Abstract
Drug abuse is a global problem of considerable concern to health. One such health concern stems from the fact that many drugs of abuse have immunosuppressive actions and consequently have the potential to increase susceptibility to infectious disease. This article is focused on the impact of the amphetamine derivative, methylenedioxymethamphetamine (MDMA; ‘Ecstasy’) on immunity. Research conducted over the last 5 years, in both laboratory animals and humans, has demonstrated that MDMA has immunosuppressive actions. Specifically, MDMA suppresses neutrophil phagocytosis, suppresses production of the pro-inflammatory cytokines tumour necrosis factor-α (TNF-α) and interleukin (IL)-1β, and increases production of the endogenous immunosuppressive cytokine (IL-10), thereby promoting an immunosuppressive cytokine phenotype. MDMA also suppresses circulating lymphocyte numbers, with CD4+ T cells being particularly affected, and alters T-cell function as indicated by reduced mitogen-stimulated T-cell proliferation, and a skewing of T-cell cytokine production in a T helper 2 (Th2) direction. For the most part, the aforementioned effects of MDMA are not the result of a direct action of the drug on immune cells, but rather caused by the release of endogenous immunomodulatory substances. Consequently, the physiological mechanisms that are thought to underlie the immunosuppressive effects of MDMA will be discussed. As many of the physiological changes elicited by MDMA closely resemble those induced by acute stress, it is suggested that exposure to MDMA could be regarded as a ‘chemical stressor’ on the immune system. Finally, the potential of MDMA-induced immunosuppression to translate into significant health risks for abusers of the drug will be discussed.
Introduction
Drug abuse is a worldwide problem that has major social, health and economic implications. Over the last two decades, investigators have documented the ability of a number of drugs of abuse, such as cocaine, opioids, cannabinoids and amphetamines, to impair many aspects of immune function, either directly, or via neuroimmune pathways.1–6 In addition, there is substantial evidence that the stress associated with morphine and cocaine withdrawal also induces an immunosuppressive state.7,8 Moreover, numerous reports in a number of experimental models indicate that the immunosuppression elicited by drugs of abuse can result in diminished host resistance to disease.9–12 Indeed, some investigators have implicated drug abuse as a cofactor in the susceptibility to infection with human immunodeficiency virus (HIV) or other viruses.10,11,13,14 This review focuses on the immunomodulatory capacity of methylenedioxymethamphetamine (MDMA; ‘Ecstasy’), an amphetamine derivative that has become a popular drug of abuse over the last two decades.15,16
Mdma: history and biological actions
MDMA is a ring-substituted phenylisopropylamine that is structurally related to both amphetamines and hallucinogens. The compound was initially developed as an appetite suppressant in 1914 by Merck Pharmaceuticals, but was never marketed for this purpose. It was later discovered that MDMA had psychoactive properties, and it was reported to confer benefit as an adjunct to psychotherapy, where it was shown to enhance communication and intimacy.16 In the 1980s MDMA became a popular drug of abuse, particularly at raves and all-night dance clubs. However, following an increase in the prevalence of its abuse, and evidence of adverse health effects, MDMA was classified as a schedule I controlled substance by the Food and Drug Administration (FDA) in 1985.
In terms of its neurochemical mechanism of action, MDMA is a potent releaser of the indolamine neurotransmitter, serotonin, and, to a lesser extent, the catecholamine neurotransmitter, dopamine, in the central nervous system (CNS) (Fig. 1).17,18 These neurochemical actions of MDMA result in psychoactive properties that have been studied both in animals and humans.19–21 In humans, MDMA produces a relaxed, euphoric state, including emotional openness, increased empathy and a decrease in inhibitions. These subjective behavioural effects elicited by MDMA have led to its widespread abuse over the last 15–20 years.
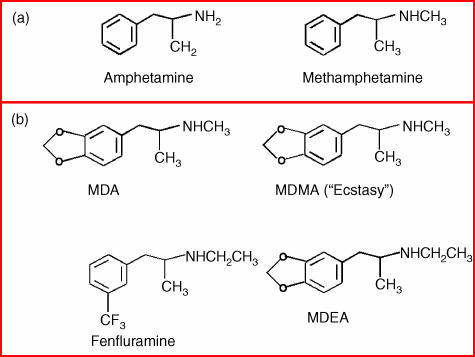
Figure 1.
An illustration of the chemical structures of (a) amphetamine and methamphetamine, both of which stimulate catecholamine release in the central nervous system (CNS), and (b) MDA, methylenedioxymethamphetamine (MDMA), MDEA and fenfluramine; substituted amphetamines, all of which are potent releasers of serotonin.
In addition to the psychoactive properties of MDMA, it produces an array of physiological actions such as hyperthermia, acute sympathomimetic effects (e.g. increased heart rate and blood pressure), a transient increase in anxiety and increased activation of the hypothalamic pituitary adrenal axis.15 Experimental studies conducted in laboratory animals22,23 and humans21 have concluded that many of these actions of MDMA occur secondary to the central release of either serotonin or dopamine.
MDMA use has been associated with a number of serious side-effects, such as cardiac arrhythmias, hyperthermia, renal failure, seizures and intracranial haemorrhage.15,16 In addition to these acute toxic effects there is substantial evidence that MDMA can result in long-term neurotoxic effects on central serotonergic neurons24,25 and that this may represent a predisposing factor to psychological disturbances/psychiatric disease.16,26
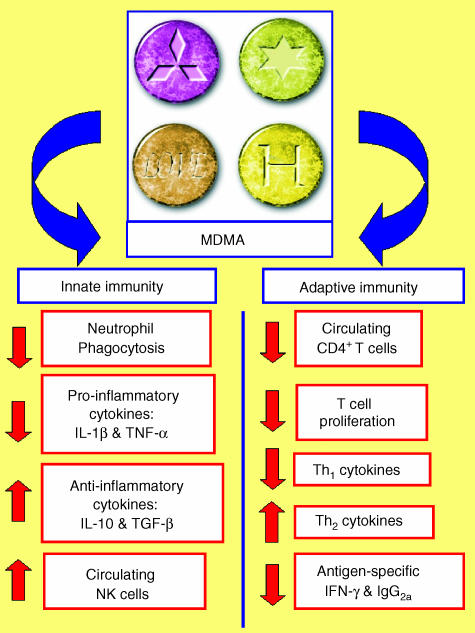
In the last few years it has become evident that like other drugs of abuse, MDMA has immunosuppressive properties. A number of studies in laboratory animals27–34 and in humans35–39 indicate that MDMA suppresses aspects of both innate and adaptive immunity (Fig. 2, Table 1). In the remainder of this review I will discuss the evidence indicating that MDMA impacts upon immune function, discuss the potential mechanisms by which MDMA elicits its immunomodulatory actions, and also discuss the potential of MDMA-induced immunosuppression to translate into significant health risks for abusers.
Figure 2.
A diagrammatic summary of the effect of in vivo administration of methylenedioxymethamphetamine (MDMA) on aspects of innate and adaptive immunity.
Table 1.
A summary of the studies conducted concerning the impact of methylenedioxymethamphetamine (MDMA) on the immune system
| Parameter | Source | Stimulant | MDMA Conc./dose | Effect | Reference |
|---|---|---|---|---|---|
| Neutrophil phagocytosis | Rat blood | Zymosan | In vivo: 10 mg/kg (i.p.)/in vitro (10 µg/ml) | Decrease | [28] |
| IL-1β production | Rat blood | LPS | In vivo: 10–20 mg/kg (i.p.) | Decrease | [30,31] |
| Rat blood | LPS | In vitro: 1–10 µg/ml | No change | [31] | |
| Human blood | PHA | In vivo: 100 mg (p.o.) | Decrease | [38] | |
| TNF-α production | Rat blood | LPS | In vivo: 5–20 mg/kg (i.p.) | Decrease | [30,31] |
| Rat blood | LPS | In vitro: 1–10 µg/ml | No change | [31] | |
| Mouse macrophages | LPS | In vitro: 0·0001–100 µm | No change | [50] | |
| IL-6 production | Mouse macrophages | LPS | In vitro: 0·0001–100 µm | No change | [50] |
| Human blood | PHA | In vivo: 100 mg (p.o.) | Decrease | [38] | |
| IL-10 production | Rat blood | LPS | In vivo: 1·25–10 mg/kg (i.p.) | Increase | [28] |
| Rat blood | LPS | In vitro: 1–10 µg/ml | No change | [28] | |
| Rat blood | Con A | In vivo: 7·5 mg/kg (i.p.) | Decrease | [32] | |
| Human blood | PHA | In vivo: 100 mg (p.o.) | Increase | [38] | |
| TGF-β1 production | Human blood | PHA | In vivo: 100 mg (p.o.) | Increase | [38] |
| IFN-γ production | Rat blood | Con A | In vivo: 7·5 mg/kg (i.p.) | Decrease | [32] |
| Human blood | PHA | In vivo: 100 mg (p.o.) | Decrease | [38] | |
| Rat splenocytes | KLH | In vivo: 10 mg/kg (i.p.) | Decrease | [29] | |
| IL-2 production | Rat blood | Con A | In vivo: 7·5 mg/kg (i.p.) | Decrease | [32] |
| Human blood | PHA | In vivo: 100 mg (p.o.) | Decrease | [38] | |
| Mouse splenocytes | Anti-CD3 | In vitro: 0·0001 µm | Increase | [50] | |
| IL-4 production | Mouse splenocytes | Anti-CD3 | In vitro: 0·0001–100 µm | Increase/decrease | [50] |
| Human blood | PHA | In vivo: 100 mg (p.o.) | Increase | [38] | |
| NK cell numbers | Human blood | None | In vivo: 75–100 mg (p.o.) | Increase | [35,37–39] |
| NK cell activity | Mouse splenocytes | None/IL-2 | In vitro: 0·0001–100 µm | Increase/decrease | [50] |
| Lymphocyte numbers | Rat blood | None | In vivo: 1·25–40 mg/kg | Decrease: lymphocytes | [32,33] |
| Human blood | None | In vivo: 75–125 mg | Decrease: CD4+ T cells | [35,37–39] | |
| T-cell proliferation | Rat blood | Con A/PHA | In vivo: 7·5–20 mg/kg | Decrease | [32–34] |
| Human blood | PHA | In vivo: 75–125 mg | Decrease | [39] | |
| CTL activity | Mouse splenocytes | None | In vitro: 100 µm | Decrease | [50] |
| B-cell proliferation | Mouse splenocytes | Anti-IgM/IL-4 | In vitro: 0·0001–100 µm | No change | [50] |
| Antibody production | Rat blood | KLH | In vivo: 5–10 mg/kg (i.p.) | IgG2a: decrease | [29] |
Con A, concanavalin A; CTL, cytotoxic T lymphocyte; IFN-γ, interferon-γ; IL, interleukin; i.p., intraperitoneal; KLH, keyhole limpet haemocyanin; LPS, lipopolysaccharide; NK, natural killer; PHA, phytohaemagglutinin; p.o., per os; TGF-β1, transforming growth factor-β1; TNF-α, tumour necrosis factor-α.
In vivo effects of mdma in animal models
Innate immunity
Neutrophils are a subset of phagocytic cells that play a key role in the innate immune response. Neutrophil activation that occurs following phagocytosis is accompanied by an oxidative burst which produces reactive oxygen species and destroys bacteria and fungi. Administration of MDMA (10 mg/kg) to rats has been shown to suppress the neutrophil oxidative burst in response to opsonized zymosan (a phagocytic stimulus), but not in response to chemical stimulation of protein kinase C by the phorbol ester, phorbol 12-myristate 13-acetate (PMA).27 The fact that the PMA-induced oxidative burst was unaltered suggests that the inhibitory effect of MDMA occurs upstream of protein kinase C activation, possibly by interfering with the phagocytic process. It is suggested that MDMA may downregulate the expression of molecules such as CR3 or FcR that are required to initiate phagocytosis of opsonized zymosan; however, further studies are required in order to study the effect of MDMA on the expression of such cell-surface molecules.
Cells of the innate immune system, such as macrophages and dendritic cells, produce proinflammatory cytokines such as interleukin (IL)-1β, tumour necrosis factor-α (TNF-α) and IL-12 in response to stimulation with bacterial products such as lipopolysaccharide (LPS). These cytokines are of strategic importance in initiating and co-ordinating a large range of immune responses against invading pathogens.40–43 MDMA administration to rats impairs the ability to respond to an in vivo immune challenge with bacterial LPS. Specifically, MDMA suppresses LPS-induced IL-1β and TNF-α production.28,30,31 The suppression of TNF-α that occurs following MDMA administration is the more profound and persists for longer than the suppression of IL-1β.31 Whilst the rat does not produce IL-12 in response to LPS (T.J. Connor, unpublished), we have recent data demonstrating that MDMA suppresses IL-12p40 and IL-12p70 production in the mouse following an in vivo LPS challenge (N. Boyle and T.J. Connor, unpublished).
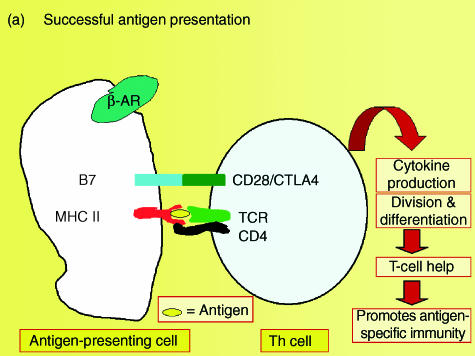
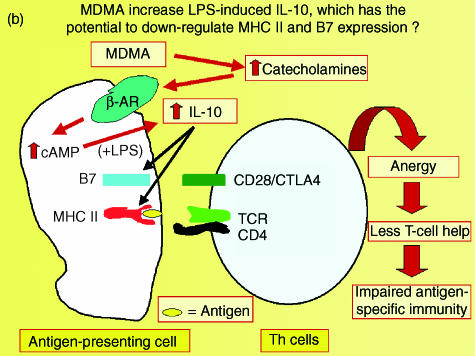
In addition to producing pro-inflammatory cytokines, cells of the innate immune system also produce IL-10, an anti-inflammatory or immunosuppressive cytokine that inhibits several macrophage functions, including pro-inflammatory cytokine production in response to LPS.44–47 In a recent study it was demonstrated that MDMA provokes a dose-dependent increase in IL-10 following an in vivo LPS challenge in rats. This increase in IL-10 production occurs at doses of MDMA as low as 1·25 mg/kg, and closely parallels the suppression of TNF-α observed in response to MDMA.28 However, the ability of MDMA to suppress LPS-induced TNF-α and IL-1β is still evident following immunoneutralization of IL-10, indicating that the suppression of these pro-inflammatory cytokines occurs independently of increased IL-10 production.28 In addition to suppressing the production of pro-inflammatory cytokines, IL-10 also downregulates the expression of the antigen-presenting molecule, major histocompatibility complex (MHC) class II, and expression of the costimulatory molecule, B7, on antigen-presenting cells, thereby inhibiting antigen presentation producing anergy in the T-cell arm of the immune response.45,46 Studies are ongoing in order to determine whether the increase in LPS-induced IL-10 production observed following MDMA administration will have a significant impact on the antigen-presenting or costimulatory capacity of macrophages or dendritic cells (Fig. 3).
Figure 3.
A schematic diagram depicting the potential of methylenedioxymethamphetamine (MDMA)-induced increases in interleukin-10 (IL-10) production to interfere with antigen presentation, and thereby produce anergy in the immune system.
Adaptive immunity
Lymphocytes are the main subset of immune cell that mediate adaptive or specific immune responses. A number of studies indicate that MDMA suppresses lymphocyte function in rats. The first study to examine the effect of MDMA on immune function demonstrated that quite a high dose of MDMA (20 mg/kg, intraperitoneally) produced a rapid (within 30 min) suppression of concanavalin A (Con A)-induced lymphocyte proliferation, and a profound reduction in the total leucocyte count in rats, both of which persisted for at least 6 hr following injection.34 In a subsequent study it was observed that MDMA, its major metabolite methylenedioxyamphetamine (MDA), and also the related amphetamine derivative, fenfluramine, suppressed circulating lymphocyte numbers, mitogen-stimulated T-cell proliferation and cytokine production, with MDA and fenfluramine being more potent than MDMA with respect to their immunosuppressive actions.32
Whilst examining mitogen-stimulated lymphocyte responses gives a useful indication of lymphocyte function, it has the limitation that under normal physiological circumstances the immune system does not encounter mitogens, but rather encounters antigens. Consequently, a study was conducted to assess the impact of MDMA administration on an antigen-specific immune response to the soluble protein antigen, keyhole limpet haemocyanin (KLH) in rats.29 In this study, KLH-specific immunoglobulin production and KLH-specific cytokine production were assessed as indices of immunocompetence. MDMA did not alter the KLH-specific immunoglobulin M (IgM) response. In contrast, MDMA (5 and 10 mg/kg) significantly suppressed KLH-specific immunoglobulin G (IgG) production.29 Therefore, whilst MDMA failed to alter the initial generation of the antibody response, it profoundly inhibited antibody class switching from IgM to IgG. Two pathways for the genetic switch from IgM to IgG production were investigated. One pathway requires the T helper 1 (Th1)-type cytokine, interferon-γ (IFN-γ), to stimulate the switch to IgG2a-secreting cells, whilst another pathway requires the T helper 2 (Th2) type cytokines IL-4 and IL-6 to stimulate the switch to IgG1-secreting cells. IgG1 and IgG2a levels were measured to determine if these two pathways were differentially affected. The results indicate that only IgG2a levels were decreased following MDMA administration. Furthermore, this decrease in IgG2a production was accompanied by decreased KLH-specific splenic IFN-γ production. Overall, these data indicate that MDMA alters the ability to switch from IgM to IgG2a production, possibly by reducing production of the Th1 cytokine, IFN-γ. These data indicated that in addition to the ability of MDMA to suppress the lymphocyte response to mitogenic stimuli, it also suppresses the Th1 arm of the immune response to an antigenic stimulus.
In vivo effect of mdma in humans
Over the last number of years, Pacifici and co-workers have clearly demonstrated that MDMA is a potent immunomodulator in humans.35–39 In these studies, either placebo or MDMA (75–100 mg) were administered orally to recreational MDMA users in a controlled setting. The effect of ethanol consumption (0·8 mg/kg) on immune function was also assessed, as was the effect of MDMA and ethanol co-administration. These studies were conducted in a double-blind manner, using a crossover (Latin Square) design. Each subject received all of the treatments in separate experimental sessions, with a 1-week washout period between each session. Blood samples were collected from the participants at a number of time-points up to 24 hr following drug administration, or up to 48 hr after drug administration in the study that assessed the impact of repeated doses of MDMA on immune function.
The first study conducted was a pilot study using four subjects. This study demonstrated that MDMA profoundly suppressed the number of circulating CD4+ T cells, suppressed mitogen-stimulated T-cell proliferation and increased the number of circulating natural killer (NK) cells.39 Although MDMA was shown to increase circulating NK-cell numbers, the activity of these NK cells was not assessed; consequently, it is difficult to predict the effect of MDMA on overall NK-cell functionality. In a follow-up study, the ability of MDMA to reduce the number of circulating T cells and increase the number of circulating NK cells was replicated. It was also reported that MDMA promoted a switch to a Th2-type cytokine profile, as indicated by reduced IFN-γ and IL-2 production, with an concomitant increase in the Th2 cytokines, IL-4 and IL-6, and the T-regulatory cytokines, IL-10 and transforming growth factor-β1 (TGF-β1)·38 These immunosuppressive effects of MDMA were maximal 3–6 hr following drug administration, and in some cases were evident 24 hr later. In some instances, the co-administration of alcohol further enhanced the immunosuppressive effects of MDMA.
In a more recent study, Pacifici and colleagues assessed cell-mediated immune responses after administration of two repeated doses of MDMA (100 mg per dose) at 4- and 24-hr intervals.37 As previously reported, MDMA produced a time-dependent decrease in the number of CD4+ T-helper cells, a decrease in the functional responsiveness of lymphocytes to mitogenic stimulation, and a simultaneous increase in the number of circulating NK cells. In the first clinical trial, two MDMA doses were given 4 hr apart, and the immune changes produced by the first dose were enhanced following administration of the second dose. Furthermore, administration of two doses of MDMA, 4 hr apart, produced longer-lasting immunological deficits than a single dose of MDMA, in that, 24 hr after treatment, significant residual effects were observed for all the altered immune parameters after the administration of two MDMA doses, when compared to a single dose. In the second clinical trial, the second MDMA dose was administered 24 hr after the first dose, and produced immunological changes significantly greater than those induced by the initial drug administration, which seemed to show a delayed onset. In addition, significant residual effects were observed for all the immune parameters as late as 48 hr after the second dose. Based on these findings, it can be concluded that repeated administration of MDMA with both a short and a long time interval between doses, increases both the magnitude and duration of MDMA-induced immunosuppression.
The clinical trials that were outlined above were conducted in a controlled environment. The same research group conducted a study in a non-controlled environment where they compared baseline immunological parameters in 30 recreational MDMA users with a sample of 24 matched control subjects.36 When baseline values were compared, the only significant difference between MDMA users and controls was in the number of NK cells; NK-cell numbers in MDMA users were reduced to 33% of that seen in control subjects. In another study, leucocyte subsets from six habitual MDMA users, from the 30 who participated in the clinical trials, were followed-up for a 2-year period and compared to eight matched control subjects.36 At the start of this study, basal levels of leucocyte subsets (total lymphocytes, T-helper cells, cytotoxic T cells and B cells) were equivalent between the six control subjects and eight MDMA abusers. In contrast, NK-cell numbers were significantly decreased in MDMA users, and remained decreased over the 2-year duration of the study. One year after the initial observation, the total number of lymphocytes, CD4+ T cells and CD19+ B lymphocytes were all significantly reduced in MDMA users compared to control subjects. Moreover, this suppression of leucocyte subpopulations in MDMA users became even more apparent 2 years following the initial observation. Based on these data, the authors concluded that permanent alterations in immunological parameters are evident in MDMA abusers.
Whilst these non-controlled studies are interesting, the results obtained are somewhat difficult to interpret, for two reasons. First, it is not possible to ascertain if the suppression of immune parameters observed in the MDMA users is a result of MDMA abuse per se. For instance, it is outlined in the methods of the study that the subjects enrolled in the clinical trials were all habitual users of cannabis, with prior experience of cocaine or methamphetamine.36 As it is well established that cannabis, cocaine and methamphetamine all have significant immunomodulatory properties in their own right,1,2,5,6 it is impossible to discount the fact that they may have contributed to the immunological changes observed in this experimental group. The second issue pertains to the experimental design adopted in the 2-year follow-up study. In this study the control subjects were not tested at 1- and 2-year intervals, thus comparisons were made using historical data. For these reasons, one must be cautious when interpreting these results. For a summary of studies examining the impact of MDMA on immune function, see Table 1.
Mechanisms by which mdma can alter immune function
Direct effects of MDMA on immune cells: evidence from in vitro studies
The molecular targets of MDMA are the transporter (uptake) sites for serotonin and dopamine. Whilst these transporter sites are located predominantly on presynaptic serotonergic and dopaminergic neurons, respectively, there is now ample evidence that cells of the immune system also express transporters for both of these neurotransmitters.48,49 Consequently, MDMA can interact directly with immune cells, and thereby has the potential to alter their activity directly.
The first study to examine the immunomodulatory potential of MDMA was published almost 10 years ago by House and co-workers, and examined the impact of in vitro exposure to MDMA (0·0001–100 µm) on a number of immune parameters in splenocytes and peritoneal macrophages from B6C3F1 mice.50 In this study, T-cell function was assessed by anti-CD3-induced IL-2 and IL-4 production, B-cell function was assessed by measuring proliferation, natural immunity was assessed by measuring NK-cell cytotoxicity, T-cell effector function was evaluated by assessing cytotoxic T-lymphocyte (CTL) activity, and macrophage function was assessed by measuring production of the pro-inflammatory cytokines IL-6 and TNF. In vitro exposure to MDMA had no effect on B-cell proliferation. In terms of T-cell function, production of the Th1 cytokine, IL-2, was enhanced by 0·0001 µm MDMA, suppressed by 100 µm MDMA, and not altered by any of the five intermediate concentrations. Production of the Th2 cytokine, IL-4, was not altered by exposure to any concentration of MDMA examined. Basal and IL-2-augmented NK-cell cytotoxicity were enhanced at MDMA concentrations between 0·0001 and 0·1 µm; however, this effect was evident only at one of the three effector : target cell ratios employed. Conversely, IL-2-stimulated NK-cell activity was significantly suppressed by MDMA (10 µm), but again this effect was evident only at one of the three effector : target cell ratios employed in the assay. CTL induction was significantly suppressed at a concentration of 100 µm, but was unaltered at any of the other concentrations used. Finally, LPS-induced macrophage IL-6 or TNF production were not significantly altered by any concentration of MDMA; however, there was a slight, but statistically non-significant suppression of TNF observed at 10 and 100 µm MDMA. In summary, the data generated by House and co-workers50 indicates that in vitro exposure to MDMA has variable, and for the most part modest effects on the immune system, depending on the dose employed and the specific immune parameter under investigation.
In a subsequent study, Connor et al.31 observed that the in vitro exposure of LPS-stimulated diluted rat blood to MDMA failed to mimic its ability to suppress the pro-inflammatory cytokines, IL-1β and TNF-α, following an in vivo LPS challenge, thereby supporting in vitro findings of House et al.50 It was also reported that the ability of MDMA to increase LPS-induced IL-10 production in vivo was not mimicked by the in vitro exposure of LPS-stimulated diluted whole-blood cultures to the drug.28 In addition, the suppressive effect of MDMA on Con A-stimulated lymphocyte proliferation that is observed in vivo cannot be mimicked by in vitro exposure to MDMA (T. Connor, unpublished observations). These data suggest that the potent immunosuppressive actions of MDMA observed following in vivo administration are not a result of the direct action of the drug on immune cells, but are probably caused by the release of endogenous immunomodulatory substances that occurs in response to MDMA. In contrast to these findings, both in vivo and in vitro exposure to MDMA elicit similar suppressive effects on the zymosan-induced oxidative burst in rat neutrophils, indicating that MDMA can elicit a direct effect on neutrophil phagocytosis.27
Indirect mechanisms by which MDMA can affect the immune system
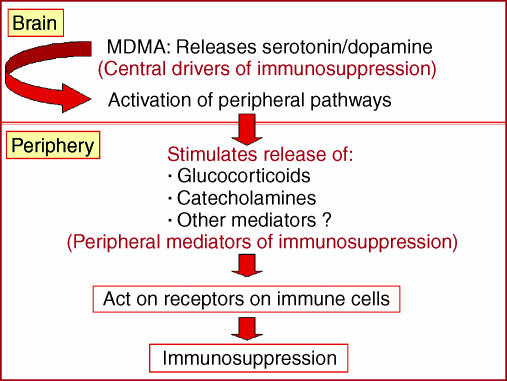
In addition to having a direct effect on immune cells, it is possible that a drug such as MDMA which alters neurotransmission within the CNS, could alter immunocompetence via a CNS-mediated mechanism. Specifically, it is well established that changes in CNS neurotransmitter function can alter immunity via changes in endocrine output and sympathetic nervous system activity.51,52 MDMA stimulates the release of the neurotransmitters serotonin and dopamine in the CNS17,18 and produces consequential downstream activation of peripheral immunomodulatory pathways, such as the hypothalamic pituitary adrenal axis and the sympathetic nervous system.23,53 Therefore, when addressing the underlying physiological mechanisms that mediate the immunosuppressive effects of MDMA, it is necessary to consider central neurotransmitters that drive downstream responses, and also peripheral neurotransmitters and hormones that are the ultimate mediators impacting on immune cells (Fig. 4).
Figure 4.
A schematic diagram outlining potential central and peripheral mediators of methylenedioxymethamphetamine (MDMA)-induced immunosuppression.
Central mediators of MDMA-induced immunosuppression
As the predominant neurochemical action of MDMA is to release serotonin within the CNS, it is logical to assume that central serotonin release may mediate the actions of MDMA on the immune system. In this regard it was demonstrated that the related amphetamine compound, fenfluramine, which is a selective releaser of serotonin, produced qualitatively similar suppressive effects to MDMA on a number of immunological measures in rats.32,54 For instance, fenfluramine suppressed circulating lymphocyte numbers, T-lymphocyte proliferation and cytokine production,32 and also production of the pro-inflammatory cytokines IL-1β and TNF-α in response to an in vivo LPS challenge.54 It was also of interest that fenfluramine was more potent than MDMA at inducing serotonergic changes, and also at provoking immunosuppressive effects.32 All of these data pointed towards serotonin release as a mediator of the immunosuppressive effects of MDMA in rats.
In order to evaluate the role of serotonin in MDMA-induced immunosuppression, two pharmacological strategies that inhibit MDMA-induced serotonin release were employed. First, the selective serotonin reuptake inhibitor paroxetine was used to prevent MDMA from entering serotonergic neurons, thereby preventing MDMA-induced serotonin release.30 In the second study, rats were pretreated with the tryptophan hydroxylase inhibitor p-chlorophenylalanine (pCPA), an agent that reduces brain serotonin concentrations by ≈90%. The impact of both of these anti-serotonin strategies on MDMA-induced suppression of IL-1β and TNF-α in rats was evaluated. Whilst paroxetine pretreatment completely blocked MDMA-induced serotonin depletion in both the frontal cortex and hypothalamus, it failed to alter the suppressive effects of MDMA on LPS-induced TNF-α secretion. It was also of interest that paroxetine treatment alone suppressed both LPS-induced IL-1 and TNF-α secretion by 27% and 50%, respectively, possibly because of its ability to increase central serotonin concentrations.30 The treatment regimen of pCPA used caused in excess of a 90% depletion of brain serotonin concentrations. However, the suppressive effects of MDMA on LPS-induced IL-1 and TNF-α were equivalent in both saline and pCPA-treated groups. All of these data indicated that the immunosuppressive effects of MDMA occured by a mechanism(s) independent of serotonin release. In a similar manner, it was observed that the suppressive effect of fenfluramine (10 mg/kg) on LPS-induced IL-1β and TNF-α production were not blocked by pretreatment with either paroxetine or pCPA.55 However, in a later study, using much lower doses of fenfluramine, it was observed that the suppressive effect of fenfluramine (1·25 and 2·5 mg/kg) on LPS-induced IL-1β production was blocked by pretreatment with pCPA, indicating that it was indeed a serotonin-dependent event.56 In a similar manner, it is also possible that serotonin may contribute to the immunosuppressive effect of low doses of MDMA, and that this may be overridden by dopaminergic influences when higher doses are employed. However, further studies are necessary in order to substantiate this hypothesis. In addition, is possible that some aspects of MDMA-induced immunosuppression may be serotonin dependent, whilst others may occur by serotonin-independent mechanisms.
In contrast to the studies outlined above that failed to elucidate a role for serotonin in MDMA-induced immunosuppression in rats,30 a recent study in humans demonstrated that pretreatment with the serotonin reuptake inhibitor paroxetine could block some of the immunosuppressive effects of MDMA. Specifically, paroxetine pretreatment partially inhibited the ability of MDMA to suppress the number of circulating CD4+ T-helper cells and to increase the number of circulating NK cells.35 In addition, paroxetine totally abolished the suppressive effect of MDMA on lymphocyte proliferation and IL-2 production induced by the T-cell mitogens Con A and PHA, and blocked the ability of MDMA to enhance PHA-stimulated production of the T-regulatory cytokines IL-10 and TGF-β. In all, these data support a role for serotonin release in mediating the suppressive effect of MDMA on human T-cell function.
In addition to the potent serotonin-releasing properties of MDMA, it is also well established that MDMA releases dopamine within the CNS, although with less potency.17,18 Thus, it is possible that dopamine release may play a role in the immunosuppressive effects of MDMA. In this regard, it was previously demonstrated that both d-amphetamine and methamphetamine; two psychostimulants that are structurally related to MDMA (see Fig. 1) and are potent dopamine releasers, elicit immunosuppressive effects in rodents.6,57,58 Future studies will evaluate the role of dopamine in MDMA-induced immunosuppression.
Does behavioural stimulation play a role in the immunosuppressive effect of mdma?
MDMA provokes a variety of euphoric effects in humans and behavioural hyperactivity in laboratory animals.15 It is of interest that in previous studies depleting serotonin concentrations with pCPA, or blockade of serotonin release by pretreatment with selective seratonin reuptake inhibitors (SSRIs), attenuated the locomotor stimulant effect of MDMA in rats.19 Therefore, whilst pretreatment with paroxetine or pCPA attenuates the behavioural effects of MDMA, the immunosuppressive effects (at least on pro-inflammatory cytokine production) still persist, indicating a dissociation between the behavioural and immunosuppressive effects of MDMA.30 In addition, the fact that the non-psychostimulant amphetamine derivative, fenfluramine, elicits similar immunosuppressive effects to MDMA,32,54,56 supports the view that the psychoactive and immunosuppressive properties of substituted amphetamines in rats are not necessarily linked.
A previous study conducted in humans, reported that treatment with the SSRI, citalopram, blocked the positive mood, extraversion and self-confidence induced by MDMA.21 In addition, some of the immunosuppressive actions of MDMA in humans are blocked by serotonin transporter blockade with the related SSRI, paroxetine.35 However, despite these coincidental effects, the exact role that the euphoric effect of MDMA plays in its ability to induce immunosuppression in humans is not clear.
Does hyperthermia play a role in the immunosuppressive effect of mdma?
As MDMA is known to induce hyperthermia, it is pertinent to address the possibility that the ability of MDMA to alter body temperature could contribute to its immunomodulatory effects. However, based on evidence from the published literature, it appears unlikely that MDMA-induced hyperthermia plays a significant role in its immunomodulatory effects. For instance, the ability of MDMA to promote an immunosuppressive cytokine phenotype (reduced TNF-α and increased IL-10), and the suppression of lymphocyte proliferation, are observed at doses as low as 1·25 mg/kg,28,33 a dose that does not alter body temperature.59 In addition, whilst both MDMA and the related amphetamine, fenfluramine, provoke similar immunosuppressive responses in rats, they provoke contrasting thermal responses, with fenfluramine eliciting a hypothermic response60 and MDMA eliciting a hyperthermic response.23
Peripheral mediators of mdma-induced immunosuppression: a role for catecholamines or glucocorticoids?
It is well established that MDMA activates both the hypothalamic pituitary adrenal axis and sympathetic nervous system23,33,53 and that the end products of these axes, namely glucocorticoids and catecholamines, have immunosuppressive properties.61,62 Therefore, it was plausible to suggest that MDMA could elicit its immunosuppressive actions by increasing the release of these endogenous negative immunoregulators. Consistent with this hypothesis, it was demonstrated that the increase in IL-10 induced by MDMA could be blocked by pretreatment with the β-adrenoceptor antagonists propranolol and nadolol, indicating that the MDMA-induced enhancement of IL-10 production was mediated by β-adrenoceptor activation, presumably in response to MDMA-induced catecholamine release.28 In contrast, the suppression of the pro-inflammatory cytokines TNF-α and IL-1β induced by MDMA occurred independently of β-adrenoceptor activation.28 However, the possibility still exists that catecholamines may mediate the suppression of pro-inflammatory cytokines induced by MDMA by acting on other adrenoceptor subtypes, such as the α1-adrenoceptor.
With respect to the role of glucocorticoids in MDMA-induced immunosuppression, it was observed that pretreatment of rats with the glucocorticoid receptor antagonist, mifepristone, failed to block the suppressive effect of MDMA on LPS-induced IL-1β and TNF-α production (T. Connor, unpublished data). Moreover, in a recent study, it was observed that adrenalectomy failed to block the ability of MDMA to suppress TNF-α and increase IL-10 production.28 These data clearly demonstrate that glucocorticoids do not mediate the ability of MDMA to promote an immunosuppressive cytokine phenotype in rats. However, further studies are required to determine whether glucocorticoids play a role in the suppressive effect of MDMA on other immunological parameters, such as mitogen stimulated T-cell responses, or on antigen-specific T-cell responses and antibody production.
The fact that MDMA maintained the ability to increase LPS-induced IL-10 production in adrenalectomized rats indicates that this response is not mediated by adrenal catecholamines, suggesting that noradrenaline may modulate LPS-induced IL-10 production by direct innervation of immune organs such as the spleen. Studies are currently underway to investigate this possibility. To date there has been a paucity of research with respect to peripheral mediators of MDMA-induced immunosuppression in humans. Whilst it has been demonstrated that MDMA activates the hypothalamic pituitary adrenal axis and sympathetic nervous system in humans,39,53 the effect of glucocoticoid receptor antagonists/synthesis inhibitors or adrenoceptor antagonists has not been examined in human studies.
A role for peripheral serotonin or dopamine in the immunosuppressive actions of mdma?
Serotonin
Immune cells have ample opportunity to be exposed to serotonin that is released from platelets in the periphery.48 In addition to platelet-derived serotonin, it has been suggested that sympathetic nerve terminals in lymphoid tissues take up serotonin, which can be released upon later stimulation of these nerves.49 Moreover, it is well established that serotonin receptors are present on a variety of immune cells48,49 and that serotonin has immunomodulatory properties, with some studies indicating that it enhances, and others indicating that it suppresses, aspects of immune function.63–65 However, despite the immunomodulatory potential of serotonin, there is no evidence to suggest that MDMA releases serotonin from peripheral stores. In fact the few studies that have examined the impact of MDMA on platelet or plasma serotonin concentrations have not observed any change following MDMA administration.66,67
Dopamine
Catecholamines, including dopamine, are present in lymphocytes, macrophages and neutrophils; the first two of which have been shown capable of synthesizing dopamine.48 In addition, noradrenergic sympathetic fibres in the spleen can take up circulating dopamine, particularly during stress, which may then be released on sympathetic activation and act upon immune cells in the vicinity of release.48 Thus, immune cells can come into contact with dopamine in the periphery. From a functional perspective, a number of studies have demonstrated the presence of dopamine receptors on various immune cells, and indicate that dopamine has immunosuppressive properties.68–71 However, despite these findings, the impact of MDMA on dopamine release in the periphery has not been studied, and therefore, although dopamine has immunosuppressive properties, it is difficult to say if it plays a role in MDMA-induced immunosuppression.
Thus, further studies are required in order to determine whether peripheral serotonin or dopamine play a role in MDMA-induced immunosuppression.
Mdma abuse: a stressor on the immune system
As many of the physiological changes elicited by MDMA closely resemble those induced by psychological or physical stress, it is suggested that exposure to MDMA could be regarded as a ‘chemical stressor’ on the immune system. Exposure to stress is known to provoke similar immunological changes, in both laboratory animals and humans, to those induced by MDMA. For instance, acute stress promotes an immunosuppressive cytokine phenotype in response to LPS.72,73 Stress also suppresses lymphocyte proliferation, promotes a Th2-type cytokine response74–79 and increases circulating NK-cell numbers.79–81
Does MDMA-induced immunosuppression translate into a significant health risk for abusers?
Based on the studies outlined above, it is clear that MDMA suppresses both innate and adaptive immunity in animals and humans. Therefore, it is possible that the immunosuppressive effects of MDMA could lead to an abnormal immune response at times of infection or illness. For instance, preclinical studies have demonstrated that a deficiency in pro-inflammatory cytokines, such as IL-1 or TNF-α, can have a significant impact on host resistance to infectious disease. Specifically, TNF-α knockout mice have a reduced host resistance to Listeria monocytogenes infection,82 and antagonism of IL-1 receptors with IL-1ra interferes with host resistance to infection with Mycobacterium avium.83 In addition, considering the important role of Th1-driven IgG2a antibody production in antiviral immunity,29 it is possible that MDMA abuse could interfere with a reduced host resistance to viral infections. Also, the long-term depletion in NK-cell numbers reported following MDMA abuse in humans gives rise to concern considering the important role that NK cells play in cell-mediated immunity and, more particularly, in tumour surveillance.84,85 This said, it is difficult to predict the impact of MDMA-induced immunosuppression on disease susceptibility, as we do not know what degree of functional reserve is present within the immune system, and also as there are no data available regarding the impact of MDMA administration on host resistance in infection models. However, it has been demonstrated that the administration of d-amphetamine (the parent compound of MDMA) resulted in a reduced host resistance to infection with influenza A virus and Listeria monocytogenes.11,57 Nonetheless, studies examining the susceptibility to bacterial/viral infections are required in order to determine whether impairments in innate and adaptive immunity, induced by MDMA, have a major impact on infectivity and disease progression.
There are very few studies in the clinical literature that have examined the incidence of infections in MDMA abusers. However, one recent report presented the results of a survey of 282 Ecstasy users who participated in an internet study.86 The sample comprised 109 novice users (one to nine occasions), 136 moderate users (10–99 occasions), and 36 heavy users (100+ occasions). In this study, yes/no responses were recorded to a series of questions covering problems experienced when drug-free, which were attributed by the respondents to their Ecstasy use. In this survey, infections were cited as one of the problems that were significantly associated with the extent of Ecstasy use.86 In addition, there have been cases where MDMA abuse in humans closely preceded the development of meningococcal meningitis.87
It is also possible that both the environment in which MDMA is consumed, and/or the psychoactive effects that MDMA induces, could synergize with the immunosuppressive effects of the drug, to result in an increased susceptibility to infectious disease. For instance, MDMA use has traditionally been associated with the rave dance club scene, a crowded environment where teenagers congregate. Such an environment is optimal for transmitting airborne infection between individuals. In addition, the results of a recent study indicated that MDMA abuse was strongly and significantly associated with high-risk sexual behaviours (unprotected anal intercourse) in a population of gay/bisexual men sampled from three New York dance clubs.88 Thus, when one combines such environmental factors with the immunosuppressive effect of MDMA, it is reasonable to suggest that MDMA users may have a higher risk of developing infectious disease than drug-free subjects.
Conclusions
Research conducted over the last number of years has clearly demonstrated that the administration of MDMA to both animals and humans has profound immunosuppressive effects, a property that it shares with other drugs of abuse.1–6 For the most part, the immunosuppressive effects of MDMA are not attributed to a direct action of the drug on immune cells, but rather to the release of endogenous immunomodulatory substances, and it is suggested that exposure to MDMA could be regarded as a ‘chemical stressor’ on the immune system. Further studies are required in order to elucidate the central and peripheral mediators of MDMA-induced immunosuppression. In addition, assessment of the effect of MDMA in preclinical host resistance models, and further clinical research, is required before any definitive statement can be made on the propensity of MDMA-induced immunosuppression to translate into increased disease susceptibility.
Acknowledgments
The author wishes to thank Enterprise Ireland, The Health Research Board, The Higher Education Authority and the Irish Research Council for Science Engineering and Technology for funding his research on the immunosuppressive effects of MDMA. The author also gratefully acknowledges NIDA, USA, for the gift of MDMA.
References
- 1.Friedman H, Newton C, Klein TW. Microbial infections, immunomodulation, and drugs of abuse. Clin Microbiol Rev. 2003;16:209–19. doi: 10.1128/CMR.16.2.209-219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein TW, Friedman H, Specter S. Marijuana, immunity and infection. J Neuroimmunol. 1998;83:102–15. doi: 10.1016/s0165-5728(97)00226-9. [DOI] [PubMed] [Google Scholar]
- 3.Mellon RD, Bayer BM. Evidence for central opioid receptors in the immunomodulatory effects of morphine: review of potential mechanism(s) of action. J Neuroimmunol. 1998;83:19–28. doi: 10.1016/s0165-5728(97)00217-8. [DOI] [PubMed] [Google Scholar]
- 4.Nunez-Iglesias MJ, Castro-Bolano C, Losada C, et al. Effects of amphetamine on cell-mediated immune response in mice. Life Sci. 1996;58:L29–33. doi: 10.1016/0024-3205(95)02272-4. [DOI] [PubMed] [Google Scholar]
- 5.Pellegrino T, Bayer BM. In vivo effects of cocaine on immune cell function. J Neuroimmunol. 1998;83:139–47. doi: 10.1016/s0165-5728(97)00230-0. [DOI] [PubMed] [Google Scholar]
- 6.Yu Q, Zhang D, Walston M, Zhang J, Liu Y, Watson RR. Chronic methamphetamine exposure alters immune function in normal and retrovirus-infected mice. Int Immunopharmacol. 2002;2:951–62. doi: 10.1016/s1567-5769(02)00047-4. [DOI] [PubMed] [Google Scholar]
- 7.Alonzo NC, Bayer BM. Antagonism of N-methyl-d-aspartate receptors reduces the vulnerability of the immune system to stress after chronic morphine. J Pharmacol Exp Ther. 2003;307:793–800. doi: 10.1124/jpet.103.053264. [DOI] [PubMed] [Google Scholar]
- 8.Avila AH, Alonzo NC, Bayer BM. Immune cell activity during the initial stages of withdrawal from chronic exposure to cocaine or morphine. J Neuroimmunol. 2004;147:109–13. doi: 10.1016/j.jneuroim.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Cabral GA, Dove Pettit DA. Drugs and immunity: cannabinoids and their role in decreased resistance to infectious disease. J Neuroimmunol. 1998;83:116–23. doi: 10.1016/s0165-5728(97)00227-0. [DOI] [PubMed] [Google Scholar]
- 10.Gavrilin MA, Mathes LE, Podell M. Methamphetamine enhances cell-associated feline immunodeficiency virus replication in astrocytes. J Neurovirol. 2002;8:240–9. doi: 10.1080/13550280290049660. [DOI] [PubMed] [Google Scholar]
- 11.Nunez MJ, Fernandez-Rial JC, Coucciro J, Suarez JA, Gomez-Fernandez DE, Rey-Mendez M, Freire-Garabal M. Effects of amphetamine on influenza virus infection in mice. Life Sci. 1993;52:PL73–78. doi: 10.1016/0024-3205(93)90520-d. [DOI] [PubMed] [Google Scholar]
- 12.Risdahl JM, Khanna KV, Peterson PK, Molitor TW. Opiates and infection. J Neuroimmunol. 1998;83:4–18. doi: 10.1016/s0165-5728(97)00216-6. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin GC, Roth MD, Tashkin DP. Acute and chronic effects of cocaine on the immune system and the possible link to AIDS. J Neuroimmunol. 1998;83:133–8. doi: 10.1016/s0165-5728(97)00229-4. [DOI] [PubMed] [Google Scholar]
- 14.Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J Neuroimmunol. 1998;83:77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- 15.Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- 16.Hegadoren KM, Baker GB, Bourin M. 3,4-Methylenedioxy analogues of amphetamine: defining the risks to humans. Neurosci Biobehav Rev. 1999;23:539–53. doi: 10.1016/s0149-7634(98)00046-3. [DOI] [PubMed] [Google Scholar]
- 17.Kankaanpaa A, Meririnne E, Lillsunde P, Seppala T. The acute effects of amphetamine derivatives on extracellular serotonin and dopamine levels in rat nucleus accumbens. Pharmacol Biochem Behav. 1998;59:1003–9. doi: 10.1016/s0091-3057(97)00527-3. [DOI] [PubMed] [Google Scholar]
- 18.Koch S, Galloway MP. MDMA induced dopamine release in vivo: role of endogenous serotonin. J Neural Transm. 1997;104:135–46. doi: 10.1007/BF01273176. [DOI] [PubMed] [Google Scholar]
- 19.Callaway CW, Wing LL, Geyer MA. Serotonin release contributes to the locomotor stimulant effects of 3,4-methylenedioxymethamphetamine in rats. J Pharmacol Exp Ther. 1990;254:456–64. [PubMed] [Google Scholar]
- 20.Cole JC, Sumnall HR. The pre-clinical behavioural pharmacology of 3,4-methylenedioxymethamphetamine (MDMA) Neurosci Biobehav Rev. 2003;27:199–217. doi: 10.1016/s0149-7634(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 21.Liechti ME, Vollenweider FX. Which neuroreceptors mediate the subjective effects of MDMA in humans? A summary of mechanistic studies. Hum Psychopharmacol. 2001;16:589–98. doi: 10.1002/hup.348. [DOI] [PubMed] [Google Scholar]
- 22.Mechan AO, Esteban B, O'Shea E, Elliott JM, Colado MI, Green AR. The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) to rats. Br J Pharmacol. 2002;135:170–80. doi: 10.1038/sj.bjp.0704442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nash JF, Meltzer HY, Gudelsky GA. Elevation of serum prolactin and corticosterone concentrations in the rat after the administration of 3,4 methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1988;245:873–9. [PubMed] [Google Scholar]
- 24.Stone DM, Stahl DC, Hanson GR, Gibb JW. The effects of 3,4-methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine on monoaminergic systems in rat brain. Eur J Pharmacol. 1986;128:41–8. doi: 10.1016/0014-2999(86)90555-8. [DOI] [PubMed] [Google Scholar]
- 25.Stone DM, Merchant KM, Hanson GR, Gibb JW. Immediate and long-term effects of 3,4-methylenedioxymethamphetamine on serotonin pathways in the brain of rat. Neuropharmacology. 1987;26:1677–83. doi: 10.1016/0028-3908(87)90117-1. [DOI] [PubMed] [Google Scholar]
- 26.Harkin A, Shanahan E, Kelly JP, Connor TJ. Methylenendioxyamphetamine produces serotonin nerve terminal loss and diminished behavioural and neurochemical responses to the antidepressant fluoxetine. Eur J Neurosci. 2003;18:1021–7. doi: 10.1046/j.1460-9568.2003.02802.x. [DOI] [PubMed] [Google Scholar]
- 27.Connor TJ, O'Shaughnessy D, Kelly JP. Methylenedioxymethamphetamine (‘MDMA; Ecstasy’) suppresses zymozan-induced oxidative burst in neutrophils. Ir J Med Sci. 2004 in press. [Google Scholar]
- 28.Connor TJ, Harkin A, Kelly JP. Methylenedioxymethamphetamine (MDMA; ‘Ecstasy’) increases LPS-induced IL-10 production via β-adrenoceptor activation. Eur Cytokine Netw. 2003;14(Suppl.):47. [Google Scholar]
- 29.Connor TJ, Connelly DB, Kelly JP. Methylenedioxymethamphetamine (MDMA; ‘Ecstasy’) suppresses antigen specific IgG2a and IFN-γ production. Immunol Lett. 2001;78:67–73. doi: 10.1016/s0165-2478(01)00231-0. [DOI] [PubMed] [Google Scholar]
- 30.Connor TJ, Dennedy MC, Harkin A, Kelly JP. Methylenedioxymethamphetamine-induced suppression of interleukin-1β and tumour necrosis factor-α is not mediated by serotonin. Eur J Pharmacol. 2001;418:147–52. doi: 10.1016/s0014-2999(01)00928-1. [DOI] [PubMed] [Google Scholar]
- 31.Connor TJ, Kelly JP, McGee M, Leonard BE. Methylenedioxymethamphetamine (MDMA; Ecstasy) suppresses IL-1β and TNF-α secretion following an in vivo lipopolysaccharide challenge. Life Sci. 2000;67:1601–12. doi: 10.1016/s0024-3205(00)00743-8. [DOI] [PubMed] [Google Scholar]
- 32.Connor TJ, Kelly JP, Leonard BE. An assessment of the acute effects of the serotonin releasers methylenedioxymethamphetamine, methylenedioxyamphetamine and fenfluramine on immunity in rats. Immunopharmacology. 2000;46:223–35. doi: 10.1016/s0162-3109(99)00180-0. [DOI] [PubMed] [Google Scholar]
- 33.Connor TJ, McNamara MG, Kelly JP, Leonard BE. 3,4-methlyenedioxymethamphetamine (MDMA; Ecstasy) administration produces dose-dependent neurochemical, endocrine and immune changes in the rat. Human Psychopharmacol. 1999;14:95–104. [Google Scholar]
- 34.Connor TJ, McNamara MG, Finn D, Currid A, O'Malley M, Redmond AM, Kelly JP, Leonard BE. Acute 3,4-methylenedioxymethamphetamine (MDMA) administration produces a rapid and sustained suppression of immune function in the rat. Immunopharmacology. 1998;38:253–60. doi: 10.1016/s0162-3109(97)00084-2. [DOI] [PubMed] [Google Scholar]
- 35.Pacifici R, Pichini S, Zuccaro P, et al. Paroxetine inhibits acute effects of MDMA on the immune system in humans. J Pharmacol Exp Ther. 2004 doi: 10.1124/jpet.103.061374. in press. [DOI] [PubMed] [Google Scholar]
- 36.Pacifici R, Zuccaro P, Farre M, et al. Cell-mediated immune response in MDMA users after repeated dose administration: studies in controlled versus non-controlled settings. Ann N Y Acad Sci. 2002;965:421–33. doi: 10.1111/j.1749-6632.2002.tb04183.x. [DOI] [PubMed] [Google Scholar]
- 37.Pacifici R, Zuccaro P, Farre M, et al. Effects of repeated doses of MDMA (‘ecstasy’) on cell-mediated immune response in humans. Life Sci. 2001;69:2931–41. doi: 10.1016/s0024-3205(01)01373-x. [DOI] [PubMed] [Google Scholar]
- 38.Pacifici R, Zuccaro P, Hernandez Lopez C, et al. Acute effects of 3,4 methylenedioxymethamphetamine alone and in combination with ethanol on the immune system in humans. J Pharmacol Exp Ther. 2001;296:207–15. [PubMed] [Google Scholar]
- 39.Pacifici R, Zuccaro P, Farre M, et al. Immunomodulating properties of MDMA alone and in combination with alcohol: a pilot study. Life Sci. 1999;65:PL309–16. doi: 10.1016/s0024-3205(99)00555-x. [DOI] [PubMed] [Google Scholar]
- 40.Beutler B. Tumor necrosis factor, immunity and inflammatory disease: lessons of the past decade. J Invest Med. 1995;43:227–35. [PubMed] [Google Scholar]
- 41.Kalinski P, Storkus WJ, Thompson AW, Lotze MT. Interleukin-12 family [IL-12, 23, 12RA, 27] In: Thompson AW, Lotze MT, editors. The Cytokine Handbook. 4. San Diego: Academic Press; 2003. pp. 383–408. [Google Scholar]
- 42.Henderson B. Interleukin-1. In: Dale MM, Foreman JC, Fan TD, editors. Textbook of Immunopharmacology. 3. Oxford: Blackwell Scientific Publications; 1988. pp. 193–9. [Google Scholar]
- 43.Tracey KJ. Tumor necrosis factor-α. In: Thompson A, editor. The Cytokine Handbook. 2. London: Academic Press; 1994. pp. 289–304. [Google Scholar]
- 44.Bogdan C, Paik J, Vodovotz Y, Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J Biol Chem. 1992;267:23301–8. [PubMed] [Google Scholar]
- 45.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage co-stimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–34. [PubMed] [Google Scholar]
- 47.Gerard C, Bruyns C, Marchant A, et al. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–50. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon J, Barnes NM. Lymphocytes transport serotonin and dopamine: agony or ecstasy? Trends Immunol. 2003;24:438–43. doi: 10.1016/s1471-4906(03)00176-5. [DOI] [PubMed] [Google Scholar]
- 49.Mossner R, Lesch KP. Role of serotonin in the immune system and in neuroimmune interactions. Brain Behav Immun. 1998;12:249–71. doi: 10.1006/brbi.1998.0532. [DOI] [PubMed] [Google Scholar]
- 50.House RV, Thomas PT, Bhargava HN. Selective modulation of immune function resulting from in vitro exposure to methylenedioxymethamphetamine (Ecstasy) Toxicology. 1995;96:59–69. doi: 10.1016/0300-483x(94)02955-t. [DOI] [PubMed] [Google Scholar]
- 51.Dantzer R, Kelley KW. Stress and immunity: an integrated view of relationships between the brain and the immune system. Life Sci. 1989;44:1995–2008. doi: 10.1016/0024-3205(89)90345-7. [DOI] [PubMed] [Google Scholar]
- 52.Serafeim A, Gordon J. The immune system gets nervous. Curr Opin Pharmacol. 2001;1:398–403. doi: 10.1016/s1471-4892(01)00069-8. [DOI] [PubMed] [Google Scholar]
- 53.Grob SC, Russell EP, Chang L, Ernst T. Psychobiologic effects of 3,4-methylenedioxymethamphetamine in humans: methodological considerations and preliminary observations. Behav Brain Res. 1996;73:103–7. doi: 10.1016/0166-4328(96)00078-2. [DOI] [PubMed] [Google Scholar]
- 54.Connor TJ, Kelly JP. Fenfluramine-induced immunosuppression: an in vivo analysis. Eur J Pharmacol. 2002;455:175–85. doi: 10.1016/s0014-2999(02)02588-8. [DOI] [PubMed] [Google Scholar]
- 55.Dennedy MC, Connor TJ, Harkin A, Kelly JP, O'Donnell JM. Does serotonin mediate the immunosuppressive effects of methylenedioxymethamphetamine and fenfluramine in rats? Ir J Med Sci. 2000;169:360. [Google Scholar]
- 56.Connor TJ, O'Mahony S, Kelly JP, Harkin A. Augmentation of serotonergic function suppresses pro-inflammatory cytokine production in response to an in vivo immune challenge. Ir J Med Sci. 2003;172(Suppl. 2):23. [Google Scholar]
- 57.Freire-Garabal M, Balboa JL, Nunez MJ, Castano MT, Llovo JB, Fernandez-Rial JC, Belmonte A. Effects of amphetamine on T-cell immune response in mice. Life Sci. 1991;49:PL107–112. doi: 10.1016/0024-3205(91)90570-2. [DOI] [PubMed] [Google Scholar]
- 58.Pezzone MA, Rush KA, Kusnecov AW, Wood PG, Rabin BS. Corticosterone-independent alteration of lymphocyte mitogenic function by amphetamine. Brain Behav Immun. 1992;6:293–9. doi: 10.1016/0889-1591(92)90050-x. [DOI] [PubMed] [Google Scholar]
- 59.Malpass A, White JM, Irvine RJ, Somogyi AA, Bochner F. Acute toxicity of 3,4-methylenedioxymethamphetamine (MDMA) in Sprague-Dawley and Dark Agouti rats. Pharmacol Biochem Behav. 1999;64:29–34. doi: 10.1016/s0091-3057(99)00116-1. [DOI] [PubMed] [Google Scholar]
- 60.Cryan JF, Harkin A, Naughton M, Kelly JP, Leonard BE. Characterization of d-fenfluramine-induced hypothermia. evidence for multiple sites of action. Eur J Pharmacol. 2000;390:275–85. doi: 10.1016/s0014-2999(00)00012-1. [DOI] [PubMed] [Google Scholar]
- 61.Bateman A, Singh A, Kral T, Solomon S. The immune-hypothalamic-pituitary-adrenal axis. Endocr Rev. 1989;10:92–112. doi: 10.1210/edrv-10-1-92. [DOI] [PubMed] [Google Scholar]
- 62.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve – an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 63.Kut JL, Young MR, Crayton JW, Wright MA, Young ME. Regulation of murine T-lymphocyte function by spleen cell-derived and exogenous serotonin. Immunopharmacol Immunotoxicol. 1992;14:783–96. doi: 10.3109/08923979209009235. [DOI] [PubMed] [Google Scholar]
- 64.Stefulj J, Cicin-Sain L, Schauenstein K, Jernej B. Serotonin and immune response: effect of the amine on in vitro proliferation of rat lymphocytes. Neuroimmunomodulation. 2001;9:103–8. doi: 10.1159/000049013. [DOI] [PubMed] [Google Scholar]
- 65.Eugen-Olsen J, Afzelius P, Andresen L, Iversen J, Kronborg G, Aabech P, Nielsen JO, Hofmann B. Serotonin modulates immune function in T cells from HIV-seropositive subjects. Clin Immunol Immunopathol. 1997;84:115–21. doi: 10.1006/clin.1997.4384. [DOI] [PubMed] [Google Scholar]
- 66.Nash JF, Arora RC, Schreiber MA, Meltzer HY. Effect of 3,4 methylenedioxymethamphetamine on [3H]paroxetine binding in the frontal cortex and blood platelets of rats. Biochem Pharmacol. 1991;41:79–84. doi: 10.1016/0006-2952(91)90013-u. [DOI] [PubMed] [Google Scholar]
- 67.Stuerenburg HJ, Petersen K, Baumer T, Rosenkranz M, Buhmann C, Thomasius R. Plasma concentrations of 5-HT, 5-HIAA, norepinephrine, epinephrine and dopamine in ecstasy users. Neuroendocrinol Lett. 2002;23:259–61. [PubMed] [Google Scholar]
- 68.Hasko G, Szabo C, Nemeth ZH, Deitch EA. Dopamine suppresses IL-12 p40 production by lipopolysaccharide-stimulated macrophages via a β-adrenoceptor-mediated mechanism. J Neuroimmunol. 2002;122:34–9. doi: 10.1016/s0165-5728(01)00459-3. [DOI] [PubMed] [Google Scholar]
- 69.Carr L, Tucker A, Fernandez-Botran R. In vivo administration of L-dopa or dopamine decreases the number of splenic IFN gamma-producing cells. J Neuroimmunol. 2003;137:87–93. doi: 10.1016/s0165-5728(03)00047-x. [DOI] [PubMed] [Google Scholar]
- 70.Ghosh MC, Mondal AC, Basu S, Banerjee S, Majumder J, Bhattacharya D, Dasgupta PS. Dopamine inhibits cytokine release and expression of tyrosine kinases, Lck and Fyn in activated T cells. Int Immunopharmacol. 2003;3:1019–26. doi: 10.1016/S1567-5769(03)00100-0. [DOI] [PubMed] [Google Scholar]
- 71.Saha B, Mondal AC, Majumder J, Basu S, Dasgupta PS. Physiological concentrations of dopamine inhibit the proliferation and cytotoxicity of human CD4+ and CD8+ T cells in vitro: a receptor-mediated mechanism. Neuroimmunomodulation. 2001;9:23–33. doi: 10.1159/000049004. [DOI] [PubMed] [Google Scholar]
- 72.Connor TJ, Brewer C, Harkin A, Kelly JP. Acute stress promotes an immunosuppressive cytokine phenotype: a role for catecholamines. Brain Behav Immun. 2003;17:168. [Google Scholar]
- 73.Meltzer JC, MacNeil BJ, Sanders V, Pylypas S, Jansen AH, Greenberg AH, Nance DM. Stress-induced suppression of in vivo splenic cytokine production in the rat by neural and hormonal mechanisms. Brain Behav Immun. in press. [DOI] [PubMed]
- 74.Engler H, Stefanski V. Social stress and T cell maturation in male rats: transient and persistent alterations in thymic function. Psychoneuroendocrinology. 2003;28:951–69. doi: 10.1016/s0306-4530(02)00117-8. [DOI] [PubMed] [Google Scholar]
- 75.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–46. [PubMed] [Google Scholar]
- 76.Matalka KZ. Neuroendocrine and cytokines-induced responses to minutes, hours, and days of mental stress. Neuroendocrinol Lett. 2003;24:283–92. [PubMed] [Google Scholar]
- 77.Elenkov IJ. Systemic stress-induced Th2 shift and its clinical implications. Int Rev Neurobiol. 2002;52:163–86. doi: 10.1016/s0074-7742(02)52009-2. [DOI] [PubMed] [Google Scholar]
- 78.Connor TJ, Kelly JP, Leonard BE. Forced swim test-induced neurochemical endocrine, and immune changes in the rat. Pharmacol Biochem Behav. 1997;58:961–7. doi: 10.1016/s0091-3057(97)00028-2. [DOI] [PubMed] [Google Scholar]
- 79.Bachen EA, Manuck SB, Marsland AL, Cohen S, Malkoff SB, Muldoon MF, Rabin BS. Lymphocyte subset and cellular immune responses to a brief experimental stressor. Psychosom Med. 1992;54:673–9. doi: 10.1097/00006842-199211000-00007. [DOI] [PubMed] [Google Scholar]
- 80.Gerra G, Monti D, Panerai AE, et al. Long-term immune-endocrine effects of bereavement: relationships with anxiety levels and mood. Psychiatry Res. 2003;121:145–58. doi: 10.1016/s0165-1781(03)00255-5. [DOI] [PubMed] [Google Scholar]
- 81.Benschop RJ, Jacobs R, Sommer B, Schurmeyer TH, Raab JR, Schmidt RE, Schedlowski M. Modulation of the immunologic response to acute stress in humans by beta-blockade or benzodiazepines. FASEB J. 1996;10:517–24. doi: 10.1096/fasebj.10.4.8647351. [DOI] [PubMed] [Google Scholar]
- 82.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in tumor necrosis factor alpha-deficient mice: a critical requirement for tumor necrosis factor alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Denis M, Ghadirian E. Interleukin-1 is involved in mouse resistance to Mycobacterium avium. Infect Immun. 1994;62:457–61. doi: 10.1128/iai.62.2.457-461.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Albertsson PA, Basse PH, Hokland M, Goldfarb RH, Nagelkerke JF, Nannmark U, Kuppen PJ. NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol. 2003;24:603–9. doi: 10.1016/j.it.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 85.Farag SS, VanDeusen JB, Fehniger TA, Caligiuri MA. Biology and clinical impact of human natural killer cells. Int J Hematol. 2003;78:7–17. doi: 10.1007/BF02983234. [DOI] [PubMed] [Google Scholar]
- 86.Parrott AC, Buchanan T, Scholey AB, Heffernan T, Ling J, Rodgers J. Ecstasy/MDMA attributed problems reported by novice, moderate and heavy recreational users. Hum Psychopharmacol. 2002;17:309–12. doi: 10.1002/hup.415. [DOI] [PubMed] [Google Scholar]
- 87.Prasad N, Cargill R, Wheeldon NM, Long CC, McDonald TM. ‘Ecstasy’ and meningococcal meningitis. Infect Dis Clin Pract. 1994;3:122–3. [Google Scholar]
- 88.Klitzman RL, Pope HG, Jr, Hudson JI. MDMA (‘Ecstasy’) abuse and high-risk sexual behaviors among 169 gay and bisexual men. Am J Psychiatry. 2000;157:1162–4. doi: 10.1176/appi.ajp.157.7.1162. [DOI] [PubMed] [Google Scholar]