Abstract
The role of mitogen-activated protein kinase (MAPK) and nuclear factor κB (NF-κB) pathways, especially NF-κB-inducing kinase (NIK)-mediated alternative pathway, in CD40-mediated interleukin (IL)-6 and IL-12 productions by immature or mature dendritic cells (DCs) was investigated. Murine myeloid DCs were matured by treatment with lipopolysaccharide. CD40 ligation induced modest or vigorous cytokine productions in immature or mature DCs, respectively. After CD40 ligation, p38 MAPK was significantly activated in either immature or mature DCs. SB203580, a p38 MAPK inhibitor, markedly decreased CD40-mediated IL-6 and IL-12 productions in immature DCs. In mature DCs, SB203580 significantly decreased CD40-mediated IL-6 but not IL-12 production. On the other hand, CD40 ligation induced vigorous activation of the NF-κB alternative pathway including p100 phosphorylation and subsequent nuclear translocations of p52, a processed form of p100, and RelB in mature but not immature DCs. The CD40-mediated phosphorylation of p100 was completely abolished in NIK-mutated mature DCs. The NIK mutation markedly reduced CD40-mediated IL-12 but not IL-6 production by mature DCs. Taken together, we concluded that IL-6 and IL-12 productions in response to CD40 ligation were controlled by p38 MAPK and NIK mediated alternative pathway, respectively, in mature DCs.
Keywords: dendritic cells, NIK, MAPK, cytokine production, signal transduction
Introduction
Dendritic cells (DCs) are potent antigen-presenting cells (APCs) and play major roles in the regulation of immune responses to various antigens.1–3 Immature DCs presenting peripheral tissues express moderate levels of major histocompatibility complex (MHC) and costimulatory molecules including CD80, CD86, and CD40 on the surface. Upon encountering pathogens, immature DC are activated and matured by maturation stimuli including pathogen products such as lipopolysaccharide (LPS).2,3 The mature DCs highly express MHC and costimulatory molecules on the surface, which are associated with the potent capability of antigen presentation.
Subsequently, the mature DCs leave the peripheral tissue and migrate to the T-cell areas of draining lymphoid organs and activate antigen-specific T cells in these areas.4,5 During the antigen presentation, DCs are also activated via interaction of the CD40 with CD40 ligand (L) expressed on T cells and thereby produce interleukin (IL)-12 and IL-6. It has been shown that the DC-produced IL-12 drives polarization of native CD4+ T cells toward T helper 1 (Th1) type, while the inflammatory cytokine IL-6 is involved in Th2 polarization.6 Recently, Dodge et al.7 reported that IL-6 produced by pulmonary DCs impeded Th1 immune responses. Thus, the balance of IL-6 and IL-12 produced by mature DCs in the T-cell area appears to influence the Th1/Th2 polarization in the subsequent acquired immunity.
One of the downstream events in CD40 signalling is activation of mitogen-activated protein kinases (MAPKs).8,9 Activation of the p38 MAPK pathway is involved in IL-12 production by DCs, 10–12 whereas activation of the extracellular signal-related kinases (ERK) pathway acts to suppress the IL-12 production. 13–15
CD40 ligation also induces activation of nuclear factor κB (NF-κB).16 Five members of the mammalian NF-κB family, p65 (RelA), RelB, c-Rel, p50/p105 (NF-κB1), and p52/p100 (NF-κB2), are present in unstimulated cells as homo- or heterodimers bound to IκB family proteins. IκB proteins prevent NF-κB translocation to the nucleus, thereby maintaining NF-κB in an inactive state.17 NF-κB signalling is generally considered to occur through either the classical or alternative pathway18. In the classic pathway, IκB kinase (IKK) phosphorylates IκB proteins which bind to p65 : p50 heterodimer and leads to degradation of IκB.19 In the alternative pathway, IKK phosphorylates p100 coupled with RelB and leads to processing of p100 to p52, resulting in formation of the p52 : RelB heterodimer. The freed NF-κB dimers such as p65 : p50 and p52 : RelB heterodimers translocate to the nucleus where they bind to specific sequences in the promoter or enhancer regions of the target genes.
The phosphorylation of p100 has, so far, been shown to be catalysed by IKK acting downstream from NF-κB inducing kinase (NIK).20,21 Evidence that NIK is an important kinase in mediating signal transduction of tumour necrosis factor receptor (TNFR) family in vivo has recently been deduced using alymphoplasia (aly) mice.22 It has been demonstrated that the genetic lesion in the aly mouse is a point mutation that results in a single amino acid substitution in the COOH terminus of NIK, and that wild-type NIK expressed in transgenic mice can restore a normal phenotype in these mice.23
It has been reported that NF-κB p50 and p65 are involved in DC development, while p50 and c-Rel are involved in CD40L- and TNF-related activation-induced cytokine (TRANCE)-induced survival and IL-12 production of DCs.16 However, a role of the NIK-mediated alternative pathway in DC functions is unclear. In the present study, we analysed the CD40-mediated signal transduction in murine myeloid DCs focusing on p38 MAPK and NF-κB pathways. We demonstrate herein that CD40-mediated IL-6 and IL-12 production are separately regulated via p38 MAPK and NIK in mature DCs.
Materials and methods
Mice
aly/aly mice, the heterozygous littermates (aly/+), and wild-type C57BL/6 (B6) mice were purchased from Clea Japan (Tokyo, Japan) and were maintained in a specific pathogen-free condition of our animal facility at Hokkaido University. The genetic background of the aly/aly and aly/+ mice is B6. Nine- to 14-week-old female mice were used throughout the study. All experiments were approved by regulations of Hokkaido University Animal Care and Use Committee.
Reagents and antibodies
Murine recombinant granulocyte–macrophage colony-stimulating factor (GM-CSF) was purchased from PeproTech (London, UK). LPS from Escherichia coli 055:B5 was obtained from Sigma Chemical Co (St Louis, MO). SB20358, a specific inhibitor of p38 MAPK, was purchased from Calbiochem (La Jolla, CA). This inhibitor was used at 20 µm based on prior studies.24–26 Anti-phospho-p38 MAPK (Thr180/Tyr182) antibody, anti-p38 MAPK antibody, anti-phospho-NF-κB2 p100 antibody, anti-NF-κB2 p52/p100 antibody, anti-NF-κB p65 antibody, anti-RelB antibody, and horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) antibody were purchased from Cell Signalling Technology (Beverly, MA). Purified anti-CD40 monoclonal antibody (mAb) (HM40-3) and hamster IgM as an isotype control (no azide/low endotoxin format) were purchased from BD Biosciences Pharmingen (San Diego, CA). Fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD86 mAb (GL1), phycoerythrin (PE)-conjugated anti-mouse CD40 mAb (3/23), biotin-conjugated anti-I-Ab mAb (AF6-120.1), biotin-conjugated anti-I-Ad mAb (AMS-32.1), and streptavidin Per-CPTM were obtained from BD Biosciences Pharmingen.
Cell culture
Iscove's modified Dulbecco's medium (IMDM; Sigma) was supplemented with 100 IU/ml penicillin, 100 µg/ml streptomycin, 600 µg/ml l-glutamine. Fibroblast supernatants from NIH-3T3 cells were collected from confluent cultures with IMDM containing 10% heat-inactivated fetal calf serum (FCS). The DC line (BC1) was generated from BALB/c mouse spleen as previously described.15,27 BC1 cells were cultured and expanded in R1 medium, IMDM containing 10% FCS, 30% culture supernatants from NIH/3T3, 10 ng/ml mouse recombinant GM-CSF, and 50 µm 2-mercaptoethanol. Unstimulated BC1 cells were used as immature DCs and BC1 cells treated with 5 µg/ml LPS for 48 hr were used as mature DCs.
Spleen-derived DCs (SDDCs) were generated by culturing aly/aly, aly/+, or wild-type B6 mouse splenocytes in R1 medium for 14–17 days as previously described.27–29 The splenocytes were plated at a density of 2·5–5 × 105 cells/ml in R1 medium. Cultures were fed every 3–4 days with fresh R1 medium. CD11c+ cells were positively selected using anti-CD11c (N418) MicroBeads and magnetic-activated cell-sorting column (Miltenyi Biotec, Bergisch Gladbach, Germany). The purified cells were >93% CD11c+ cells in purity; these purified populations were used as SDDCs. Unstimulated SDDCs were used as immature DCs and SDDCs treated with 5 µg/ml LPS for 24 h were used as mature DCs.
Flow cytometry
The cells were detached with 3 mm ethylenediaminetetra-acetic acid (3 min at 37°). These cells were incubated with 2.4G2 (rat anti-mouse FcII/III receptor, CD32) supernatant to prevent binding to FcRII/III, and then stained using FITC-, PE-, or biotin-conjugated mAb, and streptavidin-Per-CPTM. Flow cytometric analysis was performed on EPICS® XL (Coulter Co. Miami, FL), as described in a pervious study.15
Measurement of IL-6 and IL-12
Immature or mature DCs (2 × 105/ml) were incubated with 1 µg/ml anti-CD40 mAb (HM40-3) or control IgM in R1 medium for 24 hr at 37° and the culture supernatants were subjected to quantification of the protein level of IL-6 and IL-12 p40 by enzyme-linked immunosorbent assay (ELISA) using OptEIATM Set (BD Biosciences Pharmingen). The doses of anti-CD40 mAb was determined on the basis of it's maximum effect on the cytokine productions.
In some experiments, cells (2 × 105/ml) were pretreated with SB20358 (20 µm) or vehicle alone (0·1% dimethylsulphoxide) for 1 hr and then treated with 1 µg/ml anti-CD40 mAb for 24 hr in the presence of the inhibitor or vehicle alone. The culture supernatants were subjected to quantification of the cytokine levels.
Immunoblotting
BC1 cells or SDDCs were incubated in 0·5% FCS IMDM for 1 hr at a density of 1 × 106 cells/ml, and then stimulated with 1 µg/ml anti-CD40 mAb (HM40-3) or control IgM for the indicated time period. Reactions were halted by rapidly cooling on ice and washed with ice-cold phosphate-buffered saline (PBS). The whole cell lysates were prepared using cell lysis buffer (Cell Signalling Technology, Beverly, MA). The nuclear extracts were prepared using Nuclear Extract kit version C3 (Active Motif, Carlsbad, CA). The cell lysates and nuclear extracts were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis, then blotted onto an immobilon membrane (Millipore, Bedford, MA). The membrane was probed with primary antibody, and developed with horseradish peroxidase conjugated secondary antibody by enhanced chemiluminescence.
Statistical analysis
The Student's t-test was used to analyse data for significant differences. P-values less than 0·05 were regarded as significant.
Results
CD40-mediated IL-6 and IL-12 production by immature and mature DCs
We have established a murine DC line (BC1 cells) from BALB/c splenocytes, according to Winzler's method.15,27 Unstimulated BC1 cells are typical immature myeloid DCs.16 Following treatment with TNF-α or LPS, BC1 cells exhibit mature DC phenotype and functions.15,28,30–32 Using a similar in vitro differentiation system of DCs, a number of important findings have been reported and verified. 33–36
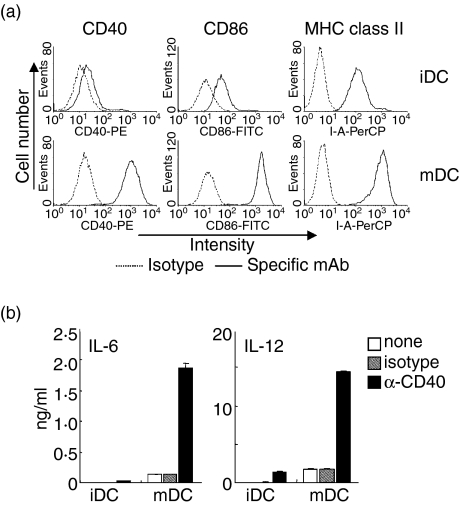
Figure 1(a) shows surface marker expressions on unstimulated BC1 cells (immature DCs) and LPS-treated BC1 cells (mature DCs). Immature DCs showed moderate level of CD40, CD86, and MHC class II expressions. These surface molecules were markedly up-regulated on mature DCs. CD40 ligation with anti CD40-mAb induced IL-6 and IL-12 productions in both immature and mature DCs (Fig. 1b). However, the CD40-mdiated cytokine productions by mature DCs were considerably higher than those by immature DCs.
Figure 1.
CD40-mediated enhancement of surface molecule expressions and IL-6 and IL-12 production by immature and mature DCs. Unstimulated or LPS-treated BC1 cells were considered as immature (iDC) or mature DCs (mDC), respectively. (a) Cell surface expressions of CD40, CD86, and MHC class II (I-Ad). iDC and mDC were analysed by flow cytometry. (b) Cytokine production by iDCs and mDCs. The iDC and mDC were treated with anti-CD40 mAb (α-CD40) or isotype-matched control-IgM (isotype) for 24 hr. The amount of IL-6 and IL-12 in the culture supernatants was measured by ELISA. Each column represents the mean ± SE of triplicate wells. Data are representative of at least three independent experiments.
CD40-mediated signal transduction in immature and mature DCs
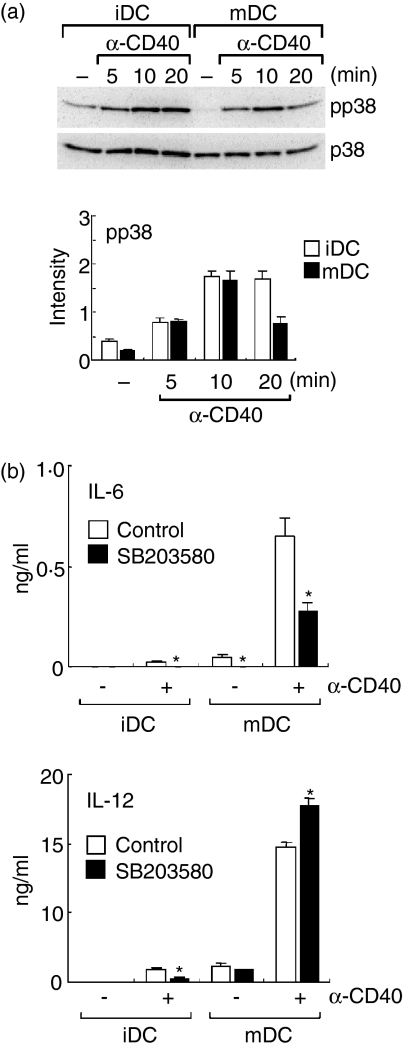
It has been reported that activation of the p38 MAPK pathway promotes IL-12 production by DCs. 10–12 We analysed the effect of CD40 ligation on p38 MAPK activation in immature DCs and mature DCs. Intracellular protein levels of the active form of p38 MAPK, phospho-p38 MAPK, in immature DCs and mature DCs were evaluated after CD40-mediated stimulation (Fig. 2a). After treatment with anti-CD40 mAb, levels of phospho-p38 in either immature or mature DCs were significantly increased at 5–20 min, peaking at 10 min (Fig. 2a, bottom).
Figure 2.
CD40-medaited p38 MAPK activation in immature and mature DCs. Unstimulated or LPS-treated BC1 cells were considered as immature (iDC) or mature DCs (mDC), respectively. (a) Phosphorylation of p38 MAPK. iDC and mDC were treated with anti-CD40 mAb (α-CD40) for 5–20 min, and then whole cell lysates were prepared. Levels of phospho-p38 MAPK (pp38) and p38 MAPK (p38) in the cell lysates were determined by immunoblotting. A representative immunoblot of three independent experiments is represented (upper). The relative intensity of the specific band in mDC is shown (lower). Each column represents the mean ± SE of three independent experiments. The effect of a p38 MAKP inhibitor, SB203580. The iDC and mDC were pretreated with SB203580 (20 µm) for 1 hr and then incubated with anti-CD40 mAb (α-CD40) in the presence of the inhibitor for 24 hr. The amount of IL-6 and IL-12 in the culture supernatants was measured by ELISA. Each column represents the mean ± SE of three independent experiments. Statistical significance was calculated by Student's t-test (*P < 0·05 versus control).
To examine roles of the CD40-mediated p38 MAPK activation in the cytokine productions by immature and mature DCs, the effect of a specific inhibitor of p38 MAPK pathway, SB203580 (20 µm), was evaluated. Immature and mature DCs were pretreated with SB203580 (20 µm). The DCs were then treated with anti-CD40 mAb in the presence of the inhibitor (Fig. 2b). CD40-mediated IL-6 production by either immature and mature DCs was significantly decreased by treatment with SB203580. CD40-mediated IL-12 production by immature DCs was also decreased by SB203580. In contrast, SB203580 failed to decrease CD40-mediated IL-12 production by mature DCs.
CD40-mediated NF-κB activation in immature and mature DCs
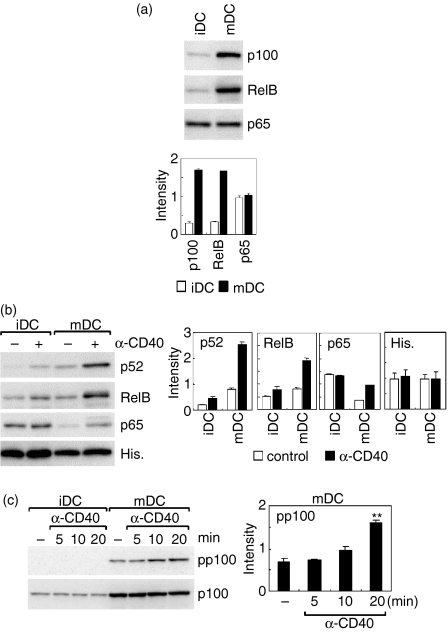
The inhibitor studies (Fig. 2) suggested that CD40-mediated IL-12 production by mature DCs is regulated by a signalling pathway other than p38 MAPK pathway. NF-κB family proteins are involved in a variety of cytokine productions. We then analysed nuclear translocation of the NF-κB proteins, which are involved in alternative (p52/p100 and RelB) or classical (p65) pathway, in immature and mature DCs after CD40 ligation. Figure 3(a) shows the levels of NF-κB family proteins in the whole cell lysates. The levels of p100 and RelB in mature DCs were considerably higher than those in immature DCs, while the p65 level showed no difference between immature and mature DCs (Fig. 3a). We next analysed nuclear translocations of these NF-κB proteins 90 min after treatment with anti-CD40 mAb. The NF-κB nuclear-levels reached a peak at 60–90 min after the CD40 ligation (data not shown). Constitutive level of nuclear p52, a processed form of p100, was higher in mature DCs than that in immature DCs (Fig. 3b). RelB showed the similar constitutive level between immature and mature DCs. Notably, the nuclear levels of p52 and RelB in mature DCs were markedly increased by CD40 ligation. Although the nuclear level of p52 also increased in immature DCs upon CD40 ligation, the level was much lower than that in mature DCs. Similarly, nuclear levels of RelB in immature DCs were slightly increased after CD40 ligation. However, this effect was not statistically significant. On the other hand, the constitutive level of nuclear p65 in immature DCs was higher than that in mature DCs. After CD40 ligation, the nuclear level of p65 was unchanged in immature DCs, whereas that in mature DCs was increased. The nuclear level of p65 in mature DCs, however, was still below the constitutive nuclear level of this protein in immature DCs. No significant differences were detected among nuclear levels of histone H3 between immature and mature DC irrespective of the CD40 ligation.
Figure 3.
Activation of alternative NF-κB pathway after CD40 ligation in mature DCs. Unstimulated or LPS-treated BC1 cells were considered as immature (iDC) or mature DCs (mDC), respectively. (a) Levels of p100, RelB, or p65 in whole cell lysates. Levels of p100, RelB, or p65 in whole cell lysates of iDC or mDC were determined by immunoblotting. A representative immunoblot of three independent experiments is shown (upper). The relative intensity of the specific band is shown (lower). Each column represents the mean ± SE of three independent experiments. (b) Nuclear translocation of p100, RelB, or p65 after CD40 ligation. Cells were treated with anti-CD40 mAb (α-CD40) or control immunoglobulin for 90 min, and then the nuclear extracts were prepared. Levels of p100, RelB, p65, or histone H3 (His) in the nuclear extracts were determined by immunoblotting. A representative immunoblot of three independent experiments is shown (left). The relative intensity of the specific band is shown (right). Each column represents the mean ± SE of three independent experiments. (c) Phosphorylation of p100 after CD40 ligation. Cells were treated with anti-CD40 mAb (α-CD40) for 5–20 min, and then the whole cell lysates were prepared. Levels of phospho-p100 (pp100) or p100 in the cell lysates were determined by immunoblotting. A representative immunoblot of three independent experiments is shown (left). The relative intensity of the specific band is shown (right). Each column represents the mean ± SE of three independent experiments. Statistical significance was calculated by Student's t-test (**P < 0·01 versus unstimulated cells).
Nuclear translocation of RelB : p52 heterodimer is induced following phosphorylation and subsequent processing of p100.18 We therefore analysed levels of p100 phosphorylation after CD40 ligation in immature DCs and mature DCs (Fig. 3c). The total p100 level in the whole cell lysate was again up-regulated in mature DCs. The level of phospho-p100 in mature DCs was significantly increased 20 min after CD40 ligation. No phospho-p100 was detected in immature DCs, irrespective of CD40 ligation.
Involvement of NIK in CD40-mediated IL-6 and IL-12 productions in DCs
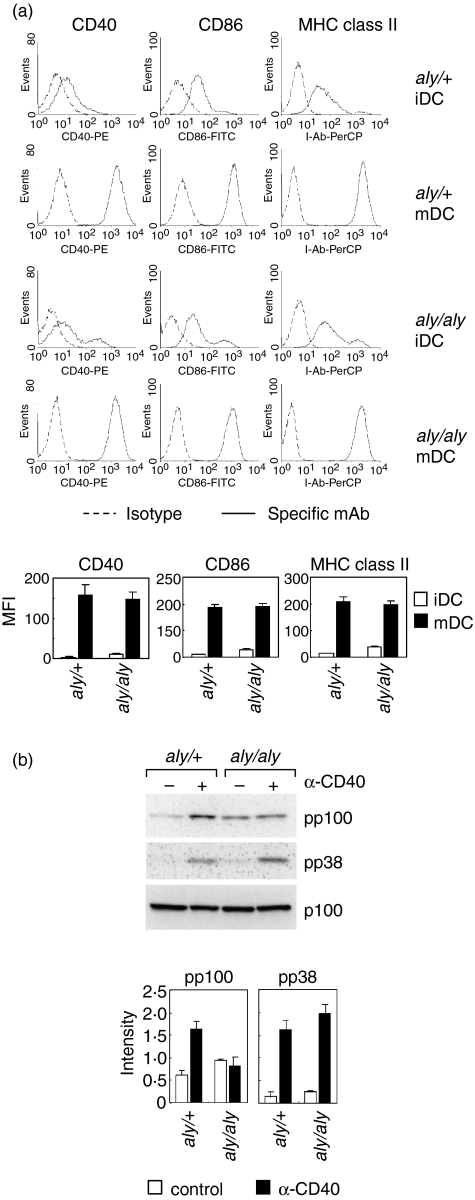
Phosphorylation of p100 has been shown to be catalysed by IKK acting downstream from NIK. Thus, it is possible that NIK is involved in the CD40-mediate phosphorylation of p100 in mature DCs. To examine this, we used a primary culture of DCs, SDDCs, from aly/aly mice which have no NIK activity because of the genetic point mutation. SDDCs were positive for CD11b and negative for CD8 and B220 (data not shown), a pattern typical of myeloid DC. Immature DCs (unstimulated DCs) from both aly/aly mice and the heterozygous littermates (aly/+) show moderate expressions of CD40, CD86, and MHC class II (I-Ab), and these expressions were markedly up-regulated after treatment with LPS irrespective of the aly/aly mutation (Fig. 4a).
Figure 4.
Impaired phosphorylation of p100 after CD40 ligation in mature DCs from NIK-mutated aly/aly mice. A primary culture of DCs, SDDCs were prepared from splenocytes from aly/+ or aly/aly mice. Unstimulated or LPS-treated SDDCs were considered as immature (iDC) or mature DCs (mDC), respectively. (a) Cell surface marker expressions. The iDC and mDC from aly/+ or aly/aly mice were stained with specific mAb against CD40, CD86, or MHC class II (I-Ab) or isotype-matched control immunoglobulin (Isotype). The cell surface expressions were analyzed by flow cytometry. A representative histogram of three independent experiments is shown (upper). The mean fluorescence intensity (MFI) of the cells is represented (lower). Each column represents the mean ± SE of three independent experiments. (b) Phosphorylation of p100, p38 MAPK. The mature DCs from aly/+ or aly/aly mice were treated with anti-CD40 mAb (α-CD40) or control immunoglobulin for 20 min, and then whole cell lysates were prepared. Levels of phospho-p100 (pp100), p100, phospho-p38 MAPK (pp38), and p38 MAPK (p38) in the cell lysates were determined by immunoblotting. A representative immunoblot of three independent experiments is shown (upper). The relative intensity of the specific band is represented (lower). Each column represents the mean ± SE of three independent experiments.
Figure 4(b) shows the effect of aly/aly mutation on CD40-mediated phosphorylation of p100 and p38 MAPK in mature DCs. CD40 ligation markedly increased the level of phospho-p100 in mature aly/+ DCs, while showing no effects on that in mature aly/aly DCs. No phospho-p100 was detected in immature DCs from both aly/+ and aly/aly mice (data not shown), consistent with the data with BC1 cells (Fig. 3c). In contrast, the level of phospho-p38 was significantly increased by CD40 ligation irrespective of the aly/aly mutation (Fig. 4b).
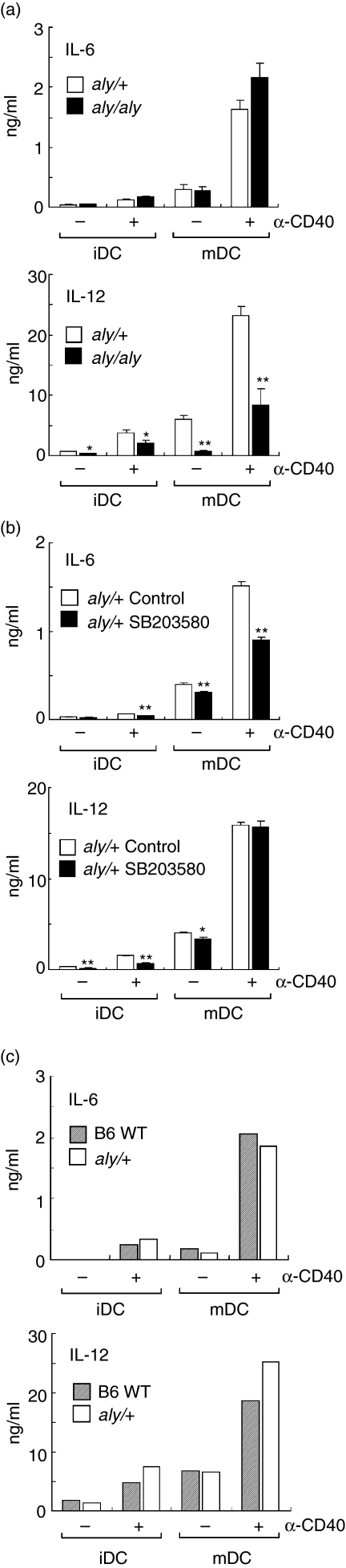
We then analysed the CD40-mediated IL-6 and IL-12 productions by DCs from aly/+ and aly/aly mice (Fig. 5a). The aly/aly mutation exerted no significant influence on constitutive and CD40-mediated IL-6 productions by immature and mature DCs. In contrast, constitutive and CD40-mediated IL-12 productions by mature DCs from aly/aly mice were markedly reduced as compared to those by mature DCs from aly/+ mice. Although IL-12 production by immature DCs from aly/aly mice were also reduced, the reduction rate by the aly/aly mutation was modest as compared with that in mature DCs.
Figure 5.
Effects of aly mutation on IL-6 and IL-12 productions by DCs. A primary culture of DCs, SDDCs, were prepared from splenocytes of aly/+, aly/aly, or B6 wild-type (WT) mice. Unstimulated or LPS-treated SDDCs were considered as immature (iDC) or mature DCs (mDC), respectively. The iDC and mDC from aly/+ or aly/aly mice were incubated with anti-CD40 mAb (α-CD40) or control immunoglobulin for 24 hr. The amount of IL-6 and IL-12 in the culture supernatants was measured by ELISA. (a) Influence of the aly/aly mutation on cytokine production. Each column represents the mean ± SE of three independent experiments. (b) The effect of a p38 MAKP inhibitor, SB203580, on cytokine production by aly/+ SDDCs. The iDC and mDC from aly/+ were incubated with anti-CD40 mAb (α-CD40) with or without SB203580 as described in Fig. 2(b). Each column represents the mean ± SE of triplicate wells. The analysis was repeated twice with similar results. (c) Cytokine production by aly/+ and WT DCs. Each column represents the mean of two independent experiments. Statistical significance was calculated by Student's t-test (*P < 0·05, **P < 0·01 versus aly/+ (a) or control (b)).
We also evaluated the effects of a specific inhibitor of p38 MAPK pathway, SB203580 (20 µm), on CD40-mediated IL-6 and IL-12 production by immature and mature SDDCs from aly/+ mice. Immature and mature DCs were pretreated with SB203580 (20 µm). The DCs were then treated with anti-CD40 mAb in the presence of the inhibitor (Fig. 5b). CD40-mediated IL-6 production by either immature and mature DCs was significantly decreased by treatment with SB203580. CD40-mediated IL-12 production by immature DCs was also decreased by SB203580. In contrast, SB203580 showed no significant effects on CD40-mediated IL-12 production by mature DCs. These results were compatible with the data from BC1 cells (Fig. 2b).
When the levels of cytokine production were compared between wild-type (B6) and aly/+ DCs, almost comparable levels of IL-6 and IL-12 were produced by aly/+ DCs as by wild-type DCs (Fig. 5c). Thus, it seemed that wild-type NIK production from the single allelic gene was sufficient for full induction of IL-12 production in response to CD40 ligation in DCs.
Discussion
When encountering pathogens, DCs capture the antigens and are matured by the pathogen products such as LPS. Subsequently, the mature DCs migrate to the T-cell area of the lymph nodes and present the antigens to T cells. During antigen presentation, the mature DCs receive signal via CD40 and produce IL-6 and IL-12. The balance of these cytokine productions appears to be crucial in determining the subsequent Th1 and Th2 immune responses.
In the present study, we focused on the molecular mechanisms underlying the CD40-mediated IL-6 and IL-12 production in immature and mature stages of murine myeloid DCs. We demonstrated herein that the regulation systems for IL-6 and IL-12 production were clearly separated in mature DCs.
It has been reported that CD40 ligation induces p38 MAPK activation in DCs, which results in promotion of IL-12 production by the DCs.10–12 However, only few reports analysed the role of p38 MAPK in IL-6 and IL-12 production among different maturational stages of DCs. Recently, we have demonstrated that blocking of the p38 MAPK pathway significantly decreases CD40-mediated IL-12 production by immature but not mature DCs.37 The functional role of p38 MAPK in the CD40-mediated IL-12 production seems to be different between immature and mature (Toll-like receptor (TLR)-stimulated) DCs.
We examined herein a role of p38 MAPK in CD40-mediated IL-6 production by immature and mature DCs in addition to IL-12 production. In agreement with our previous study,37 blocking of the p38 MAPK pathway significantly decreased CD40-mediated IL-12 production in immature but not mature DCs (Fig. 2b and Fig. 5b). In contrast, blocking of this pathway significantly decreased CD40-mediated IL-6 production in either immature or mature DCs (Fig. 2b and Fig. 5b). Thus, it seems that the role of p38 MAPK activation after CD40 ligation in mature DCs is different between IL-6 and IL-12 production.
CD40-mediated production of IL-12 by mature BC1 cells was slightly but significantly enhanced by treatment with the p38 inhibitor (Fig. 2b). Thus, p38 MAPK may be partially involved in negative regulation of the IL-12 production in this culture system. Although the effect of the inhibitor was modest, we should evaluate the precise molecular basis underlying the present finding in future studies.
It has been reported that CD40-mediated IL-12 production by human DCs is mediated via the p38 MAPK pathway.12 We showed herein that IL-12 production by immature murine DCs, but not mature DCs, was mediated via p38 MAPK. However, in the study of human DCs,12 roles of the p38 MAPK pathway in CD40-mediated IL-12 productions were examined in DCs without TLR-mediated prestimulation. Thus, the roles of p38 MAPK in human mature (prestimulated) DCs remain unclear.
Andreakos et al.38 have shown that IKK2, but not NIK, is essential for effective antigen presentation by human DCs in the allogeneic mixed lymphocyte reaction. In their study, blocking NIK in human DCs never affected T-cell proliferation against the alloantigen on the DCs. Further, TNF-α, IL-6, and IL-8 production by the NIK-inhibited DCs in response to CD40 ligation were same as those by normal DCs. Concerning CD40-mediated IL-6 production, we found that NIK mutation showed no significant effect on the IL-6 productions by murine DCs, which appears to be consistent with the report by Andreakos et al.38 However, it was shown in our study that CD40-mediated IL-12 production was NIK dependent in mature DCs. The apparent differences between the report by Andreakos et al.38 and our present study may be a result of the difference in DC condition used. Andreakos et al. did not use DCs matured by TLR-mediated stimuli before CD40 ligation, and IL-12 production by DCs was not analysed. Thus, the precise role of NIK in mature human DCs should be analysed to elucidate the functional differences in IL-12 production between murine and human DCs. It seems to us that decreased IL-12 production in NIK-deficient DCs affects Th1 development rather than T-cell proliferation.
Prior studies of aly mice, which express a non-functional NIK mutant, and NIK-knockout mice show that NIK participates selectively in the activation of NF-κB by a restricted set of ligands.23,39 Based on the characterization of cells derived from these mutant mice, NIK has been thought to participate in activation of the alternative NF-κB pathway.40 The alternative pathway involves NIK activation of IKKα and leads to phosphorylation and processing of p100, generating the p52 : RelB heterodimer.
It was shown in the present study that during DC maturation, protein levels of p100 and RelB, which are involved in the alternative pathway, were significantly increased, while that of p65, which is involved in the classical pathway, was not changed (Fig. 3a). Thus, equipment of the alternative pathway was enhanced during the DC maturation. In addition, CD40 ligation induced considerable activation of the alternative pathway, such as phosphorylation of p100 and translocation of p52 and RelB in mature DCs but not immature ones (Fig. 3b, c). Thus, the role of the alternative NF-κB pathway in DC function seems to become crucial according to the DC maturation.
In the present study, we found that CD40-mediated p100 phosphorylation was completely abolished in mature DCs of NIK-mutated aly/aly mice (Fig. 4b). CD40-mediated IL-12 production by mature DCs was also markedly reduced by the aly/aly mutation (Fig. 5a). In contrast, CD40-mediated IL-6 production was not reduced by the aly/aly mutation at all (Fig. 5a). We therefore would like to conclude that NIK-mediated activation of the NF-κB alternative pathway is responsible for IL-12 but not IL-6 production in mature DCs upon stimulation with CD40 ligation. However, it was shown that the basal level of phospho-p100 was slightly increased in aly/aly DCs compared with that in aly/+ DCs (Fig. 4b). It has been reported that aly type mutation of NIK leads to elevation of spontaneous phosphorylation of p100 but fails to increase p100 phosphorylation in response to extracellular stimuli.41 Although the mechanism underlying the spontaneous p100 phosphorylation in the presence of aly NIK is unclear, we consider that the difference in the level of spontaneous phospho-p100 seen between aly/aly and aly/+ DCs may be attributed to the nature of aly NIK products.
Garceau et al.42 have shown that NIK is involved in CD40-mediated B cell proliferation and immunoglobulin production using aly/aly mice. These authors also reported that the aly/aly mutation exerted negligible effects on CD40-mediated IL-12 production by splenic DCs. However, in their study no maturational stimuli were given to these DCs before the CD40 ligation. In the present study, we compared the effects of the aly/aly mutation on IL-6 and IL-12 productions between immature and mature DCs (Fig. 5a). The influence of the aly/aly mutation on CD40-mediated IL-12 production was modest in immature DCs as compared with that in LPS-induced mature DCs. Thus, the role of NIK-mediated pathway in IL-12 production by mature DCs seems to be more important than that by immature DCs.
Taken together, we demonstrated that IL-6 and IL-12 productions in response to CD40 ligation were controlled by p38 MAPK- and NIK-mediated alternative pathways, respectively, in mature DCs. Because the balance of IL-6 and IL-12 production by mature DCs during the antigen presentation is crucial for the subsequent Th1/Th2 balance, elucidation of the complex pathways that control production of these cytokines by mature DCs may lead to the development of clinical applications exploiting this new regulation system for the treatment of various infectious diseases and immune disorders.
Acknowledgments
We wish to thank Ms Mayumi Kondo for her assistance in the preparation of this manuscript. This study was supported in part by a Grant-in-Aid for Scientific Research (S), Grant-in Aid for Exploratory Research and a Grant-in-Aid for Young Scientists (B) from Japan Society for the Promotion of Science (JSPS), and a Grant-in-Aid for Scientific Research on Priority Areas by the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) Japan. This study was also supported by the Akiyama Foundation.
Abbreviations
- APC
antigen-presenting cells
- CD40L
CD40 ligand
- DC
dendritic cells
- ELISA
enzyme-linked immunosorbent assay
- ERK
extracellular signal-related kinases
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IKK
IκB kinase
- IMDM
Iscove's modified Dulbecco's medium
- mAb
monoclonal antibody
- LPS
lipopolysaccharide
- MAPKs
mitogen-activated protein kinases
- MHC
major histocompatibility complex
- NF-κB
nuclear factor κB
- NIK
NF-κB inducing kinase
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- SDDC
spleen-derived DCs
- TNFR
tumour necrosis factor receptor
- TRANCE
TNF-related activation-induced cytokine
References
- 1.Steinman R. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Hart DNJ. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–87. [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–51. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 5.Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–40. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- 6.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–9. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodge IL, Carr MW, Cernadas M, Brenner MB. IL-6 production by pulmonary dendritic cells impedes Th1 immune responses. J Immunol. 2003;170:4457–64. doi: 10.4049/jimmunol.170.9.4457. [DOI] [PubMed] [Google Scholar]
- 8.Yu Q, Kovacs C, Yue FY, Ostrowski MA. The role of the p38 mitogen-activated protein kinase, extracellular signal-regulated kinase, and phosphoinositide-3-OH kinase signal transduction pathways in CD40 ligand-induced dendritic cell activation and expansion of virus-specific CD8+ T cell memory responses. J Immunol. 2004;172:6047–56. doi: 10.4049/jimmunol.172.10.6047. [DOI] [PubMed] [Google Scholar]
- 9.Luft T, Maraskovsky E, Schnurr M, et al. Tuning the volume of the immune response: strength and persistence of stimulation determine migration and cytokine secretion of dendritic cells. Blood. 2004;104:1066–74. doi: 10.1182/blood-2003-12-4146. [DOI] [PubMed] [Google Scholar]
- 10.Lu HT, Yang DD, Wysk M, Gatti E, Mellman I, Davis RJ, Flavell RA. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3) -deficient mice. EMBO J. 1999;18:1845–57. doi: 10.1093/emboj/18.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacker H, Mischak H, Miethke T, et al. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230–40. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aicher A, Shu GL, Magaletti D, Mulvania T, Pezzutto A, Craxton A, Clark EA. Differential role for p38 mitogen-activated protein kinase in regulating CD40-induced gene expression in dendritic cells and B cells. J Immunol. 1999;163:5786–95. [PubMed] [Google Scholar]
- 13.Hacker H, Mischak H, Hacker G, Eser S, Prenzel N, Ullrich A, Wagner H. Cell type-specific activation of mitogen-activated protein kinases by CpG-DNA controls interleukin-12 release from antigen-presenting cells. EMBO J. 1999;18:6973–82. doi: 10.1093/emboj/18.24.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puig-Kroger A, Relloso M, Fernandez-Capetillo O, Zubiaga A, Silva A, Bernabeu C, Corbi AL. Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Blood. 2001;98:2175–82. doi: 10.1182/blood.v98.7.2175. [DOI] [PubMed] [Google Scholar]
- 15.Yanagawa Y, Iijima N, Iwabuchi K, Onoé K. Activation of extracellular signal-related kinase by TNF-α controls the maturation and function of murine dendritic cells. J Leukoc Biol. 2002;71:125–32. [PubMed] [Google Scholar]
- 16.Ouaaz F, Arron J, Zheng Y, Choi Y, Beg AA. Dendritic cell development and survival require distinct NF-κB subunits. Immunity. 2002;16:257–70. doi: 10.1016/s1074-7613(02)00272-8. [DOI] [PubMed] [Google Scholar]
- 17.Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 18.Bonizzi G, Karin M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 20.Senftleben U, Cao Y, Xiao G, et al. Activation by IKK of a second, evolutionary conserved, NF-κB signaling pathway. Science. 2001;293:1495–9. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 21.Xiao G, Fong A, Sun SC. Induction of p100 processing by NF-κB-inducing kinase involves docking IB kinase (IKK) to p100 and IKK-mediated phosphorylation. J Biol Chem. 2004;279:30099–105. doi: 10.1074/jbc.M401428200. [DOI] [PubMed] [Google Scholar]
- 22.May MJ, Ghosh S. Signal transduction through NF-κB. Immunol Today. 1998;19:80–8. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 23.Shinkura R, Kitada K, Matsuda F, et al. Alymphoplasia is caused by a point mutation in the mouse gene encoding NF-κB-inducing kinase. Nat Genet. 1999;22:74–7. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- 24.Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-α, and contact sensitizers. J Immunol. 2001;166:3837–45. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- 25.Awasthi A, Mathur R, Khan A, et al. CD40 signaling is impaired in L. major-infected macrophages and is rescued by a p38MAPK activator establishing a host-protective memory T cell response. J Exp Med. 2003;197:1037–43. doi: 10.1084/jem.20022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhuang S, Yan Y, Han J, Schnellmann RG. p38 kinase-mediated transactivation of the epidermal growth factor receptor is required for dedifferentiation of renal epithelial cells after oxidant injury. J Biol Chem. 2005;280:21036–42. doi: 10.1074/jbc.M413300200. [DOI] [PubMed] [Google Scholar]
- 27.Winzler C, Rovere P, Rescigno M, et al. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185:317–28. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanagawa Y, Onoé K. CCL19 induces rapid dendritic extension of murine dendritic cells. Blood. 2002;100:1948–56. doi: 10.1182/blood-2002-01-0260. [DOI] [PubMed] [Google Scholar]
- 29.Granucci F, Petralia F, Urbano M, Citterio S, Di Tota F, Santambrogio L, Ricciardi-Castagnoli P. The scavenger receptor MARCO mediates cytoskeleton rearrangements in dendritic cells and microglia. Blood. 2003;102:2940–7. doi: 10.1182/blood-2002-12-3651. [DOI] [PubMed] [Google Scholar]
- 30.Yanagawa Y, Onoé K. CCR7 ligands induce rapid endocytosis in mature dendritic cells with concomitant up-regulation of Cdc42 and Rac activities. Blood. 2003;101:4923–9. doi: 10.1182/blood-2002-11-3474. [DOI] [PubMed] [Google Scholar]
- 31.Kikuchi K, Yanagawa Y, Aranami T, Iwabuchi C, Iwabuchi K, Onoé K. Tumour necrosis factor-α but not lipopolysaccharide enhances preference of murine dendritic cells for Th2 differentiation. Immunology. 2003;108:42–9. doi: 10.1046/j.1365-2567.2003.01537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iijima N, Yanagawa Y, Iwabuchi K, Onoé K. Selective regulation of CD40 expression in murine dendritic cells by thiol antioxidants. Immunology. 2003;110:197–205. doi: 10.1046/j.1365-2567.2003.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villadangos JA, Cardoso M, Steptoe J, van Berkel D, Pooley J, Carbone FR, Shortman K. MHC class II expression is regulated in dendritic cells independently of invariant chain degradation. Immunity. 2001;14:739–49. doi: 10.1016/s1074-7613(01)00148-0. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–8. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- 35.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–7. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 36.Granucci F, Vizzardelli C, Pavelka N, et al. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat Immunol. 2001;2:882–8. doi: 10.1038/ni0901-882. [DOI] [PubMed] [Google Scholar]
- 37.Kikuchi K, Yanagawa Y, Iwabuchi K, Onoé K. Differential role of mitogen-activated protein kinases in CD40-mediated IL-12 production by immature and mature dendritic cells. Immunol Lett. 2003;89:149–54. doi: 10.1016/s0165-2478(03)00134-2. [DOI] [PubMed] [Google Scholar]
- 38.Andreakos E, Smith C, Monaco C, Brennan FM, Foxwell BM, Feldmann M. Iκ B kinase 2 but not NF-κB-inducing kinase is essential for effective DC antigen presentation in the allogeneic mixed lymphocyte reaction. Blood. 2003;101:983–91. doi: 10.1182/blood-2002-06-1835. [DOI] [PubMed] [Google Scholar]
- 39.Yin L, Wu L, Wesche H, Arthur CD, White JM, Goeddel DV, Schreiber RD. Defective lymphotoxin-β receptor-induced NF-κB transcriptional activity in NIK-deficient mice. Science. 2001;291:2162–5. doi: 10.1126/science.1058453. [DOI] [PubMed] [Google Scholar]
- 40.Pomerantz JL, Baltimore D. Two pathways to NF-κB. Mol Cell. 2002;10:693–5. doi: 10.1016/s1097-2765(02)00697-4. [DOI] [PubMed] [Google Scholar]
- 41.Ramakrishnan P, Wang W, Wallach D. Receptor-specific signaling for both the alternative and the canonical NF-κB activation pathways by NF-κB-inducing kinase. Immunity. 2004;21:477–89. doi: 10.1016/j.immuni.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Garceau N, Kosaka Y, Masters S, Hambor J, Shinkura R, Honjo T, Noelle RJ. Lineage-restricted function of nuclear factor κB-inducing kinase (NIK) in transducing signals via CD40. J Exp Med. 2000;191:381–6. doi: 10.1084/jem.191.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]