Abstract
Myasthenia gravis (MG) and experimental autoimmune MG (EAMG) are T cell-dependent, antibody-mediated autoimmune diseases. A dual altered peptide ligand (APL) that is composed of the tandemly arranged two single amino acid analogues of two myasthenogenic peptides, p195–212 and p259–271, was demonstrated to down-regulate in vitro and in vivo MG-associated autoreactive responses. The aims of this study were to investigate the possible role of Fas–FasL-mediated apoptosis in the down-regulatory mechanism of the dual APL. We demonstrate here the effect of the dual APL on expression of key molecules involved in the Fas–FasL pathway, in a p195–212-specific T cell line, in mice immunized with Torpedo acetylcholine receptor and in mice afflicted with EAMG (induced with the latter). In vitro and in vivo results show that the dual APL up-regulated expression of Fas and FasL on the CD4 cells. Expression of the pro-apoptotic molecules, caspase 8 and caspase 3, was significantly up-regulated, while anti-apoptotic cFLIP and Bcl-2 were down-regulated upon treatment with the dual APL. The dual APL also increased phosphorylation of the mitogen-activated protein kinases, c-Jun-NH2-terminal kinase and p-38, known to play a role in the regulation of FasL expression. Further, in the T cell line incubated with the dual APL as well as in mice of the SJL inbred strain immunized with the myasthenogenic peptide and treated concomitantly with the dual APL, the percentage of apoptotic cells increased. Results strongly indicate that up-regulation of apoptosis via the Fas–FasL pathway is one of the mechanisms by which the dual APL reverses EAMG manifestations in C57BL/6 mice.
Keywords: apoptosis, dual APL, experimental autoimmune myasthenia gravis (EAMG), Fas–FasL, immunosuppression
Introduction
Myasthenia gravis (MG) and its experimental animal model, experimental autoimmune myasthenia gravis (EAMG), are T cell-dependent autoimmune disorders characterized by muscular weakness and excessive fatigability due to antibodies against acetylcholine receptor (AChR) of skeletal muscles that cause decreased functional efficiency of the neuromuscular junction.1,2 It was demonstrated previously by this laboratory that two sequences of the α-subunit of the human AChR, namely, peptides p195–212 and p259–271, discriminated significantly between MG patients and healthy controls based on proliferation of peripheral blood lymphocytes.3 Furthermore, peripheral blood lymphocytes of seronegative MG patients responded by either proliferation or interleukin-2 (IL-2) secretion to the peptides emphasizing the importance of T cells in MG.4 The two peptides were found to be immunodominant T cell epitopes in SJL (p195–212) and BALB/c (p259–271) inbred mice.5 T cell lines specific to p195–212 and p259–271 that were inoculated into SJL and BALB/c mice, respectively, were found to be pathogenic and to induce EAMG manifested by the presence of anti-self AChR antibodies and compound muscle action — potential decrements.6
A dual altered peptide ligand (APL) composed of the tandemly arranged two single amino acid substituted analogues, designated Lys-262-Ala-207, was demonstrated to inhibit efficiently the proliferation of T cell lines specific to the myasthenogenic peptides and of lymph node cells that were primed in vivo to either of the myasthenogenic peptides.7 Most importantly, the dual APL, when administered orally, could reverse EAMG manifestations, either induced (in BALB/c mice) by the p195–212-specific T cell line7 or by immunization (of C57BL/6 mice) with the multideterminant Torpedo AChR (TAChR)8 as assessed by the clinical score, grip strength and electromyography. The inhibitory activity of the dual APL was demonstrated to be associated with a down-regulation of the Th1 type cytokines, namely, IL-2 and especially interferon-gamma (IFN-γ), which is known to be pathogenic in MG and EAMG and an up-regulation of the immunosuppressive Th3 cytokine, transforming growth factor-β (TGF-β).9 In addition, Faber-Elmann et al.10,11 showed that the dual APL acts as a partial agonist by inhibiting phospholipase C (PLC) activity induced by myasthenogenic T cell epitopes and immunomodulating T cell migration ability. Splenocytes of mice administered with the dual APL inhibited significantly and specifically the proliferation to Torpedo AChR,9 indicating that the suppressor activity of the dual APL can be transferred by cells. The dual APL was shown to induce CD4+ CD25+ T regulatory cells12 that are known to play a critical role in the maintenance of peripheral tolerance.13,14 These cells were shown further to express CD45Rblow, CTLA-4, intracellular TGF-β and forkhead box p3 (Foxp3)15,16 markers that are characteristic to the CD4+ CD25+ regulatory cell lineage.14
Down-regulation of T cell responses to the myasthenogenic peptide p195–212 was shown further to be correlated with an up-regulation in c-Jun-NH2-terminal kinase (JNK) activity and an elevation in Fas- and Fas ligand (FasL)-expressing cells in the dual APL-treated mice.16 Interaction between the Fas and FasL molecules provides the key signal to maintaining apoptosis and Fas–FasL-mediated apoptosis, or activation-induced cell death (AICD) of antigen-specific T cells, is a major mechanism of peripheral tolerance induction and of immune homeostasis.17 The importance of the Fas death pathway in immune homeostasis is illustrated by the massive accumulation of T cells and the development of systemic autoimmunity in mice and humans deficient in Fas and FasL.18 In the simplest scheme understood so far, binding of FasL to Fas induces trimerization of the Fas receptor, and Fas-associated death domain (FADD) binds to the trimerized Fas cytoplasmic region. Procaspase 8 is then recruited to FADD and this death-inducing signalling complex activates procaspase 8 (FLICE). The active caspase 8 then cleaves and activates the downstream caspase 3 resulting in characteristic apoptosis.17 Two subfamily members of the mitogen-activated protein kinase (MAPK) family, c-Jun-NH2-terminal kinase (JNK) and p-38 MAPK have also been implicated in apoptosis, as they commit T cells to apoptosis through the regulation of FasL expression.19 The whole process of apoptosis is regulated tightly by a number of gene products that block (such as Bcl-2 and cFLIP) cell death at different stages.17
Apoptosis mediated via the Fas–FasL pathway serves as a defence against autoimmunity by deleting autoreactive cells in the periphery.17 Because the progress of MG and development of advanced stages has been attributed to a down-regulation of apoptosis,20–22 in the present study we investigated the possible role of the Fas–FasL-mediated pathway in the dual APL-induced suppression of myasthenogenic-associated T cell responses. To this end we utilized a T cell line specific for the myasthenogenic peptide, p195–212, as well as C57BL/6 mice immunized with the whole macromolecule of Torpedo AChR and mice afflicted with EAMG induced by the latter. We demonstrate here the effect of the dual APL on the expression of key apoptotic molecules involved in the Fas–FasL-mediated pathway and most importantly, we show that the Fas–FasL pathway is one of the mechanisms by which the dual APL down-regulates an already established EAMG in C57BL/6 mice.
Materials and methods
Mice
SJL (The Jackson Laboratory, Bar Harbor, Maine, ME) and C57BL/6 (Harlan Breeders, Indianapolis, IN) female mice were used at the age of 8–12 weeks. The study was approved by the Animal Care and Use Committee of The Weizmann Institute of Science.
TAChR and peptide analogues
AChR was purified from Torpedo californica as described23 and was used for immunizations and in vitro studies. Peptide p195–212 (DTPYLDITYHFVMQRLPL) was synthesized and characterized as described.7 The dual APL, Lys-262-Ala-207 (VIVKLIPSTSSAVDTPYLDITYHFVAQRLPL) was synthesized (97% purity) by UCB-Bioproducts (Brussels, Belgium). A peptide synthesized in the reversed order (reversed peptide) of the dual APL (LPLRQAVFHYTIDLYPTDVASSTSPILKVIV) was used as control.7
Establishment of a p195–212-specific T cell line
Lymph node (LN) cells were harvested from SJL mice 10 days after their immunization with p195–212 [10 µg per mouse in complete Freund's adjuvant (CFA; Difco, Detroit, MI)]. The LN cells (5 × 107) were cultured in 25-ml flasks in 5 ml of enriched RPMI-1640 medium supplemented with 1% normal mouse serum and 5 µg/ml p195–212. After 4 days of incubation, cells were washed and resuspended in 5 ml of enriched RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) and 2 ng/ml of recombinant murine IL-2 (PeproTech, Rocky Hill, NJ). Cells were exposed to the stimulating peptide (5 µg/ml) presented on irradiated (3000 rad) syngeneic spleen cells every 14 days24 and were maintained between stimulations in enriched medium containing IL-2. For all the experiments, cells of the line were harvested 7 days after antigenic stimulation and stimulated with the peptide with or without the dual or the reversed dual APL, for 24 hr in the presence of irradiated syngeneic spleen cells as antigen-presenting cells (APC).
Immunization of mice and in vivo inhibition of priming of LN cells by the dual APL
SJL mice or C57BL/6 mice were injected with 10 µg per mouse of p195–212 or Torpedo AChR (TAChR), respectively, in CFA (Difco) and popliteal LN cells were harvested 10 days later. For in vivo inhibition of priming, the dual APL or its reversed form (used as a specificity control) was administered subcutaneously [(s.c.), 200 µg/0·2 ml phosphate-buffered saline (PBS)], concomitant with the immunization.
Induction of EAMG and treatment of mice
C57BL/6 mice were immunized and boosted 1 month later with 20 µg per mouse TAChR in CFA enriched with 2·5 mg/ml Mycobacterium tuberculosis H37 Ra (Difco). A second group was injected with an emulsion of CFA/PBS. The mice were observed for clinical manifestations on a daily basis and graded as follows: grade 0, no definite muscular weakness after exercise; grade 1, moderate muscular weakness after exercise; grade 2, weakness at rest; and grade 3, severe muscle weakness, paralysis, dehydration, moribund. Mice that showed clinical manifestations of EAMG were divided randomly into two groups. One group was treated with the dual APL twice a week (200 µg/mouse) and the second group was given the vehicle, PBS. The mice were treated for a period of 4 weeks.
Antibodies
The following antibodies were used in the study: anti-CD4-phycoerythrin (PE) (GK1·5), anti-CD25/IL-2R-fluorescein isothiocyanate (FITC) (7D4), and their matched isotype controls (Southern Biotechnology Associates, Birmingham, AL); hamster anti-mouse-Fas-PE (Jo2), hamster anti-mouse CD178 (FasL, CD95 ligand)-PE (MFL3) and rat anti-mouse CD4-allophycocyanin (L3T3) antibody (clone 1B8) and their matched isotype controls (Pharmingen, San Diego, CA); annexin V/FITC and propidium iodide (PI)-PE (IQ Products, Groningen, The Netherlands); anti-JNK2 (Serotec, Raleigh, NC) and anti-phosphorylated-JNK, p-JNK (Serotec), anti-phosphorylated p38 (p-p38, Thr180/Tyr182, sc-17852-R) and anti-p38 (C-20) (Santa Cruz Biotechnology, Santa Cruz, CA), anti-rabbit IgG-horseradish peroxidase and anti-mouse IgG-horseradish peroxidase (Jackson ImmunoResearch Laboratories, West Grove, PA).
Fluorescence staining of LN cells
Cells (1 × 106) from LN of tested mice or cells of the p195–212-specific T line (5 × 105) were washed with 10% FCS in PBS, incubated with the relevant antibody, washed again and analysed by fluorescence activated cell sorter (FACS; Becton Dickinson, Franklin Lakes, NY). For intracellular staining, cells were first incubated with a fixation solution, washed and resuspended in permeabilization solution (Serotec) in the presence of their respective antibodies.
Preparation of cell lysates and Western blot analysis
Cells (50 × 106) from LN of mice or peptide-specific T cells (5 × 106) were incubated in lysis buffer, pH 7·2 [containing 50 mm Hepes, 150 mm NaCl, 1·5 mm MgCl2, 1 mm ethyleneglycol tetraacetic acid (EGTA), 1% ethylenediamine tetraacetic acid (EDTA), 1% Triton X-100, 10% glycerol, 1 mm Na-orthovanadate, 30 mm Na-pyrophosphate, 1 mm phenylmethylsulphonyl fluoride (PMSF), 10 µg/ml leupeptin and 10 µg/ml aprotinin] for 10 min on ice. Supernatants were collected and protein concentrations were determined by Bradford reagent (Bio-Rad, Hercules, CA). Lysates were boiled in sample buffer and equal amounts of protein were separated on 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membrane. Following blocking, the membrane was reacted with the relevant antibody and further with the secondary antibody coupled to horseradish peroxidase (HRP). Detection was carried out by the enhanced chemiluminescence method. Protein expression was determined by photodensitometry using the National Institutes of Health image program.
Determination of mRNA levels
Levels of mRNA were analysed by quantitative real time reverse transcription–polymerase chain reaction (RT–PCR) using LightCycler (Roche, Mannheim, Germany). Reverse transcription into complementary DNA was performed using Molony murine leukemia virus reverse transcriptase (Promega, Madison, WI). Real time PCR was performed according to the manufacturer's instructions. Briefly, 20 µl of reaction volume contained 3 mm MgCl2, LightCycler HotStart DNA SYBR Green I mix (Roche), specific primer pairs and 5 µl of cDNA. PCR cycling conditions were 10 min at 95° followed by 45 cycles at 95° for 15 seconds, 60° for 15 seconds and 72° for 15 seconds. The following primers, forward and reverse, were used: caspase 8, 5′-ACATAACCCAACTCCGAA-3′ and 5′-GTGGGATAGGATACAGCAGA-3′; caspase 3, 5′-TCTCGCTCTGGTACGG-3′ and 5′-GGCAGTAGTCGCCTCT-3′; cFLIP, 5′-GTTCGCCGATATGCAAG-3′ and 5′-CTGGCTACCTAACGACT-3′; Bcl-2, 5′-GCATAGGTTGTCAACACT-3′ and 5′-AAGCACGATATGATGCC-3′; β-actin, 5′-GACGTTGACATCCGTAAAG-3′ and 5′-GGCCGGACTCATCGTA-3′.
Results
The dual APL up-regulates expression of Fas and FasL on cells of the p195–212-specific line
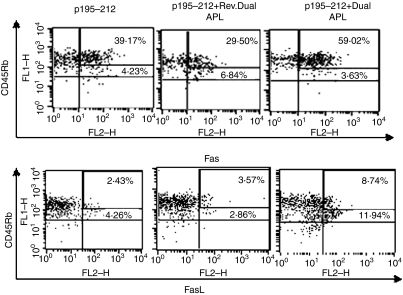
In order to determine the possible role of the Fas–FasL-mediated pathway in the mechanism of action of the dual APL we utilized a T cell line specific for p195–212 that was established from LN cells of SJL mice 10 days after their immunization with the peptide. The phenotype of the T cell line as analysed by FACS was 96% CD4+ T cells15. As the dual APL-induced CD4+ CD25+ regulatory cells were primarily the CD45Rblow cells while the CD45Rbhigh were expressed on the autoreactive cells of the p195–212-specific T cell line,15 we determined the effect of the dual APL on the expression of Fas and FasL on the CD45Rb isoforms by FACS analysis. To this end, cells of the line were stimulated with the peptide with or without the dual/reversed dual APL for 24 hr. Figure 1 shows the percentage expression of Fas and FasL on CD45Rbhigh and CD45Rblow population of cells. The cut-off settings for CD45Rb high and low cells were determined from histogram plot of CD45Rb stained cells (figure not shown) which yielded two peaks corresponding to CD45Rbhigh and CD45Rblow. As seen in Fig. 1, Fas expression on CD45Rbhigh cells of the dual APL-treated p195–212-specific T cells increased to 59·02% from 39·17% on the untreated cells or 29·5% on the reversed dual APL-treated cells. The dual APL also increased FasL expression (by about twofold) on the CD45Rbhigh when compared to the untreated cells. It is noteworthy that while the expression of Fas on the CD45Rblow cells was slightly down-regulated in the dual APL-treated cells, FasL expression was up-regulated by almost threefold when compared to the untreated or the reversed dual APL-treated cells. Levels of the latter were similar to those on the CD45Rbhigh cells. No prominent effect was observed in the reversed dual APL-treated cells.
Figure 1.
The dual altered peptide ligand (APL) up-regulates expression of Fas and FasL on p195–212-specific T cells. Cells (106 per ml) of the line were stimulated with p195–212 (5 µg per ml) with or without the dual or reversed dual APL (500 µg per ml) for 24 hr. Cells were double-stained for CD45Rb and Fas or FasL and analysed by fluorescence activated cell sorter (FACS). The results presented are after reduction of the background staining obtained with the matched isotype controls. Numbers in the upper levels correspond to CD45Rbhigh cell population and those below correspond to CD45Rb1ow cells. Shown is one of two experiments performed.
The effect of the dual APL on pro-apoptotic and anti-apoptotic molecules on cells of the T cell line
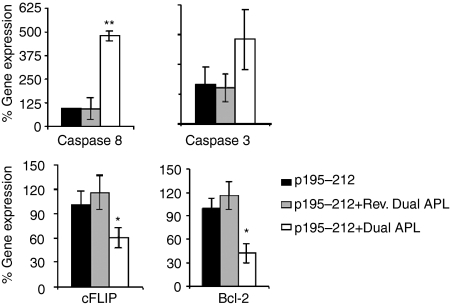
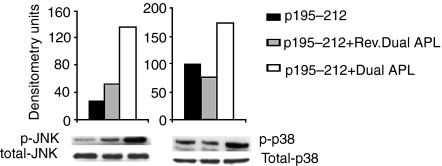
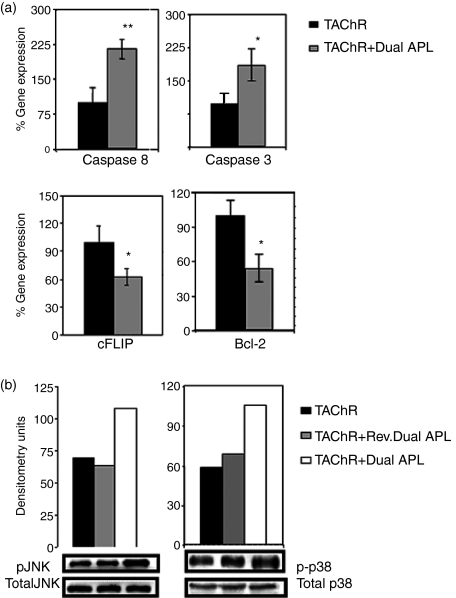
We next determined the expression of downstream key molecules of apoptosis. mRNA levels of caspase 8, caspase 3, cFLIP and Bcl-2 were determined for the treated and untreated p195–212-specific T cells of the line. To this end, total RNA was prepared from cells of the line that were stimulated with p195–212 for 24 hr with or without the dual/reversed dual APL. Levels were normalized to β-actin and presented as a percentage of the levels in untreated cells (accounted as 100%). As seen in Fig. 2, a very significant increase in caspase 8 was observed in the dual APL-treated cells. The dual APL also increased the levels of caspase 3 by about 2·5-fold when compared to the untreated or the reversed dual APL-treated cells. On the other hand, expression of the anti-apoptotic molecules, cFLIP and Bcl-2 was significantly reduced in cells treated with the dual APL when compared to the untreated T cell line. Levels of phosphorylated MAPK, reported to play a role in the regulation of Fas and FasL expression, were measured. Phosphorylated pJNK and p-38 were determined for cell lysates of the p195–212-specific T cell line stimulated with the peptide for 24 hr with or without the dual/reversed dual APL. As seen in Fig. 3, phosphorylated pJNK levels increased by more than threefold and those of p-38 increased by 1·5-fold when the line cells were incubated with the dual APL compared to the untreated cells. The control peptide did not have any effect on the cells of the line when compared to the untreated cells.
Figure 2.
Effect of the dual altered peptide ligand (APL) on the expression of apoptotic molecules by a p195–212-specific T cell line. T cells (5 × 106) of the line were stimulated with p195–212 with or without the dual or the reversed dual APL for 24 hr. Total RNA was extracted, and real time polymerase chain reaction (PCR) was performed for caspase 8, caspase 3, cFLIP and Bcl-2 as described in Materials and methods. Results are expressed as the mean percentage of the gene expression of triplicate samples ± SD values. *P < 0·05, **P < 0·0005, when gene expression in the presence of the dual APL was compared with that in the absence of the dual APL. Shown is one of two experiments performed.
Figure 3.
The dual altered peptide ligand (APL) up-regulates phosphorylation of pJNK and p-38 in p195–212-specific T cells. T cells (5 × 106) of the line were stimulated with p195–212 with or without the dual or the reversed dual APL for 24 hr. Whole-cell lysates were prepared, separated on sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to a nitrocellulose membrane that was later blotted with anti-p-JNK or anti-p-38 or their respective totals. Results are expressed as densitometry units (measured with National Institutes of Health image software). Shown is one of two experiments performed.
The dual APL up-regulates annexin V-positive cells
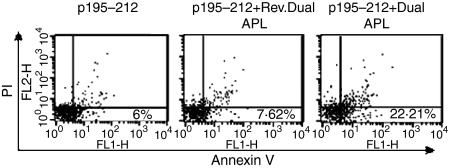
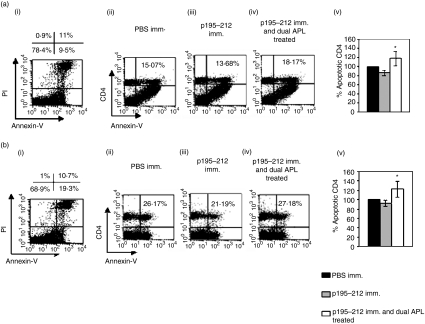
Apoptosis of cells of the p195–212-specific line was quantified by phosphatidyl serine exposure, determined by annexin V/FITC binding and FACS analysis coupled with propidium iodide staining to exclude necrotic cells. To this end, cells of the line were stimulated with the peptide for 24 hr in the presence or absence of the dual or reversed dual APL. As seen in Fig. 4, a prominent fourfold increase in levels of apoptotic cells was measured in the dual APL-treated peptide-specific T cells when compared to the untreated cells. Levels of apoptosis were also determined for splenocytes and lymph node cells of SJL mice that were either injected with PBS or p195–212 in CFA or were immunized with p195–212 and administered concomitantly with the dual APL. Because the suppressive effects of the dual APL in association with an increase in CD4+ CD25+ cells were best observed at day 10 after immunization,17 we determined the apoptotic effects that possibly correlate with the down-regulatory effect of the dual APL at the same time-point in the present study. Figure 5(aii–iv and bii–iv) demonstrates the CD4+ cells stained for annexin-v out of PI negative cells (Fig. 5a, bi). A reduction in the apoptotic CD4+ cells was determined in mice immunized with p195–212 (Fig. 5a, biii), whereas treatment with the dual APL up-regulated the population of apoptotic CD4+ cells (Fig. 5a, biv). Figure 5(a, bv) shows the mean percentages of apoptotic CD4+ cells determined in the three experiments performed (four mice per group) compared to cells of PBS immunized mice (considered 100%). It can be seen that treatment with the dual APL up-regulated significantly the apoptotic CD4+ cells.
Figure 4.
The dual altered peptide ligand (APL) up-regulates annexin V positive cells in a p195–212-specific cell line. Cells (106 per ml) of the line were stimulated with p195–212 (5 µg per ml) with or without the dual or reversed dual APL (500 µg per ml) for 24 hr. Cells were double-stained for annexin V-fluorescein isothiocyanate (FITC) and PI-PE. The results are presented after reduction of background obtained with unstained cells. Percentage of annexin V positive and PI negative cells are depicted in the lower right quadrant. Shown is one of two experiments performed.
Figure 5.
The dual altered peptide ligand (APL) up-regulates annexin V positive cells in lymph node (LN) derived CD4+ cells of SJL inbred mice immunized with p195–212. SJL mice were either immunized with p195–212 [10 µg per mouse in complete Freund's adjuvant (CFA)] and concomitantly administered subcutaneously (s.c.) with the dual APL [200 µg per mouse in phosphate-buffered saline (PBS)] or immunized with p195–212 or PBS alone. Ten days after immunization, spleen cells (a) and popliteal LN cells (b) were harvested. Cells were stained for CD4, annexin V and PI and analysed by fluorescence activated cell sorter (FACS).(a, bi) Staining for PI and annexin-V. Percentages in the upper left and right quadrants represent PI-stained necrotic cells while that in the lower right quadrant indicates annexin V positive apoptotic cells. (a, bii–iv) Percentage of CD4+ cells out of the gated annexin-V positive and PI negative cells. Shown is one of three experiments performed. (a, bv) Results are also expressed as the mean percentage of CD4 cells positive for annexin in the three experiments performed ± SD values. Percentage of apoptotic CD4+ cells of PBS immunized mice was considered as 100%. *P < 0·05 when compared with the group immunized with the peptide alone.
The effect of the dual APL on the Fas/FasL pathway in cells of mice immunized with TAChR
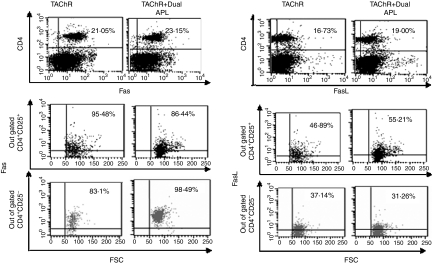
We determined the expression levels of Fas and FasL on lymph node-derived CD4+ cells of C57BL/6 mice that were immunized with TAChR and concomitantly administered with the dual APL or were immunized with TAChR alone. We have demonstrated previously that the dual APL up-regulates expression of the CD4+ CD25+ T regulatory cells in mice immunized with TAChR (Aruna et al. unpublished data). We therefore determined the expression of Fas and FasL on gated CD4+ CD25+ and CD4+ CD25− cells in C57BL/6 mice 10 days postimmunization. As seen in Fig. 6, an approximately 2–3% increase in levels of both Fas and FasL was observed on the CD4+ cells of C57BL/6 mice immunized with TAChR and treated concomitantly with the dual APL. The dual APL up-regulated Fas expression on the CD4+ CD25− cells while that on CD4+ CD25+ cells of the treated mice was slightly down-regulated when compared to the untreated mice. In contrast, while a minor down-regulation of FasL was observed on the CD4+ CD25− cells, the FasL was up-regulated on the CD4+ CD25+ cells of the dual APL-treated mice as compared to the untreated group. Further, mRNA expression levels of the downstream apoptotic molecules, caspase 8, caspase 3, cFLIP and Bcl-2 in the LN cells of the tested C57BL/6 mice was determined by real time RT–PCR and normalized to β-actin. As seen in Fig. 7(a), the dual APL significantly up-regulated the expression of the pro-apoptotic caspase 8 and caspase 3 while down-regulating the expression of the anti-apoptotic cFLIP and Bcl-2 molecules when compared to the untreated mice. We next determined the effect of the dual APL in mice immunized with the whole macromolecule of Torpedo AChR on the phosphorylation of MAP kinases by Western blot analysis of whole cell lysates. Levels of pJNK and p-p38 that have been normalized to their respective totals are shown in Fig. 7(b). It can be seen that both pJNK and p-p38 were up-regulated in the immunized and concomitantly dual APL-treated mice compared to untreated or control peptide-treated groups.
Figure 6.
The dual altered peptide ligand (APL) up-regulates Fas and FasL expression in mice immunized with Torpedo acetylcholine receptor (TAChR). C57BL/6 mice were either immunized with TAChR [10 µg per mouse in complete Freund's adjuvant (CFA)] and concomitantly administered subcutaneously (s.c.) with the dual APL/reversed dual APL [200 µg per mouse in phosphate-buffered saline (PBS)] or immunized with TAChR alone. Ten days after immunization, popliteal lymph node (LN) cells were harvested. LN cells were stained for CD4, CD25, and Fas or FasL and analysed by fluorescence activated cell sorter (FACS). The results presented are after reduction of the background staining obtained with the matched isotype controls. Shown is one of three experiments performed.
Figure 7.
Effect of the dual altered peptide ligand (APL) on the expression of apoptotic markers in lymph node (LN) cells of C57BL/6 mice immunized with Torpedo acetylcholine receptor (TAChR). C57BL/6 mice were either immunized with TAChR [10 µg per mouse in complete Freund's adjuvant (CFA)] and concomitantly administered subcutaneously (s.c.) with the dual APL/reversed dual APL [200 µg per mouse in phosphate-buffered saline (PBS)] or immunized with TAChR alone. Ten days after immunization, popliteal LN cells were harvested. (a) Total RNA was extracted from LN cells and real time polymerase chain reaction (PCR) was performed for caspase 8, caspase 3, cFLIP and Bcl-2 as described in Materials and methods. Results are expressed as the mean percentage of the gene expression of triplicates ± SD values. *P < 0·05, **P < 0·001 when gene expression in the presence of the dual APL was compared with that in the absence of the dual APL. (b) Lysates were prepared from LN cells, separated on sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane that was later blotted with anti-p-c-Jun-NH2-terminal kinase (JNK) or anti-p-38 or their respective totals. Results are expressed as densitometry units (measured with National Institutes of Health image software). Shown is one of two experiments performed.
The dual APL up-regulates Fas expression on the CD4+CD25− cells and FasL on the CD4+ CD25+ cells in C57BL/6 mice with EAMG with an increase in caspase 8 and a decrease in cFLIP expression
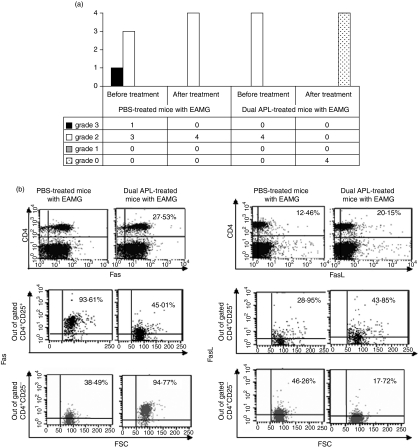
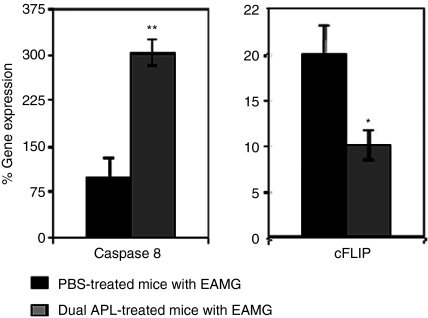
For the induction of EAMG, C57BL/6 mice were primed and boosted with the Torpedo AChR in CFA. Mice in which EAMG manifestations were determined were treated with either the dual APL (200 µg/mouse, twice a week administered s.c.) or PBS for a period of 4 weeks. At the end of treatment, LN cells were harvested from four mice of either of the treatment groups and were tested for the expression of Fas and FasL. Figure 8(a) shows the clinical score of the tested mice, before and after treatment. It can be seen that while the dual APL-treated mice did not exhibit any clinical symptoms, PBS-administered mice had clinical manifestations at the end of treatment. Figure 8(b) shows that the dual APL up-regulated the levels of both Fas and FasL on the CD4 cells of the treated mice. It was of interest to determine the expression of Fas and FasL on each of the CD4+ CD25+ and CD4+ CD25− T cell populations. Figure 8(b) also shows an analysis of Fas and FasL expression on the gated CD4+ CD25+ and CD4+ CD25− cells of the dual APL/PBS-treated C57BL/6 mice with EAMG. The dual APL markedly increased Fas expression on the CD4+ CD25− (38·5–94·8%), while that on the CD4+ CD25+ was down-regulated twofold when compared to cells of mice treated with PBS. FasL expression was increased on CD4+ CD25+ cells, while about twofold down-regulation (46·3–17·7%) was observed on cells of dual APL-treated CD4+ CD25+ mice compared to the PBS-treated mice. It is noteworthy that Fas and FasL on the CD4 CD25high population of cells exhibited the same pattern of expression as observed on the whole CD4+ CD25+ population of cells (data not shown). Expression levels of caspase 8 and cFLIP were determined using mRNA prepared from LN cells of C57BL/6 mice with EAMG, 4 weeks after treatment with either the dual APL or with PBS. Similar to observations in mice that were immunized with TAChR and treated concomitantly with the dual APL, mRNA expression of caspase 8 increased significantly, whereas the anti-apoptotic cFLIP decreased in mice with EAMG that were treated with the dual APL compared to the PBS-treated mice (Fig. 9).
Figure 8.
Effect of the dual altered peptide ligand (APL) on the expression of Fas, FasL by lymph node (LN) cells of C57BL/6 mice with experimental autoimmune myasthenia gravis (EAMG). (a) EAMG was induced in C57BL/6 mice by immunization and boost with Torpedo acetylcholine receptor (TAChR). Mice were treated for 4 weeks with either phosphate-buffered saline (PBS) (vehicle) or the dual APL (see Materials and methods). Mice were graded before and after treatment as follows: grade 3, severe muscle weakness, paralysis, dehydration, moribund; grade 2, weakness at rest; grade 1, moderate muscular weakness after exercise; and grade 0, no definite muscular weakness after exercise. (b) At the end of treatment, popliteal LN cells were harvested, stained for CD4, CD25, and Fas or FasL and analysed by fluorescence activated cell sorter (FACS). The results are from pooled samples (LN cells) of mice with EAMG that were treated with the dual APL or of diseased mice that were administered with PBS (as shown in a). Results presented are after reduction of the background staining obtained with the matched isotype controls. Shown is one of two experiments performed.
Figure 9.
Effect of the dual altered peptide ligand (APL) on the expression of apoptotic markers by lymph node (LN) cells of C57BL/6 mice with experimental autoimmune myasthenia gravis (EAMG). EAMG was induced in C57BL/6 mice by immunization with Torpedo acetylcholine receptor (TAChR). After 4 weeks of treatment with either phosphate-buffered saline (PBS) (vehicle) or the dual APL, popliteal LN cells were harvested of four mice of each of the treatment groups. Total RNA was extracted from lymph node cells and real time polymerase chain reaction (PCR) was performed for caspase 8 and cFLIP expression as described in Materials and methods. Results are expressed as mean percentage gene expression of triplicates ± SD values. *P < 0·001, **P < 0·005 when gene expression of the dual APL-treated mice was compared with that of PBS-administered mice.
Discussion
The main findings of the present study are that the Fas–FasL pathway-mediated apoptosis is one of the mechanisms by which the dual APL down-regulates myasthenogenic-associated T cell responses. The latter conclusion is based on results of in vitro studies using a T cell line specific for the myasthenogenic peptide, p195–212 and also on in vivo studies in mice immunized with p195–212 or with the whole Torpedo AChR (TAChR) molecule. Most importantly, we show that the amelioration of EAMG manifestations by the dual APL is in association with the up-regulated expression of the key apoptotic molecules that are involved in the Fas–FasL pathway.
Analyses of T cell responses to various antigens have confirmed that Fas (CD95) plays a dominant role in activation-induced cell death (AICD) of CD4+ T cells.17 Indeed, by enhancing the expression of Fas on the autoreactive CD45Rbhigh cells15 of the p195–212-specific T cell line as well as on CD4+ CD25− cells of C57BL/6 mice, the dual APL has rendered them susceptible to transduction of apoptotic signal. Fas antigen is known to play an important role in autoimmunity.25 Abnormalities in apoptosis and Fas gene expression of thymocytes of MG patients have been related to the pathogenesis and progression of MG.26 Further, Fas- or FasL-deficient mice (lpr and gld mice) are susceptible to EAMG because their T cells cannot undergo apoptosis due to Fas or FasL mutation.27 Thus the increase in sensitivity, by the dual APL, of the autoreactive CD4+ CD25− cells to Fas-mediated apoptosis may be an important regulation mechanism that limits the expansion of activated cells. Results show that Fas is also expressed by the CD4+ CD25+ regulatory cells of the dual APL-treated mice. However, regulatory CD4+ CD25+ T cells have been reported to be resistant/defective to Fas-induced apoptosis, contrary to CD4+ CD25− T cells. Further, these T regulatory cells do not produce IL-2, and IL-2 was shown to potentiate Fas-mediated AICD.28,29 Thus resistance of the dual APL-induced regulatory T cells to apoptosis may, in turn, be an important mechanism maintaining a permanent population of regulatory T cells that can control and eventually suppress activated T cells.27 CD95L or FasL, which is the natural ligand for CD95, is known to have an immunosuppressive function and permits the T cells expressing them to eliminate the neighbouring CD95-positive cells.17,30 As AICD results from interaction between Fas and FasL, up-regulation by the dual APL of FasL on the CD4+ CD25+ may cause the latter to deliver a death signal to the Fas expressing CD4+ CD25− autoreactive T cells and hence may contribute to the maintenance as well as the induction of peripheral tolerance. In support of this finding, a CD4+ CD25+ T cell line, derived from mice immunized with a peptide containing a major T cell epitope of a common allergen, was shown to exert Fas/FasL-mediated cytotoxic properties on APC and bystander T cells.31 It has been suggested that a partial peripheral deletion driven by Fas/FasL interaction could have occurred in the latter model. In another report Cavinato et al.,32 in their studies on the formation of T regulatory cells for maintenance of tolerance to organ transplantation, also showed that FasL mRNA was up-regulated in tolerant CD4+ cells when compared with naive CD4+ cells. In further support of the above, Watanabe et al.33 demonstrated CD4+ CD25+ cells that expressed FasL and were shown to behave like cytotoxic T cells. It is widely reported that cytokines such as IL-4 and IL-10 increase cell surface expression of FasL and hence enhances FasL-mediated cytotoxicity.33–36 It is possible that the levels of IL-4 and IL-10 shown to be elevated as a result of the ameliorating effects of the dual APL9 might be associated with the increased expression of FasL on the CD4+ CD25+ cells of the dual APL-treated mice when compared to the CD4+ CD25− cells. Lundy and Boros37 showed further that maximum expression of FasL on B lymphocytes is dependent on antigenic stimulation and the production of IL-4 and IL-10. Although expression of Fas and FasL were shown to be similar in C57BL/6 mice 10 days following immunization and at the end of a 4-week treatment in mice afflicted with EAMG, differences in expression levels between PBS- and dual APL-treated groups were much greater in mice with EAMG. This could possibly be attributed to differences in the kinetics of expression of Fas and FasL under the two different experimental conditions, or because at the disease state, treatment with the dual APL is much more effective.
Interaction between Fas and FasL is known to lead further to subsequent activation of the cell death proteases, caspase 8 and caspase 3, resulting in cellular fragmentation and death.17 Upon treatment with the dual APL, the increase in expression of caspase 8 and caspase 3 observed with the up-regulation of Fas and FasL suggests the apoptotic link between these molecules. Caspase 8 has been linked to homeostatic regulation of the immune system and mutations in caspase 8 have reported to lead to autoimmunity.38 Further, the parallel increase in the annexin V reactivity observed with the dual APL-treated p195–212-specific T cells as well as in SJL mice immunized and treated concomitantly with the dual APL indicates strongly that the dual APL promotes apoptosis by up-regulating the expression of Fas.
Bcl-2 and cFLIP, observed to be down-regulated by the dual APL treatment, are known to inhibit FasL induced apoptosis and prolong cell survival of autoreactive thymocytes.22,29 Thus the dual APL appears to promote apoptosis by down-regulating the expression of the anti-apoptotic molecules. It has been reported that Bcl-2 protein is up-regulated in MG thymuses and it has been indicated to suppress thymocytic apoptosis, allowing MG autoreactive T cells to escape elimination.21,22 cFLIP appears to prevent early death receptor-mediated T cell apoptosis and over-expression of cFLIP also supports the accumulation of autoreactive lymphocytes.39 In addition, high levels of cFLIP expression may shift death receptor signalling from apoptosis induction towards cell-activating signals and enhanced proliferation through activation of nuclear factor kappa-b (NF-κB).39,40 Thus, in addition to enhancing death receptor-mediated apoptosis, down-regulation of cFLIP by dual APL treatment in T cells may also limit the proinflammatory activating effect of death-receptor signalling. Further, the absence of autocrine IL-2 production in the CD4+ CD25+ T regulatory cells is known to favour the expression of anti-apoptotic proteins and apoptosis inhibitors, while autocrine IL-2 production in CD4+ CD25− T cells may up-regulate the expression of pro-apoptotic proteins and suppress inhibitor expression.28,29
Phosphorylation of the MAPKs, JNK and p38 of the p195–212-specific T cells of the line and the LN cells of C57BL/6 mice were significantly up-regulated by the dual APL. These two MAPKs are integral mediators of Fas/FasL signalling. p-38 MAPK plays a pivotal role in T cell AICD by initiating FasL expression, which then leads to the Fas–FasL-dependent activation of caspases and JNK. JNK functions downstream of caspases and together with p38 MAPK up-regulates FasL expression and elicits caspase-mediated AICD in T cells19. Thus, the up-regulation of phosphorylated MAPKs in parallel to activation of the pro-apoptotic caspases by the dual APL suggests the apoptotic link between them in the Fas–FasL-mediated pathway. It was demonstrated recently that a neurotrophic factor withdrawal by a JNK-dependent mechanism is essential for activation of FasL transcription.41 JNK activation was also demonstrated to be facilitated by TGF-β via the adaptor protein, Daxx.42 Ben-David et al.16 showed the up-regulation of TGF-β in SJL mice upon treatment with the dual APL to be associated with increased activation of JNK. Also, TGF-β is reported to activate the p38 MAPK kinase pathway to induce apoptosis.43
Failure to undergo AICD is an important underlying cause of many autoimmune diseases. Studies have reported that apoptosis plays a critical role in T cell development within the thymus and is probably the mechanism that underlies deletion of autoreactive T cell precursors.17 Thus the progress of MG and development of advanced stages have been attributed to possible lower inhibition of apoptosis that lead to enhanced cell survival, and this may be one of the mechanisms through which the autoreactive cell promoters exert their effect.22 By enhancing the myasthenogenic response-associated T cells' own suicide mechanism by the Fas–FasL pathway, the dual APL may provide an efficient therapy for the treatment of autoimmune diseases such as MG.
Acknowledgments
This study was supported by DeveloGen (Goettingen, Germany).
References
- 1.Lindstrom J, Shelton D, Fuji Y. Myasthenia gravis. Adv Immunol. 1988;42:233–84. doi: 10.1016/s0065-2776(08)60847-0. [DOI] [PubMed] [Google Scholar]
- 2.Drachman DB. Medical progress: myasthenia gravis. N Engl J Med. 1994;330:1797–810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 3.Brocke S, Brautbar C, Steinman L, Abramsky O, Rothbard J, Neumann D, Fuchs S, Mozes E. In vitro proliferative responses and antibody titers specific to human acetylcholine receptor synthetic peptides in patients with myasthenia gravis and relation to HLA class II genes. J Clin Invest. 1988;82:1894–900. doi: 10.1172/JCI113807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karni A, Zisman E, Katz-Levy Y, et al. Reactivity of T cells from seronegative patients with myasthenia gravis to T cell epitopes of the human acetylcholine receptor. Neurology. 1997;48:1638–42. doi: 10.1212/wnl.48.6.1638. [DOI] [PubMed] [Google Scholar]
- 5.Brocke S, Dayan M, Rothbard J, Fuchs S, Mozes E. The autoimmune response of different mouse strains to T-cell epitopes of the human acetylcholine receptor alpha subunit. Immunology. 1990;69:495–500. [PMC free article] [PubMed] [Google Scholar]
- 6.Kirshner SL, Katz-Levy Y, Wirguin I, Argov Z, Mozes E. Fine specificity of T-cell lines and clones that are capable of inducing autoimmune manifestations in mice. Cell Immunol. 1994;157:11–28. doi: 10.1006/cimm.1994.1201. [DOI] [PubMed] [Google Scholar]
- 7.Katz-Levy Y, Paas-Rozner M, Kirshner S, et al. A peptide composed of tandem analogs of two myasthenogenic T cell epitopes interferes with specific autoimmune responses. Proc Natl Acad Sci USA. 1997;94:3200–5. doi: 10.1073/pnas.94.7.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paas-Rozner M, Dayan M, Paas Y, Changeux J-P, Wirguin I, Sela M, Mozes E. Oral administration of two myasthenogenic T cell epitopes down-regulates experimental autoimmune myasthenia gravis in mice. Proc Natl Acad Sci USA. 2000;97:2168–73. doi: 10.1073/pnas.040554597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paas-Rozner M, Sela M, Mozes E. The nature of the active suppression of responses associated with experimental autoimmune myasthenia gravis by a dual altered peptide ligand administered by different routes. Proc Natl Acad Sci USA. 2001;98:12642–7. doi: 10.1073/pnas.221456798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faber-Elmann A, Paas-Rozner M, Sela M, Mozes E. Altered peptide ligands act as partial agonists by inhibiting phospholipase C activity induced by myasthenogenic T cell epitopes. Proc Natl Acad Sci USA. 1998;95:14320–5. doi: 10.1073/pnas.95.24.14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faber-Elmann A, Grabovsky V, Dayan M, Sela M, Alon R, Mozes E. Cytokine profile and T cell adhesiveness to endothelial selectins: in vivo induction by a myasthenogenic T cell epitope and immunomodulation by a dual altered peptide ligand. Int Immunol. 2000;12:1651–8. doi: 10.1093/intimm/12.12.1651. [DOI] [PubMed] [Google Scholar]
- 12.Paas-Rozner M, Sela M, Mozes E. A dual altered peptide ligand down-regulates myasthenogenic T cell responses by up-regulating CD25– and CTLA-4—expressing CD4+ T cells. Proc Natl Acad Sci USA. 2003;100:6676–81. doi: 10.1073/pnas.1131898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 14.Shevach EM. Certified professionals: CD4(+) CD25(+) suppressor T cells. J Exp Med. 2001;193:F41–5. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aruna BV, Sela M, Mozes E. Suppression of rnyasthenogenic responses of a T cell line by a dual altered peptide ligand by induction of CD4(+) CD25(+) regulatory cells. Proc Natl Acad Sci USA. 2005;102:10285–90. doi: 10.1073/pnas.0504578102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-David H, Sela M, Mozes E. Down-regulation of myasthenogenic T cell responses by a dual altered peptide ligand via CD4(+) CD25(+)-regulated events leading to apoptosis. Proc Natl Acad Sci USA. 2005;102:2028–33. doi: 10.1073/pnas.0409549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krammer PH. CD95's deadly mission in the immune system. Nature. 2000;407:789–95. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 18.Davidson WF, Haudenschild C, Kwon J, Williams MS. T cell receptor ligation triggers novel nonapoptotic cell death pathways that are Fas-independent or Fas-dependent. J Immunol. 2002;169:6218–30. doi: 10.4049/jimmunol.169.11.6218. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Gao JX, Salojin K, Shao Q, Grattan M, Meagher C, Laird DW, Delovitch TL. Regulation of Fas ligand expression during activation-induced cell death in T cells by p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase. J Exp Med. 2000;191:1017–30. doi: 10.1084/jem.191.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masunaga A, Arai T, Yoshitake T, Itoyama S, Sugawara I. Reduced expression of apoptosis-related antigens in thymuses from patients with myasthenia gravis. Immunol Lett. 1994;39:169–72. doi: 10.1016/0165-2478(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 21.Nagata T, Onodera H, Ohuchi M, et al. Decreased expression of c-myc family genes in thymuses from myasthenia gravis patients. J Neuroimmunol. 2001;115:199–202. doi: 10.1016/s0165-5728(01)00252-1. [DOI] [PubMed] [Google Scholar]
- 22.Salakou S, Tsamandas AC, Bonikos DS, Papapetropoulos T, Dougenis D. The potential role of bcl-2, bax, and Ki67 expression in thymus of patients with myasthenia gravis, and their correlation with clinicopathologic parameters. Eur J Cardiothorac Surg. 2001;20:712–21. doi: 10.1016/s1010-7940(01)00776-x. [DOI] [PubMed] [Google Scholar]
- 23.Elliott J, Blanchard SG, Wu W, et al. Purification of Torpedo californica post-synaptic membranes and fractionation of their constituent proteins. Biochem J. 1980;185:667–77. doi: 10.1042/bj1850667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Axelrod O, Mozes E. Analysis of the biological functions and fine specificity of (T,G)-A-L specific T cell clones. Immunobiology. 1986;172:99–109. doi: 10.1016/S0171-2985(86)80056-0. [DOI] [PubMed] [Google Scholar]
- 25.Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, de Villartay JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–9. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 26.Du Y, Zhang QY, Ruan LR, Liang CC, Li QR, He W. The relationship between abnormal apoptosis and Fas expression of thymocytes from patients with myasthenia gravis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2003;19:450–3. [PubMed] [Google Scholar]
- 27.Deng C, Goluszko E, Christadoss P. Fas/Fas ligand pathway, apoptosis, and clonal anergy involved in systemic acetylcholine receptor T cell epitope tolerance. J Immunol. 2001;166:3458–67. doi: 10.4049/jimmunol.166.5.3458. [DOI] [PubMed] [Google Scholar]
- 28.Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615–23. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 29.Papiernik M. Natural CD4+ CD25+ regulatory T cells. Their role in the control of superantigen responses. Immunol Rev. 2001;182:180–9. doi: 10.1034/j.1600-065x.2001.1820114.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen J-J, Sun Y, Nabel GJ. Regulation of the proinflammatory effects of Fas ligand (CD95L) Science. 1998;282:1714–17. doi: 10.1126/science.282.5394.1714. [DOI] [PubMed] [Google Scholar]
- 31.Janssens W, Carlier V, Wu B, VanderElst L, Jacquemin MG, Saint-Remy JM. CD4+CD25+ T cells lyse antigen-presenting B cells by Fas–Fas ligand interaction in an epitope-specific manner. J Immunol. 2003;171:4604–12. doi: 10.4049/jimmunol.171.9.4604. [DOI] [PubMed] [Google Scholar]
- 32.Cavinato RA, Casiraghi F, Azzollini N, et al. Pretransplant donor peripheral blood mononuclear cells infusion induces transplantation tolerance by generating regulatory T cells. Transplant. 2005;79:1034–9. doi: 10.1097/01.tp.0000161663.64279.6b. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe T, Yoshida M, Shirai Y, et al. Administration of an antigen at a high dose generates regulatory CD4+ T cells expressing CD95 ligand and secreting IL-4 in the liver. J Immunol. 2002;168:2188–99. doi: 10.4049/jimmunol.168.5.2188. [DOI] [PubMed] [Google Scholar]
- 34.Aung S, Graham BS. IL-4 diminishes perforin-mediated and increases Fas ligand-mediated cytotoxicity in vivo. J Immunol. 2000;164:3487–93. doi: 10.4049/jimmunol.164.7.3487. [DOI] [PubMed] [Google Scholar]
- 35.Barreiro R, Luker G, Herndon J, Ferguson TA. Termination of antigen-specific immunity by CD95 ligand (Fas ligand) and IL-10. J Immunol. 2004;173:1519–25. doi: 10.4049/jimmunol.173.3.1519. [DOI] [PubMed] [Google Scholar]
- 36.Ebert EC. IL-15 converts human intestinal intraepithelial lymphocytes to CD94 producers of IFN-gamma and IL-10, the latter promoting Fas ligand-mediated cytotoxicity. Immunology. 2005;115:118–26. doi: 10.1111/j.1365-2567.2005.02132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundy SK, Boros DL. Fas ligand-expressing B-1a lymphocytes mediate CD4(+)-T-cell apoptosis during schistosomal infection. induction by interleukin 4 (IL-4) and IL-10. Infect Immun. 2002;70:812–19. doi: 10.1128/iai.70.2.812-819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chun HJ, Zheng L, Ahmad M, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–9. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- 39.Wasem C, Arnold D, Saurer L, et al. Sensitizing antigen-specific CD8+ T cells for accelerated suicide causes immune incompetence. J Clin Invest. 2003;111:1191–9. doi: 10.1172/JCI16344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Budd RC. Death receptors couple to both cell proliferation and apoptosis. J Clin Invest. 2002;109:437–42. doi: 10.1172/JCI15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallagher SF, Peng Y, Haines K, Baksh K, Epling-Burnette PK, Yang J, Murr MM. Fas/FasL play a central role in pancreatitis-induced hepatocyte apoptosis. J Gastrointest Surg. 2005;9:467–74. doi: 10.1016/j.gassur.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Perlman R, Schiemann WP, Brooks MW, Lodish HF, Weinberg RA. TGF-beta-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat Cell Biol. 2001;3:708–14. doi: 10.1038/35087019. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez-Capelo A. Dual role for TGF-beta1 in apoptosis. Cytokine Growth Factor Rev. 2005;16:15–34. doi: 10.1016/j.cytogfr.2004.11.002. [DOI] [PubMed] [Google Scholar]