ‘indirect effects’ of oral tolerance25 because the tolerated and the second antigens do not need to be present at the same place, nor at the same time for the phenomenon to occur.26 We noticed previously that, in OVA-tolerant mice, an i.p. injection of OVA concomitant with an intravenous (i.v.) injection of Shistosoma mansoni eggs, inhibits granuloma formation around the eggs.27 The fewer and smaller granuloma found in these OVA-tolerant mice had fewer eosinophils, macrophages and lymphocytes. In addition, OVA-tolerant mice exposed to i.p. OVA concomitantly with i.v. injection of eggs from S. mansoni have less expression of CD54 around granulomas (results not published). The exposure to the tolerated antigen might have global effects and inhibit leucocyte formation and/or migration into inflammatory sites.26,27 Thus, the study of oral tolerance and the indirect effects of parenteral exposure to tolerated antigens may produce insights into the relationship of innate and adaptive immunity. To extend our study on this hypothesis we used the present model of antigen-induced peritonitis.
The present results indicate that oral tolerance to OVA interferes with several stages of the inflammatory process, such as the differentiation, release and/or migration of cells (neutrophils, eosinophils and monocytes) involved in innate mechanisms of inflammation. In orally tolerant mice, the global increase in leucocytes was weaker and the influx into the peritoneum was less intense (Figs 1,2 and 4). Tolerance hindered the intensity of migration of cells into the peritoneum, but not its kinetics. Both in immune and tolerant mice, the migration of neutrophils was high at 6 hr and plunged at 24 hr, whereas that of eosinophils was moderate at 6 hr, raised at 24 hr and was maintained at 48 hr (Fig. 2). On the other hand, an increase in peritoneal lymphocyte numbers, only significant at 24 hr, occurred both in immune and orally tolerant mice, although there were less CD3+ CD8+ and more CD45R+ lymphocytes in the peritoneum of tolerant mice (Figs 2 and 3). We also noticed a marked decrease in eosinophils in the bone marrow of tolerant mice (Fig. 4). Considering that the bone marrow actively participates in the release and migration of cells to local sites of inflammation we can predict that oral tolerance may actively interfere with these changes in bone marrow. Whether or not this is important in inflammatory diseases has not been considered, but it may turn out to be an important systemic mechanism.19
Symptoms of allergic inflammatory reactions vary according to the cell types attracted to the inflammatory site.13 The selectivity of leucocyte recruitment results from a combination of factors, including the pattern of cytokines released from local cells, the repertoire of adhesion molecules on leucocytes, as well as the pattern of adhesion molecules and chemoattractants expressed on vascular endothelium. Much attention has been given to the mechanism of eosinophil recruitment into the airways of asthmatics because of the potential damage they may cause to bronchial mucosa.14 However, other cell types are also involved in the maintenance of the inflammatory reaction and lymphocytes are especially important in chronic inflammatory diseases. It has been proposed that CD4+ T cells are required to activate eosinophil degranulation in a manner that is antigen dependent.28 These authors propose that this process would be conceptually similar to the role of IgE in mediating the discharge of mast cell contents.
In immune BALB/c mice allergen re-exposure by nasal or peritoneal routes triggers the release of eosinophils from bone marrow and their migration to inflamed sites.20,29 Also in BALB/c mice significantly increased numbers of bone marrow eosinophils were found in sensitized animals exposed to allergen, relative to unchallenged, sensitized controls.29 However, we found that sensitized B6D2F1 mice have similar numbers of bone marrow eosinophils irrespective of challenge (Fig. 4). This may be the result of strain differences as C57B1/6 mice also have this same behaviour (Gapar-Elsas, personal communication). So, mice with C57Bl/6 background, as opposed to BALB/c mice, may present persistent bone marrow eosinophilia after sensitization.
T-cell derived cytokines such as IL-5, IL-3 and granulocyte–macrophage colony-stimulating factor have been shown to stimulate eosinophilopoiesis in vitro, but IL-5 is specifically involved in eosinophil growth and maturation.29–31 On the other hand, eotaxin, a CC chemokine, stimulates the migration of eosinophils from bone marrow into tissue.32
Many studies have shown that oral tolerance blocked airway eosinophilic inflammation in different mouse strains, such as BALB/c, BP2, CBA/Ca10,15–18 bone marrow eosinophilia and the increase in IL-5 in C57Bl/6 mice.10 Blocking the generation of IL-5 by oral tolerance can play a decisive role in mediating the decrease of bone marrow production of eosinophils. However it is not clear how oral tolerance inhibits tissue eosinophilia. It may be trough blocking IL-5 production. However, although anti-IL5 antibodies block blood eosinophilia after allergen challenge, it does not alter significantly bronchial hyper-responsiveness or the late asthmatic response in atopic asthmatics33 and also this treatment only partially depletes eosinophil numbers in asthmatic airway.34
Zuani-Amorim et al.20 have shown that IL-5 release in mice PLF emerged 6 hr after OVA challenge, and preceded the rise in T-cell numbers, which was noted at 24 hr. These authors claim that the early (6 hr) recruitment of eosinophils to the peritoneum is T-cell dependent and possibly caused by the activation of resident T cells. So, the inhibition of eosinophil recruitment in tolerant mice may be caused by a change in the profiles of mediators secreted by these resident T lymphocytes.
Alternatively, or in addition, oral tolerance might affect cell types other than T lymphocytes, such as mast cells, which may be involved in the early OVA-induced IL-5 production.35 The effect of oral tolerance in mast cell may be indirect, by inhibition of antibody production. Despite the attention given recently to T-cell derived cytokines, immune complexes continue to be important mediators of inflammatory reactions by interaction with Fc-receptors on leucocytes and activation of complement cascades. In allergic inflammatory reactions mast cells and macrophages play an important role, through their ability to respond to the aggregation of receptors in their surface, such as FcγR, FcεRI and FcεRII receptors, by IgG1 or IgE molecules bridged by antigen. As we have shown (Fig. 1c and Fig. 2a), oral tolerance inhibits antibody responses and these are additional mechanisms through which oral tolerance may reduce allergic inflammation.
Herein, we show that oral tolerance to OVA significantly interferes with changes in some cell types, but not in others. Thus, it inhibited the OVA-triggered increase in neutrophils, eosinophils and macrophages in peritoneal lavage fluid (PLF) and in peripheral blood (peripheral blood), but failed to block a concomitant increase in total lymphocytes; in the bone marrow, it blocked eosinophilia but left the number of neutrophils unchanged (not shown).
The robustness of neutrophil production in the bone marrow is possibly related to their high turnover rates in normal conditions; their high rate of production may be an impediment to regulation of their involvement in inflammatory reactions by interference with the bone marrow. On the other hand, inhibition of neutrophil release from bone marrow and their migration to the peritoneal cavity of orally tolerant mice may be achieved by blocking the secretion of pro-inflammatory mediators from activated mast cells, lymphocytes and macrophages. Cytokines, such as IL-1, IL-6 and tumour necrosis factor-α, which are primarily produced by macrophages, play multiple roles in inflammation, including activation of vascular endothelium, with enhanced expression of leucocyte adhesion molecules and induction of chemokine synthesis. Oral tolerance blocks production of pro-inflammatory cytokines.36
Oral tolerance is a T-cell dependent phenomenon and although total lymphocyte numbers did not vary in PLF (Fig. 2) and in peripheral blood (Fig. 4) of tolerant mice we noted a lower proportion of CD3+ CD8+ lymphocytes and a higher proportion of CD45R+ lymphocytes in the PLF from tolerant OVA-challenged mice (Fig. 3). Interestingly, we have found that lymphocyte counts in the peripheral blood (Fig. 5) were opposed to those found in PLF (Fig. 3), i.e. a higher proportion of CD3+ CD8+ lymphocytes and a lower proportion of CD45R+ lymphocytes in tolerant OVA-challenged mice compared with immune OVA-challenged mice (Fig. 5). This may reflect differences in cell migration to inflammatory sites, i.e, if a smaller amount of CD3+ CD8+ migrate to the peritoneal cavity it remains in a higher proportion in the peripheral blood. On the contrary, if a greater number of CD45R+ migrate to the peritoneal cavity they will be proportionately lower in the peripheral blood. Cell migration depends on the expression of adhesion molecules that may be altered by activation. However we did not find correlation between the mean fluorescence intensity of CD54 on CD45R+ lymphocytes from tolerant and not tolerant mice and their differential migration. In addition, contrary to our expectation, the mean fluorescence intensity of CD18 in CD45R+ lymphocytes is significantly lower in the peripheral blood from tolerant mice. Maybe the difference between the distribution of lymphocytes in tolerant and not tolerant mice can be better understood after analyses of adhesion molecules expressed by endothelial cells.
Acknowledgments
Research supported by CNPq Brazil (N° 30.5043/2003-0) and FAPEMIG.
Abbreviations
- BM
bone marrow
- CD
cluster of differention
- DC
dentritic cell
- DTH
delayed-type hypersensitivity
- ELISA
enzyme-linked immunosorbent assay
- FACS
fluorescent activated cell sorter
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- IFN
interferon
- IL
interleukin
- i.p.
intraperitoneal
- HBSS
Hanks' balanced salt solution
- OPD
orthophenylene-diamine
- OVA
ovalbumin
- PE
phycoerythrin
- PBS
phosphate-buffered saline
- PLF
peritoneal lavage fluid
- RT
room temperature
- s.c.
subcutaneous
- TGF-β
transforming growth factor-β
- Th
T helper
- TNF-α
tumor necrosis factor-α
References
- 1.Van Der Heijden PJ, Stok W, Bianchi ATJ. Contribution of immunoglobulin secreting cells in the murine small intestine to the total ‘background’ immunoglobulin production. Immunology. 1987;62:551–5. [PMC free article] [PubMed] [Google Scholar]
- 2.Husby S, Jensenius JC, Svehag SE. Passage of undegraded dietary antigen into the blood of healthy adults. Scand J Immunol. 1985;22:83–92. doi: 10.1111/j.1365-3083.1985.tb01862.x. [DOI] [PubMed] [Google Scholar]
- 3.Verdolin BA, Ficker SM, Faria AMC, Vaz NM, Carvalho CR. Stabilization of serum antibody responses triggered by initial mucosal contact with the antigen independently of oral tolerance induction. Braz J Med Bio Res. 2001;34:211–9. doi: 10.1590/s0100-879x2001000200008. [DOI] [PubMed] [Google Scholar]
- 4.Vaz N, Faria A, Verdolin B, Carvalho C. Immaturity, ageing and oral tolerance. Scand J Immunol. 1997;46:225–9. doi: 10.1046/j.1365-3083.1997.d01-117.x. [DOI] [PubMed] [Google Scholar]
- 5.Titus RG, Chiller JM. Orally-induced tolerance. Definition at the cellular level. Int Arch Allergy App Immunol. 1981;65:323–38. [PubMed] [Google Scholar]
- 6.Khoury SJ, Lider O, al-Sabbagh A, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. III. Synergistic effect of lipopolysaccharide. Cell Immunol. 1990;131(2):302–10. doi: 10.1016/0008-8749(90)90256-q. [DOI] [PubMed] [Google Scholar]
- 7.Zhang ZY, Lee CS, Lider O, Weiner HL. Suppression of adjuvant arthritis in Lewis rats by oral administration of type II collagen. J Immunol. 1990;145(8):2489–93. [PubMed] [Google Scholar]
- 8.Pomeranz JR. Immunologic unresponsiveness following a single feeding of picryl chloride. J Immunol. 1970;104(6):1486–90. [PubMed] [Google Scholar]
- 9.Van Hoogstraten IM, Andersen KE, Von Blomberg BM, et al. Reduced frequency of nickel allergy upon oral nickel contact at an early age. Clin Exp Immunol. 1991;85(3):441–5. doi: 10.1111/j.1365-2249.1991.tb05746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo M, Jancar S, Pereira de Siqueira AL, Mengel J, Gomes E, Ficker SM, Caetano de Faria AM. Prevention of lung eosinophilic inflammation by oral tolerance. Immunol Lett. 1998;61(1):15–23. doi: 10.1016/s0165-2478(97)00155-7. [DOI] [PubMed] [Google Scholar]
- 11.Weiner HL. Current issues in the treatment of human diseases by mucosal tolerance. Ann N Y Acad Sci. 2004;1029:211–24. doi: 10.1196/annals.1309.053. [DOI] [PubMed] [Google Scholar]
- 12.Bjorksten B. Effects of intestinal microflora and the environment on the development of asthma and allergy. Springer Semin Immunopathol. 2004;25(3–4):257–70. doi: 10.1007/s00281-003-0142-2. [DOI] [PubMed] [Google Scholar]
- 13.Venge P. Monitoring the allergic inflammation. Allergy. 2004;59(1):26–32. doi: 10.1046/j.1398-9995.2003.00386.x. [DOI] [PubMed] [Google Scholar]
- 14.Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323(15):1033–9. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 15.Zemann B, Schwaerzler C, Griot-Wenk M, et al. Oral administration of specific antigens to allergy-prone infant dogs induces IL-10 and TGF-beta expression and prevents allergy in adult life. J Allergy Clin Immunol. 2003;111(5):1069–75. doi: 10.1067/mai.2003.1411. [DOI] [PubMed] [Google Scholar]
- 16.Chung Y, Cho J, Chang YS, Cho SH, Kang CY. Preventive and therapeutic effects of oral tolerance in a murine model of asthma. Immunobiology. 2002;206(4):408–23. doi: 10.1078/0171-2985-00190. [DOI] [PubMed] [Google Scholar]
- 17.Russo M, Nahori MA, Lefort J, et al. Suppression of asthma-like responses in different mouse strains by oral tolerance. Am J Respir Cell Mol Biol. 2001;24(5):518–26. doi: 10.1165/ajrcmb.24.5.4320. [DOI] [PubMed] [Google Scholar]
- 18.Haneda K, Sano K, Tamura G, Sato T, Habu S, Shirato K. TGF-beta induced by oral tolerance ameliorates experimental tracheal eosinophilia. J Immunol. 1997;159(9):4484–90. [PubMed] [Google Scholar]
- 19.Cyr MM, Denburg JA. Systemic aspects of allergic disease: the role of the bone marrow. Curr Opin Immunol. 2001;13(6):727–32. doi: 10.1016/s0952-7915(01)00286-2. [DOI] [PubMed] [Google Scholar]
- 20.Zuani-Amorim C, Créminon C, Nevers MC, Nahori MA, Vargaftig B, Pretolani M. Modulation by IL-10 of antigen-induced IL-5 generation, and CD4+ T lymphocyte and eosinophil infiltration into the mouse peritoneal cavity. J Immunol. 1996;157:377–84. [PubMed] [Google Scholar]
- 21.Mowat AM. Oral tolerance and regulation of immunity to dietary antigens. In: Ogra PL, Sroben W, Mestecky J, McGhee JR, Lamm ME, Bienenstock JE, editors. Handbook of Mucosal Immunology. San Diego: Academic Press; 1994. pp. 185–201. [Google Scholar]
- 22.Faria AM, Weiner HL. Oral tolerance: mechanisms and therapeutic applications. Adv Immunol. 1999;73:153–264. doi: 10.1016/s0065-2776(08)60787-7. [DOI] [PubMed] [Google Scholar]
- 23.Conde AA, Stransky B, Faria AM, Vaz NM. Interruption of recently induced immune responses by oral administration of antigen. Braz J Med Biol Res. 1998;31(3):377–80. doi: 10.1590/s0100-879x1998000300008. [DOI] [PubMed] [Google Scholar]
- 24.Miller A, Lider O, Weiner HL. Antigen driven bystander suppression after oral administration of antigen. J Exp Med. 1991;174:791–8. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carvalho CR, Verdolin BA, Souza AV, Vaz NM. Indirect effects of oral tolerance in mice. Scand J Immunol. 1994;39:533–8. doi: 10.1111/j.1365-3083.1994.tb03410.x. [DOI] [PubMed] [Google Scholar]
- 26.Carvalho C, Verdolin B, Vaz N. Indirect effects of oral tolerance cannot be ascribed to bystander suppression. Scand J Immunl. 1997;45:276–81. doi: 10.1046/j.1365-3083.1997.d01-394.x. [DOI] [PubMed] [Google Scholar]
- 27.Carvalho CR, Lenzi HL, Correa-Oliveira R, Vaz NM. Indirect effects of oral tolerance to ovalbumin interfere with the immune responses triggered by Schistosoma mansoni eggs. Braz J Med Biol Res. 2002;35(10):1195–9. doi: 10.1590/s0100-879x2002001000012. [DOI] [PubMed] [Google Scholar]
- 28.Shinkai K, Mohrs M, Locksley R. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825–9. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- 29.Gaspar Elsas MI, Joseph D, Elsas PX, Vargaftig BB. Rapid increase in bone-marrow eosinophil production and responses to eosinopoietic interleukins triggered by intranasal allergen challenge. Am J Respir Cell Mol Biol. 1997;17(4):404–13. doi: 10.1165/ajrcmb.17.4.2691. [DOI] [PubMed] [Google Scholar]
- 30.Walker C, Virchow J, Bruijnzeel P, Blaser K. T-cell subsets and their soluble products regulate eosinophilia in allergic and nonallergic asthma. J Immunol. 1991;146:1829–35. [PubMed] [Google Scholar]
- 31.Sanderson C. Interleukin-5, eosinophils and disease. Blood. 1992;79:3101–9. [PubMed] [Google Scholar]
- 32.Li J, Saito H, Crawford L, Inman MD, Cyr MM, Denburg JA. Haemopoietic mechanisms in murine allergic upper and lower airway inflammation. Immunology. 2005;114(3):386–96. doi: 10.1111/j.1365-2567.2005.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leckie M, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–8. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 34.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asth,atic airway. Am J Respir Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 35.Oskeritzian C, Milon G, Braquet P, Mencia-Huerta J, David B. Activated mast cell release biological activities able to support eosinophil production from mouse hemopoietic precursors. Cell Immunol. 1996;167(2):205–15. doi: 10.1006/cimm.1996.0028. [DOI] [PubMed] [Google Scholar]
- 36.Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4 and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–64. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]