Abstract
CD4+ CD45RO+ T cells could mature freshly isolated human plasmacytoid dendritic cells (PDC) in a superantigen-driven culture in a similar way to recombinant interleukin-3 (IL-3). Mature PDC expressed significantly higher levels of inducible costimulator ligand (ICOS-L), but similar levels of CD80 and CD86, when compared to mature monocyte-derived DC (moDC). We therefore directly compared the capacities of mature PDC and moDC to activate T cells. A similar T helper type 1 (Th1)/Th2 pattern of cytokines was generated in both systems, but significantly higher levels of IL-3, IL-4 and IL-10 were induced by PDC. In T cells interacting with PDC, the ICOS/ICOS-L costimulatory pathway played a pre-eminent role in the generation of IL-3 and IL-10, CD28 was central to the induction of IL-2, and both pathways were equally important for the generation of other cytokines. In cocultures with moDC, the CD28 pathway was dominant over ICOS under all circumstances, except for the ICOS-mediated release of IL-10. In general, our data demonstrate an eminent role of ICOS in the interaction of T cells with PDC, and thus modify the current paradigm of CD28 dominance for the costimulation of T cells interacting with professional antigen-presenting cells. In particular, our data highlight the role of ICOS in the generation of IL-3, a factor central to the biology of human PDC.
Keywords: co-stimulation co-stimulating molecules, dendritic cells, human studies, T cells
Introduction
Dendritic cells (DC) are professional antigen-presenting cells (APC), with a potent capacity to initiate and maintain T-cell-mediated immune responses.1–6 Classical DC, e.g. epidermal Langerhans cells, typically reside in peripheral tissues in an immature state. They efficiently take up antigen, but are poor stimulators of naive T cells because they largely lack the surface ligands (CD80, CD86) required for initial T-cell costimulation.7–9 Upon exposure to inflammatory signals, DC migrate from peripheral tissues to T-cell areas of lymphoid organs and concomitantly acquire the capacity to efficiently present antigen to naive T cells by up-regulating the major histocompatibility complex (MHC) class II molecules, CD80 and CD86.9,10 Immature and mature classical DC can be generated in vitro from monocytes11–13 and are then designated ‘monocyte-derived DC’ (moDC).
Recently, another type of DC has been identified.14–16 These DC circulate in the peripheral blood in an immature state and are distinguishable by their plasma cell-like morphology. These ‘plasmacytoid dendritic cells’ (PDC) characteristically express high levels of the interleukin-3 receptor (IL-3R) α-chain (CD123)15,17 and synthesize large amounts of IFN-α upon contact with viruses and bacteria (reviewed in ref. 18). Similar to myeloid DC, PDC do not express significant levels of CD80 and CD86 in their immature state in the peripheral blood.14–16 However, upon exposure to exogenous IL-3 and/or to an exogenous source of CD40 ligand (CD40L), PDC acquire a mature phenotype, assume a dendritic morphology and up-regulate CD80 and CD86.14,16,19 Compared to classical DC, PDC are much less characterized. In particular, PDC surface molecules involved in the activation of T cells have not been functionally examined. Moreover, the physiological source(s) of the maturation factors IL-3 and/or CD40L have not been determined.
T-cell costimulation is a complex and hierarchical process (for compilation of reviews see ref. 20). Naive T cells constitutively express CD28, an important T-cell costimulatory molecule, which interacts with its two ligands CD80 and CD86, mainly expressed on professional APC. Upon initial activation, T cells up-regulate inducible costimulator (ICOS), another central T-cell costimulator, which is structurally and functionally closely related to CD28.21 Once expressed, ICOS interacts with its sole ligand, ICOS-L, which is expressed not only on professional APC but also on a variety of peripheral tissues.20,22,23
To better understand the similarities and differences of CD28 and ICOS function in humans, we have in the past analysed their relative contributions to the costimulation of T cells interacting with classical mature DC. In these initial studies we used CD45RA+ naive T cells as responders, and also T cells expanded by IL-2, a model system representing late effector T cells.7
The present study was undertaken to directly compare the costimulatory capacity of classical moDC with that of PDC. Our results indicate at various levels that the ICOS/ICOS-L axis plays an eminent role in the costimulation of T cells interacting with PDC and is of relatively less importance for their interaction with moDC.
Materials and methods
Isolation and maturation of PDC
Peripheral blood mononuclear cells (PBMC) were isolated from the peripheral blood of healthy donors as previously described.7 PDC were sorted magnetically using the BDCA4-Isolation-Kit according to the manufacturer's instructions (Miltenyi-Biotec, Bergisch-Gladbach, Germany). Purity of the isolated PDC was routinely > 94%. Freshly sorted immature PDC (6 × 104) were matured in 96-well round-bottomed culture plates in RPMI-1640 supplemented with 10 ng/ml IL-3 (Peprotech, London, UK), in the presence of l-cells (mouse fibroblastic Ltk− cells; 1·4 × 104) transfected with human CD40L. After 2 days of culture, matured PDC were washed with phosphate-buffered saline (PBS) with 2 mm ethylenediaminetetraacetic acid, and used for experiments.
Generation of mature moDC
MoDC were generated as described elsewhere.7 Briefly, monocytes were isolated from PBMC by adherence on cell culture dishes (Corning, Corning, NY) in RPMI-1640 supplemented with 1% heat-inactivated human serum. Adherent monocytes were cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mm glutamine (both Biochrom, Berlin, Germany), 100 μg/ml streptomycin, 100 μg/ml penicillin (both PAA, Coelbe, Germany) in the presence of 800 U/ml granulocyte–macrophage colony-stimulating factor (GM-CSF; Novartis, Basel, Switzerland) and 1000 U/ml IL-4 (kindly provided by Dr Kalthoff, Vienna, Austria) for 7 days. Resulting immature moDC were matured with 250 ng/ml lipopolysaccharide (Sigma-Aldrich, Deisenhofen, Germany), 500 U/ml IL-4, 800 U/ml GM-CSF and 1 μm prostaglandin E2 (Calbiochem-Novabiochem, Schwalbach, Germany) for 2 days. After extensive washing, moDC were used in experiments. Purity, as determined by the expression of CD11c, was always > 90%. Full maturation was controlled by CD83 staining.
Isolation of CD4+CD45R0+ T cells
T cells were enriched from PBMC by passage over a nylon-wool column. CD4+ CD45RO+ T cells were obtained by a magnetodepletion procedure using the following monoclonal antibodies (mAb): BU12 (anti-CD1924), B73.1 (anti-CD1625), L243 (anti-HLA-DR), OKM 1 (anti-CD11b), 10F7MN (anti-glycophorin A), OKT 8 (anti-CD8), all from the American Type Culture Collection (ATCC, Manassas, VA, USA) and 4G11 (anti-CD45RA26), and goat anti-mouse immunoglobulin G (IgG) magnetobeads (Miltenyi-Biotec), resulting in a purity > 96%.
Cell culture systems
MoDC or PDC were cocultured with T cells in 96-well round-bottomed culture plates at a ratio of 1 : 10 with 1 × 105 T cells, or in 48-well culture plates (1 : 20 with 1 × 106 T cells), in the presence of 1 ng/ml Staphylococcus aureus enterotoxin B (SEB; Toxin Technology, Sarasota, FL). Blocking was performed with mAb HIL-131 (anti-ICOS-L7) or with a cytotoxic T-lymphocyte antigen 4—immunoglobulin (CTLA-4Ig) fusion protein (ATCC). The mAb 3G11 (own hybridoma) was used as an IgG1 isotype control. The influence of IL-3 on the survival of PDC was tested on freshly isolated naive PDC cocultured with T cells (2 × 105) in 96-well round-bottomed culture plates at a ratio of 1 : 10 in the presence of 1 ng/ml SEB. IL-3 was neutralized by mAb BVD8-3G11, IL-3 receptor α-chain was blocked by mAb 7G3 (both BD Biosciences, Heidelberg, Germany). CD40/CD40L interaction was blocked by mAb G28-5 (anti-CD40, ATCC), 18.1.16 (muIgG1)27 and 1D10 (rat IgG2a, own hybridoma) were used as isotype controls. All blocking mAb were used at a concentration of 20 μg/ml. After 3–4 days of culture, total cell number was determined using flow cytometry and perfect-count microsphere beads (Caltag, Hamburg, Germany) according to the manufacturer's instructions.
Flow cytometry
Before staining, cells were preincubated with excess human IgG (Endobulin®, Baxter, Heidelberg, Germany) to block the Fc-receptors. Dead cells were excluded by propidium iodide staining (0·33 μg/ml). The following mAb were used for staining: AC144-fluorescein isothiocyanate (FITC; anti-BDCA2), AC145-allophycocyanin (anti-CD123, both from Miltenyi-Biotec), IT2.2-FITC (anti-CD86), L307.4-phycoerythrin (PE; anti-CD80, both from BD Biosciences), L243-Cy5® (anti-HLA-DR, ATCC; Cy®5 from GE Healthcare Life Sciences, Buckinghamshire, UK), HIL-131-PE (anti-ICOS-L), 2A11-FITC/-PE/-Cy5/-allophycocyanin (murine IgG1 isotype control, own hybridoma). Immunofluorescence analysis was performed on a FACSCalibur (BD Biosciences).
Intracellular flow cytometry
Intracellular staining was performed as previously described28 with the following modifications. After 16 hr of coculture of T cells with matured PDC, brefeldin A (5 μg/ml, Sigma-Aldrich) was added for 7 hr. Cells were washed with PBS and fixed with 2% formaldehyde in PBS for 20 min. OKT 3-Cy5 (anti-CD3, ATCC) was used for staining T cells. For intracellular IL-3 detection 2·5 × 106 cells/ml were permeabilized with 0·5% saponin (Sigma-Aldrich) and stained with mAb BVD3-1F9 coupled with PE. For cold blocking, mAb BVD3-1F9 (both BD Biosciences) at a concentration of 100 μg/ml was used.
Cytokine analysis by enzyme-linked immunosorbent assay
Cytokines in supernatants of cocultures were determined by sandwich enzyme-linked immunosorbent assay (ELISA) using mAb pairs 653A10B1 and 653A7D6 (IL-3), 860A4B3 and 860F10H12 (IL-4), 677B6A2 and 505E23C7 (IL-6), 68B2B3 and 68B3C5 (tumour necrosis factor-α, all from Biosource, Camarillo, CA), 4SB3 and 7R2A4 [interferon-γ (IFN-γ), both European Collection of Cell Cultures, ECACC29,30], 5344.111 and B33-2 (IL-2), JES1-39D10 and JES1-5A10 (IL-5), JES3-19F1 and JES3-12G8 (IL-10), JES10-5A2 and B69-2 (IL-13, all BD Biosciences).
Results
ICOS-L, expressed on immature PDC, is strongly up-regulated after in vitro PDC maturation with IL-3 and CD40L
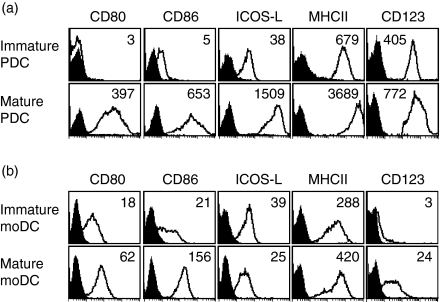
We analysed freshly isolated human PDC from the peripheral blood for the expression of a number of cell surface molecules. These ‘immature’ PDC expressed high levels of the IL-3 receptor α-chain (CD123) and MHC class-II molecules, but were almost negative for CD80 and CD86, as described previously (Fig. 1a and ref. 14–17). In contrast, ICOS-L, the ligand for ICOS, was present on immature PDC at significant levels (Fig. 1a). Maturation of PDC in the presence of a CD40L-expressing transfectant and IL-314 for 48 hr, led to a strong up-regulation of CD80, CD86, ICOS-L, and also MHC-class II molecules and CD123, with ICOS-L reaching very high surface densities (Fig. 1a). When moDC were analysed for comparison, CD80, CD86 and ICOS-L were found on immature moDC at significant levels. Maturation of moDC resulted in substantial up-regulation of CD80 and CD86, whereas ICOS-L remained at the same level or was even down-regulated (Fig. 1b and refs 7, 31, 32). CD123 was absent on immature moDC, but became detectable after maturation (Fig. 1b).
Figure 1.
High up-regulation of ICOS-L on mature PDC. (a) Human PDC were isolated from the peripheral blood and analysed by flow cytometry for the expression of CD80, CD86, ICOS-L, MHC class-II and the IL-3 receptor α-chain (CD123) immediately after isolation or after maturation in the combined presence of IL-3 and a CD40L-expressing transfectant for 48 hr. One representative experiment of five is shown. (b) Human immature monocyte-derived DC (moDC) and mature moDC were analysed for the expression of the same markers for comparison. Black histograms represent isotype control staining, inset numbers indicate the mean fluorescence over all cells minus the isotype control mean fluorescence.
Maturation of PDC also occurs on coculture with effector T cells
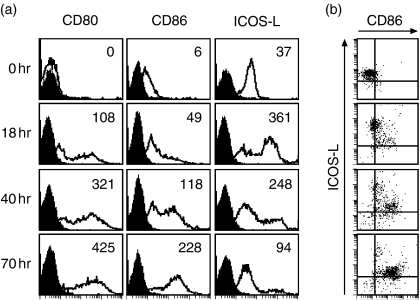
To determine whether T cells can deliver maturation signals similar to exogenous IL-3 and CD40L, freshly isolated PDC were cocultured with CD4+ CD45RO+ T cells over 70 hr in the presence of the superantigen SEB. This coculture led to an up-regulation of CD80, CD86 and ICOS-L within 18 hr (Fig. 2a). Thereafter, CD80 and CD86 were further up-regulated, whereas ICOS-L was down-regulated again (Fig. 2a). CD86 (Fig. 2b) and CD80 (not shown) up-regulation occurred only on PDC with down-regulated ICOS-L, indicating a reverse regulation of these ligands on the PDC surface. The latter observation most likely reflects the direct physical interaction of T cells with PDC. T-cell derived signals, most notably CD40L, are known to up-regulate CD80 and CD86 on APC, whereas ICOS/ICOS-L interaction leads to a down-regulation of ICOS-L.7,33 The observed up-regulation of the maturation markers CD80, CD86 and ICOS-L was marginal on coculture with CD4+ CD45RA+ T cells in the presence of SEB, and did not occur in the absence of T cells. Without T cells the survival rate of PDC dropped very quickly (data not shown).
Figure 2.
PDC mature in the presence of antigen-experienced T cells. (a) Freshly isolated PDC were cocultured with peripheral blood CD4+ CD45RO+ T cells for the indicated time periods in the presence of SEB and analysed by flow cytometry for the expression of CD80, CD86 and ICOS-L. Black histograms represent isotype control staining, the inset numbers indicate the mean-fluorescence over all cells minus the mean fluorescence of the isotype control. Shown is one representative experiment out of three. (b) Two-colour staining of the PDC for the expression of CD86 and ICOS-L. One representative experiment out of three is shown.
Mature PDC and mature moDC induce different cytokine profiles in T cells
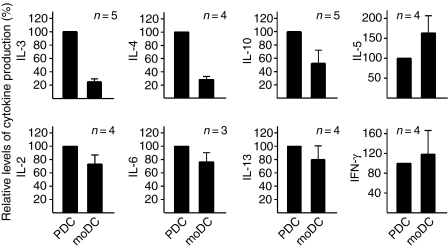
PDC were directly compared with moDC for their capacity to stimulate cytokines on coculture with T cells. In preliminary experiments, cocultures (24 hr) of CD4+CD45RA+ T cells with freshly isolated PDC in the presence of SEB did not lead to the secretion of any of the cytokines measured (data not shown). Apparently, the CD28 molecule constitutively expressed on T cells did not encounter effective CD80/CD86 levels, and the ICOS-L molecule on naive PDC did not encounter significant levels of ICOS on the T-cell surface. Cocultures of naive T cells with matured PDC only yielded low levels of IL-2, IL-6 and tumour necrosis factor-α (data not shown). We therefore focused on comparative experiments in which CD4+ CD45RO+ T cells from a given donor were cocultured with mature PDC and in parallel with mature moDC. Mature PDC induced in T cells significantly higher levels of IL-3, IL-4 and IL-10, whereas moDC were more efficient in the induction of IL-5, as shown in a summary of a series of experiments (Fig. 3). Induction of IL-2, IL-6, IL-13 and IFN-γ was generally similar for both types of DC (Fig. 3; for absolute levels of cytokines measured see legend to Fig. 3). As an additional readout for T-cell activation we compared the regulation of several cell surface markers (CD25, CD69, OX40, ICOS) by flow cytometry. The moDC were slightly superior in induction of these activation markers than mature PDC (data not shown).
Figure 3.
Relative cytokine production in cocultures of T cells with mature PDC or mature moDC. Mature PDC or mature moDC were cocultured with CD4+ CD45RO+ T cells from a given donor for 48 hr in the presence of SEB, and cytokine levels in culture supernatants were determined by ELISA. In each comparative experiment the level of each cytokine in the PDC/T-cell coculture was set as 100%. The data shown represent the average (± SEM) of three to five comparative experiments. In a typical comparative experiment the following absolute cytokine levels were measured in the T/PDC coculture: IL-3, 73 ng/ml; IL-4, 704 pg/ml; IL-10, 8 ng/ml; IL-5, 210 pg/ml; IL-2, 47 ng/ml; IL-6, 803 pg/ml; IL-13, 1371 pg/ml; IFN-γ, 1806 pg/ml; and in the T/moDC coculture: IL-3, 19 ng/ml; IL-4, 194 pg/ml; IL-10, 1·5 ng/ml; IL-5, 581 pg/ml; IL-2, 29 ng/ml; IL-6, 558 pg/ml; IL-13, 1513 pg/ml; IFN-γ, 1432 pg/ml.
ICOS, but not CD28, is important for the generation of IL-3 and IL-10 in cocultures of T cells with PDC
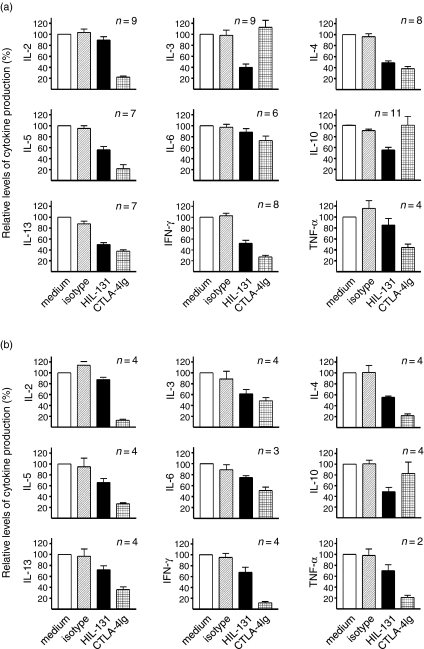
In the next step we determined the individual contribution of the CD28 and ICOS costimulatory pathways for the induction of a number of cytokines in cocultures of CD4+ CD45RO+ T cells with mature PDC versus mature moDC. In these experiments, the CD28 pathway was blocked by addition of CTLA-4Ig, the ICOS pathway was blocked by mAb HIL-131 directed to ICOS-L.7 Interestingly, in cocultures with mature PDC, the generation of IL-3 and IL-10 was significantly and exclusively reduced by the blockade of the ICOS pathway (Fig. 4a). The synthesis of IL-4, IL-5, IL-13, and IFN-γ was reduced by blocking of either the ICOS or CD28 pathways, whereas the synthesis of IL-6 remained largely unaffected. Diminished secretion of the ‘early’ cytokines IL-2 and tumour necrosis factor-α was only observed on blocking the CD28 pathway (Fig. 4a). These experiments indicated an overall equivalent importance of the CD28 and ICOS pathways for the costimulation of T cells by PDC, with an exclusive role of ICOS for the induction of IL-3 and IL-10.
Figure 4.
Relative contribution of the CD28/B7 and ICOS/ICOS-L pathways for the costimulation of T cells interacting with PDC versus moDC. CD4+ CD45RO+ T cells from a given donor were cocultured with (a) mature PDC or (b) mature moDC for 48 hr in the presence of SEB and the cytokine levels were determined by ELISA. The ICOS costimulation pathway was blocked with anti-ICOS-L mAb HIL-131, the CD28 pathway was blocked with CTLA-4Ig, an isotype control mAb was also used. In each experiment, the cytokine level observed in the medium control was set as 100%. The data shown represent two to eleven experiments.
In parallel cocultures of CD45RO+ T cells from the same donor with moDC, the production of IL-10 remained the domain of the ICOS costimulatory pathway, whereas a significant blockade of IL-3 induction could also be achieved with CTLA-4Ig (Fig. 4b). As expected by the specific role of CD28 for IL-2 gene induction, the generation of IL-2 was again inhibited only by blocking CD28 (Fig. 4b). The levels of the remaining cytokines were reduced by the blockade of either the ICOS or the CD28 pathways, but the CD28/B7 axis was generally dominant over the ICOS/ICOS-L axis for the costimulation of CD4+ CD45RO+ T cells by moDC (Fig. 4b).
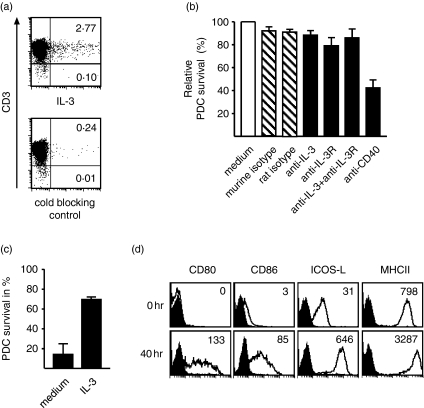
Role of IL-3 for the survival of PDC in cocultures with CD4+ CD45RO+ T cells
The pre-eminent role of the ICOS pathway for the induction of IL-3 in cocultures with PDC, which characteristically express high IL-3 receptor levels, prompted us to investigate this system further. In cocultures with PDC, IL-3 was detected by intracellular flow cytometry in T cells only (Fig. 5a), and PDC cultured in medium alone did not secrete IL-3 (data not shown). Together, these experiments indicated that T cells are the only source of IL-3 in these cocultures. When CD45RO+ T cells were cocultured with PDC and SEB in the presence of a neutralizing anti-IL-3 mAb, BVD3-3G11, and/or with the blocking anti-IL-3 receptor mAb 7G3, we did not observe a significant effect on the survival of PDC (Fig. 5b). In contrast, the inhibition of the CD40L/CD40 axis by the anti-CD40 mAb G28-5 reduced the survival of PDC by 50–60% after 3 days of coculture (Fig. 5b). On freshly isolated PDC, however, IL-3 acted as an effective survival factor (Fig. 5c and ref. 14), and was fully neutralized by either mAb BVD3-3G11 or mAb 7G3 (data not shown). Moreover, IL-3 alone potently up-regulated not only CD80, CD86 (ref. 14 and Fig. 5d), but also ICOS-L (Fig. 5d). These experiments strongly suggested that the IL-3 generated in the cocultures is not essential for the survival of PDC that are physically interacting with T cells, but is effective on isolated immature PDC.
Figure 5.
Source and biological role of IL-3 in the interaction of T cells with PDC. (a) Mature PDC were cocultured with CD4+ CD45RO+ T cells for 24 hr in the presence of SEB, brefeldin A was added for the last 7 hr, and IL-3 was detected by intracellular flow cytometry. Inset numbers indicate the percentage of positive cells. (b) Naive PDC were cocultured with CD4+ CD45RO+ T cells and SEB in the absence or presence of blocking mAb directed to IL-3, the IL-3 receptor, or CD40. PDC survival was determined after 3 days, the medium control was set to 100%. (c,d) Naive PDC were cultured in medium alone or in the presence of IL-3. After 48 hr, the survival rate of PDC (c) and the expression of CD80, CD86, ICOS-L and MHC class-II on PDC cultured in the presence of IL-3 (d) were determined. One representative experiment out of three is shown.
Discussion
After the identification of PDC in human peripheral blood as a new type of dendritic cells14–16 much of the work performed focused on the question as to whether PDC have an inherent property to induce the differentiation of T cells towards T helper type 1 (Th1) versus Th2 cells.19,34,35 In our system, the overall pattern of cytokines generated in cocultures with PDC was similar to that in cocultures with moDC. Nevertheless, there were reproducible differences. IL-4 and IL-10, two typical Th2 cytokines, were generated to clearly higher levels in cocultures with PDC, whereas higher levels of IL-5, another Th2 cytokine, were observed in cocultures with moDC. IL-2 and IFN-γ, the prototypical Th1 cytokines, were generated in both systems to comparable levels, but the same was true for IL-13, a classical Th2 cytokine. We thus could not substantiate earlier conflicting hypotheses that mature PDC induce preferentially Th1 or Th2 cells.19,34,35 It rather seems that human PDC, similar to moDC, do not have an inherent property to induce a certain pattern of cytokines in cocultures with T cells, but instead flexibly respond to Th1- or Th2-biasing maturation signals received through their pattern recognition receptors.36–38 Recent data with human39 and murine40 PDC matured by different methods, strongly support this conclusion.
Previous work had revealed not only an essential role of IL-3, but also an important contribution of the CD40L for the survival and maturation of PDC in vitro 14–16 but did not indicate the physiological sources of these factors. In our studies, we consistently observed high levels of IL-3 in cocultures of T cells with PDC and could demonstrate that IL-3 was generated by the T cells. Furthermore, we observed that CD4+ CD45RO+ T cells (but not CD4+ CD45RA+ T cells) are capable of providing all the necessary signals for PDC maturation in vitro. Interestingly, neutralization of the T-cell-derived IL-3 did not change the survival rate of cocultured PDC, suggesting that IL-3 is not essential for PDC directly contacting activated T cells. These in vitro data suggest a scenario in which initial PDC maturation in vivo is most likely induced through pathogen-derived signals18,41 and not through interaction with naive T cells. However, later in the immune response or on re-exposure, antigen-specific T cells could speed up the maturation of neighbouring PDC either through direct contact or by secreting IL-3. It is even conceivable that the T-cell-derived IL-3 exerts its biological effects in a systemic fashion.42,43
In our work we have, for the first time, attempted to determine the relative contributions of the CD28/B7 and ICOS/ICOS-L pathways for the costimulation of human T cells interacting with moDC versus PDC. A larger series of experiments allowed several conclusions to be drawn. First, the costimulation of IL-2 remained in all systems the domain of the CD28 pathway, as expected. Second, the costimulation of IL-10 was the domain of ICOS, in both the interaction with mature PDC and mature moDC. Third, the relative contribution of the ICOS/ICOS-L was clearly greater in the T/PDC interaction as compared with the T/moDC coculture system. In T/PDC interaction, the ICOS pathway generally seemed to be as important for the costimulation of T cells as the CD28 pathway, whereas in the T/moDC interaction system the CD28 pathway appeared dominant under all circumstances (with the exception of IL-10 release). The greater impact of the ICOS costimulation pathway in the interaction with PDC most likely reflects the very high levels of ICOS-L observed on matured PDC when compared to matured moDC. These data change the current paradigm of a dominant role of the CD28 pathway in all interactions of T cells with professional APC.
ICOS costimulation played a pre-eminent role for the release of IL-10, both in the interaction with PDC and moDC. Following the original observation on the preferential release of IL-10 in human in vitro studies,21 we later demonstrated in various murine in vivo models that high ICOS expression characterizes CD4+ T cells with a high probability of secreting IL-10.28,44 The cellular program linking high ICOS expression and IL-10-secreting capacity in T cells is not identified yet, but most likely explains our finding of a predominant role of ICOS for the release of IL-10 in our present studies.
On isolated triggering of the CD28 or the ICOS pathways with monoclonal antibodies, human CD4+ CD45RO+ T cells respond with a similar production of IL-3 (data not shown), excluding the possibility that only ICOS signalling can induce IL-3. Furthermore, our previous studies did not indicate a link between high ICOS expression and the capacity of T cells to release IL-3.28 There is thus no obvious explanation for the very prominent role of ICOS in the release of IL-3 in the interaction of T cells with PDC. This finding, however, once more underlines the importance of the ICOS/ICOS-L axis for CD4+ T cells interacting with PDC.
Acknowledgments
We thank Yvonne Strübing for excellent technical assistance. This work was supported by DFG grants Kr827/10-5 and 13-2.
Abbreviations
- APC
antigen-presenting cell
- CTLA-4Ig
cytotoxic T-lymphocyte antigen 4–immunoglobulin
- DC
dendritic cell
- ELISA
enzyme-linked immunosorbent assay
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- ICOS
inducible costimulator
- ICOS-L
ICOS-ligand
- IFN-γ
interferon-γ
- IgG
immunoglobulin G
- IL-3R
interleukin-3 receptor
- l-cells
mouse fibroblastic Ltk− cells
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- moDC
monocyte-derived DC
- PBS
phosphate-buffered saline
- PBMC
peripheral blood mononuclear cells
- PDC
plasmacytoid dendritic cell
- PE
phycoerythrin
- SEB
Staphylococcus aureus enterotoxin B
- Th1
T helper type 1
References
- 1.Steinman RM. Dendritic cells. In: Paul WE, editor. Fundamental Immunology. 4. New York: Lippincott-Raven; 1999. pp. 547–73. [Google Scholar]
- 2.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nature Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 3.Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–93. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–20. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 5.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–93. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 6.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 7.Witsch EJ, Peiser M, Hutloff A, et al. ICOS and CD28 reversely regulate IL-10 on re-activation of human effector T cells with mature dendritic cells. Eur J Immunol. 2002;32:2680–6. doi: 10.1002/1521-4141(200209)32:9<2680::AID-IMMU2680>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 9.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 10.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 11.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Meth. 1996;196:121–35. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 14.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL) -3 and CD40-ligand. J Exp Med. 1997;185:1101–11. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olweus J, BitMansour A, Warnke R, et al. Dendritic cell ontogeny: a human dendritic cell lineage of myeloid origin. Proc Natl Acad Sci USA. 1997;94:12551–6. doi: 10.1073/pnas.94.23.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strobl H, Scheinecker C, Riedl E, et al. Identification of CD68+lin– peripheral blood cells with dendritic precursor characteristics. J Immunol. 1998;161:740–8. [PubMed] [Google Scholar]
- 17.Facchetti F, de Wolf-Peeters C, Mason DY, Pulford K, van den Oord JJ, Desmet VJ. Plasmacytoid T cells. Immunohistochemical evidence for their monocyte/macrophage origin. Am J Pathol. 1988;133:15–21. [PMC free article] [PubMed] [Google Scholar]
- 18.Colonna M, Krug A, Cella M. Interferon-producing cells. on the front line in immune responses against pathogens. Curr Opin Immunol. 2002;14:373–9. doi: 10.1016/s0952-7915(02)00349-7. [DOI] [PubMed] [Google Scholar]
- 19.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–10. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 20.Kroczek RA, Mages HW, Hutloff A. Emerging paradigms of T-cell co-stimulation. Curr Opin Immunol. 2004;16:321–7. doi: 10.1016/j.coi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Hutloff A, Dittrich AM, Beier KC, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–6. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 22.Khayyamian S, Hutloff A, Büchner K, et al. ICOS-ligand, expressed on human endothelial cells, costimulates Th1 and Th2 cytokine secretion by memory CD4+ T cells. Proc Natl Acad Sci USA. 2002;99:6198–203. doi: 10.1073/pnas.092576699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang L, Sha WC. The right place at the right time: novel B7 family members regulate effector T cell responses. Curr Opin Immunol. 2002;14:384–90. doi: 10.1016/s0952-7915(02)00342-4. [DOI] [PubMed] [Google Scholar]
- 24.Flavell DJ, Flavell SU, Boehm DA, et al. Preclinical studies with the anti-CD19-saporin immunotoxin BU12-SAPORIN for the treatment of human-B-cell tumours. Br J Cancer. 1995;72:1373–9. doi: 10.1038/bjc.1995.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perussia B, Starr S, Abraham S, Fanning V, Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. I. Characterization of the lymphocyte subset reactive with B73.1. J Immunol. 1983;130:2133–41. [PubMed] [Google Scholar]
- 26.Morimoto C. CD45 cluster reproof (Vol. 1) In: Schlossman SF, Boumsell L, Gilks W, et al., editors. Leucocyte Typing V. Oxford: Oxford University Press; 1995. pp. 386–9. [Google Scholar]
- 27.White-Scharf ME, Imanishi-Kari T. Characterization of the NPa idiotype through the analysis of monoclonal BALB/c anti-(4-hydroxy-3-nitrophenyl) acetyl (NP) antibodies. Eur J Immunol. 1981;11:897–904. doi: 10.1002/eji.1830111109. [DOI] [PubMed] [Google Scholar]
- 28.Löhning M, Hutloff A, Kallinich T, et al. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med. 2003;197:181–93. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meager A. Antibodies against interferons – characterization of interferons and immunoassays. In: Clemens MJ, Morris AG, Gearing AJH, editors. Lymphokines and Interferon: a Practical Approach. Oxford: IRL Press; 1987. pp. 105–27. [Google Scholar]
- 30.Meager A, Parti S, Barwick S, Spragg J, O'Hagan K. Detection of hybridomas secreting monoclonal antibodies to human gamma interferon using a rapid screening technique and specificity of certain monoclonal antibodies to gamma interferon. J Interferon Res. 1984;4:619–25. doi: 10.1089/jir.1984.4.619. [DOI] [PubMed] [Google Scholar]
- 31.Aicher A, Hayden-Ledbetter M, Brady WA, et al. Characterization of human inducible costimulator ligand expression and function. J Immunol. 2000;164:4689–96. doi: 10.4049/jimmunol.164.9.4689. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Zhu G, Chapoval AI, et al. Costimulation of T cells by B7–H2, a B7-like molecule that binds ICOS. Blood. 2000;96:2808–13. [PubMed] [Google Scholar]
- 33.Hutloff A, Büchner K, Reiter R, et al. Involvement of inducible co-stimulator in the exaggerated memory B-cell and plasma cell generation in systemic lupus erythematosus. Arthr Rheum. 2004;50:3211–20. doi: 10.1002/art.20519. [DOI] [PubMed] [Google Scholar]
- 34.Rissoan MC, Soumelis V, Kadowaki N, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 35.Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 2000;95:2484–90. [PubMed] [Google Scholar]
- 36.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–93. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 37.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–9. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krug A, Towarowski A, Britsch S, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–37. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 39.Ito T, Amakawa R, Inaba M, et al. Plasmacytoid dendritic cells regulate Th cell responses through OX40 ligand and type I IFNs. J Immunol. 2004;172:4253–9. doi: 10.4049/jimmunol.172.7.4253. [DOI] [PubMed] [Google Scholar]
- 40.Boonstra A, Asselin-Paturel C, Gilliet M, et al. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J Exp Med. 2003;197:101–9. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 42.Martinez-Moczygemba M, Huston DP. Biology of common beta receptor-signaling cytokines. IL-3, IL-5, and GM-CSF. J Allergy Clin Immunol. 2003;112:653–65. doi: 10.1016/S0091. [DOI] [PubMed] [Google Scholar]
- 43.Thomson AW, Lotze MT. The Cytokine Handbook. 4. London: Academic Press; 2003. [Google Scholar]
- 44.Bonhagen K, Liesenfeld O, Stadecker MJ, et al. ICOS+ Th cells produce distinct cytokines in different mucosal immune responses. Eur J Immunol. 2003;33:392–401. doi: 10.1002/immu.200310013. [DOI] [PubMed] [Google Scholar]