Abstract
Antigen presentation by major histocompatibility complex type II (MHC II) molecules and activation of CD4+ helper T cells are critical for the generation of immunological memory. We previously described a DNA vaccine encoding human immunodeficiency virus-1 p55Gag as a chimera with the lysosome-associated membrane protein (LAMP/gag). The LAMP/gag chimera protein traffics to the MHC II compartment of transfected cells and elicits enhanced immune responses as compared to a DNA vaccine encoding native gag not targeted to the MHC II compartment. We have now investigated the long-term responses of immunized mice and show that the LAMP/gag DNA vaccine promotes long-lasting B cell- and CD4+ and CD8+ T-cell memory responses and elicits a potent Gag-specific CD8+ recall response to challenge with vaccinia virus encoding gag, even 11 months after immunization. In contrast, the immune responses induced by DNA encoding non-targeted Gag decay rapidly and elicit very low or undetectable levels of Gag-specific CD4+ and CD8+ memory cells. A single priming immunization with LAMP/gag DNA is sufficient to generate T-cell memory. Following this initial priming immunization with LAMP/gag DNA, booster immunizations with native gag DNA or the LAMP/gag chimera are equally efficient in eliciting B- and T-cell secondary responses, results in accordance with observations that secondary expansion of CD8+ cells in the boost phase does not require additional CD4+ help. These findings underscore the significance of targeting DNA-encoded vaccine antigens to the MHC II processing compartments for induction of long-term immunological memory.
Keywords: vaccines: DNA LAMP/Gag, prime/boost immunizations; immunological memory: T-cell memory; MHC II: trafficking
Introduction
A critical goal in the application of prophylactic vaccines is the development of immunological memory and the resulting accelerated responses to pathogen exposures.1,2 There is increasing evidence that, in a primary response to an antigen, the acquisition of an effective B- and T-cell memory population requires antigen presentation by the major histocompatibility complex type II (MHC II) pathway with the resulting activation of CD4+ T-cell help.3–7 This is particularly true in the case of DNA vaccines, which elicit a much lower innate immune response as compared to infectious pathogens. Immunization with plasmid DNA is known to induce cellular and humoral responses and is a promising strategy in the development of vaccines.8–10 However, because access to MHC II pathways is conventionally mediated by endocytosis or phagocytosis of exogenous antigens, it is possible that DNA-encoded, endogenously synthesized antigens do not efficiently access these pathways and are limited in the activation of CD4+ T cells.11,12 Several studies have demonstrated that the efficacy of DNA immunizations can be augmented by the co-administration of plasmids expressing adjuvants, such as interleukin (IL)-2,8 granulocyte–macrophage colony-stimulating factor (GM-CSF)13 or other cytokines, as surrogates of appropriate CD4+ T-helper activation. Nevertheless, MHC II presentation of antigenic epitopes and activation of CD4+ T cells are required for the development of CD8+ responses, and DNA vaccination systems have demonstrated an impaired ability to generate CD8+ T cells unless a CD4+ response is also stimulated.14–16 In order to overcome the problem of endogenous antigen access to the MHC II compartment, we have made use of the lysosomal-associated membrane proteins (LAMPs), major lysosomal membrane glycoproteins that contain a cytoplasmic tail targeting sequence that directs the trafficking of the molecule through an endosome/lysosome pathway,17 including cellular compartments where it is co-localized with MHC II molecules.18–22 We and others have demonstrated that a variety of DNA vaccine chimeras encoding an antigen and the targeting sequences of LAMP, induce antigen-trafficking to MHC II compartments23,24 and increase the immune responses to those antigens.13,24–31
Human immunodeficiency virus-1 (HIV-1) vaccines, in particular, require efficient sustained cellular, as well as humoral, immune responses for the control of infection.32–34 The importance of CD8+ cytotoxic T cells has been extensively documented.35,36 In experiments with monkeys chronically infected with simian immunodeficiency virus (SIV), the suppression of CD8+ T cells resulted in a rapid and marked increase in viremia, and accelerated progression to acquired immune-deficiency syndrome (AIDS),37 and the presence of HIV-specific cytotoxic T lymphocytes (CTLs) has been observed in exposed uninfected individuals.38–40 Additionally, an inverse correlation between viral loads and HIV-specific CTL responses has been observed, especially anti-Gag CTLs.41 CD4+ helper T cells also have critical regulatory roles, and increased numbers of HIV-specific CD4+ cells are correlated with lower viral titres and slower disease progression.32–34 Moreover, the decline in the number and function of anti-HIV-specific CD4+ T cells appears to be related to an impaired CD8+ CTL activity.42 Our current studies include p55Gag, one of the most conserved proteins of HIV-1, and the importance of an anti-Gag immune response for the control of HIV infection has been demonstrated.34 We have described a new form of an HIV-1 p55gag DNA vaccine, with the gag sequence incorporated into the complete LAMP cDNA sequence and with the coding sequences bracketed by the inverted terminal repeat sequences (ITR) of the adeno-associated virus. The LAMP/Gag protein chimera co-localized with the endogenous MHC II of transfected cells, and LAMP/gag DNA vaccine elicited stronger cellular and humoral immune responses of immunized mice as compared to the response to DNA encoding native gag, with a 10-fold increase in CD4+ responses, a four- to fivefold increase in CD8+ T-cell responses, and antibody titres 50–100-fold higher.24
In the present study, we conducted a 1-year analysis of the response of mice to the LAMP/gag vaccine, with particular reference to the generation of a memory anti-Gag response. We observed that immunization with LAMP/gag DNA vaccine promoted a long-lasting immune response, associated with a sustained activation of B lymphocytes as well as CD8+ and CD4+ T cells. Furthermore, a single LAMP/gag immunization induced the generation of Gag-specific memory T cells that elicited a potent recall response to DNA encoding native Gag protein and to recombinant vaccinia virus expressing Gag. These results substantiate the application of this vaccination strategy as a means to establish memory T- and B-cell responses capable of responding to HIV-1 virus infection.
Materials and methods
Plasmids
HIV-Gag plasmids were constructed by cloning the p55Gag sequence from the HXB2 strain of HIV (nucleotides 1–1503; GenBank K03455; HIV sequence Database, 1997, Los Alamos National Laboratory Theoretical Biology and Biophysics, Los Alamos, NM) into the mammalian expression vector, pITR,43 which contains a cytomegalovirus (CMV) promoter and ITR sequences from adeno-associated virus (AAV) flanking the expression elements. The mouse LAMP-1 sequence (GenBank J03881) was also cloned in the same vector. For construction of the LAMP/gag plasmid chimera, the p55Gag sequence was inserted between the lumenal domain and the transmembrane and cytoplasmic tail of LAMP-1 (Fig. 1). Both the LAMP and ITR sequences have been found to increase Rev-independent expression of Gag by cells transfected in vitro.24 For example, in a representative experiment, 500 and 2200 pg of Gag/mg of cell extract protein was present in cells transfected with pcDNA3.1/LAMP/gag and pITR/LAMP/gag, respectively (data not shown). All the plasmids were produced by transforming DH5αEscherichia coli (Invitrogen, Calsbad, CA) and were purified using endotoxin-free columns (Qiagen Inc., Valencia, CA).
Figure 1.
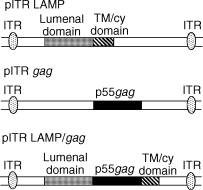
Schematic representation of the plasmids used in this study. The plasmid vectors contain the adeno-associated virus inverted terminal repeats (ITRs) flanking the expression elements. The grey box indicates the open reading frame (ORF) of the lysosome-associated membrane protein (LAMP) luminal domain and the striped box indicates the transmembrane (TM) and cytoplamic (cy) ORFs of LAMP. The black box indicates the gag ORF.
Mice immunization
Female BALB/c mice, 6–8 weeks of age, were obtained from Charles River (Kingston, NY). Mice in groups of eight were each immunized, intradermally (i.d.), with 50 µl of the indicated plasmid containing 50 µg of DNA. In some experiments, the mice received a second dose of the DNA plasmids encoding either pITR/LAMP/gag or pITR/native gag, or were challenged with VDK-1 vaccinia virus containing native Gag sequence (107 plaque-forming units; NIH AIDS Research and Reference Reagent Program, Rockville, MD). The immunization schedule is indicated in each figure.
Antibody response
Mice serum was obtained from the tail vein before the first immunization and periodically thereafter, at the indicated days after immunization. The serum immunoglobulin G (IgG) levels were measured by enzyme-linked immunosorbent assay (ELISA) as follows: 96-well plates were coated with 50 µl of HIVIIIB lysate at 5 µg/ml (ABI, Rockville, MD) and incubated at 4°, overnight. The plates were blocked with phosphate-buffered saline (PBS), containing 10% fetal calf serum (FCS) for 2 hr at 37°, washed with PBS containing 0·05% Tween-20 and the serum samples, added in serial dilutions, were incubated at 4° overnight. The plates were then incubated with horseradish peroxidase (HRP)-conjugated anti-mouse IgG (1 : 2000 dilution; Cappel, Durham, NC) for 2 hr at 37°, washed and developed by tetramethylbenzidine (TMB) substrate reagent set (Pharmingen, San Diego, CA). After 30 min, the reaction was stopped with 1 m H2SO4 and the plates were read at 450 nm using an ELISA reader (Bio-Rad Laboratories Inc., Hercules, CA).
Frequency of interferon-γ (IFN-γ)-producing CD4+ or CD8+ T cells
The frequency of IFN-γ-producing CD4+ or CD8+ T cells of immunized mice was measured by ELISPOT assays, using the IFN-γ ELISPOT set from BD-Biosciences Pharmingen (San Diego, CA) according to the manufacturer's protocol. Initially, ELISPOT plates were coated with anti-IFN-γ immunoglobulin at 5 µg/ml and incubated at 4° overnight. After blocking with RPMI-1640, containing 10% FCS, for 2 hr at room temperature, total splenocytes (106 cells/well) were cultured with RPMI-1640 supplemented with 100 U/ml penicillin/streptomycin, 2 mm l- glutamine, 50 µm 2-mercaptoethanol, and 1 m HEPES, in the presence of recombinant baculovirus HIVSF2p55 Gag protein (5 µg/ml; NIH AIDS Research and Reference Reagent Program); or with the MHC I-restricted Gag epitope AMQMLKETI65−73, 10 µg/ml, as indicated in the results. After 16 hr of culture, the plates were washed and incubated with biotinylated anti-IFN-γ for 2 hr at room temperature, followed by HRP-conjugated avidin for 1 hr at room temperature. The reaction was developed with AEC substrate (Calbiochem-Novabiochem Corporation, San Diego, CA). Analysis of the IFN-γ levels was performed using the Immunospot series Analyser software (BD-Biosciences). The data indicate the number of spot-forming cells (SFC)/106 cells.
Analysis of IFN-γ secretion by capture ELISA
Total splenocytes (106 cells/well) from immunized mice were cultured under the conditions, described above, for 72 hr at 37° in 5% CO2. Afterwards, culture supernatants were harvested and IFN-γ secretion was analysed by capture ELISA, according to the manufacturer's protocol (ELISA kit, OPTEIA; Pharmingen).
CD8+ recall response, tetramer binding and intracellular IFN-γ staining
The protocol for assay of CD8+ T cells utilized in vivo expansion induced by vaccinia-Gag challenge and CTL stimulation by a Gag-specific peptide epitope, followed by ex vivo assays to measure tetramer binding, IFN-γ production and CTL responses, as previously described.24 Groups of mice were primed with empty vector, or plasmids encoding gag or LAMP/gag, and 3 weeks later were injected with VDK-1 vaccinia Gag/Pol or a control strain of vaccinia. Five days after challenge, the mice were stimulated, in vivo, by injection with 10 µg of p55Gag-derived AMQMLKETI peptide. After 2 hr, the tetramer binding and intracellular IFN-γ-positive cells were analysed ex vivo. Splenocytes were harvested, washed with PBS containing 10% FCS and blocked with anti-CD16/CD32 immunoglobulin at 10 µg/ml (Pharmingen) for 10 min at 4°. The cells were then incubated with phycoerythrin (PE)-conjugated H-2Kd/AMQMLKETI tetramer (1 : 100; National Institute of Allergy and Infectious Disease, Atlanta, GA) and fluorescein isothiocyanate (FITC)-conjugated anti-CD8 (Pharmingen) for 1 hr at 4°. After several washes, the cells were analysed by FACScan (Becton Dickson Immunocytometry Systems; BD Biosciences, San Jose, CA) and the results were expressed as the percentage of tetramer+/CD8+ cells. The levels of intracellular IFN-γ among CD8+ cells were measured using the Cytofix/Cytoperm Plus™ kit (Pharmingen). Briefly, total splenocytes were incubated with GolgiStop™, containing monensin, for 2 hr at 37°, were then washed and blocked as described above, and incubated with FITC-conjugated anti-CD8 immunoglobulin for 30 min at 4°. The cells were then washed and incubated with Cytofix/Cytoperm solution for 20 min at 4°, washed with Perm/Wash solution containing saponin, incubated with PE-conjugated anti-IFN-γ immunoglobulin (Pharmingen) for 30 min at 4°, and then analysed by FACScan. The results were expressed as the percentage of IFN-γ+/CD8+ cells. Repeated studies have shown that none of the mice challenged with control vaccinia showed any anti-Gag-specific responses, while the mice primed with either gag or LAMP/gag plasmids showed significant CD8+ responses ex vivo. The group injected with empty vector control did not show any detectable anti-Gag response 5 days after vaccinia-gag challenge. Similar control experiments were also performed with irrelevant control peptide and it was determined that the intravenous (i.v.) peptide injection was required for the ex vivo CTL assay.
Results
Sustained CD4+- and CD8+ T-cell responses of mice immunized with the LAMP/gag DNA vaccine
Activation of CD4+ helper T cells is required for an effective and sustained immune response to DNA vaccines and for the development of antigen-specific CD8+ T-cell memory.14,16 Previous studies have shown that early immune responses induced by immunization with the MHC II-targeted LAMP/gag chimera were characterized, as compared to the non-targeted Gag, by a six- to 10-fold increase in CD4-mediated IL-2, IL-4 and IFN-γ cytokine production and by a three- to fivefold increase in the Gag-specific CD8+ T-cell population.24 In the experiments reported herein, we have analysed the effect of LAMP targeting on the duration of the CD4+ and CD8+ T-cell responses.
Three groups of mice were immunized twice, at a 3-week interval, with pITR plasmids encoding: control LAMP without antigen; native gag without LAMP; and the LAMP/gag chimera. The frequency of Gag-specific IFN-γ-producing cells was analysed by ELISPOT assay. Freshly collected spleen cells were cultured in the presence of recombinant baculovirus-produced HIVSF2p55 Gag protein for 16 hr, the plates were washed and the cells then stained with anti-IFN-γ immunoglobulin. We had previously determined that cytokine secretion stimulated by intact Gag protein is mainly related to the activation of CD4+ cells.24 Moreover, the short time of culture with Gag protein reflects the activation of previously primed cells. The activation of cells from each mouse was assayed, both at the early peak of the response (10 days after boosting) and 120 days after boosting, in the presence and absence of antigen (Fig. 2). Consistent with our previous data, cells obtained 10 days after the two immunizations with LAMP/gag contained a large number of Gag-specific CD4+ IFN-γ SFC (≈ 2500 SFC/106 cells), whereas the number of CD4+ cells obtained from mice immunized with DNA encoding native gag was not significant. At 120 days after the two immunizations, the number of IFN-γ SFC from mice immunized with LAMP/gag remained elevated (≈ 600 SFC/106 cells). Thus, the MHC II-targeted LAMP/gag not only increased the helper T-cell activation at the peak of the response, but also promoted the long-term (4 months) survival of a significant fraction of the Gag-specific CD4+ T cells.
Figure 2.

Enhanced and sustained CD4+ T-cell response following immunization with lysosome-associated membrane protein (LAMP)/gag DNA. Mice were immunized twice with 50 µg of plasmid DNA encoding LAMP (control), native gag, or the LAMP/gag chimera, as indicated. Ten (a) or 120 (b) days after immunization, splenocytes were prepared and cultured overnight in the presence or absence of p55Gag protein, and the frequency of interferon-γ (IFN-γ)-producing cells was analysed by ELISPOT assay. The data represent the measurements obtained from each mouse group, shown as average and standard deviation.
Parallel studies were performed by measuring the IFN-γ secretion in response to an MHC I-restricted Gag epitope – AMQMLKETI(65−73)– which has been demonstrated as the immunodominant Gag epitope in BALB/c mice.44 Similarly to the CD4+ response, there was a strong CD8+ response (≈ 1200 SFC/106 cells) to the LAMP/gag DNA vaccine at 10 days after the immunization, as compared to the absence of a significant response of mice immunized with DNA encoding native gag (Fig. 3). A considerable fraction of the cells stimulated by LAMP/gag DNA (≈ 120 SFC/106 cells) remained positive on day 120.
Figure 3.
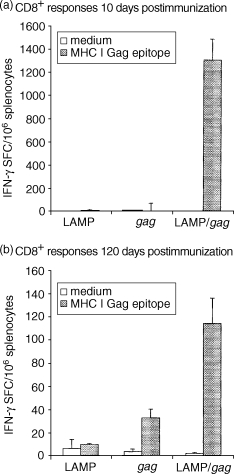
Enhanced and sustained CD8+ T-cell response following immunization with lysosome-associated membrane protein (LAMP)/gag DNA. Mice were immunized as described in the legend to Fig. 2. Ten (a) or 120 (b) days after immunization, splenocytes obtained from these mice were cultured overnight in the presence or absence of the Gag major histocompatibility complex type I (MHC I) epitope, AMQMLKETI, and the frequency of interferon-γ (IFN-γ)-producing cells was analysed by ELISPOT assay. The data represent the measurements obtained from each mouse group, shown as average and standard deviation. SFC, spot-forming cells.
Long-lasting antibody response following immunization by DNA encoding LAMP/gag
We have previously shown that mice immunized twice with a DNA vaccine encoding the MHC II-targeted LAMP/gag chimera quickly developed a large increase in antibody secretion.24 In the present study, we followed the duration of the antibody response. Mice were immunized with the LAMP control, native gag or LAMP/gag plasmids, and blood was collected before the immunization and periodically after two DNA doses. The amount of serum IgG specific to an HIVIIIB lysate was analysed (Table 1). Both the gag and LAMP/gag-immunized mice presented a positive antibody response 15 days after the second immunization, but the IgG secretion induced by the LAMP/gag chimera was much stronger (five- to tenfold greater) and longer lasting than the response of mice immunized with DNA encoding native Gag. An arbitrary cut-off value of 0·4 (absorbance at 450 nm) was used for titre definition. While decreasing significantly from the peak titre observed on day 15 (>2700 dilution), the anti-HIV response elicited by LAMP/gag was sustained at a titre greater then 1 : 900 at 120 days after immunization. In contrast, in the group immunized with the native gag plasmid, no positive antibody response was detected from day 45 and thereafter.
Table 1.
Enhanced and sustained antibody response following immunization with lysosome-associated membrane protein (LAMP)/gag DNA plasmid
| Reverse IgG titre (days after immunization) | |||||
|---|---|---|---|---|---|
| Vaccine | 15 | 45 | 60 | 90 | 120 |
| LAMP | <100 | <100 | <100 | <100 | <100 |
| gag | 300 | <100 | <100 | <100 | <100 |
| LAMP/gag | >2700 | >2700 | >2700 | >2700 | 900 |
Mice were immunized twice with 50 µg of plasmid DNA encoding LAMP (control), native gag or LAMP/gag chimera, as indicated, and blood samples were collected on the indicated days after immunization. The threefold serial serum dilutions, starting from 1 : 100, were incubated with human immunodeficiency virus (HIV)IIIB lysate, and bound immunoglobulin G (IgG) was quantified by enzyme-linked immunosorbent assay (ELISA).
LAMP/gag DNA elicits a potent CD8+ memory recall response to a vaccinia/gag virus challenge 11 months after immunization
Eleven months following the two DNA plasmid immunizations, mice were given a vaccinia/gag challenge. Five days later, the mice were stimulated in vivo by injection with the AMQMLKETI(65–73) MHC I Gag epitope and, 2 hr later, the spleen cells were harvested and tetramer-specific and IFN-γ-producing CD8+ cells were assayed ex vivo. LAMP/gag-immunized mice presented strong CD8+ tetramer and intracellular IFN-γ responses (Fig. 4), indicating that immunization with DNA encoding the LAMP/gag chimera generated a population of memory T cells capable of producing a potent recall response to challenge with an antigen-containing virus at very late time-points after the immunization. Mice immunized with DNA encoding native Gag did not show a significant CD8+ response.
Figure 4.
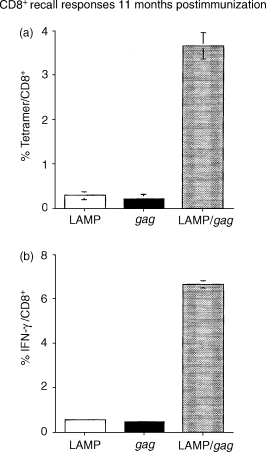
Lysosome-associated membrane protein (LAMP)/gag DNA immunization promotes a potent CD8-recall response to vaccinia-gag challenge. Mice were immunized as described in the legend to Fig. 2. Eleven months later they were challenged with VDK-1 vaccinia/gag. After 5 days, the cells were stimulated in vivo by an intravenous (i.v.) injection of the major histocompatibility complex type I (MHC I)-restricted Gag epitope, AMQMLKETI65−73. Splenocytes were harvested 2 hr later and (a) stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD8 immunoglobulin and phycoerythrin (PE)-conjugated H-2Kd/AMQMLKETI tetramer, or (b) cultured for 2 hr with Golgi-stop containing monensin, and then stained with FITC-conjugated anti-CD8 immunoglobulin, followed by intracellular staining with PE-conjugated anti-IFN-γ, as described in the Materials and methods. The results indicate the percentage of tetramer- or IFN-γ-positive cells among CD8+ cells. The data represent the measurements obtained from each mouse group, shown as average and standard deviation.
A single dose of LAMP/gag DNA is sufficient to prime both B- and T-cell memory responses to a subsequent boost with DNA encoding native gag
It has been shown that during the primary response to non-inflammatory immunogens, CD4+ T-cell help is required for the generation of memory CD8+ cells capable of efficient recall responses, whereas the secondary expansion of memory CD8+ cells in the boost phase does not require additional CD4+ help.3–6 In this case, it could be predicted that MHC II-targeting of Gag in the initial priming immunization is more relevant for the development of memory cells than it is in the second or booster immunization. To analyse this hypothesis, groups of mice were immunized on days 1 (prime) and 30 (boost) with DNA vaccines, as follows:
Prime and boost with control LAMP plasmid lacking antigen.
Single prime inoculation with DNA encoding native gag.
Prime and boost with native gag.
Single prime inoculation with LAMP/gag.
Prime with LAMP/gag and boost with native gag.
Prime and boost with LAMP/gag (Fig. 5).
Figure 5.

A single dose of lysosome-associated membrane protein (LAMP)/gag DNA is sufficient to prime both B- and T-cell responses to a subsequent boost with DNA encoding native Gag. Mice were injected with one dose of DNA plasmids (50 µg) encoding lysosome-associated membrane protein (LAMP) control, native gag, or LAMP/gag chimera (L/g), as indicated. On day 30, some groups of mice received a booster DNA injection of the indicated plasmids. On day 40 after the first immunization, the mice were killed and (a) splenocytes were cultured with medium alone or p55Gag protein for 3 days and supernatant interferon-γ (IFN-γ) levels were analysed by enzyme-linked immunosorbent assay (ELISA); and (b) the amount of anti-Gag serum immunoglobulin G (IgG) of blood samples was analysed by ELISA at a serum dilution of 1 : 300. The data represent the measurements obtained from each mouse group, shown as average and standard deviation.
The Gag-specific CD4+-mediated IFN-γ secretion and antibody responses were measured on day 40. Mice receiving one immunization with DNA encoding native gag did not produce detectable levels of IFN-γ or antibody on day 40. A second inoculation with the native gag DNA construct resulted in a significant boost of the CD4+ response but had little effect on the antibody responses. In contrast, mice receiving a single dose of the LAMP/gag DNA construct showed increased CD4+ and greatly increased antibody responses following the booster inoculation, notably with DNA encoding native gag of LAMP/gag. Thus, LAMP/gag DNA priming was sufficient to generate a population of Gag-specific cells capable of producing a potent secondary response to native Gag in the absence of LAMP-mediated Gag trafficking to the MHC II compartment.
An additional experiment was conducted with a single immunization of LAMP/gag, after which the T-cell response was measured on days 40 and 120 after immunization (Fig. 6). CD4+ T-cell assays showed a strong response, with high levels of IFN-γ on day 40 and a persisting, but lower, level on day 120 after immunization. After 4 months, the mice were treated by a vaccinia/gag challenge and CD8+ epitope stimulation. A very large fraction of CD8+ cells (20–25%), were found to be positive for IFN-γ staining, of which ≈ 1·5% were positive for the specific Gag epitope tetramer binding. Taken together, these data indicate that while repeated injections of the LAMP/gag DNA induce greater activation of CD4+ and CD8+ T cells, a single dose is sufficient to promote the generation of CD8+ memory cells that develop an anti-Gag T-cell response when stimulated by Gag expressed in the cytoplasm, its normal cellular compartment.
Figure 6.
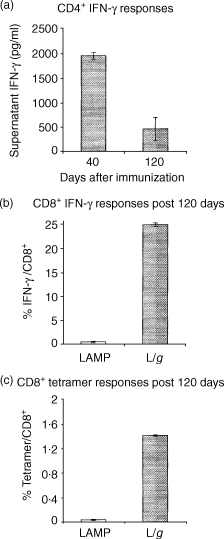
A single dose of lysosome-associated membrane protein (LAMP)/gag DNA induces the generation of memory T cells. Mice were injected with one dose of DNA plasmids (50 µg) encoding LAMP (control) or LAMP/gag chimera. (a) CD4+ response: the mice were killed on day 40 or day 120, as indicated, splenocytes were harvested and cultured with p55Gag for 3 days, and the supernatant interferon-γ (IFN-γ) concentration was analysed by enzyme-linked immunosorbent assay (ELISA). (b) and (c) The CD8+ response on day 120 after immunization was analysed as described in Fig. 4 for tetramer binding (b) or intracellular IFN-γ (c). The results indicate the percentage of tetramer- or IFN-γ-positive cells among CD8+ cells. The data represent the measurements obtained from each mouse group, shown as average and standard deviation.
Discussion
This study demonstrated that a DNA vaccine encoding a LAMP/Gag antigen chimera with the unique property of LAMP-mediated antigen trafficking to the cellular compartment containing MHC II shows an effective, long-term response of both B and T cells in immunized mice. The LAMP/gag DNA vaccine induced a strong and sustained antibody response that remained elevated for 4 months. Additionally, the early strong T-cell response was also maintained, with Gag-specific CD4+ and CD8+ T cells detected by IFN-γ ELISPOT assay 4 months after immunization. It was evident that LAMP trafficking of the antigen to the MHC II compartment and the generation of memory cells was required only during the primary response following the initial immunization. Mice primed with LAMP/gag DNA were able to respond to a boost with native gag with the same efficiency as the response to a second immunization with LAMP/gag. Furthermore, the cells primed by a single dose of LAMP/gag chimera were able to produce a potent CD8+ recall response to vaccinia/gag challenge at 4 months after immunization. Moreover, 11 months after immunization, the LAMP/gag-immunized mice mediated a potent CD8+ recall response when challenged with vaccinia/gag virus, with 3–4% of the CD8+ cells positive for Gag tetramer binding and over 6% positive in the Gag-specific IFN-γ assay.
In these experiments, the same pITR plasmid construct encoding native Gag in the absence of the LAMP sequences was singularly ineffective when used as the prime immunization as compared to immune response to the LAMP/gag construct. In contrast, when administered as a booster immunization following the initial priming with LAMP/gag, the immune responses were similar to or even stronger than those resulting from a LAMP/gag boost. Thus, the critical difference between the LAMP/gag and native gag DNA vaccine constructs is judged to be the inability of the endogenously expressed native Gag to access the MHC II compartment and activate CD4+ T cells. One caveat to this conclusion is the difference in protein expression between cells transfected by the two plasmids, with a known low expression of native Gag in transfected cells in vitro in the absence of Rev. The addition of ITR sequences to the plasmid construct increases the expression of native Gag but it is still reduced as compared to the amount of Gag expressed by the LAMP/Gag chimera. In current experiments, the amount of HIV p24 produced in vitro by transfections with pITRgag and pITR LAMP/gag are 500 and 2200 pg/mg of protein, respectively. The effect on the immune response of this possible difference in Gag expression in vivo is not known. First, the level of expression of native Gag in cells transfected in vivo is unknown and a reduced expression, based on the in vitro data, is assumed. Second, the immune response is determined by the processing of the protein in the transfected cells, and the effect of protein expression levels is conditioned by the type of cell transfected. In the case of the LAMP/Gag chimera, our model is that the expressed protein in all transfected cells will traffick to lysosomes where Gag will be degraded. The critical cell in this model is the professional antigen-presenting cell (APC) containing MHC II, where co-localizaton of MHC II and LAMP-targeted Gag is hypothesized to enhance antigen presentation to CD4+ T cells. The number of transfected dendritic cells and other professional APCs is unknown but is presumed to be a small fraction in comparison to muscle and other cells. The processing and presentation of Gag is completely different. As a cytoplasmic protein, it presumably will be processed and presented by MHC I in the majority of transfected cells. Thus, it should be more effective in eliciting an MHC I than an MHC II response. These models are in accordance with the experimental data. The LAMP/gag chimera vaccine is critically effective in eliciting a CD4+ response in the primary immunization and also promotes a CTL response. In contrast, native Gag, with no demonstrated access to the MHC II compartment of APCs, elicits fewer CD4+-mediated responses but is equally effective in a booster immunization. The role of protein expression levels in these various processes, and the possible lower expression of native Gag than LAMP/Gag, is unknown. It has been shown, however, in several short-term studies comparing immunization with native antigens and LAMP/antigens, that the LAMP/antigen chimera elicits stronger immune responses despite equal amounts of expressed antigen; these include studies of a DNA dengue virus vaccine,23 vaccinia vector delivery of HIV-1 gp16026,30 and papilloma virus E729,31 genetic vaccines, and transfection of dendritic cells with RNA encoding carcinoembryonic antigen and telomerase.27,28 These studies indicate that access to the MHC II compartment is a critical requirement of endogenous vaccines, irrespective of the mode of gene delivery and the expression of comparable amounts of antigen.
In addition, it is noteworthy that LAMP/gag immunization promoted a strong CD8+ as well as a CD4+ response, indicating efficient antigen presentation by both class I and class II molecules. This is despite the fact that the vaccine construct contains an endoplasmic reticulum (ER) translocation signal sequence which directs the protein product into an endosomal/lysosomal vesicular trafficking pathway in transfected cells. Thus, there is a question of how the LAMP/Gag chimera protein accesses the conventional cytoplasmic proteosome system for processing of MHC I epitope. While we have no specific information of protein processing in cells transfected in vivo, a possible explanation of Gag epitope access to the MHC I molecules is peptide cross-presentation.45,46 Many different mechanisms of cross-presentation have been reported, including the exit of exogenous antigens from the endocytic compartments and its processing in the cytosol,47,48 and the recycling of MHC I molecules through the endosomal/lysosomal pathway and transfer of processed peptides to the endosomal compartments.49,50 Helper CD4+ T cells may exert a role in the efficiency of these cross-presentation mechanisms, because presentation of exogenous antigens by the MHC I of dendritic cells appears to be dependent on activated CD4+ T cells51–53 and can be upregulated by CD40 ligand or anti-CD40 immunoglobulin.54 The requirement of CD4+ T-cell activation in CD8+ responses to a DNA vaccine was also shown in studies of influenza virus epitope minigene vaccine.15
DNA encoding a dominant CD8+ epitope from influenza virus targeted to the ER induced sustained levels of specific CD8+ CTLs, which was no longer detectable when the ER targeting signal was removed.15 Also, the addition of a CD4+ T-helper epitope in the plasmid construct induced a five- to 10-fold higher memory response to the MHC I-restricted epitope. Other studies of lymphocytic choriomeningitis virus (LCMV) have also demonstrated that immunization with a plasmid expressing a CTL epitope elicited only a short-lived immune protection to challenge with LCMV, and the addition of CpG immune stimulatory sequences did not promote the development of CD8+ memory.55,56
Our previous studies of CD4+ activation have shown, besides the increase of IFN-γ, a sixfold increase of IL-2 and a much greater upregulation of IL-4 mRNA,23,24 cytokines that have been shown to be associated with the homeostasis of memory T cells.57,58 Although other mechanisms may be involved, we attribute the enhanced memory response to the LAMP/gag vaccine construct and to the greater CD4+ T-cell activation and cytokine secretion induced by the LAMP targeting. Additionally, we now demonstrate that LAMP/gag immunization promoted the generation of memory cells during the primary response because mice primed with a single dose of LAMP/gag chimera were able to produce a potent CD8+ recall response to vaccinia/gag challenge at 4 months after immunization. These data are consistent with other studies showing that the CD4+ help is essential for the development CD8+ memory T cells only during the priming phase.
These results provide evidence that the mechanisms of processing, cross-priming and cross-presentation achieved by the LAMP/Gag chimera result in an efficient stimulation of multiple arms of the immune system and in the development of immunological memory. The data further support the hypothesis that a critical element of genetic vaccines is the trafficking of an endogenous antigen to the MHC II compartment for antigen processing and presentation to CD4+ T cells, which is normally accessed by receptor-mediated endocytosis of exogenous antigens by dendritic and other professional APCs.59–61 Co-localization of LAMP with the MHC II compartment has been extensively demonstrated18–22 and we have previously shown that antigens encoded as LAMP chimeras also co-localize with MHC II.23,24 The enhanced MHC II presentation and CD4+ T-cell activation may be particularly critical for effective HIV vaccine and for maintaining functional CD8+ responses to HIV-Gag during chronic viral infections, as Gag-specific CD4+ and CD8+ T-cell proliferative responses are related to lower viral loads.34,41,62 In conclusion, we propose that antigen targeting to an optimal processing and presentation pathway may promote a more efficient activation of memory T cells and may be essential to HIV vaccine development.
Acknowledgments
We thank Betty Earls Hart and Dolores Henson for their excellent technical assistance. Several reagents were obtained through the AIDS Research Reagents Program, Division of AIDS, NIAID, NIH: recombinant vaccinia virus Gag-Pol (vDK1), and purified p55 Gag protein. The H-2Kd/Gag tetramer was obtained through the NIAID MHC Tetramer Core Facility, Atlanta, GA. We also thank Dr Diane Griffin (The Johns Hopkins Bloomberg School of Public Health) for the use of the CTL Immunospot series 2 Analyzer. This research was supported by NIAID grants R37-AI41908 and R21 AI44317 from the National Institute of Allergy and Infectious Disease (National Institutes of Health). Luciana B. Arruda was also supported by CAPES, Brazil.
Abbreviations
- CTL
cytotoxic T lymphocyte
- ELISA
enzyme-linked immunosorbent assay
- HIV-1
human immunodeficiency virus-1
- IFN-γ
interferon-γ
- IL
interleukin
- IgG
immunoglobulin G
- ITR
internal terminal repeats
- LAMP
lysosome-associated membrane rotein
- L/g
LAMP/gag plasmid
- MHC
major histocompatibility complex
- SFC
spot-forming cells
References
- 1.Zinkernagel RM. On natural and artificial vaccinations. Annu Rev Immunol. 2003;21:515–46. doi: 10.1146/annurev.immunol.21.120601.141045. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–62. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 3.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–6. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 4.Shedlock DJ, Whitmire JK, Tan J, MacDonald AS, Ahmed R, Shen H. Role of CD4 T cell help and costimulation in CD8 T cell responses during Listeria monocytogenes infection. J Immunol. 2003;170:2053–63. doi: 10.4049/jimmunol.170.4.2053. [DOI] [PubMed] [Google Scholar]
- 5.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 6.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–42. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley LM. CD4(+) cell memory: the enigma of Th1 cells. Trends Mol Med. 2003;9:186–8. doi: 10.1016/s1471-4914(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 8.Barouch D, Santra S, Schmitz J, et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–92. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 9.Amara R, Villinger F, Altman J, et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki A, Torres CAT, Ohashi PS, Robinson HL. The dominant role of bone-marrow derived cells in CTL induction following plasmid DNA immunization at different sites. J Immunol. 1997;159:11–4. [PubMed] [Google Scholar]
- 11.Yewdell JW, Bennink JR. The binary logic of antigen processing and presentation to T cells. Cell. 1990;62:203–6. doi: 10.1016/0092-8674(90)90356-j. [DOI] [PubMed] [Google Scholar]
- 12.Zhong G, Castellino F, Romagnoli P, Germain RN. Evidence that binding site occupancy is necessary and sufficient for effective major histocompatibility complex (MHC) class II transport through the secretory pathway redefines the primary function of class II-associated invariant chain peptides (CLIP) J Exp Med. 1996;184:2061–6. doi: 10.1084/jem.184.5.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raviprakash K, Marques ETA, Ewing D, et al. Synergistic neutralizing antibody response to a dengue virus type 2 DNA vaccine by incorporation of lysosome-associated membrane protein sequences and use of plasmid expressing GM-CSF. Virology. 2001;290:74–82. doi: 10.1006/viro.2001.1136. [DOI] [PubMed] [Google Scholar]
- 14.Chan K, Lee DJ, Schubert A, Tang CM, Crain B, Schoenberger SP, Corr M. The roles of MHC class II, CD40, and B7 costimulation in CTL induction by plasmid DNA. J Immunol. 2001;166:3061–6. doi: 10.4049/jimmunol.166.5.3061. [DOI] [PubMed] [Google Scholar]
- 15.Langlade-Demoyen P, Garcia-Pons F, Castiglioni P, Garcia Z, Cardinaud S, Xiong S, Gerloni M, Zanetti M. Role of T cell help and endoplasmic reticulum targeting in protective CTL response against influenza virus. Eur J Immunol. 2003;33:720–8. doi: 10.1002/eji.200323287. [DOI] [PubMed] [Google Scholar]
- 16.Maecker HT, Umetsu DT, DeKruyff RH, Levy S. Cytotoxic T cell responses to DNA vaccination: dependence on antigen presentation via class II MHC. J Immunol. 1998;161:6532–6. [PubMed] [Google Scholar]
- 17.Guarnieri FG, Arterburn LM, Penno MB, Cha Y, August JT. The motif Tyr-X-X-hydrophobic residue mediates lysosomal membrane targeting of lysosome-associated membrane protein 1. J Biol Chem. 1993;268:1941–6. [PubMed] [Google Scholar]
- 18.Kleijmeer MJ, Morkowski S, Griffith JM, Rudensky AY, Geuze HJ. Major histocompatibility complex class II compartments in human and mouse B lymphoblasts represent conventional endocytic compartments. J Cell Biol. 1997;139:639–49. doi: 10.1083/jcb.139.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters PJ, Neefjes JJ, Oorschot V, Ploegh HL, Geuze HJ. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991;349:669–76. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- 20.Geuze HJ. The role of endosomes and lysosomes in MHC class II functioning. Immunol Today. 1998;19:282–7. doi: 10.1016/s0167-5699(98)01269-9. [DOI] [PubMed] [Google Scholar]
- 21.Drake JR, Lewis TA, Condon KB, Mitchell RN, Webster P. Involvement of MIIC-like late endosomes in B cell receptor-mediated antigen processing in murine B cells. J Immunol. 1999;162:1150–5. [PubMed] [Google Scholar]
- 22.Turley SJ, Inaba K, Garrett WS, Ebersold M, Unternaehrer J, Steinman RM, Mellman I. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288:522–7. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Raviprakash K, Leao IC, et al. Dengue 2 PreM-E/LAMP chimera targeted to the MHC class II compartment elicits long-lasting neutralizing antibodies. Vaccine. 2003;21:2178–89. doi: 10.1016/s0264-410x(03)00009-4. [DOI] [PubMed] [Google Scholar]
- 24.Marques ET, Jr, Chikhlikar P, De Arruda LB, et al. HIV-1 p55Gag encoded in the lysosome-associated membrane protein-1 (LAMP-1) as a DNA plasmid vaccine chimera is highly expressed, traffics to MHC II compartment, and elicits enhanced immune responses. J Biol Chem. 2003;278:37926–36. doi: 10.1074/jbc.M303336200. [DOI] [PubMed] [Google Scholar]
- 25.Bonini C, Lee SP, Riddell SR, Greenberg PD. Targeting antigen in mature dendritic cells for simultaneous stimulation of CD4+ and CD8+ T cells. J Immunol. 2001;166:5250–7. doi: 10.4049/jimmunol.166.8.5250. [DOI] [PubMed] [Google Scholar]
- 26.Ruff AL, Guarnieri FG, Staveley-O'Carroll KF, Siliciano RF, August JT. The enhanced immune response to the HIV gp160/LAMP chimeric gene product targeted to the lysosome membrane protein trafficking pathway. Proc Natl Acad Sci USA. 1997;272:8671–8. doi: 10.1074/jbc.272.13.8671. [DOI] [PubMed] [Google Scholar]
- 27.Nair SK, Boczkowski D, Morse M, Cumming RI, Lyerly HK, Gilboa E. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol. 1998;16:364–9. doi: 10.1038/nbt0498-364. [DOI] [PubMed] [Google Scholar]
- 28.Su Z, Vieweg J, Weizer AZ, et al. Enhanced induction of telomerase-specific CD4(+) T cells using dendritic cells transfected with RNA encoding a chimeric gene product. Cancer Res. 2002;62:5041–8. [PubMed] [Google Scholar]
- 29.Wu T, Guarnieri F, Staveley-O'Carroll K, et al. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc Natl Acad Sci USA. 1995;92:11671–5. doi: 10.1073/pnas.92.25.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowell JF, Ruff AL, Guarnieri FG, Staveley-O'Carroll K, Lin X, Tang J, August JT, Siliciano RF. Lysosome-associated membrane protein-1-mediated targeting of the HIV-1 envelope protein to an endosomal/lysosomal compartment enhances its presentation to MHC class II-restricted T cells. J Immunol. 1995;155:1818–28. [PubMed] [Google Scholar]
- 31.Lin K, Guarnieri F, Staveley-O'Carroll K, Levitsky H, August JT, Pardoll D, Wu T. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–6. [PubMed] [Google Scholar]
- 32.Klein MR, Vanbaalen CA, Holwerda AM, et al. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–72. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath MJ. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–74. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–50. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 35.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–10. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–5. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–60. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 38.Rowland-Jones S, Sutton J, Ariyoshi K, et al. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 39.Rowland-Jones SL, Dong T, Dorrell L, et al. Broadly cross-reactive HIV-specific cytotoxic T-lymphocytes in highly-exposed persistently seronegative donors. Immunol Lett. 1999;66:9–14. doi: 10.1016/s0165-2478(98)00179-5. [DOI] [PubMed] [Google Scholar]
- 40.Rowland-Jones S. Long-term, non-progression in HIV infection: clinico pathological issues. J Infect. 1999;38:67–70. doi: 10.1016/s0163-4453(99)90070-1. [DOI] [PubMed] [Google Scholar]
- 41.Ogg GS, Jin X, Bonhoeffer S, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–6. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 42.Appay V, Nixon DF, Donahoe SM, et al. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kessler PD, Podsakoff GM, Chen X, McQuiston SA, Colosi PC, Matelis LA, Kurtzman GJ, Byrne BJ. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93:14082–7. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mata M, Travers PJ, Liu Q, Frankel FR, Paterson Y. The MHC class I-restricted immune response to HIV-gag in BALB/c mice selects a single epitope that does not have a predictable MHC-binding motif and binds to Kd through interactions between a glutamine at P3 and pocket D. J Immunol. 1998;161:2985–93. [PubMed] [Google Scholar]
- 45.Schirmbeck R, Reimann J. Alternative processing of endogenous or exogenous antigens extends the immunogenic, H-2 class I-restricted peptide repertoire. Mol Immunol. 2002;39:249–59. doi: 10.1016/s0161-5890(02)00105-0. [DOI] [PubMed] [Google Scholar]
- 46.Jondal M, Schirmbeck R, Reimann J. MHC class I-restricted CTL responses to exogenous antigens. Immunity. 1996;5:295–302. doi: 10.1016/s1074-7613(00)80255-1. [DOI] [PubMed] [Google Scholar]
- 47.Carbone FR, Kurts C, Bennett SR, Miller JF, Heath WR. Cross-presentation: a general mechanism for CTL immunity and tolerance. Immunol Today. 1998;19:368–73. doi: 10.1016/s0167-5699(98)01301-2. [DOI] [PubMed] [Google Scholar]
- 48.Norbury CC, Chambers BJ, Prescott AR, Ljunggren HG, Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Immunol. 1997;27:280–8. doi: 10.1002/eji.1830270141. [DOI] [PubMed] [Google Scholar]
- 49.Castellino F, Boucher PE, Eichelberg K, Mayhew M, Rothman JE, Houghton AN, Germain RN. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J Exp Med. 2000;191:1957–64. doi: 10.1084/jem.191.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gromme M, Uytdehaag FG, Janssen H, et al. Recycling MHC class I molecules and endosomal peptide loading. Proc Natl Acad Sci USA. 1999;96:10326–31. doi: 10.1073/pnas.96.18.10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heath WR, Carbone FR. Cytotoxic T lymphocyte activation by cross-priming. Curr Opin Immunol. 1999;11:314–8. doi: 10.1016/s0952-7915(99)80050-8. [DOI] [PubMed] [Google Scholar]
- 52.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 53.Heath WR, Carbone FR. Cross-presentation in viral immunity and self-tolerance. Nat Rev Immunol. 2001;1:126–34. doi: 10.1038/35100512. [DOI] [PubMed] [Google Scholar]
- 54.Delamarre L, Holcombe H, Mellman I. Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentially regulated during dendritic cell maturation. J Exp Med. 2003;198:111–22. doi: 10.1084/jem.20021542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oehen S, Junt T, Lopez-Macias C, Kramps TA. Antiviral protection after DNA vaccination is short lived and not enhanced by CpG DNA. Immunology. 2000;99:163–9. doi: 10.1046/j.1365-2567.2000.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rush C, Mitchell T, Garside P. Efficient priming of CD4+ and CD8+ T cells by DNA vaccination depends on appropriate targeting of sufficient levels of immunologically relevant antigen to appropriate processing pathways. J Immunol. 2002;169:4951–60. doi: 10.4049/jimmunol.169.9.4951. [DOI] [PubMed] [Google Scholar]
- 57.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–79. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 58.Jaleco S, Swainson L, Dardalhon V, Burjanadze M, Kinet S, Taylor N. Homeostasis of naive and memory CD4(+) T cells: IL-2 and IL-7 differentially regulate the balance between proliferation and Fas-mediated apoptosis. J Immunol. 2003;171:61–8. doi: 10.4049/jimmunol.171.1.61. [DOI] [PubMed] [Google Scholar]
- 59.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 60.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 61.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–50. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 62.Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–6. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]


