Abstract
Macrophages are decisive cells for the course of leprosy as they phagocytose Mycobacterium leprae and have the potential to influence the specific immune response. Expression and release of the myeloid-related protein (MRP) 8 and MRP14 (S100A8 and S100A9) characterize a proinflammatory subtype of macrophage that is prominent in, for example, murine infection with lack of a T helper 1 cell response and in certain highly active chronic inflammations of mice and humans. We investigated cutaneous biopsies of the different forms of leprosy (41 untreated patients) including leprosy reaction type 1 (reversal reaction) and type 2 (erythema nodosum leprosum) (n = 18) for expression of MRP8 and MRP14 by subtypes of macrophages. Concomitantly we determined serum levels of MRP8 and MRP14 by sandwich enzyme-linked immunosorbent assay. Expression of MRP8 and MRP14 by CD68-positive macrophages was low in tuberculoid leprosy and rose significantly in borderline tuberculoid leprosy and especially in multibacillary forms, there being expressed by mycobacteria-loaded foam cells. A significant rise of MRP8 and MRP14 expression also occurred in lepra reactions compared to the corresponding non-reactional forms. In type 2 reactions this additional increase was associated with a sigificant elevation of serum levels. In type 1 it was associated with expression of MRP8 and MRP14 by epitheloid and giant cells, which so far were considered not to express both proteins. In conclusion, we present evidence that the two prominent proteins MRP8 and MRP14 can be re-expressed in vivo by tissue macrophages in chronic infection, that their increased expression is characteristic for a macrophage subtype associated with high inflammatory but low antimycobacterial activity in the absence of a T helper 1 response, and that their significant rise in serum during erythema nodosum leprosum bears diagnostic and pathophysiological relevance.
Introduction
The macrophage lineage comprises a system of cells of large diversity with respect to their morphological, functional and metabolic properties. Depending on their stage of differentiation and activation monocytes/macrophages secrete pro- or anti-inflammatory mediators and may be involved in propagation as well as in suppression of inflammatory reactions or infections.1–6 They play a particular role in those infections that involve intracellular microbial pathogens, such as leishmaniasis, tuberculosis and leprosy. Here they present the decisive effector cells which may either harbour or eliminate the invading microbes and they may have an impact on directing the T-cell response by creating an appropriate cytokine milieu and by presenting antigen.
An instructive model for the dual roles of macrophages and their subtypes in intracellular infections has been the infection of mice with Leishmania major, i.e. experimental leishmaniasis. Here macrophages are involved in initiating and especially in executing the specific immune response, which is directed by a pathogen-specific T helper 1 (Th1) cell response mediating resistance or by a Th2 response leading to susceptibility.
We have revealed that susceptibility in contrast to resistance is associated with a significantly higher percentage of certain subtypes of macrophages6 that are less efficient in killing L. major.7 These macrophages were characterized by expression of the myeloid-related protein 8 (MRP8) (S100 A8) and MRP14 (S100 A9). MRP8 and MRP14 are two calcium-binding proteins belonging to the S100 family.8,9 In vitro their expression in murine and human leucocytes has been restricted to neutrophils and early differentiation stages of monocytes.8,10 In vivo they are expressed also by macrophages in inflammatory infiltrates, but not by resident tissue macrophages.11,12
MRP8 and MRP14 form non-covaIently bound complexes and are secreted by human monocytes via an alternative pathway.13 In certain inflammatory conditions such as cystic fibrosis, inflammatory bowel disease or rheumatoid arthritis, serum levels of MRP8 and MRP14 are markedly raised in close correlation to disease activity, indicating that both proteins are released in the course of these inflammatory processes.4,11,14–17
Similar to experimental leishmaniasis macrophages fulfil distinct roles also in infection of humans with Mycobacterium leprae.18 As in experimental leishmaniasis the outcome of leprosy in humans is genetically controlled as well as dependent on development of a pathogen-specific T-cell response.18–20 Thus leprosy presents not only a still relevant and threatening disease, but also an immunologically important model for the specific and innate immune response.19,20 Although M. leprae has also a tropism for Schwann cells of the peripheral nerves and has been found within endothelial cells, it is primarily encountered in macrophages.19,20 The latter prevail over other potential host cells in terms of cell number and phagocytic capacity. They are endowed with efficient mechanisms to eliminate M. leprae, dependent on sufficient activation by T-cell-derived cytokines18,19 and probably also on the kind of subtype in the infiltrate. Resistance to infection with M. leprae is linked to an M. leprae-specific Th1 response whereas susceptibility is due to a specific anergy towards M. leprae.18,19 When an efficient Th1-cell-mediated immune response is generated, clinically apparent leprosy infection is limited to one granulomatous lesion in tuberculoid leprosy (TT) or to a restricted number of granulomas in borderline tuberculoid leprosy (BT). With the fading of efficient cellular immunity in midborderline leprosy (BB) polymorphic lesions arise at the expense of granuloma formation. From borderline lepromatous leprosy (BL) to anergic lepromatous leprosy (LL) increasing dissemination of bacteria in the skin is associated with diffuse accumulation of foam cells and a lower percentage of T cells.18–21
The polar forms of leprosy, namely paucibacillary TT and multibacillary LL, remain usually stable, whereas the borderline forms BT, BL and especially BB may move within the spectrum towards one of the poles. An acute inflammatory reaction in patients with borderline forms, which is associated with either an upgrading or, less commonly, a downgrading shift in cellular immunity (e.g. from BT to TT or from BB to BL), is seen in so-called reversal or type 1 lepra reaction (RR).22 In type 2 lepra reaction, also known as erythema leprosum nodosum (ENL), the chronic multibacillary disease state in BL or LL is disturbed by a sudden up-regulation of inflammatory mediators associated with fever, elevated C-reactive protein and involvement of systemic organs.23,24
As the different forms of leprosy present a complete spectrum in humans from resistance to susceptibility and from granuloma formation in Th1-cell-associated TT to rather diffuse accumulations of macrophages in the highly susceptible LL form, we wondered if these immunologically distinct stages would correlate with the appearance of distinct subtypes of macrophages expressing MRP8 and MRP14. As the distinct leprosy forms and especially leprosy reactions are known to be associated with a different degree of systemic inflammatory activation we also speculated that these stages would be reflected by different levels of MRP8 and MRP14 in serum.
In particular we tested (1) if appearance of MRP8- and MRP14-expressing macrophages is linked to a state of higher inflammatory activity or susceptibility, (2) if such conditions would also be associated with raised serum levels of MRP8 and MRP14, and (3) if accumulations of this subtype of leucocytes could be linked to distinct expression patterns of the endothelial adhesion molecules E-selectin, intercellular adhesion molecule type 1 (ICAM-1) and vascular cell adhesion molecule type 1 (VCAM-1).
Materials and methods
Patients
Biopsies of 41 untreated patients with diagnosis of non-reactional leprosy were studied immunohistochemically prior to treatment. The patients ranged in age from 16 to 75 years (mean 37·0 years). They were classified according to the Ridley−Jopling scale25 as nine LL, 10 BL, four BB, 10 BT and eight TT. As we did not collect more than four biopsies from BB we did not include this form in our statistical analysis.
In addition, we included biopsies of eight patients with a type 2 lepra reaction [erythema nodosum leprosum (ENL)] and 10 patients with reaction type 1 (RR); prior to development of RR, five of these patients were classified as BT, three as BB and two as BL; patients with ENL had either LL (n = 4) or BL (n = 6) and had not yet been submitted to therapy with corticosteroids or thalidomide, while five of them also had not received antileprotic therapy. Neither were the five patients with RR from BT treated with immunosuppressive therapy prior to obtaining biopsy and serum samples.
Serum samples were also obtained from a number of patients, i.e. from seven with TT, five with BT, one with BB, seven with BL, seven with LL, five with RR and 16 with ENL. We also included serum samples of 19 patients without leprosy (clinically healthy blood donors without laboratory signs of inflammation or of hepatitis or human immunodeficiency virus infection) whose blood was drawn at the same hospital (Department of Dermatology, Federal University of São Paulo) and prepared and stored under the same conditions.
Patients were enrolled after signing an informed consent that was approved by a medical ethics committee (São Paulo, 13 March 1995).
Immunohistochemistry
Biopsies of cutaneous leprotic lesions were obtained for diagnostic reasons. For immunohistochemical analysis they were cut longitudinally in two halves for preparation of paraffin and cryostat sections. For immunohistochemical staining the following antibodies were used: mouse monoclonal antibody KP1 against CD68 antigen (this antibody recognizes an epitope on a 110 000 MW transmembrane glycoprotein which is found exclusively on human monocytes and tissue macrophages, but not on granulocytes26,27) (Dako Diagnostika, Hamburg, Germany); rabbit antisera against human MRP8 and MRP14, produced as described previously8,28 (all these antibodies were applied on both paraffin and cryopreserved tissues; statistical analysis is given for evaluation of paraffin sections); antibodies for cryopreserved tissue were mouse monoclonal antibodies against adhesion molecules E-selectin (R&D Systems, Wiesbaden, Germany), ICAM-1 (Dianova, Hamburg, Germany), VCAM-1 (Dianova); rabbit antiserum against factor-VIII-related antigen (Behring, Marburg, Germany) or murine monoclonal antibody against CD31 (R&D Systems) as general markers for endothelial cells were applicable on corresponding cryopreserved sections only. For negative control, specific antibodies were replaced by isotype-matched antibodies of irrelevant specificity or by preimmune rabbit serum.
Tissue sections were subjected to immunoperoxidase assay as described elsewhere.6,12 For double-labelling experiments tissue samples were incubated successively for 1 hr with 1% bovine serum albumin, 10% normal goat serum in phosphate-buffered saline, MRP14 rabbit antiserum, peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) F(ab)2 and 1·5 mm 3-amino-9-ethylcarbazole (Sigma, Taufkirchen, Germany) as well as 0·02 mm H2O2 in acetate buffer (pH 5·2) (10 min) for colour reaction. Thereafter monoclonal antibody KP1 (Dianova) against CD68, phosphatase-conjugated goat anti-mouse IgG F(ab)2, 0·02% (w/v) naphthol AS-MX phosphate, 0·1% (w/v) levamisole, 0·1% (w/v) and fast blue RR salt (all Sigma) in 0·1 m Tris buffer pH 8·2 as substrate (30 min) were applied. Slides were not counterstained after double-labelling procedures.
Microscopic evaluation
Sections were examined at 160-fold magnification using an ocular endowed with an eyepiece grid. The percentage of positive cells was obtained as described earlier,6 i.e. by relating the number of positive cells to the number of total cells counted in a defined area. In order to be able to compare percentages of positive vessels we determined the ratio of vessels positive for adhesion molecules to vessels positive for the pan vessel markers CD31 or factor-VIII-related antigen. Results were controlled by two other observers (V.N. and J.T.-Y.) and were expressed as arithmetic means (n = 5–11) of the percentages.
Determination of MRP8/MRP14 concentrations in serum by sandwich enzyme-linked immunosorbent assay (ELISA)
Serum samples from leprosy patients with and without reactions and normal individuals were collected and kept frozen (−20°) until use.
Concentrations of complexes of MRP8 and MRP14 in serum were determined by a sandwich ELISA system established in our laboratory as described previously.4,13 MRP8 and MRP14 form non-covalently associated complexes in the presence of extracellular calcium which are detected by our ELISA system.4,13 We therefore calibrate our ELISA with the different amounts (0·5–500 ng/ml) of the native MRP8/MRP14 complex (isolated from human granulocytes as described previously)29 and present our data as ng/ml MRP8/MRP14 instead of giving values for the single monomers. The assay has a sensitivity of less than 0·5 ng/ml. Values above two standard deviations of the mean of the control group (i.e. above 800 ng/ml), were considered to be significantly elevated.4
Statistical analysis
The Student's t-test for small samples was performed to determine significant differences between the percentages of positive cells in tissue sections of the different forms of leprosy (except for BB as n <5). Values of P >0·05 were considered not to be significant. As concentrations of MRP8/MRP14 in serum have not yet been confirmed sufficiently often to be normally distributed and as they showed high variations we used the U-test according to Mann and Whitney for investigating significant differences between the groups. Values of P >0·05 were considered not to be significant.
Results
In multibacillary forms of leprosy macrophages express MRP8 and MRP14 significantly more frequently than in paucibacillary forms
The infiltrates of TT and BT showed the typical formation of non-caseating granulomas with primarily epitheloid cells, but also some smaller macrophages and giant cells in the centre, surrounded by moderately dense infiltrates composed of lymphocytes (in TT more than in BT) and few macrophages. From BB to LL there was a continuing reduction in the number of lymphocytes and a markedly increasing aggregation of foamy macrophages. Granulocytes were not observed in TT or BT and only scarcely present in BL or LL.
In lesions of RR a dense infiltrate encompassed lymphocytes, macrophages and some neutrophils. In ENL lesions inflammatory infiltrates were composed of foam cells admixed with neutrophils, eosinophils and lymphocytes.
CD68 was expressed by macrophages or macrophage-derived cells, including giant (Langerhans') cells and epitheloid cells in TT and BT (Fig. 1a) or foam cells in BL and LL (Fig. 1b). Owing to the high accumulation of lymphocytes in paucibacillary forms, the percentage of macrophages (CD68+ cells) among all cells in the infiltrate amounted to 17·7% in TT (Fig. 2). In BT it rose significantly to 32% (Fig. 2) (P = 0·05) and continued to increase, reaching 52% in LL (Figs 1b, 2). In these forms positive cells were spread throughout the diffuse or nodular infiltrates.
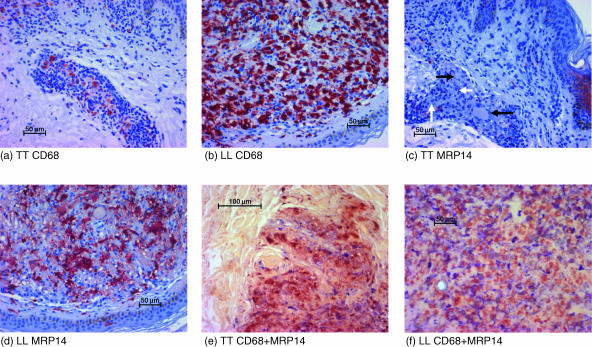
Figure 1.
Demonstration of CD68+, MRP8+ and MRP14+ cells in TT and LL. Leprosy sections from TT (a, c, e) and LL (b, d, f) were stained immunohistochemically for CD68 (a, b) or MRP14 (c, d) or double-stained for MRP14 (red) and CD68 (blue) (e, f) (without counterstaining). Whereas the number of MRP14-expressing (CD68-positive) macrophages is low in TT, the number of MRP14-expressing (CD68-positive) cells is higher in LL. Epitheloid cells (white arrows) and multinucleated giant cells (black arrows) are negative for MRP14.
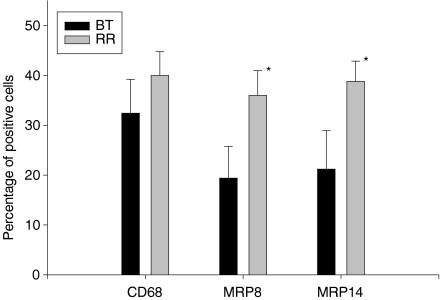
Figure 2.
Graph depicting percentages of CD68+, MRP8+ and MRP14+ cells in the infiltrate of different forms of leprosy (BB was not included because the sample number n was lower than 5). One asterisk (*) denotes a significant increase from TT to BT, two asterisks indicate a significant increase from BT to BL (values of P > 0·05 were considered not to be significant).
The expression of MRP8 and MRP14 was markedly lower than that of CD68 (Fig. 1c). In TT the average percentages of MRP8 or MRP14 were 2·9% or 4·8% respectively (Fig. 2). Thus, the percentage of CD68+ macrophages expressing MRP8 and MRP14 was low (Table 1; also shown by double-staining in Fig. 1e). Large epitheloid cells and giant cells were usually negative for MRP8 or MRP14 (Fig. 1c). There was no significant difference between cells expressing MRP8 and cells expressing MRP14 with regard to their number or localization in corresponding tissue sections.
Table 1.
Ratios of MRP8+ cells to CD68+ cells and of MRP14+ cells to CD68+ cells in the different forms of leprosy
| MRP8/CD68 | MRP14/CD68 | |
|---|---|---|
| TT | 16·6 ± 15·3 | 27·4 ± 19·3 |
| BT | 64·3 ± 26·4 | 70·3 ± 30·3 |
| BB | 91·4 ± 33·4 | 84·1 ± 36·1 |
| BL | 98·9 ± 30·8 | 107·6 ± 34·3 |
| LL | 76·6 ± 30·9 | 86·6 ± 33·4 |
| RR (type 1) from BT | 89·4 ± 4·8 | 89·6 ± 8·0 |
| ENL (type 2) from BL and LL | 96·9 ± 9·5 | 104·5 ± 13·2 |
From TT to BT there occurred a significant rise (P = 0·05) in the overall percentage of both MRP8+ and MRP14+ cells (Fig. 2). This rise was markedly more prominent than the increase of CD68+ cells as demonstrated by a climbing MRP8/CD68 ratio (from 16% to 64%) and MRP14/CD68 ratio (from 27% to 70%) (Table 1) as well as by a rise of double-labelled, MRP14+/CD68+ cells (data not shown). By double-staining we did not reliably detect cells which were MRP8+ or MRP14+ but not CD68+ (Fig. 1e). Therefore the significant increase of MRP8+ and MRP14+ cells was mostly due to expression of MRP8 and MRP14 by CD68+ cells. These findings indicate that the change from TT to BT is associated not only with an increase in the number of granulomas (according to classification by the Ridley−Jopling scale)25 but also with qualitative changes within the granulomas with regard to their macrophage population.
The percentage of MRP8- or MRP14-expressing cells continued to increase in BB and BL where it reached a plateau as there was no further rise in LL (Fig. 2). The increasing ratios of MRP8/CD68 as well as of MRP14/CD68 revealed that a rising number of CD68+ cells started to express MRP8 and MRP14 (Table 1). This was confirmed by double-labelling (Fig. 1f) and by morphological evaluation as a high percentage of macrophages (foam cells) were positive for MRP8 and MRP14 (Fig. 1d). There was a small percentage of cells which were MRP8+ or MRP14+ but on double-labelling were not unambiguously positive for CD68. Their percentage was below 5% in both BL and LL. Morphologically they could include polymorphonuclear cells or a subtype of macrophages that express MRP8 or MRP14 but not CD68.
There was also occasional focal expression of MRP8 and MRP14 in the epidermis of patients with leprosy (Fig. 1c) which was more marked in some cases of BL or LL, but it was not further quantitated for this study.
In summary, from paucibacillary to multibacillary forms there is a significant increase of the pro-inflammatory MRP8+ and MRP14+ subtypes of macrophages.
Up-regulation of MRP8 and MRP14 in lepra type 1 and 2 reactions (RR and ENL)
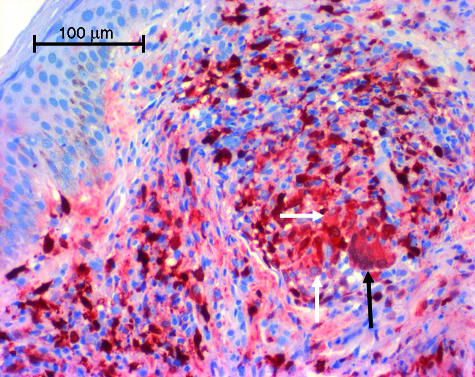
In RR (lepra type 1 reaction) and in ENL (lepra type 2 reaction) there was a rise of CD68+ cells compared to corresponding forms without reaction, but significance was not reached due to variations in values. In contrast the percentage of MRP8+ and MRP14+ cells in RR rose significantly in patients with BT (n = 5 for patients who developed RR from BT, P = 0·05). Whereas 19% of cells expressed MRP8 and 21% of cells MRP14 in non-reactional BT, the percentages rose to 36% and 38% respectively in RR (Fig. 3), resulting also in a high percentage of CD68+ cells expressing MRP8 and MRP14 (Table 1). In clear contrast to non-reactional TT or BT MRP8 and MRP14 were now expressed by several epitheloid cells and even giant cells (Fig. 4). A small group of cells which on double-stained sections were positive for MRP8 or MRP14 but not for CD68 amounted to 7% or less of the total number of stained cells and morphologically comprised either polymorphonuclear cells or macrophages.
Figure 3.
Graph depicting percentages of CD68+, MRP8+ and MRP14+ cells in lesions of RR (lepra reaction type 1) from patients with BT compared to percentages of positive cells in non-reactional BT. Asterisks denote significantly higher expression of MRP8 and MRP14 in RR (values of P > 0·05 were considered not to be significant).
Figure 4.
Expression of MRP14 in lepra reaction type 1 (RR). Immunohistochemical staining reveals marked expression of MRP14 by epitheloid cells (white arrow) and giant cells (black arrow). Both cell types were negative in non-reactional BT.
The percentage of MRP8+ and MRP14+ cells also rose in RR of patients with BB or BL, but due to the lower number of samples (n < 5) we cannot claim significance. Yet, we have direct evidence for up-regulation of MRP8 and MRP14 in the case of one patient with BL and also in one with BT as we had biopsies taken both prior to and during RR. Both patients showed a marked rise in expression of MRP8 and MRP14 in the type 1 reaction (with regard to MRP14 from 25% to 41% in the patient with BT and from 50% to 62% in the patient with BL).
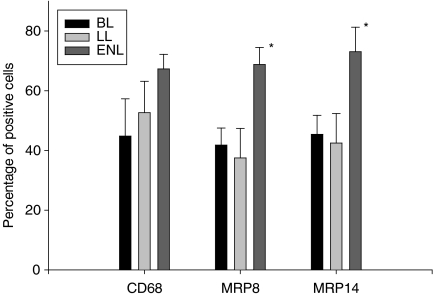
In leprosy type 2 reaction infiltrates of MRP8+ and MRP14+ cells were sometimes concentrated around vessels and contained several strongly positive macrophages which were not foam cells. Their percentage was significantly higher than in non-reactional multibacillary forms (Fig 5, P ≤ 0·05). In RR and ENL we also detected some MRP8+ and MRP14+ granulocytes, but they were not solely responsible for the significant increase in positive cells. However, as they do not express CD68, they are a minor factor in the increase of the MRP8/CD68 and MRP14/CD68 ratios (Table 1), whereas they were not in non-reactional forms.
Figure 5.
Graph depicting percentages of CD68+, MRP8+ and MRP14+ cells in lesions of ENL (lepra reaction type 2) from patients with BL or LL compared to percentages of cells in non-reactional BL or LL. Asterisks denote significantly higher expression of MRP8 and MRP14 in ENL (values of P > 0·05 were considered not to be significant).
High percentages of MRP8/MRP14-expressing macrophages are not associated with increased expression of E-selectin, VCAM-1 and ICAM-1 by vessels
We analysed endothelial expression of adhesion molecules E-selectin, VCAM-1 and ICAM-1 in corresponding cryostat sections in order to find out whether the distinct appearance of macrophage subtypes was associated with a distinct expression of these adhesion molecules. Despite a high number of infiltrating cells and denser infiltrates in LL the percentage of vessels expressing E-selectin (ratio E-selectin+ vessels to CD31+ vessels) was significantly higher in TT than in LL (Table 2). The rise was already marked in BB and BL, but not significant, due to a low number of samples (BB) or to a low number of total vessels in two of our cryostat sections from BL. Similar results were obtained with regard to endothelial expression of VCAM-1 and ICAM-1 (Table 2). Our study revealed that there was no expression pattern of these three adhesion molecules which would directly correlate with percentage of MRP8-, MRP14- or CD68-expressing phenotypes of macrophages. However, as the three molecules mediate adhesion also of lymphocytes, their strong expression in TT and BT could be one step in the course of an intact T-cell response during cell-mediated immunity.
Table 2.
Percentages of E-selectin+, VCAM-1+ and ICAM-1+ vessels (ratio to the total number of CD31+ vessels in corresponding sections) in the two polar forms of leprosy: TT and LL
| TT | LL | |
|---|---|---|
| E-selectin+/CD31+ | 19·8 ± 10·4 | 5·7 ± 4·0 |
| VCAM-1+/CD31+ | 42·5 ± 14·1 | 22·6 ± 10·1 |
| ICAM-1+/CD31+ | 26·5 ± 2·0 | 5·01 ± 3·1 |
Increased serum levels of MRP8/MRP14 are a marker for lepra type 2 reactions
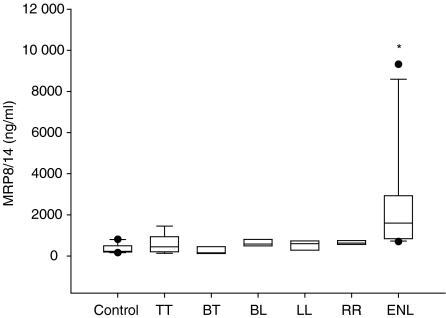
We wondered if multibacillary forms with their high percentage of MRP8- and MRP14-expressing cells would be characterized by higher serum levels of MRP8/MRP14 than paucibacillary forms. MRP8/MRP14 serum levels in TT were similar to serum levels in control persons (Fig. 6). In BL and LL the mean was only insignificantly higher than in TT or BT. Only two of 15 patients with non-reactional multibacillary form (LL, BL, BB) and one of 12 patients with paucibacilllary form (BT, TT) had MRP8/MRP14 levels over 800 ng/ml, i.e. higher than two standard deviations of control sera (Fig. 6). Thus, despite the markedly higher numbers of MRP8- and MRP14-expressing cells in their infiltrates, patients with BL and LL could not be distinguished from BT and TT by higher serum levels of these proteins. Also, in RR (type 1 reaction) serum levels of MRP8/MRP14 did not significantly surpass normal levels. In marked contrast, in ENL the mean was 2529 ng/ml, and 13 of 16 patients (81%) showed MRP8/MRP14 serum levels of 800 ng/ml or higher (Fig. 6). Thus, increase of serum levels for MRP8/MRP14 in patients with leprosy was a significant (P = 0·05) as well as a characteristic and almost exclusive sign of ENL.
Figure 6.
Serum levels for MRP8/MRP14 in the different forms of leprosy, determined by sandwich ELISA and statistically evaluated by the U-test. Plotted are the median, 10th, 25th, 75th and 90th percentiles as vertical boxes with error bars. Error bar values are column means. An asterisk (*) denotes significant elevation in ENL (U-test according to Mann and Whitney; values of P > 0·05 were considered not to be significant).
Discussion
Monocytes and macrophages do not represent a homogeneous cell population but different subpopulations sometimes exhibiting antagonistic inflammatory properties.1,2,30 Our study has revealed that emerging susceptibility to leprosy with rising bacterial counts (multibacillary forms) or with higher inflammatory activity in the lepra reactions is associated with up-regulation of MRP8 and MRP14.
MRP8 or MRP14 are known to be regularly expressed by granulocytes and infiltrating monocytes in the acute phase of human or murine inflammation6,8,10–12,31 and by macrophages in acute flares of certain chronic inflammations such as rheumatoid arthritis or inflammatory bowel disease.4,15–17,31,32 They are absent in resident macrophages and are down-regulated in inflammatory infiltrates after the acute phase.10,33 Whereas induction of MRP8 and MRP14 in vitro was observed in certain cell lines,10,34,35 only one study so far has reported induction of MRP8, but not of MRP14, in primary cultures of murine mature macrophages after stimulation with lipopolysaccharide.36
The high percentage of MRP8 and MRP14 expression among so-called foam cells in the multibacillary forms of leprosy is remarkable as it demonstrates that these proteins are expressed in vivo also by long-standing macrophages in chronic infiltrates. A novel finding was the capability of long-standing macrophages within chronic infiltrates to re-express MRP8 and MRP14 after appropriate stimulation. This could be deduced from the appearance of MRP8+ and MRP14+ epitheloid and giant cells during RR (type 1 reactions), as these cells were negative in non-reactional forms and as they do not present newly recruited MRP8+ and MRP14+ monocytes.
This investigation thus shows that expression is distinctly regulated within the spectrum of one disease. High expression of the two proteins was exclusively detected in multibacillary forms and in the highly inflammatory lepra reactions whereas their expression in paucibacillary forms of leprosy was low. Expression of MRP8 and MRP14 significantly rose in BT, indicating qualitative changes within the macrophage population. This increase of expression therefore presents a new characteristic for BT which otherwise does not show marked differences to TT with regard to histological structure of individual granulomas.
Previous studies have revealed that an MRP8+ and MRP14+ subtype of CD68+ macrophages prevails in acute arthritic infiltrates during highly inflammatory states of rheumatoid arthritis4 or in areas of degeneration in inflammatory myopathies,37 whereas after subsidence of acute bouts of inflammation or during chronic inflammation a CD68+ subtype was observed which did not express MRP8 and MRP14.
The possible functions of MRP8 and MRP14 are under intensive investigation. In the murine system MRP8+ and MRP14+ cells present the predominant subtype in mice susceptible to L. major.6 Infiltrating cells positive for MRP8 and MRP14 often colocalize with areas of high parasite load.7 In vitro these cells appear to be less efficient in eliminating intracellular parasites than macrophages not expressing MRP8 and MRP14.7 Thus, there is a striking resemblance to human leprosy where expression of MRP8 and MRP14 is associated with bacteria-loaded foam cells and where it is more frequent in the multibacillary forms. Expression of MRP8 and MRP14 therefore appears to indicate a decreased ability to eliminate intracellular microbes. Whereas the complex was shown to have microbicidal activity against Candida species and some bacteria,38,39 it was not active against Leishmania.7 In addition MRP8+ and MRP14+ cells induce or maintain a high inflammatory activity and are supposed to contribute to the damage observed in tissues with very high expression of these proteins.4,11,31,40 The correlation in both RR and ENL between clinically harmful reactions and significantly increased appearance of MRP8+ and MRP14+ cells adds evidence to the apparent hazardous effects of these cells. The mechanisms leading to tissue damage have not been fully elucidated, but the extracellular complexes have been shown to induce apoptosis and inhibition of proliferation in several cell types in vitro and in vivo when present in high concentrations.37,41 They also have the capacity to perpetuate the inflammatory process as they promote adhesion of phagocytes when binding to activated endothelial cells.9
Although much emphasis has been put on the role of T cells in determining the different forms of leprosy, one has to consider that in BL and especially LL there is an anergy towards M. leprae. Accumulation of cells expressing MRP8 and MRP14 thus is likely to present the innate, macrophage-controlled immune response towards pathogens without the influence of T cells. We cannot rule out at this point that high expression of MRP8 and MRP14 might even play a role in the inability to mount a T-cell-mediated immune response.
In leprosy high serum levels of MRP8 and MRP14 were almost exclusively associated with type 2 lepra reaction (ENL), thus emerging as a marker for the presence of ENL and for the generalized high inflammatory activity in this process. Serum levels of MRP8 and MRP14 have previously been found to be markedly raised in certain inflammations, i.e. cystic fibrosis, inflammatory bowel disease and rheumatoid arthritis, where both proteins are likely to be released from monocytes and granulocytes during inflammatory activation.4, 14, 16, 17, 32, 42–44 Release of MRP8 and MRP14 by monocytes is a tightly regulated process involving activation of protein kinase C13 and interaction with tumour necrosis factor (TNF) stimulated endothelial cells, but not elicitable by resting endothelium.4 As a rise of TNF-α and interleukin-1β in serum as well as an increased expression of E-selectin have been observed in ENL,45 this could be evidence for TNF-α-induced activation of endothelial cells, which together with activation of macrophages would present the required constellation for inducing secretion of MRP8/MRP14 in ENL. The other forms of leprosy, in contrast, do not show marked activation of both endothelial cells and macrophages, and would therefore not fulfil the requirements for release of MRP8 and MRP14.
As there was not a particular pattern of endothelial adhesion molecules whose expression would strictly correlate with the distinct appearance of the MRP8+ and MRP14+ macrophage subpopulation, their distinct presence is dependent on additional expression of other endothelial membrane proteins or chemotactic factors during the stage of diapedesis or on differential expression of MRP8 and MRP14 by monocytes after diapedesis. However, we found that the percentage of vessels expressing E-selectin, VCAM-1 and ICAM-1 was significantly higher in TT or BT, thus relating this increased expression to those forms of leprosy which are characterized by an intact antigen-specific T-cell response. This is consistent with the finding that T cells are able to sustain expression of E-selectin in an antigen-specific manner during, for example, experimental leishmaniasis.46 Such differences in endothelial expression of adhesion molecules between the polar forms of leprosy were not detectable when total numbers of positive vessels were compared47 instead of percentages of positive vessels, as there is a markedly higher number of vessels in the huge infiltrates of BL and LL.
In conclusion, the different forms of leprosy are established by lymphocytes which regulate the direction of the immune response, but also by macrophages which are decisive effector cells due to their strong phagocytic as well as potential mycobactericidal capacity and due to their capacity to release pro- or anti-inflammatory mediators. MRP8- and MRP14-expressing cells in anergic LL appear to be the phenotype that dominates the innate immune response without the support of M. leprae-specific Th1 cells. As they may not only represent a bacteria-loaded safe target cell, but also a cell responsible for the rise in inflammatory activity, as deduced from the up-regulation in lepra reactions, expression of MRP8 and MRP14 characterizes an activated but ineffective macrophage response in infectious diseases with intracellular pathogens.
Acknowledgments
We thank Dr E. Hultsch from the Institute for Informatics and Biomathematics, University of Münster, for assistance with statistical analysis. We are indebted to Mrs K. Fischer and Mrs E. Nattkemper for their skilful technical assistance.
This work was supported by grants to C. Su of the German Research Association (SFB293/A8) and the Interdisciplinary Center of Clinical Research in conjunction with the German Ministry of Research (IZKF-C2).
Abbreviations
- BB
midborderline leprosy
- BL
borderline lepromatous leprosy
- BT
borderline tuberculoid leprosy
- ELISA
enzyme-linked immunosorbent assay
- ENL
erythema nodosum leprosum, lepra reaction type 2
- ICAM-1
intercellular adhesion molecule type 1
- Ig
immunoglobulin
- LL
lepromatous leprosy
- MRP
myeloid-related protein
- RR
reversal reaction, lepra reaction type 1
- Th
t helper
- TNF
tumour necrosis factor
- TT
tuberculoid leprosy
- VCAM-1
vascular cell adhesion molecule type 1
References
- 1.Rutherford MS, Witsell A, Schook LB. Mechanisms generating functionally heterogeneous macrophages: chaos revisited. J Leukoc Biol. 1993;53:602–18. doi: 10.1002/jlb.53.5.602. [DOI] [PubMed] [Google Scholar]
- 2.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–22. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 3.Bresnihan B, Alvaro-Gracia JM, Cobby M, et al. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41:2196–204. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Frosch M, Strey A, Vogl T, et al. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2000;43:628–37. doi: 10.1002/1529-0131(200003)43:3<628::AID-ANR20>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 5.Rugtveit J, Brandtzaeg P, Halstensen TS, Fausa O, Scott H. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut. 1994;35:669–74. doi: 10.1136/gut.35.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sunderkotter C, Kunz M, Steinbrink K, Meinardus-Hager G, Goebeler M, Bildau H, Sorg C. Resistance of mice to experimental leishmaniasis is associated with more rapid appearance of mature macrophages in vitro and in vivo. J Immunol. 1993;151:4891–901. [PubMed] [Google Scholar]
- 7.Steinbrink K, Schonlau F, Rescher U, Henseleit U, Vogel T, Sorg C, Sunderkotter C. Ineffective elimination of Leishmania major by inflammatory (MRP14-positive) subtype of monocytic cells. Immunobiology. 2000;202:442–59. doi: 10.1016/s0171-2985(00)80103-5. [DOI] [PubMed] [Google Scholar]
- 8.Odink K, Cerletti N, Bruggen J, et al. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330:80–2. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- 9.Roth J, Vogl T, Sorg C, Sunderkotter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003;24:155–8. doi: 10.1016/s1471-4906(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 10.Roth J, Goebeler M, van den Bos C, Sorg C. Expression of calcium-binding proteins MRP8 and MRP14 is associated with distinct monocytic differentiation pathways in HL-60 cells. Biochem Biophys Res Commun. 1993;191:565–70. doi: 10.1006/bbrc.1993.1255. [DOI] [PubMed] [Google Scholar]
- 11.Hessian PA, Edgeworth J, Hogg N. MRP-8 and MRP-14, two abundant Ca(2+)-binding proteins of neutrophils and monocytes. J Leukoc Biol. 1993;53:197–204. [PubMed] [Google Scholar]
- 12.Roth J, Sunderkotter C, Goebeler M, Gutwald J, Sorg C. Expression of the calcium-binding proteins MRP8 and MRP14 by early infiltrating cells in experimental contact dermatitis. Int Arch Allergy Immunol. 1992;98:140–5. doi: 10.1159/000236177. [DOI] [PubMed] [Google Scholar]
- 13.Rammes A, Roth J, Goebeler M, Klempt M, Hartmann M, Sorg C. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J Biol Chem. 1997;272:9496–502. doi: 10.1074/jbc.272.14.9496. [DOI] [PubMed] [Google Scholar]
- 14.Barthe C, Figarella C, Carrere J, Guy-Crotte O. Identification of ‘cystic fibrosis protein’ as a complex of two calcium-binding proteins present in human cells of myeloid origin. Biochim Biophys Acta. 1991;1096:175–7. doi: 10.1016/0925-4439(91)90057-g. [DOI] [PubMed] [Google Scholar]
- 15.Hammer HB, Kvien TK, Glennas A, Melby K. A longitudinal study of calprotectin as an inflammatory marker in patients with reactive arthritis. Clin Exp Rheumatol. 1995;13:59–64. [PubMed] [Google Scholar]
- 16.Veale DJ, Barnes L, Rogers S, FitzGerald O. Immunohistochemical markers for arthritis in psoriasis. Ann Rheum Dis. 1994;53:450–4. doi: 10.1136/ard.53.7.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tibble J, Teahon K, Thjodleifsson B, et al. A simple method for assessing intestinal inflammation in Crohn's disease. Gut. 2000;47:506–13. doi: 10.1136/gut.47.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abulafia J, Vignale RA. Leprosy: accessory immune system as effector of infectious, metabolic, and immunologic reactions. Int J Dermatol. 2001;40:673–87. doi: 10.1046/j.1365-4362.2001.01259.x. [DOI] [PubMed] [Google Scholar]
- 19.Haimanot RT, Melaku Z. Leprosy. Curr Opin Neurol. 2000;13:317–22. doi: 10.1097/00019052-200006000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson RR, Krahenbuhl JL. Leprosy. Lancet. 1999;353:655–60. doi: 10.1016/S0140-6736(98)06322-3. [DOI] [PubMed] [Google Scholar]
- 21.Abulafia J, Vignale RA. Leprosy: pathogenesis updated. Int J Dermatol. 1999;38:321–34. doi: 10.1046/j.1365-4362.1999.00650.x. [DOI] [PubMed] [Google Scholar]
- 22.Verhagen CE, Wierenga EA, Buffing AA, Chand MA, Faber WR, Das PK. Reversal reaction in borderline leprosy is associated with a polarized shift to type 1-like Mycobacterium leprae T cell reactivity in lesional skin: a follow-up study. J Immunol. 1997;159:4474–83. [PubMed] [Google Scholar]
- 23.Murphy GF, Sanchez NP, Flynn TC, Sanchez JL, Mihm MC, Jr, Soter NA. Erythema nodosum leprosum: nature and extent of the cutaneous microvascular alterations. J Am Acad Dermatol. 1986;14:59–69. doi: 10.1016/s0190-9622(86)70008-x. [DOI] [PubMed] [Google Scholar]
- 24.Meyerson MS. Erythema nodosum leprosum. Int J Dermatol. 1996;35:389–92. doi: 10.1111/j.1365-4362.1996.tb03016.x. [DOI] [PubMed] [Google Scholar]
- 25.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255–73. [PubMed] [Google Scholar]
- 26.Pulford KA, Rigney EM, Micklem KJ, Jones M, Stross WP, Gatter KC, Mason DY. KP1: a new monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J Clin Pathol. 1989;42:414–21. doi: 10.1136/jcp.42.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993;81:1607–13. [PubMed] [Google Scholar]
- 28.Roth J, Burwinkel F, van den Bos C, Goebeler M, Vollmer E, Sorg C. MRP8 and MRP14, S-100-like proteins associated with myeloid differentiation, are translocated to plasma membrane and intermediate filaments in a calcium-dependent manner. Blood. 1993;82:1875–83. [PubMed] [Google Scholar]
- 29.van den Bos C, Rammes A, Vogl T, Boynton R, Zaia J, Sorg C, Roth J. Copurification of P6, MRP8, and MRP14 from human granulocytes and separation of individual proteins. Protein Expr Purif. 1998;13:313–8. doi: 10.1006/prep.1998.0917. [DOI] [PubMed] [Google Scholar]
- 30.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–12. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 31.Goebeler M, Roth J, Burwinkel F, Vollmer E, Bocker W, Sorg C. Expression and complex formation of S100-like proteins MRP8 and MRP14 by macrophages during renal allograft rejection. Transplantation. 1994;58:355–61. [PubMed] [Google Scholar]
- 32.Berntzen HB, Olmez U, Fagerhol MK, Munthe E. The leukocyte protein L1 in plasma and synovial fluid from patients with rheumatoid arthritis and osteoarthritis. Scand J Rheumatol. 1991;20:74–82. doi: 10.3109/03009749109165280. [DOI] [PubMed] [Google Scholar]
- 33.Goebeler M, Roth J, Henseleit U, Sunderkotter C, Sorg C. Expression and complex assembly of calcium-binding proteins MRP8 and MRP14 during differentiation of murine myelomonocytic cells. J Leukoc Biol. 1993;53:11–8. doi: 10.1002/jlb.53.1.11. [DOI] [PubMed] [Google Scholar]
- 34.Sellmayer A, Krane SM, Ouellette AJ, Bonventre JV. 1α,25-(OH)2 vitamin D3 enhances expression of the genes encoding Ca(2+)-binding proteins MRP-8 and MRP-14. Am J Physiol. 1992;262:C235–42. doi: 10.1152/ajpcell.1992.262.1.C235. [DOI] [PubMed] [Google Scholar]
- 35.Kuruto R, Nozawa R, Takeishi K, Arai K, Yokota T, Takasaki Y. Myeloid calcium binding proteins: expression in the differentiated HL-60 cells and detection in sera of patients with connective tissue diseases. J Biochem Tokyo. 1990;108:650–3. doi: 10.1093/oxfordjournals.jbchem.a123257. [DOI] [PubMed] [Google Scholar]
- 36.Hu SP, Harrison C, Xu K, Cornish CJ, Geczy CL. Induction of the chemotactic S100 protein, CP-10, in monocyte/macrophages by lipopolysaccharide. Blood. 1996;87:3919–28. [PubMed] [Google Scholar]
- 37.Seeliger S, Vogl T, Engels IH, Schröder M, Sorg C, Sunderkötter C, Roth J. Expression of calcium-binding proteins MRP8 and MRP14 in inflammatory muscle diseases. Am J Pathol. 2003;163:947–56. doi: 10.1016/S0002-9440(10)63454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinbakk M, Naess-Andresen CF, Lingaas E, Dale I, Brandtzaeg P, Fagerhol MK. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336:763–5. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- 39.Sohnle PG, Hunter MJ, Hahn B, Chazin WJ. Zinc-reversible antimicrobial activity of recombinant calprotectin (migration inhibitory factor-related proteins 8 and 14) J Infect Dis. 2000;182:1272–5. doi: 10.1086/315810. [DOI] [PubMed] [Google Scholar]
- 40.Rugtveit J, Haraldsen G, Hogasen AK, Bakka A, Brandtzaeg P, Scott H. Respiratory burst of intestinal macrophages in inflammatory bowel disease is mainly caused by CD14+ L1+ monocyte derived cells. Gut. 1995;37:367–73. doi: 10.1136/gut.37.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yui S, Nakatani Y, Mikami M. Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biol Pharm Bull. 2003;26:753–60. doi: 10.1248/bpb.26.753. [DOI] [PubMed] [Google Scholar]
- 42.Roth J, Teigelkamp S, Wilke M, Grun L, Tummler B, Sorg C. Complex pattern of the myelo-monocytic differentiation antigens MRP8 and MRP14 during chronic airway inflammation. Immunobiology. 1992;186:304–14. doi: 10.1016/S0171-2985(11)80259-7. [DOI] [PubMed] [Google Scholar]
- 43.Brun JG, Jonsson R, Haga HJ. Measurement of plasma calprotectin as an indicator of arthritis and disease activity in patients with inflammatory rheumatic diseases. J Rheumatol. 1994;21:733–8. [PubMed] [Google Scholar]
- 44.Hamann W, Floter A, Schmutzler W, Zwadlo-Klarwasser G. Characterization of a novel anti-inflammatory factor produced by RM3/1 macrophages derived from glucocorticoid treated human monocytes. Inflamm Res. 1995;44:535–40. doi: 10.1007/BF01757358. [DOI] [PubMed] [Google Scholar]
- 45.Sarno EN, Grau GE, Vieira LM, Nery JA. Serum levels of tumour necrosis factor-α and interleukin-1β during leprosy reactional states. Clin Exp Immunol. 1991;84:103–8. [PMC free article] [PubMed] [Google Scholar]
- 46.Sunderkotter C, Steinbrink K, Henseleit U, Bosse R, Schwarz A, Vestweber D, Sorg C. Activated T cells induce expression of E-selectin in vitro and in an antigen-dependent manner in vivo. Eur J Immunol. 1996;26:1571–9. doi: 10.1002/eji.1830260725. [DOI] [PubMed] [Google Scholar]
- 47.Moncada B, Torres-Alvarez MB, Gonzalez-Amaro R, Fuentes-Ahumada C, Baranda L, Delgado SP, Garcia R. Lack of expression of intercellular adhesion molecule ICAM-1 in lepromatous leprosy patients. Int J Lepr Other Mycobact Dis. 1993;61:581–5. [PubMed] [Google Scholar]