Abstract
The role of type I interferon (IFN-αβ) in modulating innate or adaptive immune responses against mycobacterial infection in the lung is unclear. In this study we investigated the susceptibility of IFN-αβ-receptor-deficient (IFN-αβR–/–) mice to pulmonary infection with aerosolized Mycobacterium bovis bacillus Calmette–Guérin (BCG). During early infection (2–3 weeks), enhanced growth of BCG was measured in the lungs of IFN-αβR–/– mice compared to wild-type mice. However, during late infection the burden of BCG was similar in the lungs of IFN-αβR–/– and wild-type mice. Although control of BCG growth was delayed, recruitment and activation of T and natural killer cells, production of IFN-γ, and cytokine expression were all similar in wild-type and IFN-αβR–/– mice. However, decreased expression of nitric oxide in bronchoalveolar lavage fluids from IFN-αβR–/– mice correlated with enhanced growth of BCG. Bone marrow-derived macrophages from IFN-αβR–/– mice also produced less nitric oxide following infection with BCG in vitro. These findings suggest that IFN-αβ contributes to innate immunity to pulmonary mycobacterial infection by augmenting production of nitric oxide.
Keywords: lung, mouse, mycobacteria, nitric oxide, type I interferon
Introduction
Type I interferon (IFN-αβ) is produced by human and mouse dendritic cells and macrophages infected with mycobacteria or exposed to mycobacterial antigens. 1–3 However, it is not clear whether IFN-αβ has a protective or exacerbating function in the immune response to mycobacteria. In support of a disease-exacerbating effect, IFN-αβ can inhibit mycobactericidal activity of human macrophages against Mycobacterium bovis bacillus Calmette–Guérin (BCG).4 Also, intranasal IFN-αβ can enhance the growth of a virulent clinical strain of M. tuberculosis (MTB) in murine lungs.2 Since MTB inhibits signal transduction downstream of the IFN-αβ receptor5 and impairs IFN-α-mediated dendritic cell maturation,6 MTB may subvert or repress IFN-αβ-mediated responses to promote its survival within host macrophages. In contrast, a beneficial role for IFN-αβ has been suggested by enhanced growth of virulent MTB in IFN-αβ-receptor-deficient (IFN-αβR–/–) mice7 and by clinical improvements observed in tuberculosis patients treated with aerosolized IFN-α.8–10 Since IFN-αβ activates nitric oxide synthase-2 (NOS-2)11,12 beneficial effects of IFN-αβ during tuberculosis may involve direct activation of NOS-2 in human alveolar macrophages,13,14 which are the initial cellular targets of aerosolized droplets containing MTB.
Activation of NOS-2 occurs in response to mycobacterial infection or exposure to mycobacterial antigens13,15 and is important for, but may be non-essential to, mycobactericidal immunity.7,16,17 Involvement of NOS-2 is most evident when T-cell responses develop and IFN-γ is expressed.7,17,18 During the adaptive phase of antigen-specific immune control, IFN-γ contributes to the activation of NOS-2 and nitric oxide production by macrophages.16 IFN-αβ also directly promotes T-cell activation,19 dendritic cell maturation and IFN-γ production, which are crucial elements of antimycobacterial immunity.16,20,21 However, it remains unclear whether IFN-αβ contributes to the development of protective mycobacterial antigen-specific T-cell responses.
During pulmonary MTB infection in mice, recruitment and activation of natural killer (NK) cells may be enhanced by expression of IFN-αβ.20,22–25 Furthermore, NK cells are capable of killing human macrophages infected with MTB.26 Although depletion of NK cells in mice does not appear to affect the control of MTB infection in the lung,24 NK-cell depletion exacerbates splenic M. avium infection.27–29 Thus, a precise role for NK cells in host defence against mycobacterial infections has not been well defined.
We investigated the role of IFN-αβ in protection against aerosolized BCG infection, which is characterized by an early phase that limits bacterial growth, and a late phase when infection resolves.30 In IFN-αβR–/– mice, we observed an initial phase of enhanced growth in the lungs. However, BCG growth was eventually controlled at bacterial burdens similar to those detected in wild-type mice. Recruitment and activation of NK and T cells and production of IFN-γ were essentially normal in the IFN-αβR–/– mice. However, despite higher bacterial burdens, bronchoalveolar nitrite levels were lower in IFN-αβR–/– mice compared to controls, suggesting a defect in NOS-2 induction. We also determined that nitric oxide production was significantly lower in IFN-αβR–/– bone-marrow-derived macrophages (BMM) infected with BCG in vitro. While not required for development of protective T-cell immunity during BCG infection, IFN-αβ contributes to the production of nitric oxide and early control of BCG growth in the murine lung.
Materials and methods
Mice
Wild-type male and female mice were housed under specific pathogen-free conditions using microisolator cages (Laboratory Products, Inc., Maywood, NJ) and fed a standard rodent diet and water ad libitum. IFN-αβR–/– A129 mice on the 129S6/SvEv background (H-2b) were purchased from B & K Universal (Hull, East Yorkshire, UK). Homozygous deletions were confirmed by polymerase chain reaction using primers 5′-AAGATGTGCTGTTCCCTTCCTCTGCTCTGA-3′ and 5′-ATTATTAAAAGAAAAGACGAGGCGAAGTGG-3′. Wild-type control 129S6/SvEv mice (formerly designated as 129/SvEv Tac) were purchased from Taconic (Germantown, NY). For each experiment, mice were age-matched and similar numbers of female and male mice were used. The studies were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University.
Bacteria and aerosol infections
Mycobacterium bovis BCG Connaught (ATCC #35745) was grown at 37° for 18–21 days in 7H9 medium supplemented with albumin-dextrose-catalase (ADC) (Difco, Detroit, MI). During mid-log growth, the culture was supplemented with glycerol (6% v/v), divided into aliquots and stored at −70° until further use. Bacterial titres were determined by plating serial 10-fold dilutions on 7H10 agar and counting colony-forming units (CFUs) after 2–3 weeks of incubation. BCG retained its viability for at least 2 years.
Inocula for aerosol infections were prepared as follows: 2 ml of BCG stock cultures was retrieved from −70° and grown to mid-log phase in 140 ml 7H9 medium containing ADC and 0·05% Tween-80 (Sigma, St Louis, MO). After 5 days growth, bacteria [optical density at 595 nm (OD595 nm) = 0·850–0·950] were pelleted (1800 g for 15 min) and resuspended in 10 ml of sterile lipopolysaccharide (LPS)-free water (#W3500, Sigma). Bacterial clumps were disrupted by three sequential aspirations through a sterile 26G hypodermic needle. This high-dose inoculum was placed in the glass nebulizer chamber of the Inhalation Exposure System (Glas Col, Terre Haute, IN) and mice were exposed to a bacterial aerosol for 50 min with input and output airflow rates set at 7 l/min and 24 l/min, respectively. Low-dose BCG aerosols were generated using one-half of a 5-day mid-log inoculum prepared as described above. The number of BCG implanted in the lung after 1 day of infection was determined as previously described30 and is specified for each experiment.
Bronchoalveolar lavage and lung cell preparation
At designated intervals after infection, mice were anaesthetized with a lethal dose of tribromoethanol (240 mg/kg). The abdominal cavity was incised and the mice were exsanguinated by splenectomy and transection of the left renal artery and vein. Before removal of the lungs, the trachea was exposed and the chest was decompressed through a transverse incision at the level of the xiphoid. Bronchoalveolar lavage (BAL) was performed by inserting an 18-gauge Angiocath intravenous catheter (Becton Dickinson, Sandy, UT) through a 1-mm incision at the first tracheal ring. A total of 1·2 ml phosphate-buffered saline (PBS) was instilled in three separate 0·4-ml lavages. BAL cells were centrifuged at 2000 g for 10 min at 4°, resuspended in RPMI-1640 (BioWhittaker, Walkersville, MD) and counted in 0·4% Trypan blue. Serial dilutions were prepared and plated on 7H10 agar to determine the number of CFUs associated with BAL cells. BAL fluids were stored at −70° for subsequent chemokine and cytokine analysis. CFUs were not detected in BAL supernatants. Cytospin slides of 10 × 104 to 15 × 104 cells/BAL/mouse were prepared using a Cytospin 3 centrifuge (40 g for 8 min; Shandon, Pittsburgh, PA) and stained with Diff-Quik (Dade Diagnostics, Aguada, PR) for differential cell counts.
Lung cell homogenates were prepared as previously described.30 Briefly, after BAL, lungs (all five lobes) were perfused with 10 ml PBS injected into the right ventricle and then dissected from mainstem bronchi. Lungs were minced with scalpels in 4 ml of RPMI-1640 and digested with 125 units/ml of type IV collagenase and 30 units/ml DNase (#C5138 and #D4527, Sigma) for 1·5 hr at 37°. Tissue was further disrupted by sequential passage through an 18G needle, clarified by filtration through sterile cotton and centrifuged (800 g for 15 min). The cells were resuspended in red blood cell lysis buffer (containing 10 mm Tris–HCl and 0·83% ammonium chloride), incubated at room temperature for 5 min and pelleted (800 g for 15 min). Lung cells were resuspended in PBS and held on ice for staining and flow cytometric studies. In addition, lung cells (4 × 106/ml) were cultured for 72 hr in complete medium [Dulbeccos' modified Eagle's minimal essential medium (DMEM), 10% fetal calf serum, 0·05 mm 2-mercaptoethaol, 2 mm HEPES, 1 mm sodium pyruvate, 100 mm non-essential amino acids, 100 U/ml penicillin and 0·1 mg/ml streptomycin]. Culture supernatants were stored at −70° until analysed for cytokine, chemokine and nitrite expression.
CFU determination
Aliquots of lung tissue homogenates were serially diluted in PBS containing 0·05% Tween-20 and plated on Middlebrook 7H10 agar enriched with 10%o-ADC (Difco) and 0·5% glycerol, and incubated at 37° for 2–3 weeks. Colonies were counted and total tissue CFU was calculated based on the volume of homogenate obtained from each organ/mouse.
Cell staining and flow cytometry
For analysis of T-cell and NK-cell numbers and activation, the following antibodies were used: allophycocyanin-anti-CD3e (clone 145–2C11), biotin-anti-CD49b (clone DX5), fluorescein isothicyanate (FITC)-anti-CD94 (clone 18d3), APC-hamster immunoglobulin G (IgG), biotin-rat IgM, and streptavidin-APC-Cy7, from eBiosciences (San Diego, CA); and phycoerythrin (PE)-Cy7-anti-CD69 (clone H1.2F3), biotin-anti-CD25 (clone 7D4), FITC-anti-Ly49D (clone 4E5), anti-CD16/CD32 (clone 2.4G2) and PE-anti-TRAIL (clone N2B2) from BD Biosciences (San Diego, CA). After BAL, lung cell homogenates were prepared as described above, resuspended at 1 × 107−2 × 107 cells/ml in staining buffer (PBS containing 2% fetal bovine seurm and 0·1% sodium azide) and kept in the dark on ice.
To stain lung T cells and NK cells, Fc receptors were blocked for 5 min with anti-CD16/CD32 (5 μg/ml). Specific antibodies and isotype controls were then added at 5 μg/ml. After 30 min, cells were washed in staining buffer and streptavidin–APC-Cy7 (1 : 100) was added for 15 min. Next, cells were washed, re-suspended in 0·3 ml PBS containing 2% paraformaldehyde, stored at 4° and analysed within 3 days. Data were acquired using an LSR II system flow cytometer (BD Biosciences, San Diego, CA). To assess activation markers on NK-cell subsets, at least 105 ungated events were acquired. Post-acquisition compensation and analysis was performed using FlowJo software (Tree Star, Ashland, OR). Cells were analysed within a lymphocyte gate based on typical forward scatter and side scatter properties. Expression of activation markers CD69 and CD25 was analysed on CD3+ CD4+ T cells. DX5+ CD3– NK cells and DX5+ CD3– NK cells expressing the phenotypic markers Ly49D or CD94 were analysed for expression of CD69 and TRAIL. To express data as total number of cells/lung, the per cent of total ungated events was multiplied by the total number of lung cells harvested.
Interferon-γ ELISPOT
Sterile ELISPOT plates (Unifilter plate #7770–0006, Whatman, Clifton, NJ) were precoated overnight at 4° with 5 μg/ml anti-IFN-γ antibody (Pharmingen #551216) diluted in PBS and washed five times with PBS. Next, 1 × 106, 5 × 105 and 2·5 × 105 lung cells from individual mice were cultured in duplicate and either incubated in complete medium alone or with exogenous BCG (at a ratio of 1 : 1). After a 48 hr incubation at 37°, cells were washed away (four times) with PBS containing Tween-20 (0·05%) and 0·1 ml of biotinylated-anti-IFN-γ (XMG1.2, Pharmingen #554410) diluted to 0·5 mg/ml in PBS (containing 0·05% Tween-20 and 1% bovine serum albumin) was added to each well. After 4 hr at room temperature, plates were washed four times and bound IFN-γ was detected using streptavidin–alkaline phosphatase according to the manufacturer's instructions (R & D Elispot Blue Color Module #SEL002). Plates were dried at room temperature for 24 hr and spots were counted and analysed using the Immunospot reader and software (CTL Analyzers, LLC, Cleveland, OH). Results were expressed as the mean number of spot-forming units (± SEM) per 106 total lung cells from three different mice. The Wilcoxon rank sum test was used to determine statistical differences between infected wild-type and IFN-αβR–/– mice.
Preparation and antigen stimulation of BMM
Bone marrow from mouse femurs was plated directly into 48-well tissue culture dishes (Costar #3548, Corning, NY) at a proportion of one femur per 10 wells. Bone marrow cells were cultured in complete medium supplemented with 20% L929 cell-conditioned medium with medium changes every 2 days as previously described.31 After 7 days, BMM had reached confluency (251 800 ± 22 500 cells/well, n = 5) and the medium was replaced with complete DMEM (without L929 cell conditioned medium) and incubated for 24 hr. On day 9, BMM were stimulated in 0·125 ml (2 × 106 cells/ml) of medium alone or medium containing BCG at a multiplicity of infection (MOI) of 1, 5 and 10 bacilli/macrophage. After 72 hr, supernatants were collected and stored at −70° until analysed for nitrite, chemokine or cytokine production.
Nitrite assay
Nitric oxide activity in BAL and cell culture supernatants was measured using the Griess Reagent. In brief, 0·05 ml of sodium nitrite standard (Sigma, S-2252), BAL fluid or cell culture supernatant was mixed with 0·05 ml Griess Reagent [1 : 1 mixture of sulphanilamide (1·5 g/100 ml of 1 m HCl, Sigma #S-9251) and N-(1-naphthyl)-ethylenediamine dihydrochloride (0·15 g/100 ml, Sigma S-2252)] in 96-well plates. The colour change developed for 15 min at room temperature and absorbance was read at 530 nm using an automated plate reader (Versamax, Molecular Devices, Sunnydale, CA). Nitrite concentrations were determined using sodium nitrite standards and a lower limit of detection of 6·5 μm nitrite.
Cytokine and chemokine measurements
BAL and lung cell culture supernatants were analysed for tumour necrosis factor-α (TNF-α; Pharmingen #558874), IFN-α (R & D #42100–1), IFN-γ (R & D #MAB758 and BAF485), interleukin-10 (IL-10; Pharmingen #555252), IL-12 p40 (R & D #M1240), IL-12 p70 (R & D #M1270), macrophage inflammatory protein-2 (R & D MM200) and monocyte chemotactic protein-1 (R & D MJE00) according to the manufacturers' instructions. At each time-point, BAL and lung culture supernatants from three or four wild-type and IFN-αβR–/– mice were analysed. For each experiment, mean values (± SEM) of cytokine or chemokine were determined from standard curves and comparisons between wild-type and IFN-αβR–/– mice were made using the Student's t-test.
Statistical analysis
The Wilcoxon rank sum test or the Student's t-test was used where indicated. Significant differences are indicated (P ≤ 0·05).
Results
Early resistance to BCG infection is defective in IFN-αβR–/– mice
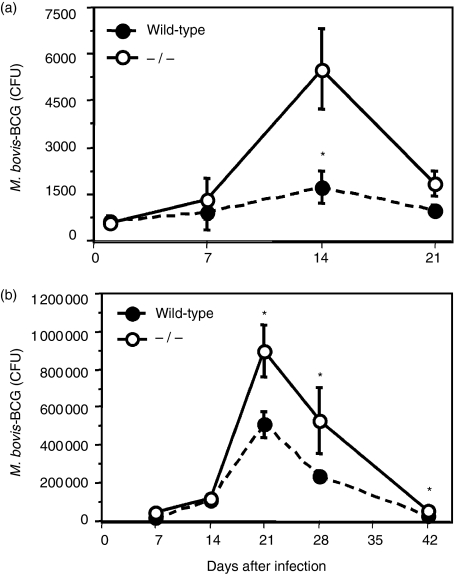
Since IFN-αβ induces dendritic cell maturation, activates T cells and enhances NK-cell functions20 we studied the growth of BCG in IFN-αβR–/– mice, which are unresponsive to IFN-αβ.32 Initially, we studied BCG growth after a low-dose aerosol infection in the lung (500–1500 CFU). As shown in Fig. 1(a), wild-type 129SvEv mice resisted and controlled pulmonary infection with aerosolized BCG and thus were relatively resistant (bcgR) to BCG infection as previously suggested.33–35 In contrast, BCG growth in the lungs of IFN-αβR–/– mice was significantly higher (1·5–3·0-fold, P < 0·05) 14 days after a low-dose aerosol infection, indicating enhanced susceptibility to BCG infection. However, by 21 days, control of BCG growth was achieved and CFUs were comparable between wild-type and IFN-αβR–/– mice after 4 weeks of infection (P ≥ 0·05). Although a statistically significant increase in CFU was detected in IFN-αβR–/– following a low-dose infection, the degree of cellular inflammation was low (data not shown). Thus, we used a higher dose of BCG (3000–5000 CFU) to establish an infection that was not cleared as readily in wild-type, ‘resistant’ mice (Fig. 1a). As shown in Fig. 1(b), following high-dose infection, the magnitude of bacterial growth was greater and a similar statistically significant (two- to threefold) increase in BCG growth occurred during the first 3 weeks of infection (Fig. 1b) in IFN-αβR–/– mice and was associated with more inflammatory cell infiltrates (data not shown). In addition, compared to low-dose infections, peak BCG growth occurred later (day 21 versus day 14, Fig. 1a) (n = 3) and statistically significant increases in CFU persisted through 42 days of infection (P < 0·05). Nonetheless, bacterial numbers decreased progressively in the lung, indicating control of BCG infection in IFN-αβR–/– mice. Enhanced BCG growth also was detected in BAL of IFN-αβR–/– mice (data not shown). Thus, although IFN-αβR–/– mice were more susceptible to enhanced early mycobacterial growth (days 14–21), mechanisms for eliminating BCG during the late adaptive phase of the immune response (days 28–42) were apparently normal.
Figure 1.
Control of early M. bovis BCG growth in the lung is dependent on IFN-αβ receptor expression: 8–10-week-old C57BL/6 mice were infected with either (a) a low dose (595 ± 32 CFU, n = 3) or (b) a high dose (4087 ± 1103 CFU, n = 4) aerosol of M. bovis BCG using the Inhalation Exposure System (Glas-Col). At designated times, lung homogenates were prepared and CFUs were determined as previously described.30 Three or four mice were analysed at each time-point and statistically significant differences are shown (*P ≤ 0·05 by Wilcoxon Rank Sum test). Data are representative of three independent experiments with low- and high-dose infections.
IFN-αβ is not required for T-cell and NK-cell activation or IFN-γ production in the lung during BCG infection
Since T cells and IFN-γ are critical for controlling mycobacterial infection, we investigated whether delayed control of BCG growth in IFN-αβR–/– mice was the result of a decrease or delay in development of T-cell responses and BCG-specific IFN-γ expression. Using flow cytometry, we measured numbers of lung T cells in wild-type and IFN-αβR–/– mice following high-dose BCG infection. We first used flow cytometry to measure lung T cells at weekly intervals in wild-type and IFN-αβR–/– mice. T-cell recruitment and activation paralleled BCG growth but was more reliably measured during peak BCG growth.30 Thus, as shown in a representative experiment, similar numbers of CD4+ (Fig. 2a,c), and CD8+ T cells (data not shown) were detected during peak infection (day 22) of both wild-type and IFN-αβR–/– mice. At earlier time-points, we also did not detect significant differences in T-cell recruitment and activation (data not shown), which was low and corresponded to lower numbers of BCG (Fig. 1a). However, we were unable to rule out differences in early T-cell activation that were transient or below the level of detection using flow cytometry.
Figure 2.
T-cell and NK-cell activation during M. bovis BCG infection were not deficient in IFN-αβR–/– lungs. Mice were infected by aerosol with 3000–4500 BCG and lung cells were prepared for flow cytometry. (a–d) Lung T cells from wild-type (a, b) and IFN-αβR–/– mice (c,d) were stained for CD3, CD4, CD69 and CD25. CD3+/CD4+ T cells, shown within the gates of scatter plots from individual mice (a,c), were analysed for expression of CD69 (b,d) and CD25 (not shown). Histograms using data from three wild-type or IFN-αβR–/– mice are shown from day 22 post-infection (b,d), when T-cell numbers were maximal. Each open histogram represents a single mouse, and shaded histograms are isotype controls. The mean (± SD) total number of cells/lung (× 10−6) is indicated for CD4+ T cells (a,c) and CD4+ CD69+ T cells (b,d) and were not significantly different between wild-type and IFN-αβR–/– mice. Similar results were observed for two separate high-dose infection experiments. (e–h) Lung NK cells from wild-type (e,f) and IFN-αβR–/– mice (g,h) were stained for CD3, DX5 and CD69. CD3– DX5+ NK cells, shown within the gates of representative scatter plots from individual mice (e,g), were analysed for expression of CD69 (f,h). Histograms from an experiment with three wild-type and IFN-αβR–/– mice are shown for day 21 post-infection. Each open histogram represents a single mouse, and shaded histograms are isotype controls. The mean (± SD) total number of cells/lung (× 10−6) is indicated for NK cells (CD3– DX5+, e,g) and CD69+ NK cells (f,h) and were not significantly different between wild-type and IFN-αβR–/– mice. Similar results were observed for two separate high-dose infection experiments.
Although CD4+ T cells can be partially activated by IFN-αβ19 we also found no significant differences in surface expression of the activation marker CD69 (Fig. 2b,d) on lung CD4+ T cells (gated as shown, Fig. 2a,c) between BCG-infected wild-type and IFN-αβR–/– mice. In addition, similar numbers of CD25+ CD4+ T cells/lung were also detected (0·06 ± 0·01 × 106 for wild-type and 0·05 ± 0·02 × 106 for IFN-αβR–/–). In addition, similar numbers of bronchoalveolar lymphocytes were detected in wild-type and IFN-αβR–/– mice (data not shown). Thus, T-cell number and activation appeared unaffected by the absence of IFN-αβ receptor.
Since NK cells are a source of IFN-γ and when activated by IFN-αβ can kill MTB-infected macrophages26,36,37 we also analysed lung NK-cell recruitment and activation during BCG infection. Lung cells (105/animal) were analysed by flow cytometry and NK cells were identified as DX5+ CD3– cells. Similar to T cells, changes in NK-cell populations were not detectable before 2–3 weeks postinfection when they represented less than 1% of the total lung cell population; and before the BCG burden had peaked. As shown in Fig. 2(e–h), no significant differences in NK-cell number or CD69 expression were detected between wild-type (Fig. 2e,f) and IFN-αβR–/– (Fig. 2g,h) lungs after 21 days. We also measured TNF-related apoptosis-inducing ligand (TRAIL) expression on NK cells, which is induced by IFN-αβ and may be involved in NK-mediated cell killing.38 However, only a small percentage (1·7% ± 0·1%) of NK cells expressed TRAIL in the lungs of both wild-type and IFN-αβR–/– mice during BCG infection (data not shown). CD69 and TRAIL expression was also the same on DX5+ CD3– NK-cell subsets further characterized by the phenotypic markers CD94 and Ly49D, which were expressed on 30–40% of the DX5+ CD3– NK cells (data not shown). Thus, NK-cell activation appeared intact, suggesting that a NK-cell defect did not account for enhanced early growth of BCG in IFN-αβR–/– mice.
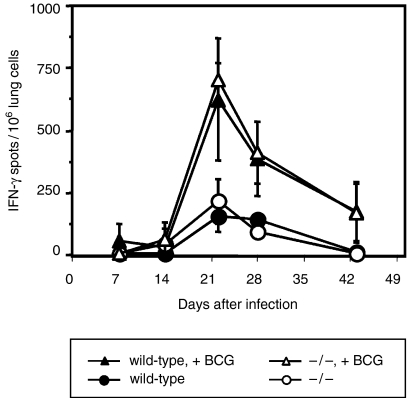
Since the numbers of T and NK cells were similar in infected wild-type and IFN-αβR–/– mice, we further examined T and NK-cell effector functions using a sensitive IFN-γ Elispot assay. In our hands, this assay is capable of detecting as few as 1/100 000 IFN-γ-secreting cells. As shown in Fig. 3, after 1–2 weeks of infection, the frequency of IFN-γ-secreting lung parenchymal cells (which includes both T and NK cells) was similar in wild-type and IFN-αβR–/– cells whether they had been cultured in the presence [closed and open triangles (range 5–50/106 cells)] or absence [closed and open circles (range 5–10/106 cells)] of additional BCG. After 21 days, the mean frequency of IFN-γ-expressing lung cells following re-stimulation with BCG increased but remained statistically similar (P ≥ 0·05) in both groups of mice (624 ± 247/106 in wild-type and 700 ± 70/106 in IFN-αβR–/– mice). Thus, total lung cell IFN-γ expression further correlated with flow cytometry data, revealing similar expression of activation markers on CD4+ and NK cells in wild-type and IFN-αβR–/– mice (Fig. 2a–h). In addition, despite enhanced growth of BCG in IFN-αβR–/– mice, we did not detect significant differences in TNF-α, IL-10, IL-12 (p40 and p70) or IFN-γ expression in BAL fluids or lung cell culture supernatants prepared from IFN-αβR–/– mice (data not shown). Thus, although lung CFUs were higher in IFN-αβR–/– mice during early infection, sufficient T-cell and NK-cell activation and IFN-γ production developed and probably contributed to control of BCG growth similar to that observed in wild-type mice.
Figure 3.
IFN-αβ receptor expression is not required for development of antigen-specific IFN-γ production by lung cells during M. bovis BCG infection. Mice were infected by aerosol with BCG (4087 ± 1102 CFU) and lung cells prepared 1–6 weeks after infection. IFN-γ production by total lung was measured by Elispot. Lung cells from wild-type mice (•, ▴) and IFN-αβR–/– mice (○, ▵) were incubated without (•, ○) or with additional BCG bacilli (moi = 1, ▴, ▵) for 48 hr. Cellular IFN-γ production was determined by Elispot. Mean spot number per 106 lung cells from three mice is shown at each time-point for a representative experiment (n = 3). No significant differences in IFN-γ spot forming units were detected (using Student's t-test) in three independent experiments with or without BCG restimulation in vitro. Fewer than 5–10 spots per 106 lung cells were detected from uninfected mice. Positive control responses to concanavalin A (4 μg/ml) were always too numerous to count.
Decreased nitric oxide expression is associated with enhanced BCG growth in IFN-αβR–/– mice
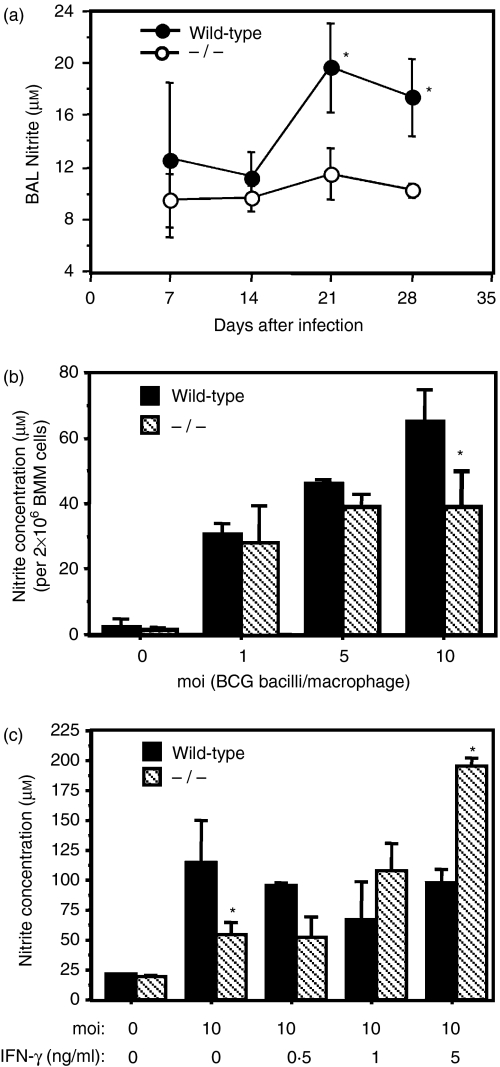
Although nitric-oxide-dependent killing of mycobacteria by macrophages is important for controlling mycobacterial growth after adaptive immunity develops, it is unclear if NOS-2 expression is important during early pulmonary mycobacterial infection.7,16,39 Since IFN-αβ induces nitric oxide production, we infected wild-type and IFN-αβR–/– mice with BCG and measured nitrite expression in BAL and lung cell culture supernatants during early infection (2–4 weeks). As shown in Fig. 4(a), local bronchoalveolar production of nitrite was significantly lower in IFN-αβR–/– mice. Since significantly higher numbers of bronchoalveolar macrophages/monocytes were detected in IFN-αβR–/– mice (178 333 ± 28 915) compared to wild-type mice (80 000 ± 10 408, P ≤ 0·05), decreased BAL nitrite levels could not be attributed to defective alveolar macrophage/monocyte recruitment in BCG-infected IFN-αβR–/– mice. However, we could not exclude a defect in BAL nitrite expressed by respiratory epithelial cells.40 In wild-type mice, maximal nitrite production occurred when both BAL CFUs and BAL cell numbers were maximal (Fig. 1b). However, despite the two- to threefold higher CFU burden in IFN-αβR–/– mice (Fig. 1), BAL nitrite levels remained low (Fig. 4a). In contrast, low levels of nitrite in lung cell culture supernatants were measured at the lower limit of detection (6–10 μm using the Griess reagent) and therefore we were unable to definitively rule out differences in nitrite expression by parenchymal lung cells isolated from wild-type and IFN-αβR–/– mice (data not shown). The low levels of nitrite in lung cell cultures may also have reflected the lower proportion of macrophages present in the lung cell homogenates compared to BAL where the bronchoalveolar macrophages represent the predominant cell type (65–95%) during BCG infection.41 Thus, high levels of nitrite in BAL fluids expressed by bronchoalveolar macrophages, recruited monocytes or respiratory epithelial cells40 may be unusually dependent upon IFN-αβ receptor coactivation during BCG infection.
Figure 4.
IFN-αβ receptor deficiency is associated with a decrease in nitric oxide expression in response to M. bovis BCG infection. (a) Wild-type (•) and IFN-αβR–/– (○) mice were infected with BCG (3000–4500 bacilli). Bronchoalveolar lavage fluids were harvested 1–4 weeks after infection and analysed for nitrite. Three mice per time point were analysed and mean levels of nitrite (± SD) are shown. The experiment is representative of three independent experiments with high-dose aerosol BCG infection. (b,c) Bone marrow-derived macrophages (2 × 106/ml) were stimulated with or without BCG in the absence (b) or presence (c) of IFN-γ at the indicated concentrations. Culture supernatants were collected after 72 hr. Nitrite concentration in triplicate cultures was measured using the Griess Reagent and the mean concentration (± SD) is shown at each time-point. The results are representative of six independent experiments. Significant differences are designated (*P ≤ 0·05, by Student's t-test).
Since the majority of bronchoalveolar cells during aerogenic BCG infection are alveolar macrophages or recruited monocytes41 we inferred that macrophages in IFN-αβR–/– mice produced less nitrite during BCG infection. Thus, we studied nitric oxide production in vitro using BMM isolated from wild-type and IFN-αβR–/– mice. BMM were infected with BCG and cumulative nitrite production in culture supernatants was measured after 72 hr. In six independent experiments, we observed significantly less nitrite production by IFN-αβR–/– macrophages as compared to wild-type cells. As shown in a representative experiment (Fig. 4b), at a MOI of 10, nitrite expression by IFN-αβR–/– BMM was 40% lower than wild-type control levels (64·9 ± 9·9 μm versus 39·1 ± 10·8 μm; P ≤ 0·05). In three independent experiments, a 53·8 ± 18·8% (mean ± SD) difference in nitrite was measured. In contrast, diminished NOS-2 activity in BCG infected IFN-αβR–/– BMM was not observed at lower MOI. These data suggest that at a low MOI (i.e. 1–5) BCG infection induces nitrite production independent of IFN-αβ expression and that at higher infection ratios (MOI 10), BCG induces higher levels of IFN-αβ expression that could augment nitric oxide production in wild-type BMM but not in IFN-αβR–/– BMM.
Since IFN-γ is expressed in the lung during BCG infection30,41 and enhances nitrite expression in BCG-infected macrophages42 we further determined if IFN-γ induced nitrite expression in infected IFN-αβR–/– BMM. As shown in Fig. 4(c), IFN-γ (5 ng/ml) significantly enhanced nitric oxide expression in BCG infected IFN-αβR–/– BMM (P ≤ 0·05). However, treatment of wild-type BMM with IFN-γ did not enhance nitrite expression that may have been maximally induced by BCG infection alone. In parallel cultures we also observed that IFN-γ alone did not significantly induce nitrite expression after 72 hr (data not shown). To further characterize the defect in NOS-2 activity, we also examined the ability of LPS to induce nitrite. Interestingly, in four experiments we determined that LPS-induced nitrite expression was significantly lower in IFN-αβR–/– BMM (7·4 ± 2·6 μm/4 × 106 cells) compared to wild-type levels (63·5 ± 11·3 μm). Furthermore, in a representative experiment (n = 3), nitrite expression in IFN-αβR–/– BMM (7·9 ± 2·6 μm) was restored to wild-type levels (102·0 ± 3·8 μm) in the presence of IFN-γ (1 ng/ml). Since both BCG- and LPS-induced nitrite expression was diminished in IFN-αβR–/– BMM and rescued by IFN-γ, we hypothesized that NOS-2 activity was partially dependent on IFN-αβ expression.
Although similar levels of IFN-γ were detected in BAL fluids of BCG-infected wild-type and IFN-αβR–/– mice (625·6 ± 113·8 pg/ml versus 792·3 ± 282·7 pg/ml, respectively, n = 5), significant BAL nitrite expression was not detected in IFN-αβR–/– mice (Fig. 4a). Thus, IFN-γ expression in vivo did not appear to enhance or rescue BAL nitrite expression in IFN-αβR–/– mice as observed in vitro for IFN-αβR–/– BMM. In contrast, similar low levels of nitrite and IFN-γ were detected in lung cell culture supernatants (data not shown) suggesting that induction of nitric oxide by interstitial lung macrophages in IFN-αβR–/– mice may have been intact. These data further suggest that different regulatory pathways may be involved in the induction of nitric oxide in bronchoalveolar and interstitial macrophages infected with mycobacteria and exposed to IFN-γ. Thus, enhancement of nitric oxide expression by bronchoalveolar macrophages exposed to IFN-γ may be more dependent on IFN-αβ signalling compared to BMM or dependent on other factors (e.g. cytokines) present in BAL fluids and capable of modulating nitric oxide expression by bronchoalveolar macrophages during BCG infection.
Discussion
Although originally discovered for its potent antiviral properties, IFN-αβ also plays a protective role against a variety of non-viral pathogens.43 In mice, induction of IFN-αβ occurs following intravenous exposure to mycobacterial cell wall fractions and in the lung during aerosol infection with MTB.2,44 Also, infection of human dendritic cells in vitro results in IFN-αβ production.4 Furthermore, while some success has been seen in treating multidrug-resistant tuberculosis patients with aerosolized IFN-α, in combination with standard chemotherapy, IFN-αβ administration also has been shown to enhance the virulence of a clinical isolate of MTB in mice.8,9 Thus, the functional role of IFN-αβ in the immune response to mycobacteria is not well defined. To further investigate the role of IFN-αβ in antimycobacterial defence in the lung, we compared immune responses of wild-type 129S6/SvEv and IFN-α/βR–/– mice during BCG infection.
After both low- and high-dose infections, BCG growth was minimal and readily controlled in wild-type mice. These data suggest that the wild-type 129S6/SvEv mouse strain is relatively resistant to BCG infection,34,35 controls early growth of aerosolized BCG, and clears bacteria from both bronchoalveolar and interstitial spaces. In contrast, 1–2 weeks after infection, BCG growth was greater in the lungs of IFN-αβR–/– mice. Enhanced BCG growth persisted for 2–3 weeks after peak growth, but control was eventually achieved and bacterial numbers were reduced to levels detected in wild-type mice during late infection. Thus, IFN-αβR–/– mice displayed a susceptible phenotype that was best evaluated during early high-dose BCG infection. Like BCG, early growth of virulent MTB (Erdman strain) was also detected in IFN-αβR–/– mice during the first 2–6 weeks of a low-dose aerosol infection.7 However, after 10–12 weeks, IFN-αβR–/– mice controlled the growth of virulent MTB and maintained a similar chronic high level infection as seen in wild-type mice. Thus, although IFN-αβ appears to partially limit early growth of mycobacteria in the lung, IFN-αβ is not essential for either the clearance of BCG or containment of high MTB burdens present during late infection.7
The enhanced early growth of BCG suggested a possible defect in IFN-αβ-dependent innate immunity. Since IFN-αβ recruits and activates NK cells, which can kill human macrophages infected with MTB,26 we investigated whether enhanced early growth of BCG in IFN-αβR–/– mice was associated with a defect in NK-cell-mediated immunity. Contrasting studies have suggested both essential and non-essential roles for NK cells in mice infected with M. avium.27–29 Furthermore, although depletion of NK cells with polyclonal anti-asialo-GM1 antibody does not affect MTB growth in the lungs of C57BL/6 mice,24 DX5-expressing NK cells remain after this depletion, leaving the possibility that specific subsets of NK cells could still play a role.27 However, we found no significant difference in pulmonary DX5+ CD3– NK-cell number or activation as determined by expression of CD69 or TRAIL.38 In addition, we found no significant differences in the numbers or activation status of DX5+ CD3– NK-cell subsets expressing the phenotypic markers Ly49D or CD94 (data not shown). These observations suggest that an NK-cell defect does not account for the increased growth of BCG in IFN-αβR–/– mice.
Since IFN-αβ facilitates dendritic cell maturation, which is critical for development of T-cell responses, we also hypothesized that a defect or delay in adaptive immunity would be detected in IFN-αβR–/– mice during BCG infection. However, we found no differences in either the number or activation status (CD69 or CD25 expression) of CD4+ T cells in the lung after infection. Furthermore, as determined by ELISPOT analysis, the frequency of BCG-specific IFN-γ expression was not different in lung cells (including NK and T cells) of IFN-αβR–/– mice. Thus, T-cell- and NK-cell-mediated immunity appeared intact in IFN-αβR–/– mice and probably resulted in resolution of BCG infection 2–3 weeks after peak growth. Despite the importance of IFN-αβ in Toll-like-receptor-mediated dendritic cell maturation, T-cell responses appear to develop through IFN-αβ-independent mechanisms in mice during pulmonary BCG infection. Interestingly, the magnitude of the T-cell responses was identical in the wild-type mice that displayed a resistant phenotype and restricted BCG growth from the beginning of infection. This suggests that T-cell responses in the IFN-αβR–/– mice are not driven directly by enhanced early bacterial growth, and that similar T-cell responses are capable of controlling a range of bacterial growth in either wild-type or IFN-αβR–/– mice.
Since mycobacterium-specific adaptive immunity appears intact in IFN-αβR–/– mice infected with BCG or MTB7 a defect in innate macrophage immunity could explain the enhanced mycobacterial growth during early infection. IFN-αβ activates NOS-2 in macrophages12,14 and this has been shown to be essential for immune responses to dermal Leishmania major infection.45 Similarly, in vitro studies have confirmed that IFN-αβ is required for NOS-2 induction by L. major or LPS.11,46,47 We found decreased nitrite expression in the BAL fluid of BCG-infected IFN-αβR–/– mice and BMM infected in vitro with BCG. These findings indicate that, as for L. major-induced NOS-2, IFN-αβ plays a role in activation of NOS-2 by macrophages infected with BCG. Alternatively, it has been shown that constitutive IFN-αβ signalling is required for a rapid and normal responses to IFN-γ.48 However, we determined that IFN-γ enhanced nitrite expression by BCG-infected IFN-αβR–/– BMM, indicating that IFN-γ responsiveness is intact. Although IFN-γ was expressed in the BAL during BCG infection, BAL nitrite levels remained low in IFN-αβR–/– mice. Thus, although IFN-γ rescued nitrite expression by IFN-αβR–/– BMM in vitro, our findings suggest that IFN-γ levels in vivo are unable to rescue in vivo nitrite expression within bronchoalveolar spaces. Whether these data reflect inhibitory actions of substances in the BAL or differences in IFN-γ receptor expression and responsiveness by different subsets of murine lung macrophages is not known. Furthermore, although human alveolar macrophages appear to produce more nitric oxide in response to MTB (H37Ra) infection than peripheral monocytes,14 it is not known if the effect is dependent on IFN-αβ. Enhanced expression of nitrite has been described for BMM derived from other BcgR mice, but a link between BCG resistance and IFN-αβ expression has not been previously described.49 Since induction of nitric oxide in MTB-infected J774 cells also was significantly enhanced by IFN-γ42 different macrophage populations appear to have distinct pathways for NOS-2 activation.
In this study we found that IFN-αβR–/– mice are more susceptible to early BCG growth and that this enhanced growth correlates with decreased BAL nitrite expression. Conversely, the relative resistance of wild-type mice correlates with higher levels of bronchoalveolar nitrite. However, the effect is transient and BCG is cleared, probably by adaptive T-cell responses. This study suggests that although not required for development of mycobacterium-specific adaptive immunity in the lung, IFN-αβ plays a role in the early control of BCG growth by augmenting production of nitric oxide.
Acknowledgments
This work was supported by National Institutes of Health Grants K8-HL04299 (to S.A.F.), AI47255 and AI34343 (to C.V.H.), and HL55967 (to W.H.B.).
Abbreviations
- BAL
bronchoalveolar lavage
- BCG
Mycobacterium bovis bacillus Calmette–Guérin
- BMM
bone marrow-derived macrophages
- IFN-αβR–/–
interferon-αβ-receptor-deficient
- MTB
Mycobacterium tuberculosis
- NK
natural killer
- NOS-2
nitric oxide synthase-2
- PBS
phosphate-buffered saline
References
- 1.Giacomini E, Iona E, Ferroni L, Miettinen M, Fattorini L, Orefici G, Julkunen I, Coccia EM. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J Immunol. 2001;166:7033–41. doi: 10.4049/jimmunol.166.12.7033. [DOI] [PubMed] [Google Scholar]
- 2.Manca C, Tsenova L, Bergtold A, et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-α/β. Proc Natl Acad Sci USA. 2001;98:5752–7. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Remoli ME, Giacomini E, Lutfalla G, et al. Selective expression of type I IFN genes in human dendritic cells infected with Mycobacterium tuberculosis. J Immunol. 2002;169:366–74. doi: 10.4049/jimmunol.169.1.366. [DOI] [PubMed] [Google Scholar]
- 4.Bouchonnet F, Boechat N, Bonay M, Hance AJ. Alpha/beta interferon impairs the ability of human macrophages to control growth of Mycobacterium bovis BCG. Infect Immun. 2002;70:3020–5. doi: 10.1128/IAI.70.6.3020-3025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prabhakar S, Qiao Y, Hoshino Y, Weiden M, Canova A, Giacomini E, Coccia E, Pine R. Inhibition of response to alpha interferon by Mycobacterium tuberculosis. Infect Immun. 2003;71:2487–97. doi: 10.1128/IAI.71.5.2487-2497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariotti S, Teloni R, Iona E, Fattorini L, Romagnoli G, Gagliardi MC, Orefici G, Nisini R. Mycobacterium tuberculosis diverts alpha interferon-induced monocyte differentiation from dendritic cells into immunoprivileged macrophage-like host cells. Infect Immun. 2004;72:4385–92. doi: 10.1128/IAI.72.8.4385-4392.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper AM, Pearl JE, Brooks JV, Ehlers S, Orme IM. Expression of the nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect Immun. 2000;68:6879–82. doi: 10.1128/iai.68.12.6879-6882.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giosue S, Casarini M, Alemanno L, et al. Effects of aerosolized interferon-α in patients with pulmonary tuberculosis. Am J Respir Crit Care Med. 1998;158:1156–62. doi: 10.1164/ajrccm.158.4.9803065. [DOI] [PubMed] [Google Scholar]
- 9.Giosue S, Casarini M, Ameglio F, Zangrilli P, Palla M, Altieri AM, Bisetti A. Aerosolized interferon-α treatment in patients with multi-drug-resistant pulmonary tuberculosis. Eur Cytokine Netw. 2000;11:99–104. [PubMed] [Google Scholar]
- 10.Palmero D, Eiguchi K, Rendo P, Castro Zorrilla L, Abbate E, Gonzalez Montaner LJ. Phase II trial of recombinant interferon-α2b in patients with advanced intractable multidrug-resistant pulmonary tuberculosis: long-term follow-up. Int J Tuberc Lung Dis. 1999;3:214–18. [PubMed] [Google Scholar]
- 11.Jacobs AT, Ignarro LJ. Lipopolysaccharide-induced expression of interferon-β mediates the timing of inducible nitric-oxide synthase induction in RAW 264.7 macrophages. J Biol Chem. 2001;276:47950–7. doi: 10.1074/jbc.M106639200. [DOI] [PubMed] [Google Scholar]
- 12.Hilkens CMU, Schlaak JF, Kerr IM. Differential responses to IFN-α subtypes in human T cells and dendritic cells. J Immunol. 2003;171:5255–63. doi: 10.4049/jimmunol.171.10.5255. [DOI] [PubMed] [Google Scholar]
- 13.Nozaki Y, Hasegawa Y, Ichiyama S, Nakashima I, Shimokata K. Mechanism of nitric oxide-dependent killing of Mycobacterium bovis BCG in human alveolar macrophages. Infect Immun. 1997;65:3644–7. doi: 10.1128/iai.65.9.3644-3647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rich EA, Torres M, Sada E, Finegan CK, Hamilton BD, Toossi Z. Mycobacterium tuberculosis (MTB) -stimulated production of nitric oxide by human alveolar macrophages and relationship of nitric oxide production to growth inhibition of MTB. Tub Lung Dis. 1997;78:247–55. doi: 10.1016/s0962-8479(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 15.Chan ED, Morris KR, Belisle JT, Hill P, Remigio LK, Brennan PJ, Riches DW. Induction of inducible nitric oxide synthase-NO* by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-kappaB signaling pathways. Infect Immun. 2001;69:2001–10. doi: 10.1128/IAI.69.4.2001-2010.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 17.Jung YJ, LaCourse R, Ryan L, North RJ. Virulent but not avirulent Mycobacterium tuberculosis can evade the growth inhibitory action of a T helper 1-dependent, nitric oxide synthase 2-independent defense in mice. J Exp Med. 2002;196:991–8. doi: 10.1084/jem.20021186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia I, Guler R, Vesin D, Olleros ML, Vassalli P, Chvatchko Y, Jacobs M, Ryffel B. Lethal Mycobacterium bovis Bacillus Calmette–Guérin infection in nitric oxide synthase 2-deficient mice: cell-mediated immunity requires nitric oxide synthase. Laboratory Invest. 2000;80:1385–97. doi: 10.1038/labinvest.3780146. [DOI] [PubMed] [Google Scholar]
- 19.Sun S, Zhang X, Tough DF, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J Exp Med. 1998;188:2335–42. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biron CA. Interferons α and β as immune regulators – a new look. Immunity. 2001;14:661–4. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- 21.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 22.Saxena RK, Weissman D, Saxena QB, Simpson J, Lewis DM. Kinetics of changes in lymphocyte sub-populations in mouse lungs after intrapulmonary infection with M. bovis (Bacillus Calmette–Guérin) and identity of cells responsible for IFNγ responses. Clin Exp Immunol. 2002;128:405–10. doi: 10.1046/j.1365-2249.2002.01839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umemura M, Nishimura H, Hirose K, Matsuguchi T, Yoshikai Y. Overexpression of IL-15 in vivo enhances protection against Mycobacterium bovis bacillus Calmette–Guérin infection via augmentation of NK and T cytotoxic 1 responses. J Immunol. 2001;167:946–56. doi: 10.4049/jimmunol.167.2.946. [DOI] [PubMed] [Google Scholar]
- 24.Junqueira-Kipnis AP, Kipnis A, Jamieson A, Juarrero MG, Diefenbach A, Raulet DH, Turner J, Orme IM. NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. J Immunol. 2003;171:6039–45. doi: 10.4049/jimmunol.171.11.6039. [DOI] [PubMed] [Google Scholar]
- 25.Lande R, Giacomini E, Grassi T, Remoli ME, Iona E, Miettinen M, Julkunen I, Coccia EM. IFN-αβ released by Mycobacterium tuberculosis-infected human dendritic cells induces the expression of CXCL10: selective recruitment of NK and activated T cells. J Immunol. 2003;170:1174–82. doi: 10.4049/jimmunol.170.3.1174. [DOI] [PubMed] [Google Scholar]
- 26.Brill KJ, Li Q, Larkin R, Canaday DH, Kaplan DR, Boom WH, Silver RF. Human natural killer cells mediate killing of intracellular Mycobacterium tuberculosis H37Rv via granule-independent mechanisms. Infect Immun. 2001;69:1755–65. doi: 10.1128/IAI.69.3.1755-1765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Florido M, Correia-Neves M, Cooper AM, Appelberg R. The cytolytic activity of natural killer cells is not involved in the restriction of Mycobacterium avium growth. Int Immunol. 2003;15:895–901. doi: 10.1093/intimm/dxg089. [DOI] [PubMed] [Google Scholar]
- 28.Harshan KV, Gangadharam PR. In vivo depletion of natural killer cell activity leads to enhanced multiplication of Mycobacterium avium complex in mice. Infect Immun. 1991;59:2818–21. doi: 10.1128/iai.59.8.2818-2821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saunders BM, Cheers C. Intranasal infection of beige mice with Mycobacterium avium complex: role of neutrophils and natural killer cells. Infect Immun. 1996;64:4236–41. doi: 10.1128/iai.64.10.4236-4241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fulton SA, Martin TD, Redline RW, Boom WH. Pulmonary immune responses during primary Mycobacterium bovis-Calmette–Guérin bacillus infection in C57BL/6 mice. Am J Respir Cell Mol Biol. 2000;22:333–43. doi: 10.1165/ajrcmb.22.3.3776. [DOI] [PubMed] [Google Scholar]
- 31.Noss EH, Harding CV, Boom WH. Mycobacterium tuberculosis inhibits MHC class II antigen processing in murine bone marrow macrophages. Cell Immunol. 2000;201:63–74. doi: 10.1006/cimm.2000.1633. [DOI] [PubMed] [Google Scholar]
- 32.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–21. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 33.Malo D, Vogan K, Vidal S, et al. Haplotype mapping and sequence analysis of the mouse Nramp gene predict susceptibility to infection with intracellular parasites. Genomics. 1994;23:51–61. doi: 10.1006/geno.1994.1458. [DOI] [PubMed] [Google Scholar]
- 34.Vidal S, Malo D, Vogan K, Skamene E, Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993;73:469–85. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- 35.Pelletier M, Forget A, Bourassa D, Gros P, Skamene E. Immunopathology of BCG infection in genetically resistant and susceptible mouse strains. J Immunol. 1982;129:2179–85. [PubMed] [Google Scholar]
- 36.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 37.Lee CK, Rao DT, Gertner R, Gimeno R, Frey AB, Levy DE. Distinct requirements for IFNs and STAT1 in NK cell function. J Immunol. 2000;165:3571–7. doi: 10.4049/jimmunol.165.7.3571. [DOI] [PubMed] [Google Scholar]
- 38.Sato K, Hida S, Takayanagi H, et al. Antiviral response by natural killer cells through TRAIL gene induction by IFN-α/β. Eur J Immunol. 2001;31:3138–46. doi: 10.1002/1521-4141(200111)31:11<3138::aid-immu3138>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 39.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–8. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roy S, Sharma S, Sharma M, Aggarwal R, Bose M. Induction of nitric oxide release from the human alveolar epithelial cell line A549: an in vitro correlate of innate immune response to Mycobacterium tuberculosis. Immunology. 2004;112:471–80. doi: 10.1046/j.1365-2567.2004.01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fulton SA, Reba SM, Martin TD, Boom WH. Neutrophil-mediated mycobactericidal immunity in the lung during Mycobacterium bovis BCG infection in C57BL/6 mice. Infect Immun. 2002;70:5322–7. doi: 10.1128/IAI.70.9.5322-5327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peteroy-Kelly M, Venketaraman V, Connell ND. Effects of Mycobacterium bovis BCG infection on regulation of l-arginine uptake and synthesis of reactive nitrogen intermediates in J774.1 murine macrophages. Infect Immun. 2001;69:5823–31. doi: 10.1128/IAI.69.9.5823-5831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogdan C. The function of type I interferons in antimicrobial immunity. Curr Opin Immunol. 2000;12:419–24. doi: 10.1016/s0952-7915(00)00111-4. [DOI] [PubMed] [Google Scholar]
- 44.Nakane A, Minagawa T. Induction of alpha and beta interferons during the hyporeactive state of gamma interferon by Mycobacterium bovis BCG cell wall fraction in Mycobacterium bovis BCG-sensitized mice. Infect Immun. 1982;36:966–70. doi: 10.1128/iai.36.3.966-970.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diefenbach A, Schindler H, Donhauser N, et al. Type 1 interferon (IFNα/β) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity. 1998;8:77–87. doi: 10.1016/s1074-7613(00)80460-4. [DOI] [PubMed] [Google Scholar]
- 46.Vadiveloo PK, Vairo G, Hertzog P, Kola I, Hamilton JA. Role of type I interferons during macrophage activation by lipopolysaccharide. Cytokine. 2000;12:1639–46. doi: 10.1006/cyto.2000.0766. [DOI] [PubMed] [Google Scholar]
- 47.Mattner J, Schindler H, Diefenbach A, Rollinghoff M, Gresser I, Bogdan C. Regulation of type 2 nitric oxide synthase by type 1 interferons in macrophages infected with Leishmania major. Eur J Immunol. 2000;30:2257–67. doi: 10.1002/1521-4141(2000)30:8<2257::AID-IMMU2257>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 48.Takaoka A, Mitani Y, Suemori H, Sato M, Yokochi T, Noguchi S, Tanaka N, Taniguchi T. Cross talk between interferon-γ and -α/β signaling components in caveolar membrane domains. Science. 2000;288:2357–60. doi: 10.1126/science.288.5475.2357. [DOI] [PubMed] [Google Scholar]
- 49.Barrera LF, Kramnik I, Skamene E, Radzioch D. Nitrite production by macrophages derived from BCG-resistant and -susceptible congenic mouse strains in response to IFN-gamma and infection with BCG. Immunology. 1994;82:457–64. [PMC free article] [PubMed] [Google Scholar]