Abstract
The complement regulatory proteins CD55 and CD59 are expressed on the plasma membrane of human spermatozoa, whereas CD46 is only on the inner acrosomal membrane (IAM) which becomes surfaced exposed after the acrosome reaction when sperm assume fertilisation-competence. CD55 & CD59, two glycosylphosphatidylinositol (GPI)-anchored proteins, have been detected previously in some studies also in the acrosomal region of chemically fixed spermatozoa but never demonstrated at this site on unfixed spermatozoa. Dual labelling immunofluorescence and confocal microscopy on fresh unfixed spermatozoa, with minimal subsequent time to fixation, has shown CD55 to be markedly expressed on the IAM, more than on the plasma membrane. However, unlike for CD46, CD55 displayed patchy staining over the acrosome, with some variation between individual spermatozoa. All IAM-associated CD55 was localised within GM1-containing lipid rafts. CD59 was expressed also on the IAM, but in a pronounced granular pattern with more variation observed from one spermatozoa to another. Both CD55 & CD59 were released from the IAM by PI-PLC, demonstrating them to be GPI-anchored. Analysis of acrosome-reacted spermatozoal CD55 by Western blotting revealed a novel single 55 kDa protein lacking significant oligosaccharides susceptible to glycosidases. Antibody-induced membrane rafting and release of CD55 & CD59 in vitro may have influenced previous results. Significant coexpression of CD55 & CD46 on the IAM suggests some functional cooperation at this site.
Keywords: spermatozoa, acrosome, CD46, CD55, CD59
Introduction
Membrane cofactor protein (CD46), decay accelerating factor (CD55) and CD59 are three widely expressed human membrane-bound complement regulatory proteins (mCRPs) that function to protect autologous tissues from complement (C)-mediated lysis at two distinct levels of the C cascade. While CD46 and CD55 regulate C at the level of C3/C5 convertases1–3 CD59 prevents the assembly of the membrane attack complex4. These mCRPs connect with the cell membrane by either a glycosylphosphatidylinositol (GPI)-anchor (CD55, CD59) or a transmembrane domain (CD46)5 In addition to their role as C inhibitors, CD46, CD55 and CD59 all serve as cell surface receptors for a variety of diverse pathogens6–10.
Spermatozoa gain fertilising ability while residing in the female reproductive tract, where they undergo a process termed capacitation which is associated with hyperactivated motility as well as reorganisation of plasma membrane lipids and proteins preparing spermatozoa for the acrosome reaction, an essential step for fertilisation11–13. The acrosome reaction is a calcium-dependant exocytotic event involving fusion and vesiculation of the plasma membrane with the outer acrosomal membrane of spermatozoa, thereby releasing the acrosomal content and exposing proteins on the inner acrosomal membrane (IAM) that mediate spermatozoa-egg binding and fusion12. Spermatozoa that have released only part of their acrosomal contents are considered partially acrosome reacted, while spermatozoa that have released all their acrosomal contents are considered fully acrosome reacted.14,15
It is well established that all three mCRPs are expressed by human spermatozoa and may be involved in spermatozoa function, survival in the female reproductive tract and clearance of redundant spermatozoa3. Acrosome-reacted (AR)-spermatozoa activate C, and C3b is deposited on the IAM independent of CD4616. However, CD46 is known to be expressed as a novel hypoglycosylated form restricted to the IAM and not found on the plasma membrane of spermatozoa17,18. This has led to speculation that CD46 may be involved in spermatozoa-egg binding, independent of its role as a mCRP. Interestingly, rodents express CD46 only on the IAM of spermatozoa and not on any other cell types3 and the systemic role of CD46 as a mCRP is taken up instead by the rodent-specific regulator, Crry19 Moreover, somatic cells of New World monkeys express an alternatively spliced isoform of CD46 lacking short consensus repeat 1 (SCR1), whereas SCR1 has been retained on spermatozoal CD4620 suggesting a role for this domain in fertilisation. In support of this hypothesis, antibodies to the SCR1 domain of CD46 have been shown to inhibit human spermatozoa binding to both hamster and human eggs17,21–23. However, CD46-knockout mice are fertile but have an accelerated spontaneous acrosome reaction24.
The precise localisation of both CD55 and CD59 on human spermatozoa remains unclear. Previous investigators using different immunocytochemical methodologies have variously reported CD55 to be localised on the plasma membrane of the head and tail of spermatozoa25,26 on the acrosomal region and tail and on the anterior segment of the sperm head27–29 and solely on the acrosomal region30. In particular, detection of CD55 on the acrosomal region has been successfully demonstrated only on chemically fixed spermatozoa28,30 and not on spermatozoa stained in suspension30. Similarly, CD59 has variously been reported to be localised on the midpiece and the plasma membrane of the head and tail26,29 on the midpiece and the plasma membrane of the head27 on the plasma membrane of the head25 and on the acrosomal region, midpiece and tail28,31 of spermatozoa.
There has been no systematic study of surface and fixed cell staining for CD55 and CD59 on acrosome-reacted (AR) and acrosome-unreacted (AUR) human spermatozoa. In order to address this, we have used dual staining confocal fluorescent microscopy using CD46 as a marker for AR-spermatozoa and different staining protocols to determine the precise localisation of CD55 and CD59 on spermatozoa.
Materials and methods
Reagents
Sperm Wash Modified — Human Tubular Fluid (SWM-HTF) medium (containing 5% human serum albumin was obtained from Conception Technologies (San Diego, USA). Rhodamine-conjugated wheat germ agglutinin (R-WGA) was obtained from Vector Laboratories (Peterborough, UK). Phosphatidylinositol-specific phospholipase C (PI-PLC) was obtained from Molecular Probes (Leiden, The Netherlands). Unless stated otherwise, all other media and reagents were obtained from Sigma-Aldrich (Dorset, UK).
Antibodies
Fluorescein isothiocyanate (FITC)-conjugated antihuman CD46 (E4.3) and CD55 (IA10) murine monoclonal antibodies (mAbs), as well as unconjugated antihuman CD46 (E4.3), CD55 (IA10) and CD59 (H319) mAbs, together with FITC-conjugated IgG1 and IgG2a isotype controls, were obtained from BD Biosciences (Oxford, UK). The murine mAb 18–6, which recognises an acrosome-associated antigen32 was kindly provided by Professor Harry Moore (University of Sheffield, UK). An unrelated mAb prepared in-house, H317, was also used as a negative control33. Texas Red (TR)-conjugated antimouse IgG was obtained from Molecular Probes (Europe). Rabbit polyclonal antibody (pAb) to human CD46 (H-294) and horseradish peroxidase (HRP)-conjugated donkey antirabbit IgG, as well as HRP-conjugated goat antimouse IgG, were obtained from Santa Cruz Biotechnology (California, USA).
Blood cells, cell lines and spermatozoal preparations
Local Ethical Committee approval was granted for this study, and informed consent obtained from all healthy volunteer donors.
Human peripheral blood mononuclear cells (PBMCs) and red blood cells (RBCs) were prepared by centrifugation over Ficoll-Paque (Amersham Biosciences, Buckinghamshire, UK) from 10 to 15 mL heparinised blood obtained by venepuncture, washed twice by centrifugation and viable cells assessed using a counting chamber and trypan blue exclusion. The human HeLa cervical carcinoma cell line was obtained from European Collection of Animal Cell Cultures (ECACC, Salisbury, UK).
Freshly ejaculated human semen samples were obtained from a panel of healthy volunteer donors of unknown fertility, and allowed to liquefy at 37 °C for 30 min. Motile spermatozoa were recovered from semen by a direct swim-up procedure34. Briefly, semen (0.25 mL) was layered under 1 mL of SWM-HTF medium in multiple tubes and incubated at 37 °C for 1 h at an angle of about 45°. Motile spermatozoa were recovered by removal of the top two-thirds of SWM-HTF medium. Spermatozoa concentration and viability were assessed using a counting chamber and trypan blue exclusion.
Capacitation and induction of the acrosome reaction
Capacitation was achieved by incubation of spermatozoa in SWM-HTF medium for a further 1 h at 37 °C. The acrosome reaction was subsequently induced by addition of the calcium ionophore A23187 (Sigma-Aldrich, Dorset, UK) to a final concentration of 10 μm for 1 h at 37 °C. For control cells, capacitated spermatozoa were incubated without ionophore. Spermatozoa were then washed twice with isotonic phosphate-buffered saline (PBS), pH 7.4, and centrifuged at 500 g for 8 min.
Dual staining fluorescent microscopy
Both capacitated and AR-spermatozoa (5 × 106 cells/mL) were either smeared onto glass slides and then immediately air-dried and stained (prefix samples), or stained in the fluid phase and then air-dried on slides (postfix samples). Unless stated otherwise, all mAbs were diluted 1: 50 in PBS and either incubated for 30 min followed by two washes by centrifugation (500 g for 8 min) for postfix spermatozoa samples or incubated for 1 h with three subsequent rinses in PBS for prefix samples.
To assess the acrosomal status of postfix capacitated spermatozoa, CD46 was used as a positive marker for those spermatozoa that had spontaneously acrosome-reacted (AR-spermatozoa) since it is uniformly distributed exclusively on the inner acrosomal region (IAM)17,35. WGA, which binds to the plasma membrane of spermatozoa36 was used as a positive control for acrosome-unreacted (AUR)-spermatozoa. FITC-conjugated cholera toxin B (CTB), which binds the GM1 ganglioside37 was used as a marker for lipid rafts.
For postfix samples, freshly isolated swim-up spermatozoa were incubated for 30 min in suspension with 5% goat serum before centrifugation and resuspension with unconjugated mAbs, either anti-CD55 or anti-CD59, followed by incubation with the secondary TR-conjugated antimouse IgG antibody (diluted 1: 200 in PBS). Spermatozoa were further blocked with 5% mouse serum before addition of a second mAb, FITC-conjugated anti-CD46, then immediately air-dried on slides. When spermatozoa were labelled with anti-CD55 mAb followed by incubation with TR-conjugated antimouse IgG and either R-WGA or FITC-cholera toxin B (CTB; 10 µg/mL), the second block with mouse serum was omitted.
For prefix samples, spermatozoa were air-dried on slides then stained as for postfix samples. An irrelevant mAb (H317) was included as a negative control for unconjugated mAbs, as well as a FITC-conjugated isotype control mAb, for both prefix and postfix spermatozoa.
Slides were mounted with Vectashield (Vector Laboratories, Peterborough) and confocal laser scanning microscopy (CLSM) carried out using a Zeiss LSM 510 microscope.
Treatment of cells with phosphatidylinositol-specific phospholipase (PI-PLC)
PBMCs, RBCs and AR-spermatozoa (1 × 106 cells/mL) were treated either with or without PI-PLC (1.0 U/mL) for 20 min at 4 °C. Cells were washed with PBS and centrifugation, and localisation of CD46, CD55 and CD59 evaluated on cell suspensions by dual immunofluorescence staining as described above for postfix samples.
Preparation of cell lysates
Cell lysates were prepared by resuspending cells (2 × 107 cells/mL) in ice-cold lysis buffer (10 mm Tris pH 7.2, 150 mm NaCl, 1% NP-40, 0.05% SDS) supplemented with 10% protease inhibitor cocktail. After 30 min incubation on ice, insoluble material was removed by centrifugation (10 000 g, 10 min, 4 °C) and supernatants stored at −80 °C until use.
Western blotting
Cell lysates were separated in a 10% SDS-polyacrylamide gel using a Bio-Rad MiniProtean 3 cell (Bio-Rad) according to Laemmli38 under nonreducing conditions. Following electrophoresis, the separated proteins were transferred onto a nitrocellulose membrane (Schleicher & Schuell, London, UK) and blots blocked overnight at 4 °C with 5% (w: v) nonfat dry milk in PBS. Blots were probed for 1 h at room temperature with either rabbit antihuman CD46 pAb (H-294; 1: 800) or anti-CD55 mAb (IA10; 1: 1000) diluted in PBS containing 0.05% Tween 20 (PBS-T), washed three times with PBS-T and incubated with either HRP-conjugated donkey antirabbit IgG or HRP-conjugated goat antimouse IgG (1: 30 000) for 30 min. Following a further three washes with PBS-T, protein bands were detected using an enzyme chemoluminescence Western blotting detection kit (Amersham Biosciences, Buckinghamshire, UK) and captured on autoradiographic film (Hyperfilm ECL, Amersham Biosciences).
Enzymatic deglycosylation
Deglycosylation of AR-spermatozoa and HeLa cell lysates was performed according to the manufacturers' instructions both with individual glycosidase enzymes and with a defined mixture of enzymes. Both endo-α-N-acetylgalactosaminidase (Calbiochem, Nottingham, UK) and O-glycosidase (Roche, Mannheim, Germany) were used separately to cleave the disaccharide (Galβ1,3GalNAc) of O-glycans linked to a serine or threonine residues39 and N-glycosidase F (Roche, Mannheim, Germany) was used to cleave all types of asparagine-bound N-glycans. Briefly, 25 µL of cell lysate was mixed with 25 µL nonreducing buffer (10% SDS, 10% glycerol, 0.1% bromophenol blue in 0.3M Tris-HCl, pH 6.8) and boiled for 10 min. Endo-α-N-acetylgalactosaminidase (0.25 mU) or O-glycosidase (0.25 mU) and/or N-glycosidase (0.5 U) were added to diluted lysate and incubated at 37 °C for 24 h. Neuramidase (25 mU; Roche) was included in all O-deglycosylation tubes to remove terminal disaccharide. Samples were then separated on SDS-PAGE, Western blotted and blots probed with anti-CD55 mAb, as described previously.
Results
CD55 is associated with both the plasma membrane & acrosome of human spermatozoa
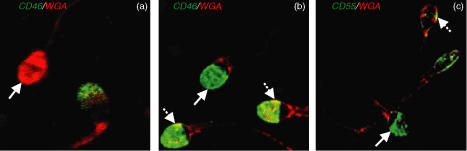
Evaluation of the acrosomal status of postfix capacitated spermatozoa samples, containing both acrosome-unreacted (AUR)-spermatozoa and spontaneous acrosome-reacted (AR)-spermatozoa, was determined by two-colour cell surface fluorescent staining with anti-CD46 mAb and R-WGA followed by postfixation (Figs 1a, b). Localisation of CD55 was evaluated also by two-colour fluorescent staining on postfix capacitated spermatozoa using R-WGA and anti-CD55 (Fig. 1c).
Figure 1.
Confocal laser scanning microscopy (CLSM) images of dual immunofluorescent localisation of CD46 (green) & WGA binding (red) (a#x0026;b) and CD55 (green) & WGA binding (red) (c) on postfix capacitated spermatozoa. Single staining for WGA in (a), CD46 in (b), and CD55 in (c) is marked with a solid arrow. Co-localisation signals as yellow pixels (broken arrow) for CD46 & WGA (b) and CD55 & WGA (c). Final magnification: × 1700 (a,b) and × 1200 (c).
Spermatozoa displaying intense binding with WGA and no anti-CD46 binding on the inner acrosomal region were considered AUR-spermatozoa (Fig. 1a; solid arrow). In contrast, spermatozoa displaying strong staining with anti-CD46 mAb and no binding with WGA were considered completely AR-spermatozoa (Fig. 1b; solid arrow). However, spermatozoa displaying both binding of WGA and anti-CD46 mAb reactivity were considered partially AR-spermatozoa; colocalisation for both WGA-binding and CD46 signals as yellow pixels (Fig. 1b; broken arrow). Variation in intensity and localisation of WGA-binding, particularly along the tail and neck regions, was observed from one spermatozoa to another. These observations indicate that CD46 is a reliable marker of the acrosome reaction and that WGA detects not only AUR-spermatozoa but also partially AR-spermatozoa.
Similarly, for spermatozoa where CD55 was localised primarily in the acrosomal region, no binding of WGA was observed at that region (Fig. 1c; solid arrow), although WGA-binding was observed on the neck region and along the tail. However, some spermatozoa displayed both anti-CD55 mAb reactivity and WGA-binding. Co-localisation for both WGA-binding and CD55 signals as yellow pixels (Fig. 1c; broken arrow), indicating that CD55 is localised on the acrosomal region of fully or partially AR-spermatozoa.
There was no positive staining with relevant isotype-matched controls (data not shown).
CD55 & CD59 are expressed on the IAM & their identification is influenced by time to fixation
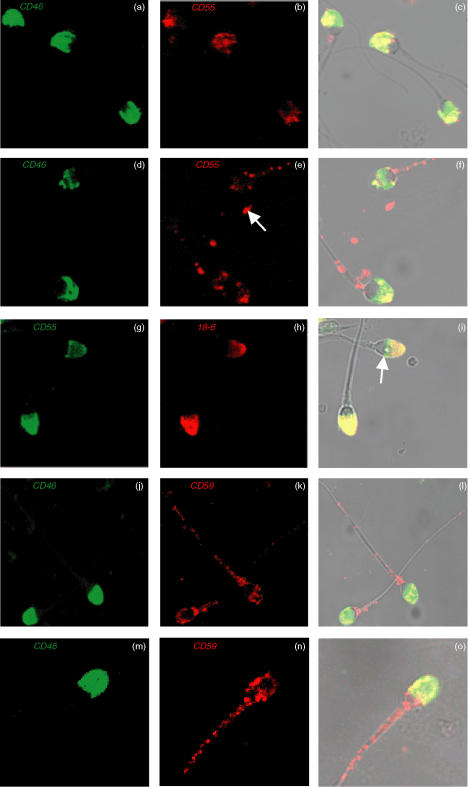
The distribution of CD55 and CD59 on AR-spermatozoa was assessed by two-colour immunofluorescent labelling with anti-CD46 mAb (Figs 2a,d,j,m) and either anti-CD55 (Figs 2b,e) or anti-CD59 mAbs (Figs 2k,n). Fresh AR-spermatozoa were stained either prefixed (Figs 2a–c,j–l) or postfixed (Figs 2d–f,m–o).
Figure 2.
CLSM images of dual immunofluorescent localisation of CD46 (green) & CD55 (red) on prefix (a–c) & postfix (d–f) AR-spermatozoa. Arrow indicates small structures not associated with intact spermatozoa (e). CD55 (green) & 18-6 mAb (red) on prefix AR-spermatozoa (g–i); arrow indicates spermatozoal equatorial segment (i). CD46 (green) & CD59 (red) expression on both prefix (j–l) and postfix (m–o) AR-spermatozoa. Co-localisation signals as yellow pixels for CD46 & CD55 (c & f), CD55 & 18–6 mAb (i) and CD46 & CD59 (l&o). Final magnification: × 1200 (a–i), × 1000 (j–l) and ×1500 (m–o).
Reactivity observed with the anti-CD46 mAb was intense and localised exclusively to the IAM of both prefix and postfix AR-spermatozoa; typically, this was approximately 90% of ionophore-treated spermatozoa. All spermatozoa were positive for both CD55 and CD59, although CD55 was primarily localised to the IAM of prefix AR-spermatozoa. However, unlike CD46, which displayed a relatively uniform pattern of staining on the IAM, CD55 was localised on the IAM of prefix AR-spermatozoa in a patchy speckled pattern. In contrast, postfix AR-spermatozoa had a significantly greater time to fixation and subsequently displayed a markedly granular pattern of staining for CD55 localised on the IAM, with similar granular staining of the neck region and along the tail. In addition, CD55 alone appeared localised on small structures not associated with intact spermatozoa (Fig. 2e; solid arrow), which may represent released membrane vesicles. On postfix spermatozoa, some variation in intensity and localisation of CD55 was observed from one spermatozoa to another.
Dual immunofluorescence staining for CD55 and binding of the 18–6 mAb, which recognises an acrosome-associated antigen, confirmed the presence of CD55 on the IAM of prefix AR-spermatozoa (Fig. 2g–i). Like for CD46, reactivity with the 18–6 mAb was intense and restricted to the IAM. Co-localisation of 18–6 mAb binding and CD55 signalled as yellow pixels (Fig. 2i). It was occasionally noted that CD55 exhibited pronounced reactivity in the equatorial segment in comparison to 18–6 mAb binding (Fig. 2i; solid arrow).
CD59 was expressed also on the IAM of both prefix and postfix AR-spermatozoa although, in contrast to CD55, a much more granular staining pattern was observed for both prefix and postfix AR-spermatozoa, with pronounced localisation as well in the neck region and on the tail (Fig. 2j–o). CD55 exhibited a higher degree of variation than CD59 in its localisation from one spermatozoa to another for both prefix and postfix AR-spermatozoa samples.
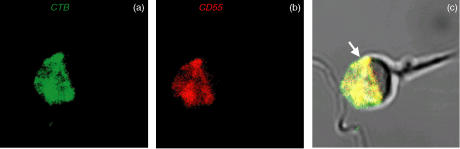
Dual immunofluorescence staining of prefix AR-spermatozoa with CTB and anti-CD55 mAb demonstrated that all IAM-associated CD55 is localised within GM1-containing lipid rafts (Fig. 3a–c).
Figure 3.
CLSM images of dual immunofluorescent localisation of CTB binding (green) and CD55 (red) on prefix AR-spermatozoa (a & b) Co-localisation signals as yellow pixels (solid arrow) for CD55 and CTB binding (c). Final magnification: × 3000.
There was no positive staining with relevant isotype-matched controls (data not shown).
Spermatozoal CD55 and CD59 are sensitive to cleavage by PI-PLC
The presence of a GPI-anchor on human spermatozoal IAM and plasma membrane CD55 and CD59 was confirmed by treating AR-spermatozoa with PI-PLC and subsequent dual staining with fluorochrome-conjugated anti-CD55 and anti-CD59 mAbs. Anti-CD46 was used both as a positive control for AR-spermatozoa and as a negative control for a non-GPI-anchored protein.
PI-PLC cleaved nearly all CD55 and CD59 from the surface of both spermatozoa and PBMCs (Table 1), demonstrating that both mCRPs are attached via a GPI-anchor as previously shown for other cell types; however, the CD55 GPI-anchor expressed by RBC is substituted with a fatty acyl chain on inositol, thus rendering them insensitive to cleavage by PI-PLC40,41.
Table 1. Sensitivity to cleavage by PI-PLC for CD46, CD55 and CD59 on AR-spermatozoa and human control cell types.
| Cell type | Without PI-PLC | With PI-PLC | |
|---|---|---|---|
| AR-spermatozoa | CD46 | +++ acrosome | +++ acrosome |
| CD55 | +++ acrosome | − acrosome | |
| ++ tail | + tail | ||
| CD59 | +++ acrosome | + acrosome | |
| ++ tail | + tail | ||
| PBMCs | CD46 | + | + |
| CD55 | +++ | − | |
| CD59 | +++ | + | |
| RBCs | CD46 | − | − |
| CD55 | +++ | +++ | |
| CD59 | +++ | +++ |
Key: + + + strong positive, + + positive, + weak positive, − negative.
Characterisation of CD55 on AR-spermatozoa by Western blotting
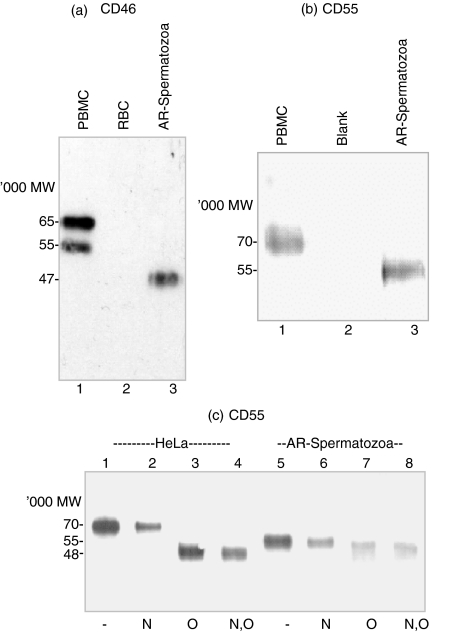
Western blot analysis of CD46 protein expressed on PBMC demonstrated a characteristic two-band pattern with Mr values of approximately 65 and 55 kDa (Fig. 4a, lane 1). However, AR-spermatozoal CD46 displayed a single band of Mr 45 kDa (Fig. 4a, lane 3). RBC, which do not express CD46, were negative (Fig. 4a, lane 2).
Figure 4.
Western blot analysis of CD46 (a) & CD55 (b & c) isoforms. (a) PBMC (lane 1), RBC (lane 2) and AR-spermatozoa (lane 3) cell lysates separated by SDS-PAGE and reacted with anti-CD46 pAb. (b) PBMC (lane 1), blank (lane 2) and AR-spermatozoa (lane 3) cell lysates. (c) Deglycosylation analysis of HeLa cell (lanes 1–4) and AR-spermatozoa (lanes 5–8) cell lysates, either untreated (lanes 1 & 5) or treated with N-glycosidase (N: lanes 2 & 6), O-glycosidase and neuramidase (O: lanes 3 & 7) or N-glycosidase, O-glycosidase and neuramidase (N,O: lanes 4 & 8), separated by SDS-PAGE and reacted with anti-CD55 mAb.
A single band with a Mr of approximately 55 kDa was detected by anti-CD55 mAb in AR-spermatozoa cell lysates (Fig. 4b, lane 3), some 15 kDa lower than that expressed by PBMC (Fig. 4b, lane 1). Because HeLa cell lysates produced more consistent deglycosylated CD55 protein bands, these were used in deglycosylation experiments rather than PBMC lysates. Treatment of AR-spermatozoa cell lysates with N-glycosidase F (Fig. 4c, lane 6) had no effect on the CD55 Mr However, treatment of AR-spermatozoa with endo-α-N-acetylgalactosaminidase (Fig. 4c, lane 7) or both glycosidases (Fig. 4c, lane 8) produced a small reduction of Mr of approximately 3 kDa; similarly, O-glycosidase had the same effect (data not shown). N-glycosidase F also had no effect on the Mr of CD55 in HeLa cell lysates (Fig. 4c, lane 2). In contrast, HeLa cell lysates treated with endo-α-N-acetylgalactosaminidase alone (Fig. 4c, lane 3) or with both glycosidases (Fig. 4c, lane 4) produced a decrease of 22 kDa in the CD55 Mr.
Discussion
A rapid two-colour cell surface fluorescence staining technique has been employed in this study to clarify the localisation of CD55 and CD59 on human spermatozoa, using also CD46 as an accurate indicator of AR-spermatozoa and WGA-binding as a positive indicator of either AUR or partially AR-spermatozoa. Of previous reports25–31 only three have identified CD55 or CD5928,30,31 on the acrosomal region. The present study has confirmed that both CD55 and CD59 are expressed on the IAM of prefix AR-spermatozoa. Unlike Cervoni et al.30 however, we were able to extend these findings to postfix cell surface staining of AR-spermatozoa showing that CD55 displayed a more granular pattern of staining on the IAM than the patchy speckled pattern of staining for prefix spermatozoa.
In addition, we have been able to demonstrate that IAM CD55 is associated with GM1-enriched lipid raft microdomains, as reported for many GPI-anchored proteins37. Hence, in vitro antibody-induced cell surface GPI-anchored protein redistribution and vesicle release may have influenced previous results for both AR- or AUR-spermatozoa in suspension. Differences in spermatozoa processing and staining could have led to the subsequent patching and potential release of CD55 and CD59 from the cell surface, as reported for GPI-anchored mCRP on other cell types42. Our experiments were designed to minimise such loss of CD55 and CD59 from the cell surface by reducing incubation and centrifugation times. Interestingly, small CD55-positive fluorescent particles, probably released membrane vesicles, were detected by fluorescent microscopy in some samples.
Spermatozoal plasma membrane GPI-anchored proteins can also be acquired from other cells or structures, e.g. prostasomes43. However, the presence of CD55 and CD59 on the IAM indicates that these mCRPs are intrinsically synthesised in germ cells. We found further that, unlike transmembrane CD46, both CD55 and CD59 were cleaved from the IAM of spermatozoa and PBMCs following incubation with PI-PLC, confirming that these proteins are attached to the IAM via a GPI-anchor.
There have been consistent reports that spermatozoal CD59 exists as a single 20 kDa protein, the same as expressed by other cell types27,28,31. In contrast, Western blot analysis of spermatozoal CD46 has shown a single 47 kDa band compared to the two band (65 and 55 kDa) profile expressed by PBMCs which represents the BC1/2 and C1/2 CD46 isoforms, the difference in molecular weight of these forms being mostly due to O-glycosylation in the STP-B region44. However, AR-spermatozoal CD46 is thought to be a hypoglycosylated C2 form and the reduced molecular weight is due to trimming of N-linked oligosaccharides20. Similarly, we have shown that AR-spermatozoal CD55 displays a single band with a Mr of approximately 55 kDa, some 15 kDa lower that that expressed by PBMC or HeLa cells. In contrast to Bozas et al.27 but in agreement with both Cervoni et al.30 and Simpson et al.28 it appears that, as well as CD46, human spermatozoa also express a unique isoform of the structurally related complement regulatory protein CD55. Treatment with N-glycosidase had no effect on the Mr for both AR-spermatozoa and HeLa cell CD55. However, in contrast to the unillustrated report of Cervoni et al.30 AR-spermatozoa treated with endo-α-N-acetylgalactosaminidase or O-glycosidase gave a small reduction in Mr of approximately 3 kDa, whereas HeLa cell lysates treated with these glycosidases produced a decrease of approximately 22 kDa in the CD55 Mr; this is compatible with a core nonglycosylated CD55 protein of 48 kDa45. It is not clear why CD55-associated glycoconjugates may be resistant to enzymatic release, although we can speculate that spermatozoal CD55 lacks mature O-linked oligosaccharides.
Spermatozoal CD46, CD55 and CD59 have been shown to be functional, providing protection from C-mediated damage in the female genital tract where there are significant levels of C proteins together with C cleavage enzymes and potential C activators3. However, it has often been assumed in the literature that CD55 and CD59 are not expressed on the IAM, and therefore provide protection of spermatozoa from C-mediated damage only in the lower regions of the female genital tract, while CD46 provides protection during the final steps of fertilisation25,46. However, our data have shown that all three mCRP are present on the IAM, and the strong coexpression of CD46 and CD55 could suggest some functional cooperativity immediately prior to fertilisation.
The levels of expression of the mCRPs, CD46, CD55 and CD59, on the spermatozoal IAM may be unexpected. However, in addition to its conventional role as a regulator of C, data have accumulated to indicate that CD46 participates also in spermatozoal events leading to fertilisation1,17,20–24. Much less is known on the potential role of spermatozoal CD55 and CD59. Complement-independent defective spermatogenesis has been reported in knockout mice lacking spermatozoal CD5947. In somatic cells, CD55 and CD59 as well as CD46 have been shown to be involved in cell adhesion and contribute to intracellular signalling46,48,49. In particular, crosslinking GPI-anchored mCRP into lipid rafts can contribute to activating T cells through the tyrosine kinase pathway; such lipid raft-associated events lead to signalling that can result in changes in intracellular calcium concentrations50,51. Cholesterol is a major player in maintaining the integrity of lipid rafts, and removal of raft cholesterol leads to dissociation of most proteins from rafts and renders them nonfunctional52. Spermatozoal capacitation involves a diverse series of biochemical and physical changes that enables the subsequent acrosome reaction. The first event in capacitation is cholesterol efflux leading to elevation of intracellular calcium concentration mediated also by the tyrosine kinase pathway13,53. This influx of calcium is essential for fusion of the plasma membrane with the outer acrosomal membrane, initiating the acrosome reaction54. Cholesterol depletion has been shown to destabilise lipid rafts and suppress capacitation55. CD55 may therefore be involved in the signalling machinery for this event. In support of this, antibodies to CD55 have been reported to inhibit the acrosome reaction induced by the oocyte-cumulus complex but not by calcium ionophore46.
In summary, an unusual isoform of GPI-anchored CD55 is markedly expressed on the IAM of human spermatozoa, more than on the surface plasma membrane. Co-localisation experiments revealed also that IAM CD55 is associated with lipid rafts, further supporting the concept that CD55 could be involved in intracellular signalling events prior to fertilisation. Its exact role, however, remains to be determined.
Abbreviations
- AR
acrosome-reacted
- AUR
acrosome-unreacted
- C
complement
- CTB
cholera toxin B
- FITC
fluorescein isothiocyanate
- GPI
glycosylphosphatidylinositol
- HRP
horseradish peroxidase
- IAM
inner acrosomal membrane
- mAb
monoclonal antibody
- mCRP
membrane complement regulatory protein
- pAb
polyclonal antibody
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- PBS-T
PBS with Tween 20
- PI-PLC
phosphoinositol-specific phospholipase C
- R
rhodamine
- RBC
red blood cells
- SCR
short consensus repeat
- SDS-PAGE
sodium dodecyl sulphate – polyacylamide gel electrophoresis
- SWM-HTF
sperm wash modified – human tubular fluid
- TR
Texas Red
- WGA
wheat germ agglutinin
References
- 1.Hourcade D, Holers VM, Atkinson JP. The regulators of complement activation (RCA) gene cluster. Adv Immunol. 1989;45:381–416. doi: 10.1016/s0065-2776(08)60697-5. [DOI] [PubMed] [Google Scholar]
- 2.Lublin DM, Atkinson JP. Decay accelerating factor. biochemistry, molecular biology, and function. Ann Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- 3.Harris CL, Mizuno M, Morgan BP. Complement and complement regulators in the male reproductive system. Mol Immunol. 2006;43:57–67. doi: 10.1016/j.molimm.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Davies A, Lachmann PJ. Membrane defence against complement lysis. the structure and biological properties of CD59. Immunol Res. 1993;12:258–75. doi: 10.1007/BF02918257. [DOI] [PubMed] [Google Scholar]
- 5.Liszewski MK, Farries TC, Lublin DM, Rooney IA, Atkinson JP. Control of the complement system. Adv Immunol. 1996;61:201–83. doi: 10.1016/s0065-2776(08)60868-8. [DOI] [PubMed] [Google Scholar]
- 6.Cattaneo R. Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as pathogens' magnet. J Virol. 2004;78:4385–8. doi: 10.1128/JVI.78.9.4385-4388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell S. CD46. a complement regulator and pathogen receptor that mediates links between innate and acquired immune function. Tissue Antigens. 2004;64:111–8. doi: 10.1111/j.1399-0039.2004.00277.x. [DOI] [PubMed] [Google Scholar]
- 8.Maurer K, Krey T, Moennig V, Thiel HJ, Rumenapf T. CD46 is a cellular receptor for bovine viral diarrhea virus. J Virol. 2004;78:1792–9. doi: 10.1128/JVI.78.4.1792-1799.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodfellow IG, Powell RM, Ward T, Spiller OB, Almond JW, Evans DJ. Echovirus infection of rhabdomyosarcoma cells is inhibited by antiserum to the complement control protein CD59. J General Virol. 2000;81:1393–401. doi: 10.1099/0022-1317-81-5-1393. [DOI] [PubMed] [Google Scholar]
- 10.Bernet J, Mullick J, Singh AK, Sahu A. Viral mimicry of the complement system. J Biosci. 2003;28:249–64. doi: 10.1007/BF02970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanagimachi R. The movement of golden hamster spermatozoa before and after capacitation. J Reprod Fertil. 1970;23:193–6. doi: 10.1530/jrf.0.0230193. [DOI] [PubMed] [Google Scholar]
- 12.Wassarman PM. Early events in mammalian fertilization. Ann Rev Cell Biol. 1987;3:109–42. doi: 10.1146/annurev.cb.03.110187.000545. [DOI] [PubMed] [Google Scholar]
- 13.Cross NL. Role of cholesterol in sperm capacitation. Biol Reprod. 1998;59:7–11. doi: 10.1095/biolreprod59.1.7. [DOI] [PubMed] [Google Scholar]
- 14.Jaiswal BS, Cohen-Dayag A, Tur-Kaspa I, Eisenbach M. Sperm capacitation is, after all, a prerequisite for both partial and complete acrosome reaction. FEBS Lett. 1998;427:309–13. doi: 10.1016/s0014-5793(98)00455-4. [DOI] [PubMed] [Google Scholar]
- 15.Jaiswal BS, Eisenbach M, Tur-Kaspa I. Detection of partial and complete acrosome reaction in human spermatozoa. which inducers and probes to use? Mol Hum Reprod. 1999;5:214–9. doi: 10.1093/molehr/5.3.214. [DOI] [PubMed] [Google Scholar]
- 16.Riley-Vargas RC, Lanzendorf S, Atkinson JP. Targeted and restricted complement activation on acrosome-reacted spermatozoa. J Clin Invest. 2005;115:1241–9. doi: 10.1172/JCI23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson DJ, Michaelson JS, Johnson PM. Trophoblast/leukocyte-common antigen is expressed by human testicular germ cells and appears on the surface of acrosome-reacted sperm. Biol Reprod. 1989;41:285–93. doi: 10.1095/biolreprod41.2.285. [DOI] [PubMed] [Google Scholar]
- 18.Riley RC, Kemper C, Leung M, Atkinson JP. Characterization of human membrane cofactor protein (MCP; CD46) on spermatozoa. Mol Reprod Dev. 2002;62:534–46. doi: 10.1002/mrd.10144. [DOI] [PubMed] [Google Scholar]
- 19.Li B, Sallee C, Dehoff M, Foley S, Molina H, Holers VM. Mouse Crry/p65. Characterization of monoclonal antibodies and the tissue distribution of a functional homologue of human MCP and DAF. J Immunol. 1993;151:4295–305. [PubMed] [Google Scholar]
- 20.Riley RC, Tannenbaum PL, Abbott DH, Atkinson JP. Inhibiting measles virus infection but promoting reproduction. an explanation for splicing and tissue-specific expression of CD46. J Immunol. 2002;169:5405–9. doi: 10.4049/jimmunol.169.10.5405. [DOI] [PubMed] [Google Scholar]
- 21.D'Cruz OJ, Lambert H, Haas GG., Jr Expression of CD15 (Lewisx) antigen on human sperm and its role in sperm–egg interaction. Am J Reprod Immunol. 1997;37:172–83. doi: 10.1111/j.1600-0897.1997.tb00209.x. [DOI] [PubMed] [Google Scholar]
- 22.Okabe M, Nagira M, Kawai Y, Matzno S, Mimura T, Mayumi T. A human sperm antigen possibly involved in binding and/or fusion with zona-free hamster eggs. Fertil Steril. 1990;54:1121–6. doi: 10.1016/s0015-0282(16)54015-1. [DOI] [PubMed] [Google Scholar]
- 23.Taylor CT, Biljan MM, Kingsland CR, Johnson PM. Inhibition of human spermatozoon–oocyte interaction in vitro by monoclonal antibodies to CD46 (membrane cofactor protein) Hum Reprod. 1994;9:907–11. doi: 10.1093/oxfordjournals.humrep.a138615. [DOI] [PubMed] [Google Scholar]
- 24.Inoue N, Ikawa M, Nakanishi T, et al. Disruption of mouse CD46 causes an accelerated spontaneous acrosome reaction in sperm. Mol Cell Biol. 2003;23:2614–22. doi: 10.1128/MCB.23.7.2614-2622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rooney IA, Davies A, Morgan BP. Membrane attack complex (MAC) -mediated damage to spermatozoa. protection of the cells by the presence on their membranes of MAC inhibitory proteins. Immunology. 1992;75:499–506. [PMC free article] [PubMed] [Google Scholar]
- 26.D'Cruz OJ, Haas GG., Jr The expression of the complement regulators CD46, CD55, and CD59 by human sperm does not protect them from antisperm antibody- and complement-mediated immune injury. Fertil Steril. 1993;59:876–84. doi: 10.1016/s0015-0282(16)55875-0. [DOI] [PubMed] [Google Scholar]
- 27.Bozas SE, Kirszbaum L, Sparrow RL, Walker ID. Several vascular complement inhibitors are present on human sperm. Biol Reprod. 1993;48:503–11. doi: 10.1095/biolreprod48.3.503. [DOI] [PubMed] [Google Scholar]
- 28.Simpson KL, Holmes CH. Differential expression of complement regulatory proteins decay accelerating factor (CD55), membrane cofactor protein (CD46) and CD59 during human spermatogenesis. Immunology. 1994;81:452–61. [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor CT, Johnson PM. Complement-binding proteins are strongly expressed by human preimplantation blastocysts and cumulus cells as well as gametes. Mol Hum Reprod. 1996;2:52–9. doi: 10.1093/molehr/2.1.52. [DOI] [PubMed] [Google Scholar]
- 30.Cervoni F, Oglesby TJ, Fenichel P, et al. Expression of decay accelerating factor (CD55) of the complement system on human spermatozoa. J Immunol. 1993;151:939–48. [PubMed] [Google Scholar]
- 31.Fenichel P, Cervoni F, Hofmann P, et al. Expression of the complement regulatory protein CD59 on human spermatozoa: characterization and role in gametic interaction. Mol Reprod Dev. 1994;38:338–46. doi: 10.1002/mrd.1080380316. [DOI] [PubMed] [Google Scholar]
- 32.Moore HD, Smith CA, Hartman TD, Bye AP. Visualization and characterization of the acrosome reaction of human spermatozoa by immunolocalization with monoclonal antibody. Gamete Res. 1987;17:245–9. doi: 10.1002/mrd.1120170308. [DOI] [PubMed] [Google Scholar]
- 33.Johnson PM, Molloy CM. Localization in human term placental bed and amniochorion of cells bearing trophoblast antigens identified by monoclonal antibodies. Am J Reprod Immunol. 1983;4:33–7. doi: 10.1111/j.1600-0897.1983.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 34.Harris SJ, Milligan MP, Masson GM, Dennis KJ. Improved separation of motile sperm in asthenospermia and its application to artificial insemination homologous (AIH) Fertil Steril. 1981;36:219–21. doi: 10.1016/s0015-0282(16)45682-7. [DOI] [PubMed] [Google Scholar]
- 35.D'Cruz OJ, Hass GG., Jr Flow cytometric quantitation of the expression of membrane cofactor protein as a marker for the human sperm acrosome reaction. Fertil Steril. 1992;58:633–6. [PubMed] [Google Scholar]
- 36.Kallajoki M, Malmi R, Virtanen I, Suominen J. Glycoconjugates of human sperm surface. A study with fluorescent lectin conjugates and lens culinaris agglutinin affinity chromatography. Cell Biol Int Rep. 1985;9:151–64. doi: 10.1016/0309-1651(85)90089-x. [DOI] [PubMed] [Google Scholar]
- 37.Kenworthy AK, Petranova N, Edidin M. High-resolution FRET microscopy of cholera toxin B-subunit and GPI-anchored proteins in cell plasma membranes. Mol Biol Cell. 2000;11:1645–55. doi: 10.1091/mbc.11.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 39.Bhavanandan VP, Umemoto J, Davidson EA. Characterization of an endo-alpha-N-acetyl galactosaminidase from Diplococcus pneumoniae. Biochem Biophys Res Commun. 1976;70:738–45. doi: 10.1016/0006-291x(76)90654-9. [DOI] [PubMed] [Google Scholar]
- 40.Davitz MA, Low MG, Nussenzweig V. Release of decay-accelerating factor (DAF) from the cell membrane by phosphatidylinositol-specific phospholipase C (PIPLC). Selective modification of a complement regulatory protein. J Exp Med. 1986;163:1150–61. doi: 10.1084/jem.163.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walter EI, Roberts WL, Rosenberry TL, Ratnoff WD, Medof ME. Structural basis for variations in the sensitivity of human decay accelerating factor to phosphatidylinositol-specific phospholipase C cleavage. J Immunol. 1990;144:1030–6. [PubMed] [Google Scholar]
- 42.Kooyman DL, Byrne GW, McClellan S, et al. In vivo transfer of GPI-linked complement restriction factors from erythrocytes to the endothelium. Science. 1995;269:89–92. doi: 10.1126/science.7541557. [DOI] [PubMed] [Google Scholar]
- 43.Rooney IA, Heuser JE, Atkinson JP. GPI-anchored complement regulatory proteins in seminal plasma. An analysis of their physical condition and the mechanisms of their binding to exogenous cells. J Clin Invest. 1996;97:1675–86. doi: 10.1172/JCI118594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seya T, Hirano A, Matsumoto M, Nomura M, Ueda S. Human membrane cofactor protein (MCP, CD46). multiple isoforms and functions. Int J Biochem Cell Biol. 1999;31:1255–60. doi: 10.1016/s1357-2725(99)00092-8. [DOI] [PubMed] [Google Scholar]
- 45.Lublin DM, Krsek-Staples J, Pangburn MK, Atkinson JP. Biosynthesis and glycosylation of the human complement regulatory protein decay accelerating factor. J Immunol. 1986;137:1629–35. [PubMed] [Google Scholar]
- 46.D'Cruz OJ. Adhesion molecules in human sperm–oocyte interaction: relevance to infertility. Front Biosci. 1996;1:161–76. doi: 10.2741/a123. [DOI] [PubMed] [Google Scholar]
- 47.Qin X, Dobarro M, Bedford SJ, et al. Further characterization of reproductive abnormalities in mCd59b knockout mice: a potential new function of mCD59 in male reproduction. J Immunol. 2005;175:6294–302. doi: 10.4049/jimmunol.175.10.6294. [DOI] [PubMed] [Google Scholar]
- 48.Morgan BP, van den Berg CW, Davies EV, Hallett MB, Horejsi V. Cross-linking of CD59 and of other glycosyl phosphatidylinositol-anchored molecules on neutrophils triggers cell activation via tyrosine kinase. Eur J Immunol. 1993;23:2841–50. doi: 10.1002/eji.1830231118. [DOI] [PubMed] [Google Scholar]
- 49.Stefanova I, Horejsi V, Ansotegui IJ, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–9. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- 50.Lund-Johansen F, Olweus J, Symington FW, et al. Activation of human monocytes and granulocytes by monoclonal antibodies to glycosylphosphatidylinositol-anchored antigens. Eur J Immunol. 1993;23:2782–91. doi: 10.1002/eji.1830231110. [DOI] [PubMed] [Google Scholar]
- 51.van den Berg CW, Cinek T, Hallett MB, Horejsi V, Morgan BP. Exogenous glycosyl phosphatidylinositol-anchored CD59 associates with kinases in membrane clusters on U937 cells and becomes Ca (2+)-signaling competent. J Cell Biol. 1995;131:669–77. doi: 10.1083/jcb.131.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J Clin Invest. 2002;110:597–603. doi: 10.1172/JCI16390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breitbart H. Signaling pathways in sperm capacitation and acrosome reaction. Cell Mol Biol. 2003;49:321–7. [PubMed] [Google Scholar]
- 54.Yanagimachi R, Usui N. Calcium dependence of the acrosome reaction and activation of guinea pig spermatozoa. Exp Cell Res. 1974;89:161–74. doi: 10.1016/0014-4827(74)90199-2. [DOI] [PubMed] [Google Scholar]
- 55.Shadan S, James PS, Howes EA, Jones R. Cholesterol efflux alters lipid raft stability and distribution during capacitation of boar spermatozoa. Biol Reprod. 2004;71:253–65. doi: 10.1095/biolreprod.103.026435. [DOI] [PubMed] [Google Scholar]