Abstract
In neutrophils, as in most other cell types, Ca2+ signalling is important for a number of cellular activities. Although inositol(1,4,5)trisphosphate-mediated release of Ca2+ from intracellular stores is a necessary prelude, it is the Ca2+ influx that is responsible for many of the neutrophil responses. We report here that although elevations of cytosolic Ca2+ do not accompany Fas-mediated apoptosis in neutrophils, the Ca2+ influx component of the response to N-formyl-methionyl-leucyl-phenylalanine (FMLP) becomes selectively inactived as the neutrophils progress towards accelerated apoptosis induced by Fas (CD95) cross-linking. After 4 hr incubation at 37°, untreated neutrophils display an exaggerated Ca2+ influx phase in response to FMLP. This was absent in neutrophils that had been Fas-activated at the same time. No Ca2+ influx component was demonstrable by the removal of extracellular Ca2+ or by Ca2+ channel blockade with Ni2+ and no Mn2+ influx was detectable. The defect could not be attributed to a decrease in receptor sensitivity, receptor coupling or receptor number because the release of stored Ca2+ remained constant during incubation and was unaffected by Fas activation. Ca2+ influx became uncoupled from store release before detectable gross morphological changes or phosphatidyl serine externalization and was also insensitive to caspase 3 and 8 inhibitors. These results suggest a mechanism other than caspase-mediated proteolytic damage to components important for Ca2+ influx.
Introduction
In neutrophils, as in most other cell types, Ca2+ signalling is important for a number of cellular activities, including the generation of oxidants and the release of proteases.1,2 The agonist-evoked Ca2+ signal is caused by inositol (1,4,5)trisphosphate (IP3)-mediated release of Ca2+ from intracellular stores and the secondary sustained phase of the Ca2+ signal is the result of Ca2+ influx across the plasma membrane activated by depletion of intracellular Ca2+ stores.1–3 While the initial Ca2+ store release is a necessary prelude, it is the Ca2+ influx that is responsible for many of the neutrophil responses.1,2 Neutrophils are highly susceptible in vitro to rapid apoptosis by Fas (CD95) cross-linking.4 The role of the Ca2+-signalling pathway in Fas-mediated neutrophil apoptosis is unknown. We report here that elevations of cytosolic Ca2+ do not accompany Fas-mediated apoptosis in neutrophils, but that the Ca2+ influx becomes increasingly uncoupled from store release. Furthermore, this Ca2+ influx shutdown was insensitive to caspase 3 and 8 inhibitors showing that Fas (CD95) cross-linking causes Ca2+ influx shutdown in neutrophils by a caspase-independent mechanism.
Materials and methods
Neutrophil isolation
Neutrophils were isolated from the heparinized blood of healthy volunteers as previously described.5 Following dextran separation, hypotonic lysis of red cells and centrifugation through Ficoll–Paque, neutrophils were resuspended in Krebs buffer (12+0 mm NaCl, 4·9 mm KCl, 1·2+ mm KH2+PO4, 1·2+ mm MgSO4, 1·3 mm CaCl2+, 2+5 mm HEPES and 0·1% bovine serum albumin, adjusted to pH 7·4 with NaOH).
Simultaneous cytosolic free Ca2+ and phase contrast imaging
Neutrophils were loaded with the Ca2+ indicator, fura2+-AM as previously described6 or with fura2+-dextran by micro-injection7 and were allowed to adhere to glass coverslips maintained at 37° on a temperature- and CO2+-controlled microscope stage system. Two excitation wavelengths 340 nm and 380 nm were sequentially transmitted to an inverted microscope (Nikon Eclipse) with an oil immersion 100× objective using a rapid monochromator (Delta RAM, PTI, Surbiton, UK). Phase-contrast images were taken simultaneously under far-red illumination (690 nm) using an appropriate dichroic mirror and a red-sensitive CCD camera. The fluorescent images were collected using a CCD camera (IC100 PTI, Surbiton, UK) and 340/380 nm ratio images were calculated using imagemaster software (PTI, UK). For the longest time-course, either illumination was continuous while continuous data was collected from regions of interest which corresponded to individual cells at one or two section intervals, or the illumination was discontinuous over periods of 4–6 hr and images were collected at defined intervals. The latter approach minimized the damage caused to cells by continuous illumination over extended times but necessarily had poor time resolution. Both strategies were used and combined so that high time resolution data could be obtained during key events such as during the cross-linking of Fas antibody or when the cell under observation was becoming morphologically apoptotic.
Micro-injection of fura2+-dextran
The large molecular weight conjugate of fura2+, fura2+-dextran (Molecular Probes, Eugene, OR; molecular weight 10 000), was employed to measure cytosolic free Ca2+ concentration with reduced diffusion of the fura2–Ca2+ complex within the cell. The fura2+-dextran was micro-injected into neutrophils by the simple, previously described,7 lipid-assisted micro-injection technique (SLAM) using premade SLAM pipettes (Cell Engineering Ltd, Swansea, UK). The probe was dissolved in intracellular medium (KCl, 150 mm, HEPES, 2+5 mm, pH 7·0) to give a final concentration of 500 μm and loaded into a micropipette (tip diameter 0·5 μm). On contact of the micropipette with the neutrophil, the transfer of fura2+-dextran into the cell was monitored by an increase in fluorescence at 360 nm to give intracellular concentrations of fura2+-dextran of between 10 and 50 μm. After successful micro-injection, the neutrophils remain fully functional, able to undergo phagocytosis in response to challenge8 appear morphologically normal, and maintain low cytosolic Ca2+ (100 nm).7
Fas stimulation
Neutrophils (5 × 106 cells/ml) were treated with either anti-human Fas monoclonal antibody (1 μg/ml, DX2+; Oncogene, Boston, MA) or control antibodies (Sigma-Aldrich, Gillingham, Dorset, UK) for 5 min at 37°. The cells were allowed to adhere to glass coverslips, which were mounted in specially prepared chambers on a microscope stage, or in a two-chambered coverglass system (Nalge Nunc, Naperville, IL). The medium was replaced with RPMI-1640, containing fetal calf serum (10%) and cross-linking antibody (1 μg/ml, RaM Ab, Sigma-Aldrich) was added. The cells were maintained at 37° either in an incubator or on the microscope stage whilst maintaining the 5% CO2+ gas phase. All images were taken using an oil immersion 100× objective.
Results
Induction of apoptosis by Fas cross-linking in absence of Ca2+ signal
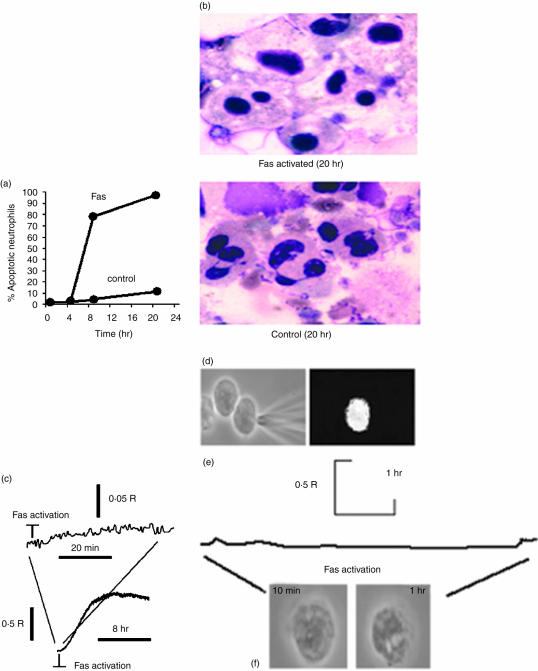
Cross-linking the irrelevant antibody has no significant effect on the spontaneous rate of apoptosis (Fig. 1a). However, cross-linking anti-Fas antibody caused a dramatic acceleration in the apoptotic rate, from apoptosis being barely detectable at 4 hr to being found in 80% of neutrophils by 8 hr (Figs 1a,b). Cross-linking anti-Fas antibody (Fas activation) had no detectable immediate effect on cytosolic Ca2+, measured over the first 30 min (Fig. 1c). There was also no significant difference in the fura2+ signals from populations of Fas-triggered and untreated neutrophils over longer periods (up to 2+ hr). However, fura2+ leakage over this time period was very significant under both experimental conditions and prevented further Ca2+ monitoring (Fig. 1c). To extend the period over which cytosolic Ca2+ could be followed, fura2+-conjugated to high molecular weight dextran (10 000) was micro-injected into individual neutrophils (Fig. 1d). This indicator was retained within neutrophils for at least 6 hr, during the time when morphological evidence of apoptosis could be demonstrated. However, there were no significant Ca2+ changes observable over the complete time-course (Fig. 1e). It was therefore concluded that elevations of cytosolic Ca2+ were not triggered by Fas activation and that an elevation of cytosolic Ca2+ was not required for progression to apoptosis by this accelerated route.
Figure 1.
Ca2+ signalling induced by Fas activation. (a) The graph shows the acceleration towards morphological apoptosis induced by cross-linking anti-Fas antibodies on human circulating neutrophils. Morphological apoptosis was identified by the darkly stained, condensed nucleus and cytoplasmic vacuolation. The rate of apoptosis was significantly different from the untreated apoptotic rate shown here. (b) The images show typical apoptotic neutrophils 2+0 h without (control) and with Fas activation. The effect was similar whether neutrophils were adherent to glass or kept in suspension on plastic. (c) The traces show the changes in cytosolic free Ca2+ in a suspension cell population after Fas activation. The upper trace shows a close-up view of the lack of a detectable Ca2+ change immediately after Fas activation and the lower trace shows a complete time course over many hours. The elevation in fura2+ ratio observed between 1 and 6 hr was attributed to leakage of fura2+ into the extracellular medium containing Ca2+ (1·3 mm, 37°). (d) The images show a typical experiment in which fura2+-dextran was micro-injected into single neutrophils, the image on the left showing the moment of micro-injection and the image on the right the resultant intracellular fura2+-dextran image (excited at 380 nm). (e) The graph shows a typical cytosolic free Ca2+ monitored by fura2+-dextran over 4 hr in individual neutrophils (5% CO2+, 37°), which was observed on at least three separate occasions. (f) The images show phase contrast images of fura2+ loaded neutrophils after Fas activation (40 min and 4 hr).
Changes in Ca2+ signalling during progression towards apoptosis
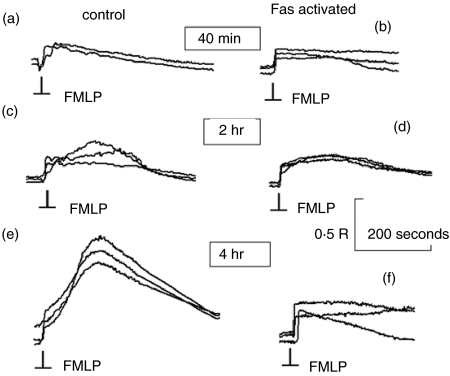
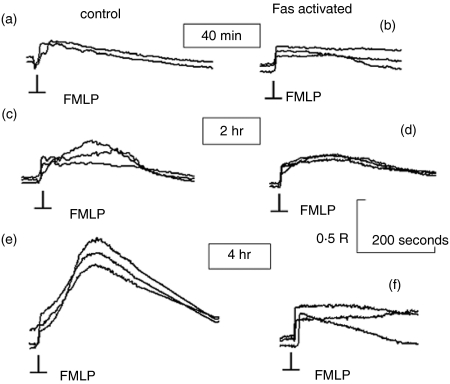
To investigate the integrity of the Ca2+ signalling pathway during progression to apoptosis, N-formyl-methionyl-leucyl-phenylalanine (FMLP; 100 nm) was used as a standard challenge. This stimulus acts on the seven conventional transmembrane receptors1,2 and signals Ca2+ through the generation of IP3 and capacitative Ca2+ influx.2,3 The Ca2+ signal generated was initially the classic single-peaked response (as a result of release of Ca2+ from stores) followed by an exponential decay (as a result of ATP-requiring pumping of Ca2+ out of the cell) to a slightly elevated plateau set by the equilibrium between Ca2+ influx and Ca2+ pumping (Fig. 2a). However, in untreated neutrophils, the second phase of the Ca2+ response became increasingly pronounced, developing a characteristic ‘kyphotic’ pattern in more than 90% of neutrophils by 4 hr (Fig. 2e). This ‘kyphotic’ Ca2+ signal was dependent on Ca2+ influx and the effect on cytosolic free Ca2+ probably arose from decreased Ca2+ extrusion as a result of the depletion of cellular ATP in the ageing neutrophils, whose ATP production relies on anaerobic respiration.9 Indeed, under the conditions in the experiments (i.e. with fura2+ loading), ATP levels fell to 2+5% of the initial value.10 However, in Fas-triggered neutrophils, by 4 hr, more than 90% of neutrophils retained their responsiveness to FMLP, but their Ca2+ responses did not show any kyphotic effect. The initial phase of the Ca2+ signal, which is related to the release of Ca2+ from intracellular stores, remained unaffected and was not significantly different from that observed at earlier times or in the controls at any times (Fig. 2). Furthermore, the rate of Ca2+ release in this phase remained faster than the time resolution of the imaging system (0·65–1 seconds) in both Fas-treated and control neutrophils, demonstrating that the signalling for IP3 generation remained unaffected. In some experiments, the initial Ca2+ signal also began to diminish in magnitude compared to that in neutrophils in the control group after 6 hr. These results show that the Ca2+ signalling pathway in Fas-treated cells becomes increasingly modified as the cells progress to apoptosis and is consistent with an early defect in coupling to Ca2+ influx.
Figure 2.
Changes in Ca2+ signalling during progress towards apoptosis. The traces show the cytosolic free Ca2+ change within individual neutrophils in response to FMLP (100 nm, added at the point indicated ‘FMLP’) after incubation at 37° for the times shown without (control) or with Fas activation. The results shown are from a typical experiment, with the responses from three individual neutrophils superimposed. Traces (a, c, e) show the responses of untreated neutrophils and traces (b, d, f) show the responses from Fas-activated neutrophils at 40 min, 2 hr and 4 hr. The magnitude is shown as fura-2+340 nm/380 nm ratio (R) with the calibrated signals for the controls in this example corresponding to approximately 300 nm Ca2+ for the signal at 40 min and over 1 μm for the signal at 4 hr. The effect was observed in more than 90% of Fas-treated neutrophils on at least 10 other occasions.
Selective shutdown of Ca2+ influx pathway
To confirm that the changes induced by ageing of neutrophils and by Fas ligation were the result of an effect on the Ca2+ influx pathway, the effects of the absence of extracellular Ca2+ and of Ca2+ channel blockade were examined. In the absence of extracellular Ca2+ (less than 10 nm, EGTA 1 mm), the differences between 4 hr control and 4 hr Fas-treated signals were abolished and there was no significant difference between the Ca2+ signals (Fig. 3). As, under these conditions, the only source of Ca2+ for the cytosolic Ca2+ signal was from within the cell, i.e. within Ca2++ stores, this confirmed that the coupling of steps between receptor occupancy to Ca2+ store release were not affected by Fas ligation. As removal of the Ca2+ influx component removed the differential effect, these results also showed that in Fas-treated cells Ca2+ influx was uncoupled from the Ca2+ store release.
Figure 3.
Dependence of ‘apoptotic’ Ca2+ signals on extracellular Ca2+. The traces show the cytosolic free Ca2+ change within individual neutrophils in response to FMLP (1 μm, added at the pointed indicated ‘FMLP’) after incubation (> 4 hr, 37°) in the presence and absence of extracellular Ca2+. Traces (a and b) show the responses from untreated neutrophils and traces (c and d) from Fas-activated neutrophils. Traces (b and d) show the Ca2+ responses in the absence of extracellular Ca2+ (labelled EGTA). In all traces, ionomycin (2+ μm) was added at the point marked ‘iono’ which confirmed the absence of extracellular Ca2+. The small and transient changes in cytosolic free Ca2+ were observed after addition of ionomycin in the absence of extracellular Ca2+ and attributed to the release of Ca2+ from sites within the neutrophils.
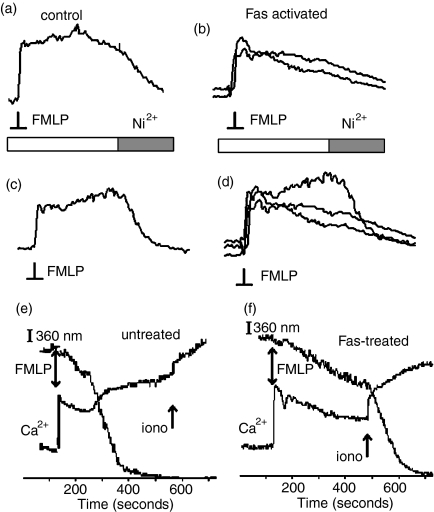
This conclusion was further confirmed by the addition of Ni2+ (1 mm), an ion which blocks Ca2+ channels on neutrophils but does not enter the cytosol (where it would quench fura2+ fluorescence). Stimulating neutrophils in the presence of Ni2+ removed the kyphotic phase of the Ca2+ signal induced by FMLP stimulation in 4-hr aged control cells (Fig. 4). In contrast, the presence of extracellular Ni2+ had no significant effect on the shape or magnitude of the response to FMLP in Fas-activated neutrophils (Fig. 4). Furthermore, by adding Ni2+ to neutrophils which were stimulated with FMLP, there was a clear reduction in the Ca2+ signal in control cells, showing that the Ca2+ channels were open at that time (Fig. 5a). In contrast, the addition of Ni2+ had no effect on the Ca2+ signal in 4-hr Fas-treated cells when added at the time of the plateau phase (Fig. 5b). However, in Fas-treated neutrophils which still had a kyphotic Ca2+ signal, the addition of Ni2+ reduced the Ca2+ signal to the levels observed in the non-kyphotic cell. Fura2+ quenching by an influx of Mn2+ ions (1 mm) was also absent in Fas-treated neutrophils (Figs 5e,f). As the influx of Mn2+ was also inhibited by Ni2+, these results confirm that Ca2+ influx channels, which were also permeable to Mn2+ and blocked by Ni2+, that normally open after stimulation which FMLP remained closed in the Fas-treated neutrophils.
Figure 4.
Effect of blockade of Ca2+ influx signals by Ni2+. The traces show the cytosolic free Ca2+ change in response within individual neutrophils to FMLP (1 μm, added at the pointed indicated ‘FMLP’) after incubation (> 4 hr, 37°) in the presence and absence of the Ca2+ channel blocking ion Ni2+ (1 mm). (a) and (b) show the responses from untreated neutrophils and traces, (c and d) show those from Fas-activated neutrophils. Traces (b and d) show the Ca2+ responses in the presence of Ni2+.
Figure 5.
Absence of Ni2+-dependent Ca2+ influx or Mn2+ influx in Fas-treated neutrophils. The traces show the cytosolic free Ca2+ change within individual neutrophils in response to FMLP (1 μm, added at the pointed indicated ‘FMLP’) after incubation (3·5 hr, 37° after Fas activation). Ni2+(1 mm) was added at the point indicated on the bar accompanying each trace. The results show a typical experiment. The traces show the effect of the addition of Ni2+ (1 mm) on the FMLP-induced Ca2+ response from (a) untreated neutrophils after incubation (control) and (b) Fas-activated neutrophils, and trace (d) shows the data from traces (b and c) superimposed to demonstrate that Ca2+ channel blockade by Ni2+ converted the ‘kyphotic’ response to the Ca2+ influx shutdown state. (e, f) In the presence of Mn2+ (0·1 mm), quenching of the fura2+ signal monitored at 360 nm (I360nm) was used as a monitor of Mn2+ influx. In untreated neutrophils at 4–6 hr, an increase in Mn2+ influx was triggered with the increase in cytosolic free Ca2+-stimulated by FMLP (e) .However, in neutrophil treatment with Fas, FMLP failed to cause an increased influx of Mn2+ despite triggering a cytosolic free Ca2+± signal (f) .In both examples, which were typical of at least four others, ionomycin (2+ µm, added at the point marked ‘iono’) produced a large influx of divalent cations giving the maximum fura-2+340/380 ratio (Ca2+) and the maximum fura-2+360 nm quenching (I360) as calibration points.
The effect of inhibition of caspases on Ca2+ influx shutdown
One possible mechanism for the inability to signal Ca2+ influx was that a protein, important for Ca2+ influx, such as the Ca2+ channel itself, was inactivated by the proteolytic activity of caspases that have a known role in apoptosis. The effect of caspase inhibition was therefore investigated. Neutrophils were preincubated for 1 hr with either caspase-3 inhibitor II (100 μm, Z-DEVD-FMK, Calbiochem, UK), or the caspase-8 inhibitor II (100 μm, Z-IETD-FMK, Calbiochem, UK). Although these treatments have an effect on the later morphological changes, Ca2+ influx shutdown in Fas-treated cells was unaffected by either caspase inhibitor (Fig. 6). These results show that Ca2+ influx shutdown was not caspase-dependent and it was concluded to be unlikely to result from protease-mediated intracellular ‘damage’. The Ca2+ influx shutdown was also demonstrable much earlier than gross morphological or nuclear changes on phase contrast microscopy or externalization of phosphatidyl serine (PS) as detectable by annexin V binding (see also Fig. 1).
Figure 6.
Effect of caspase inhibition on apoptotic Ca2++ influx shutdown. The traces show the cytosolic free Ca2++ change within individual neutrophils in response to FMLP (1 μm, added at the pointed indicated ‘FMLP’) after incubation (> 4 hr, 37° after Fas activation), with and without inhibition of caspases. The traces show the data from a typical experiment. Traces (a and b) shows the response from untreated (control) and Fas-activated neutrophils, respectively. Traces (c and d) show the effect of caspase 8 (Z-IETD-FMK, 1 hr, 100 μm) and caspase 3 (Z-DEVD-FMK, 1 hr, 100 μm) inhibition, respectively, on the response from Fas-activated neutrophils. Neither inhibitor generated the ‘kyphotic’ response, which would indicate effective Ca2++ channel opening.
Discussion
The data presented here show that during accelerated apoptosis of human circulating neutrophils induced by Fas (CD95) cross-linking, the Ca2+ influx pathway triggered by FMLP becomes increasingly ineffective, and was absent in Fas-activated neutrophils by 4 hr. The Ca2+ influx shut-down could not be attributed to a decrease in receptor sensitivity, receptor coupling or receptor number because the release of stored Ca2+ remained constant during incubation and was unaffected by Fas activation. It would be expected that uncoupling Ca2+ influx from activating stimuli may have a profound effect on the responsiveness of neutrophils. Not only would they be unable to mount potentially pathogenic actions, such as extracellular oxidant and protease liberation, but phagocytosis, which requires Ca2+ influx11 would also be impeded. These effects may underlie the previously reported decreases in neutrophil responsiveness on approach to apoptosis.12 Physiologically, it may be important that apoptotic neutrophils have reduced sensitivity to stimuli and it could be argued that such a reduction in sensitivity is in line with the ‘purpose’ of neutrophil apoptosis in reducing the number of responsive cells at an inflammatory site which are potentially pathogenic. As we also show that Ca2+ signalling shutdown is an early event en route to apoptosis and is not dependent on caspase activity, it unlikely to be the result of apoptotic morphological changes or PS externalization. If PS externalization is the key signal for phagocytosis of apoptotic neutrophils13 events which occur after this are unlikely to have significant physiological consequences. However, Ca2+ signalling shutdown is one of the earliest events detectable and so would render neutrophils less responsive before full apoptosis and phagocytic clearance.
The effect we report here is similar to that reported for T cells. In T cells, Lepple-Wienhues et al.14 have shown that Fas-stimulation of T cells also blocked Ca2+ influx triggered by ligation of CD3. As the effect of Fas activation on Ca2+ influx was prevented in T cells lacking in acidic sphingomyelinase, and was restored by transfection with the enzyme, it was concluded that this enzyme was important for the Ca2+ influx pathway.14,15 Fas may not be the only member of the superfamily of tumour necrosis factor (TNF) receptors to inhibit Ca2+ channels in the plasma membrane through activation of acid sphingomyelinase. TNF receptor ligands TNF-α and NGF have also been reported to attenuate Ca2+ influx in thyroid and mast cells, respectively.16,17 However, in T cells, the production of IP3 triggered by ligation of CD3 may also be inhibited by Fas ligation.18 This could not account for the uncoupling effect we report here for neutrophils, as the release of stored Ca2+ by FMLP, which is totally dependent on IP3 binding to stores and can be inhibited by intracellular heparin or antibodies to IP3 receptors,3 was unaffected at times when Ca2+ influx was abolished (see Figs 2,·3, 4,·5). In T cells, it has also been suggested that there may be an effect on membrane potential, a negative membrane potential providing the electrical component for Ca2+ influx. It has been shown that activation of Fas or the addition of ceramide inhibits N-type K+ channels through activation of a protein kinase, p561ck.19 Inhibition of K+ channels would cause membrane depolarization and would consequently reduce the electrochemical gradient and inhibit Ca2+ influx. Such an effect is unlikely to be relevant to neutrophils, as the oxidase is electrogenic and would already exert a large electrochemical restraint on Ca2+ influx.20
Although Ca2+ influx is common to a number of non-excitable cells, the mechanism by which it occurs has yet to be fully resolved and may involve different channels in different cell types.21,22 At least two mechanisms have been suggested, one involving conformational coupling of IP3 receptors with a Ca2+ channel located in the plasma membrane23 and another involving a diffusible second messenger released in response to Ca2+ store emptying and which causes Ca2+ channel opening.24,25 Although the molecular identity is unknown, the latter factor may be a small (mol. wt < 1000) phosphorylated molecule25 a similar activity having been isolated from Ca2+ store depleted neutrophils.26 Although the mechanism for neutrophil Ca2+ influx shutdown is unclear, the possibility exists that it may involve residue mitochondria found in neutrophils. In basophils, it has recently been shown that ‘energized’ mitochondria are crucial for coupling Ca2+ store release to Ca2+ influx.27 As mitochondria are known to take-up Ca2+ from the cytosol and are often placed close to the endoplasmic reticulum where Ca2+ release is occurring, a possible new role for mitochondria is implicated as the generator of the signal for Ca2+ influx.27 In the neutrophil, released Ca2+ may thus be taken up by nearby mitochondria having significant membrane potentials, with a generation of the signal for Ca2+ influx. Although neutrophils have few mitochondria, which do not play a role in ATP production, the mitochondria often have membrane potentials.28 Furthermore, recently it has been shown that an early event in the progress towards spontaneous neutrophil apoptosis, which precedes externalization of PS, is loss of mitochondrial membrane potential.28 This may thus provide the link to Ca2+ influx shutdown demonstrated here.
Acknowledgments
We thank volunteers registered with the Neutrophil Signalling Group, UWCM, Cardiff for donating blood. K.A. was supported in part by University of Wales College of Medicine (Research Initiative Award, 2001–2).
References
- 1.Scharff O, Foder B. Regulation of cytosolic calcium in blood cells. Physiol Rev. 1993;73:547–82. doi: 10.1152/physrev.1993.73.3.547. [DOI] [PubMed] [Google Scholar]
- 2.Hallett MB, Lloyds D. Molecular and Ionic Signalling of Neutrophils. Heidelberg Springer-Verlag and New York: Chapman & Hall; 1997. [Google Scholar]
- 3.Davies-Cox EV, Laffafian I, Hallett MB. Control of Ca2+ influx in human neutrophils by inositol 1,4,5-trisphosphate (IP3) binding: differential effects of micro-injected IP3 receptor antagonists. Biochem J. 2001;355:139–43. doi: 10.1042/0264-6021:3550139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med. 1996;184:429–40. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Randriamampita C, Tsien RY. Emptying of intracellular Ca2++ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993;364:809–14. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- 6.Davies EV, Hallett MB. A soluble cellular factor directly stimulates Ca2+ entry in neutrophils. Biochem Biophys Res Commun. 1995;206:348–54. doi: 10.1006/bbrc.1995.1048. [DOI] [PubMed] [Google Scholar]
- 7.Glitsch MD, Bakowski D, Parekh AB. Store-operated Ca2+ entry depends on mitochondrial Ca2+ uptake. EMBO J. 2002;2+1:6744–54. doi: 10.1093/emboj/cdf675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fossati G, Moulding DA, Spiller DG, Moots RJ, White MRH, Edwards SW. The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. J Immunol. 2003;170:1964–72. doi: 10.4049/jimmunol.170.4.1964. [DOI] [PubMed] [Google Scholar]
- 9.Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest. 1982;70:550–7. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Mohanna FA, Hallett MB. The use of fura 2+ to determine the relationship between intracellular free Ca2+ and oxidase activation in rat neutrophils. Cell Calcium. 1998;8:17. doi: 10.1016/0143-4160(88)90034-6. [DOI] [PubMed] [Google Scholar]
- 11.Dewitt S, Hallett MB. Cytosolic free Ca2+ changes and calpain activation are required for beta integrin-accelerated phagocytosis by human neutrophils. J Cell Biol. 2002;159:181–9. doi: 10.1083/jcb.200206089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whyte MK, Meagher LC, MacDermot J, Haslett C. Impairment of function in aging neutrophils is associated with apoptosis. J Immunol. 1993;150:5124–34. [PubMed] [Google Scholar]
- 13.Fadok VA, Bratton DL, Frasch SC, Warner ML, Henson PM. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998;5:551–62. doi: 10.1038/sj.cdd.4400404. [DOI] [PubMed] [Google Scholar]
- 14.Lepple-Wienhues A, Belka C, Laun T, et al. Stimulation of CD95 (Fas) blocks T lymphocyte calcium channels through sphingomyelinase and sphingolipids. Proc Natl Acad Sci USA. 1999;96:13795–800. doi: 10.1073/pnas.96.24.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hueber AO. CD95: more than just a death factor? Nat Cell Biol. 2000;2:E23–5. doi: 10.1038/35000092. [DOI] [PubMed] [Google Scholar]
- 16.Mathes C, Fleig A, Penner R. Calcium release-activated calcium current (ICRAC) is a direct target for sphingosine. J Biol Chem. 1998;273:25020–30. doi: 10.1074/jbc.273.39.25020. [DOI] [PubMed] [Google Scholar]
- 17.Tornquist K, Molm AM, Pasternack M, Kronquist R, Bjorklund S, Tuominen R, Slotte JP. Tumor necrosis factor-alpha, sphingomyelinase, and ceramide inhibit store-operated calcium entry in thyroid FRTL-5 cells. J Biol Chem. 1999;274:9370–7. doi: 10.1074/jbc.274.14.9370. [DOI] [PubMed] [Google Scholar]
- 18.Kovacs B, Tsokos GC. Cross-linking of the Fas/APO-1 antigen suppresses the CD3-mediated signal transduction events in human T lymphocytes. J Immunol. 1995;155:5543–9. [PubMed] [Google Scholar]
- 19.Szabo I, Gulbins E, Apfel H, et al. Tyrosine phosphorylation-dependent suppression of a voltage-gated K+ channel in T lymphocytes upon Fas stimulation. J Biol Chem. 1996;271:2+0465–9. doi: 10.1074/jbc.271.34.20465. [DOI] [PubMed] [Google Scholar]
- 20.Hallett MB. Holding back neutrophil aggression; the oxidase has potential. Clin Exp Immunol. 2003;132+:181–4. doi: 10.1046/j.1365-2249.2003.02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatachalam K, van Rossum DB, Patterson RL, Ma HT, Gill DL. The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol. 2002;4:E263–72. doi: 10.1038/ncb1102-e263. [DOI] [PubMed] [Google Scholar]
- 22.Putney JW, Jr, Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J Cell Sci. 2001;114:23–9. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- 23.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–25. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 24.Parekh AB, Terlau H, Stuhmer W. Depletion of InsP3 stores activates a Ca2+ and K+ current by means of a phosphatase and a diffusible messenger. Nature. 1993;364:814–8. doi: 10.1038/364814a0. [DOI] [PubMed] [Google Scholar]
- 25.Hallett MB, Davies EV, Campbell AK. Oxidase activation in individual neutrophils is dependent on the onset and magnitude of the Ca2+ signal. Cell Calcium. 1990;11:655–63. doi: 10.1016/0143-4160(90)90020-u. [DOI] [PubMed] [Google Scholar]
- 26.Hallett MB, Davies EV, Pettit EJ. Fluorescent methods for measuring and imaging cytosolic free Ca2+ in neutrophils. Methods. 1996;9:591–606. doi: 10.1006/meth.1996.0066. [DOI] [PubMed] [Google Scholar]
- 27.Laffafian I, Hallett MB. Lipid-assisted microinjection: introducing material into the cytosol and membranes of small cells. Biophys J. 1998;75:2558–63. doi: 10.1016/S0006-3495(98)77700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dewitt S, Laffafian I, Hallett MB. Phagosomal oxidative activity during β2 integrin (CR3) -mediated phagocytosis by neutrophils is triggered by a non-restricted Ca2+ signal: Ca2+ controls time not space. J Cell Sci. 2003;116:2857–65. doi: 10.1242/jcs.00499. [DOI] [PubMed] [Google Scholar]