Abstract
The contributions of the classical (CP) and alternative (AP) pathways of complement activation to the spontaneous deposition of C3 fragments and the formation of membrane attack complexes (MAC) on human B lymphocytes, were assessed by incubating peripheral blood mononuclear cells with autologous serum in the absence and presence of selective inhibitors of the AP and CP, respectively. While the total amount of C3 fragments deposited was relatively unaffected by blocking either pathway individually, deposition was virtually abrogated by their combined blockade. A marked difference was observed, however, in the nature of the fragments deposited as a result of CP and AP activation: C3b fragments deposited via the CP were extensively (∼ 90%) converted to the terminal degradation product, C3dg, whereas about 50% of those deposited by the AP persisted as C3b/iC3b fragments. The extent of MAC formation was also found to be highly pathway dependent, with the AP being about 15-fold more efficient at initiating this process than the CP. A model accounting for the effectiveness of the AP in both preserving C3 fragment integrity and initiating MAC is presented.
Human B lymphocytes, by virtue of their expression of the complement receptors CR1 (CD35) and CR2 (CD21), are capable of activating the complement cascade, resulting in deposition of C3 fragments and membrane attack complex (MAC) formation at the cell surface.1,2 Activation occurs both via calcium-dependent (classical/lectin, CP/LP) and calcium-independent (alternative, AP) pathways, where the latter appears to play a predominant role.3 AP activation has been shown to be mediated primarily by CR21,4 as a result of the receptor's ability to bind the hydrolysed form of C3 (C3i).5 While CR2 is capable of initiating the AP in its own right3,6 CR1 assists this process (1) by rapidly binding C3i, generated in the fluid phase, for presentation to CR2, and (2) by stabilizing the C3i–CR2 interaction through forming a ternary complex with both molecules.7 The bound C3i captures factor B (B) from the fluid phase to generate the alternative C3 convertase, upon factor D cleavage of B.5 C3b fragments generated by the convertase then become covalently attached to CR2 itself and possibly to other acceptor molecules in the locality.4,5,8 Many of the deposited C3b fragments are subsequently degraded via iC3b to C3dg, in a process dependent on CR1's unique role as cofactor in the factor I-mediated cleavage of iC3b6,9 whilst others, by attaching to C3 convertases generated via CP/LP and/or AP, convert these to C5 convertases and thereby initiate MAC formation. Thus, CR1 appears to play a dual role at the B-lymphocyte surface: as a member of the ternary complex it supports complement activation while, as a free entity, it exerts a regulatory effect as cofactor in C3 fragment degradation.
The contribution of the CP/LP to complement activation on B lymphocytes has hitherto been established only by inference, i.e. from the observation that calcium chelation reduces slightly the extent of the activation seen, compared to that with untreated serum. The purpose of the present study therefore was to establish unequivocally, which calcium-dependent pathway(s) (CP and/or LP) is(are) involved in the activation of complement on human B lymphocytes, and to examine directly their contribution to both C3-fragment deposition and MAC formation.
In order to assess the contribution of the LP to complement activation on human B lymphocytes, peripheral blood mononuclear cells (PBMC) from healthy volunteers were incubated with 30% autologous serum in the presence or absence of 50 mm mannose or 50 mmN-acetyl-glucosamine (GlcNAc), to block the activation of mannan-binding lectin and the L-/H-ficolins, respectively,10,11 and 5 μg/ml rabbit anti-human factor D (either alone or combined), or 20 mm ethylenediaminetetraacetic acid (EDTA). The cells were then probed with fluorescein isothiocyanate (FITC)-conjugated rabbit anti-human C3c or anti-human C3d antibody, or FITC-labelled mAb E11, which recognizes a neoepitope expressed by C9 incorporated in MAC.12 B lymphocytes in the preparation were identified by inclusion of phycoerythrin (PE) -conjugated anti-CD19. Mannose and GlcNAc, whether employed alone or in combination with the AP-blocking anti-factor D, were completely without effect on either the deposition of C3 fragments or the formation of MAC (see Table 1), thereby precluding the LP as a contributor to the activation process.
Table 1.
The effect of blocking the lectin/ficolin pathways of complement activation on the deposition of C3 fragments and MAC formation on B lymphocytes
| % of control for | Total C3 fragment deposition* | C3b/iC3b fragment deposition* | MAC formation† |
|---|---|---|---|
| NHS (control) | 100 | 100 | 100 |
| + mannose (M) | 98.4 ± 14.7 | 101 ± 30.9 | 92.1 ± 30.4 |
| + GlcNAc (G) | 95.9 ± 7.6 | 99.2 ± 25.3 | 103 ± 20.3 |
| + G + M | 96.7 ± 22.1 | 92.3 ± 30.8 | 98.1 ± 31.0 |
| NHS + anti-FD (control) | 100 | 100 | 100 |
| + M | 102 ± 12.1 | 110 ± 8.42 | 119 ± 32.0 |
| + G | 106 ± 14.8 | 115 ± 8.3 | 102 ± 44.9 |
| + M + G | 101 ± 13.3 | 119 ± 11.0 | 96.2 ± 39.2 |
Mean ± SD for four estimates.
Mean ± SD for three estimates.
Human PBMC from healthy donors were prepared by centrifugation of their citrated blood over Lymphoprep (Nycomed, Oslo, Norway) and their sera were harvested by holding blood collected in anticoagulant-free tubes at room temperature for 1 hr, before centrifugation at 400 g for 5 min. The cells were washed twice in 10 ml VBS (4 mm sodium barbiturate, 145 mm NaCl, pH 7.4, supplemented with 0.8 mm MgCl2) and suspended at a density of 106 cells per ml, in low-absorbing polypropylene tubes (Life Technologies, Paisley, UK) containing 30% v/v autologous serum with or without 5μg/ml rabbit anti-human factor D in VB. Mannose (Man) and/or N-acetylglucosamine (Glc-NAc), both at a final concentration of 50 mm, were added to some of the samples and complement activation was effectuated by incubating the cells at 37° for 30 min The reaction was stopped by adding 2 ml of cold EDTA (20 mm) in phosphate-buffered saline (PBS). After three washes with PBS containing 0.05% NaN3, 0.5% bovine serum albumin (BSA) and 10 mm EDTA (PBS/BSA), the cells were incubated for 2 hr on ice with FITC-conjugated rabbit anti-human C3c or -C3d (Dako A/S, Glostrup, Denmark), or FITC-E11 (murine monoclonal antibody recognizing a C9 neoepitope in MAC)12 in 400 μl PBS/BSA containing 5 mg/ml human immunoglobulin G (Biovitrum, Stockholm, Sweden); 5 μl PE anti-human CD19 (BDBiosciences, Brøndby, Denmark) was included in the mixture to identify the B lymphocytes.
Analyses were performed with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) using cell quest software. Data were acquired on 3000 B lymphocytes by combined live gating on the Forward Scatter versus. Side Scatter dot plot and the PE histogram. Complement activation was measured as the difference in mean Fl1 signal (net MFI) between cells incubated in NHS under the various conditions, and cells incubated in VBS alone. Activation in the presence of Man and Glc-NAc and expressed as a percentage of the signals obtained in NHS (± anti-FD) without the sugars is given. The results are given as means ± 1 SD.
The next question we chose to address was that of the role played by the CP and AP individually in the deposition of C3 fragments on the B-lymphocyte surface. To this end we exposed human leucocytes to in vitro complement activation with 30% autologous serum in the absence or presence of 4.4 mmol/l MgCl2/20 mmol/l, 0,0′-Bis(2-aminoethyl)ethyleneglycol-N,N,N′,N′-tetraacetic acid (Mg/EGTA) or anti-factor D to block selectively the activation via the CP and the AP, respectively. The extent and nature of C3 fragment deposition under these conditions was determined by probing the cells with both FITC-anti-human C3c and -C3d antibody, as above. Blockade of either the AP or CP alone did not significantly reduce the extent of deposition of anti-C3d-reactive fragments (i.e. C3b, iC3b and C3dg), whereas combined blockade led to almost complete inhibition of this deposition (Table 2). Thus it would appear that the two processes operate essentially independently of each other, with consumption of complement or saturation of available acceptor sites as possible limiting factors. On the other hand, probing with anti-C3c, to detect C3b/iC3b deposition, revealed marked differences in the nature of the fragments deposited following AP or CP activation. Thus, blockade of the AP resulted in a halving of the number of detectable C3b/iC3b fragments, relative to activation with untreated serum, whilst the proportion of these fragments was increased by about 65% upon blockade of the CP (Table 2). Based on our previous observation that the C3 fragments deposited on Raji cells are predominantly in the form of iC3b and display an anti-C3c to anti-C3d binding ratio of 1.41: 113 and assuming that the efficiency of binding of anti-C3d was unaffected by the type of C3 fragment involved, the ratios obtained in the present study were used to estimate the extent of C3b/iC3b degradation accompanying total complement activation at the B-cell surface, as well as that arising from activation via the AP or CP alone. In untreated serum, the ratio of anti-C3c to anti-C3d reactivity (0.391 ± 0.061: 1) was consistent with degradation of 72.2 ± 4.3% of the deposited C3, while the degree of degradation following AP and CP activation was estimated to be 49.6 ± 1.34 and 88.2 ± 2.37%, respectively (Table 3).
Table 2.
The influence of AP and CP activation on the nature of the deposited C3 fragments and on MAC formation (mean ± SD)
| Total C3 deposition* (n = 10) | C3b/iC3b deposition† (n = 5) | MAC formation‡ (n = 8) | |
|---|---|---|---|
| Response in NHS | |||
| Alone | 100 | 100 | 100 |
| + anti-FD | 105 ± 32.3 | 47.6 ± 12.1 | 13.6 ± 6.5 |
| + Mg/EGTA | 117 ± 52.1 | 165 ± 24.1 | 200 ± 66.0 |
| + anti-FD + | 6.0 ± 3.9 | 0 | 6.5 ± 5.6 |
| Mg/EGTA | |||
| + EDTA | 1.8 ± 3.7 | 0.5 ± 0.3 | 2.4 ± 6.9 |
Probing with FITC-rabbit anti-human C3d.
Probing with FITC-rabbit anti-human C3c.
Probing with FITC-E11.
Human PBMC from healthy donors were isolated and incubated in 30% v/v autologous serum, with or without 20 mm EGTA/4.4 mm MgCl2 (Mg/EGTA), 20 mm EDTA (EDTA) and 5 μg/ml rabbit anti-human factor D (anti-FD) as described in Table 1. Complement activation was measured as the difference in mean Fl1 signal (net MFI) between cells incubated in NHS under the various conditions, and cells incubated in VBS alone, and expressed as a percentage of the signal obtained in NHS alone. The results are given as means ± 1 SD.
Table 3.
The degradation of C3 fragments following deposition via the AP and CP (means ± SD)
| Anti-C3c: Anti-C3d reactivity ratio (R) | Estimated % C3b/iC3b degradation* | |
|---|---|---|
| NHS | 0.391 ± 0.061: 1 | 72.2 ± 4.3 |
| NHS + anti-FD | 0.166 ± 0.033: 1 | 88.2 ± 2.4 |
| NHS + Mg/EGTA | 0.709 ± 0.021: 1 | 49.6 ± 1.3 |
| Raji cells in NHS† | 1.41 ± 0.41: 1 | 0 |
% degradation of C3b/iC3b = 100 × (1–RB cells/RRaji cells).
see ref. 13.
The net MFI signals observed with anti-human C3c and -C3d on B lymphocytes, following in vitro complement activation of peripheral B lymphocytes as described in Table 1, were expressed as a C3c: C3d ratio for each of the five experiments performed with separate donors.
The efficiency of the two activation pathways in initiating MAC formation was then tested by incubating PBMC with 30% autologous serum under the conditions described above and probing FITC-conjugated monoclonal antibody E11. MAC formation was found to be overwhelmingly dependent on the AP convertase, in that blockade of this pathway resulted in its reduction to 13.6 ± 6.5% of the level seen in the untreated serum control, while combined blockade of the AP and CP led to almost complete inhibition (Table 1). By contrast, blockade of the CP alone resulted in marked enhancement (200 ± 66% of untreated serum control) of MAC formation (Table 2). Thus the AP was found to be approximately 15-fold more effective in inducing MAC formation on B lymphocytes than the CP.
The crucial findings in this study are that B-lymphocyte activation of the complement cascade via the AP results in the deposition of C3b fragments at sites where many of them are protected from total degradation to C3dg, and that MAC formation is primarily initiated via this pathway. By contrast, activation via the CP results in the deposition of C3 fragments, which are extensively degraded to C3dg, and gives rise to only minimal MAC formation. Indeed, the CP appears to play a regulatory role, in that blockade of this pathway leads to both a reduction in the CR1-assisted late cleavage of C3 fragments and an enhancement in the formation of MAC, compared to activation where both pathways are operational. Given that the total amount of C3 fragment deposition is apparently unaffected by blockade of either pathway alone, it would appear that availability of C3 may be the determining factor for the extent of this deposition. If the CP were consuming substantial quantities of C3, and thereby limiting the degree of AP-associated C3 and C5 convertase formation, this would explain the observed inhibitory effect of the CP on MAC formation.
The difference in the degree of C3 fragment degradation to the terminal product, C3dg, following deposition via the AP and CP, respectively, presumably reflects differences in the acceptor sites primarily engaged by the nascent C3b fragments. Given that CR2 has previously been described as a primary acceptor site for C3b generated via the AP4,5 and that AP C3 convertase formation occurs most efficiently in the context of a ternary (CR1–C3i–CR2) complex7 we propose that AP-generated C3b primarily deposit on elements of the complex (e.g. CR1, CR2, or the C3 convertase) and are thereby protected from cleavage, because of the inability of CR1, within the complex, to recruit factor I. On the other hand, C3b fragments generated via the CP (as well as some of the AP-generated C3b) may deposit on a variety of other acceptor structures on the B-cell surface, where they may encounter free CR1 possessing the full cofactor activity for Factor I and thereby become efficiently degraded.
Although many of the C3b fragments deposited via the AP may be rapidly converted to iC3b, under the influence of serum factor H or decay accelerating factor (DAF)/membrane cofactor protein (MCP) the correlation between the limitation of C3 fragment decay and MAC formation is fully in keeping with C3b's role in transforming C3 convertases to C5 convertases by binding covalently to the former. The above data strongly suggest that the C3 convertase generated via the AP, rather than the CP, is the primary target for this transformation.
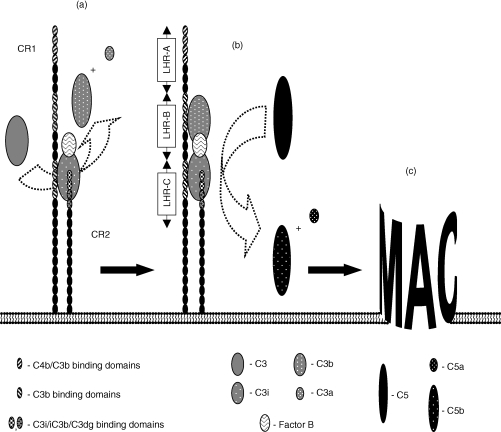
We and others have previously shown that CR2 is the primary site for generating an AP C3 convertase, and that CR1 supports this process by rapidly capturing hydrolysed C3 (C3i), which is generated in trace amounts in serum, and by entering into a stable ternary complex with both C3i and CR2. The capacity of CR1 to perform this role relies on its ability to engage both C3i and CR2 at independent sites. CR1, like CR2, is comprised of a linear array of small homologous domains (short consensus repeats, SCR), with the most common human allelic form consisting of 30 of these units. Twenty-eight of the SCR, in this form of CR1, are arranged in four ‘long homologous regions’ (LHR), each consisting of seven SCR, where the first three LHR display binding activity with specificity for C4b (in LHR-A) and/or C3i and C3b (in LHR-A, -B and -C).14 If CR1 and CR2 line up in a parallel fashion, when they associate with each other, the LHR-C of CR1 would be ideally positioned to present a captured C3i molecule to CR2 (Fig. 1). Correspondingly, LHR-B (or LHR-A) would be suitably placed to capture nascent C3b, generated by the resulting C3 convertase, and thus enhance the probability of C3b fragment incorporation by the C3 convertase. Support for this C3b capture model, in promoting AP C3 to C5 convertase transformation, is derived from our earlier studies with the CR1-negative Raji cells. Although these cells generate C3 convertases with an efficiency equal to, or greater than, that of B lymphocytes13 their ability to induce MAC formation is less by a factor of 2 or more3 presumably because they lack the C3b capture mechanism afforded by CR1. Complement activation via the CP, by contrast, remains largely uncharacterized, although the presence of active C1 on the surface of human peripheral lymphocytes has previously been reported.15 More precise definition of the structure(s) involved in C1 uptake, as well as the sites of formation of the CP convertases is required.
Figure 1.
The contribution of CR1 to AP activation and MAC formation at the B-lymphocyte surface. CR1 exerts a double influence on the CR2-mediated complement activation taking place on B lymphocytes. It facilitates the formation of a C3 convertase by rapidly capturing hydrolysed C3 (C3i) from the fluid phase and participating in a stable complex with C3i and CR2 where all three macromolecules interact with each other via distinct sites (a). While the capture of C3i may involve any of the multiple ligand-binding sites on CR1, the optimal site for engagement in a ternary complex, from a steric point of view, would be that lying in the LHR-C region of CR1. Nascent C3b, generated by a convertase located to the CR1–CR2 complex, may be readily captured by the other binding sites on CR1 (b). The site in LHR-B would be suitably placed to direct C3b to the Bb–C3i complex (the AP C3 convertase), as a potential target for nucleophilic attack by the thiolester group in C3b, resulting in its conversion to a C5 convertase and thus ensuring effective MAC formation (c).
In conclusion, it would appear that B lymphocytes, by virtue of their expression of both CR1 and CR2, are especially well equipped to capture hydrolysed C3 from the fluid phase and utilize it in the formation of C3 and C5 convertases, resulting both in the deposition of C3 fragments and the formation of MAC at the cell surface. While the biological consequences of these events still remain to be established, our recent studies have ruled out the possibility that MAC formation exerts a detrimental effect on B-lymphocyte viability (unpublished observations). Therefore, the options remain that these events contribute either to the priming of B lymphocytes for subsequent stimulation by antigen, or that they are involved in enhancing the contact of B lymphocytes, as antigen-presenting cells, with antigen-specific T lymphocytes.16 With regard to the latter role, the recent finding that naïve T lymphocytes are driven to become regulatory T cells by C3b engagement of CD46 on their surface17 may be of some interest. Given that deposited C3 fragments on the antigen-presenting B lymphocytes are capable of engaging CD46 on the naïve T lymphocytes, this would help to explain an earlier observation that primary antigen presentation by B lymphocytes to T lymphocytes suppresses an immune response.18 This possibility is currently under investigation in our laboratory.
Acknowledgments
This work was supported by grants from the Danish Arthritis and Rheumatism Council, Novo Nordisk Fund, The Fund for the Advancement of Medical Sciences, King Christian the Tenth's Fund, Director Jacob Madsen's Fund, Grocer Sven Hansen's Fund, Katherine and Viggo Skovgaard's Fund, Ingemann O. Bucks Fund, Former Director Leo Nielsen and spouse Karen Margrethe Nielsen's Legate, Mimi and Viktor Larsen's Fund, A. J. Andersen and spouse Fund, Helen and Einar Bjørnow's Fund, Henny and Holger Holgersen's Memorial Legate. We thank Mrs Nanna Bøgesvang for excellent technical assistance.
Abbreviations
- AP
alternative pathway (of complement activation)
- C3
complement component 3
- CR1 and CR2
complement receptor types 1 and 2
- CP
classical pathway (of complement activation)
References
- 1.Marquart HV, Svehag S-E, Leslie RGQ. CR2 is the primary acceptor site for C3 during alternative pathway activation of complement on human peripheral B lymphocytes. J Immunol. 1994;153:307–15. [PubMed] [Google Scholar]
- 2.Nielsen CH, Marquart HV, Prodinger WM, Leslie RGQ. CR2-mediated activation of the complement alternative pathway results in formation of membrane attack complexes on the human B lymphocyte surface. Immunology. 2001;104:418–22. doi: 10.1046/j.1365-2567.2001.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielsen CH, Pedersen ML, Marquart HV, Prodinger WM, Leslie RGQ. The role of complement receptors type 1 (CR1, CD35) and 2 (CR2, CD21) in promoting C3 fragment deposition and MAC formation on normal peripheral human B lymphocytes. Eur J Immunol. 2002;32:1359–67. doi: 10.1002/1521-4141(200205)32:5<1359::AID-IMMU1359>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 4.Mold C, Nemerow GR, Bradt BM, Cooper NR. CR2 is a complement activator and the covalent binding site for C3 during alternative pathway activation by Raji cells. J Immunol. 1988;140:1923–9. [PubMed] [Google Scholar]
- 5.Schwendinger MG, Spruth M, Schoch J, Dierich MP, Prodinger WM. A novel mechanism of alternative pathway complement activation accounts for the deposition of C3-fragments on CR2-expressing homologous cells. J Immunol. 1997;158:5455–63. [PubMed] [Google Scholar]
- 6.Leslie RGQ. The influence of complement receptor type 1 (CD35) and decay accelerating factor (CD55) on complement receptor type 2 (CD21) -mediated alternative pathway activation by B lymphocytes. Immunology. 1999;97:371–3. doi: 10.1046/j.1365-2567.1999.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leslie RGQ, Prodinger WM, Nielsen CH. Complement receptors type 1 (CR1, CD35) and 2 (CR2, CD21) cooperate in the binding of the hydrolysed form of complement factor 3 (C3I) to human B lymphocytes. Eur J Immunol. 2003;33:3311–21. doi: 10.1002/eji.200324330. [DOI] [PubMed] [Google Scholar]
- 8.Olesen E, Johnson AA, Damgaard G, Leslie RGQ. The Requirement of localised, CR2-mediated alternative pathway (AP) activation of complement for covalent deposition of C3 fragments on normal B lymphocytes. Immunology. 1998;93:177–83. doi: 10.1046/j.1365-2567.1998.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross GD, Lambris JD, Cain JA, Newman SL. Generation of three different fragments of bound C3 with purified factor I or serum. I. Requirements for factor H vs. CR1 cofactor activity. J Immunol. 1982;129:2051–60. [PubMed] [Google Scholar]
- 10.Weis WL, Drickamer K, Hendrickson WA. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature. 1992;360:127–34. doi: 10.1038/360127a0. [DOI] [PubMed] [Google Scholar]
- 11.Matsushita M, Endo Y, Taira S, Sato Y, Fujita T, Ichikawa N, Nakata M, Mizuochi T. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J Biol Chem. 1996;271:2448–54. doi: 10.1074/jbc.271.5.2448. [DOI] [PubMed] [Google Scholar]
- 12.Mollnes TE, Lea T, Harboe M, Tschopp J. Monoclonal antibodies recognising a neoantigen of poly(C9) detect the human terminal complement complex in tissue and plasma. Scand J Immunol. 1985;22:183–95. doi: 10.1111/j.1365-3083.1985.tb01870.x. [DOI] [PubMed] [Google Scholar]
- 13.Marquart HV, Olesen EH, Johnson AA, Damgaard G, Leslie RGQ. A comparative study of normal B cells and the EBV-positive Burkitt's lymphoma cell line, Raji, as activators of the complement system. Scand J Immunol. 1997;46:246–53. doi: 10.1046/j.1365-3083.1997.d01-122.x. [DOI] [PubMed] [Google Scholar]
- 14.Klickstein LB, Bartow TJ, Miletic V, Rabson LD, Smith JA, Fearon DT. Identification of distinct C3b and C4b recognition sites in the human C3b/C4b receptor (CR1, CD35) by deletion mutagenesis. J Exp Med. 1988;168:1699–717. doi: 10.1084/jem.168.5.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Füst G, Erdei A, Sármay G, Medgyesi GA, Gergely J. Functionally active C1 on the surface of human peripheral lymphocytes, its role in the complement-mediated inhibition of Fc receptors on B lymphocytes. Clin Immunol Immunopathol. 1976;5:377–87. doi: 10.1016/0090-1229(76)90047-7. [DOI] [PubMed] [Google Scholar]
- 16.Kerekes K, Prechl J, Bajtay Z, Mihály J, Erdei A. A further link between innate and adaptive immunity: C3 deposition on antigen-presenting cells enhances the proliferation of antigen-specific T cells. Internat Immunol. 1998;10:1923–30. doi: 10.1093/intimm/10.12.1923. [DOI] [PubMed] [Google Scholar]
- 17.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ T cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–92. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Nature. 1992;258:1156–9. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]