Abstract
The recognition of pathogen-associated molecular patterns (PAMPs) by the innate immune system is a crucial step in inducing effective immune responses. Double-stranded RNA [mimicked by polyinosinic-polycytidylic acid (poly(I:C)], synthesized by various types of viruses, represents one important member of these immunostimulatory microbial components. Here we report that poly(I:C) has potent γδ T-cell costimulatory capacity. Within peripheral blood mononuclear cells, poly(I:C)-stimulated γδ T cells expressed increased levels of CD69 and exhibited significantly enhanced antigen-mediated proliferation in response to isopentenylpyrophosphate (IPP). Among several recombinant cytokines tested, type I interferons (IFN-α, IFN-β) and interleukin-15 (IL-15) showed a similar activation pattern of γδ T cells. γδ T-cell clones and purified γδ T cells did not respond to poly(I:C), indicating indirect effects of this compound. Depletion of CD11c+ dendritic cells (DC), which express Toll-like receptor 3 (TLR3), known to recognize poly(I:C), abrogated poly(I:C)-mediated stimulation of γδ T cells. In addition, the supernatant of poly(I:C)-treated CD11c+ DC was able to mimic the stimulatory effects of poly(I:C) on γδ T cells. Experiments with neutralizing antibodies indicated that type I IFNs, but not IL-15, contributed to the poly(I:C)-mediated activation of γδ T cells. In conclusion, γδ T-cell activation by immunostimulatory double-stranded RNA, such as poly(I:C), is indirectly mediated via type I IFNs derived from TLR3-expressing CD11c+ DCs. These results suggest that upon confrontation with certain viruses, γδ T cells can be rapidly activated by type I interferons and may contribute to effective antiviral responses.
Keywords: poly(I:C), γδ T cell, type I interferons, dendritic cell
Introduction
γδ T cells are known to play an important role in connecting the innate and acquired immunity while sharing several features of αβ T cells and natural killer (NK) cells. Human γδ T cells expressing the Vγ9Vδ2-encoded T-cell receptor (TCR), which represent the major subset in the peripheral blood of adults, recognize non-peptide, low-weight phosphate-containing molecules (phosphoantigens) and synthetic aminobisphosphonates in the absence of major histocompatibility complex (MHC) restriction or antigen processing.1,2 After this unique antigen recognition, Vγ9Vδ2+ T cells promptly release pro-inflammatory cytokines [preferentially interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α)]3 and chemokines,4 proliferate polyclonally and become broadly reactive cytotoxic effector cells.1 Thus, γδ T cells are suggested to have a sentinel function by participating in the early host response against bacterial, parasitic and viral infections, and in linking the innate and acquired immunity by providing the first barrier until antigen-specific αβ T cells have been recruited and expanded.5
The exact role of γδ T cells in viral infections remains unknown. However, the in vivo expansion of this T-cell subset during several viral infections, and the in vitro response to virus-infected cells, indicates a role of these lymphocytes during antiviral immune responses. For example, acute Epstein–Barr virus (EBV) infection in humans is associated with an increased proportion of peripheral blood Vγ9Vδ2+ T cells that exhibit an activated phenotype.6 This in vivo Vγ9Vδ2+ cell response resamples the in vitro reactivity of this T-cell subset against the EBV-associated Burkitt's lymphoma-derived cell lines, such as Daudi.7 In murine models, γδ T cells protect mice from lethal encephalitis induced by herpes simplex virus-1 (HSV-1) infection, supporting their important contribution to the immune response against HSV infection.8 It has been shown that γδ T cells are broadly reactive against different viruses, such as herpes viruses (HSV, cytomegalovirus, human herpes virus-6), vaccinia virus, influenza virus, coxsackie B virus and human or simian immunodeficiency virus (HIV/SIV).9 In addition, the cytotoxic activity of γδ T cells was found to be MHC-unrestricted and not dependent on the infecting virus type.10 Therefore, the antiviral effector function of γδ T cells seems not to be directed against specific viral antigens.
Double-stranded RNA (dsRNA) is a viral product generated during the proliferation cycle of many pathogenic viruses. Viral dsRNA and its synthetic mimetic, polyinosinic-polycytidylic acid [poly(I:C)], are both strong inducers of type I IFNs (IFN-α and -β) in vitro and in vivo, which function as key cytokines in antiviral host defence. Previously, dsRNA was thought to activate only intracellular targets, including the IFN-inducible dsRNA-activated protein kinase R (PKR).11,12 However, recent studies have shown that the Toll-like receptor 3 (TLR3) recognizes dsRNA and transduces signals which activate the nuclear factor-kappaB (NF-κB) and the IFN-β promoter.13,14 TLR3 is structurally related to TLR7, -8 and -9, which are members of a TLR subfamily recognizing microbial nucleic acid derivates.15 In contrast to other TLRs, expression of TLR3 mRNA is restricted to dendritic cell (DC) subsets, fibroblasts and intestinal epithelial cells.14,16,17 Among different DC subsets, only CD11c+ DC were reported to respond to poly(I:C), while CD4+ CD11c– type 2 DC precursors (plasmacytoid DC) are stimulated by immunostimulatory bacterial DNA (CpG DNA) via TLR9.18
In the current study, we focused on characterizing the effects of the synthetic dsRNA poly(I:C) on human γδ T cells. Our results show that:
(1) poly(I:C) is able to activate γδ T cells similarly to recombinant type I IFNs and interleukin (IL)-15.
(2) poly(I:C) is a potent costimulatory agent for antigen-induced proliferation of Vγ9Vδ2+ T cells.
(3) poly(I:C)-induced stimulation of γδ T cells is dependent on type I IFNs derived from CD11c+ DCs.
Materials and methods
eagents and cytokines
Poly(I:C), isopentenylpyrophosphate (IPP), phytohaemagglutinin (PHA) and phorbol 12-myristate 13-acetate (PMA) were purchased from Sigma (Deisenhofen, Germany), human recombinant IFN-α2a (Roferon®) from Hoffmann-La Roche AG (Grenzach-Wyhlen, Germany), human recombinant IFN-β, human IL-12, human granulocyte–macrophage colony-stimulating factor (GM-CSF) and human IL-4 from TEBU (Frankfurt, Germany), human recombinant IFN-γ (Polyferon 50®) from Dr Rentschler Arzneimittel GmbH & Co (Laupheim, Germany), human recombinant IL-2 (Proleukin®) from Euro Cetus GmbH (Frankfurt, Germany), human recombinant IL-15 from Endogen (Biozol, München, Germany) and human recombinant TNF-α from Sigma.
Cell isolation and culture
Heparinized peripheral blood was obtained from different healthy donors after obtaining their informed consent. Mononuclear cells [peripheral blood mononuclear cells (PBMC)] were isolated by leukapheresis or from heparinized blood by Ficoll–Hypaque density centrifugation (Amersham Pharmacia, Uppsala, Sweden). A total of 5 × 104 PBMC were cultured in RPMI-1640 (Gibco, Paisley, UK) supplemented with 10% pooled human AB serum (PAA, Coelbe, Germany), 2 mm l-glutamine (Gibco), 100 U/ml penicillin and 100 µg/ml streptomycin (Seromed, Berlin, Germany) in 96-well round-bottom microtiter plates (Greiner, Solingen, Germany) for the indicated time intervals (1–8 days) at 37° in a humidified atmosphere (5% CO2). As indicated, PBMC were cultured in medium alone (negative control), or in the presence of different concentrations of poly(I:C) or various cytokines. For evaluation of γδ T-cell expansion, PBMC were harvested after an 8-day culture period and analysed using two-colour flow cytometry with fluorescein isothiocyanate (FITC)-conjugated anti-pan γδ TCR and phycoerythrin (PE)-conjugated anti-CD3 monoclonal antibody (mAb). To calculate the expansion of absolute cell numbers, the number of viable cells per well was counted. Viability was confirmed by the Trypan blue exclusion test.
Purification of γδ T cells from PBMC was performed by positive selection according to the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, PBMC were incubated with hapten-conjugated anti-γδ TCR mAb for 15 min at 4°, washed and then subsequently incubated with FITC-conjugated anti-hapten immunomagnetic beads, and then passed through a strong magnetic field. The isolated γδ T cells yielded a purity of at least 98%. Depletion of CD11c+ cells from PBMC was performed by a negative-selection procedure using PE-conjugated anti-CD11c mAb (Becton Dickinson, Heidelberg, Germany) and anti-PE immunomagnetic beads (Miltenyi Biotec). The negative-selected population contained < 0·1% CD11c+ cells, as assessed by flow cytometry. The viability of positive- or negative-selected cell populations was confirmed by the Trypan blue exlusion test and forward/side-scatter gating.
Immature DC (iDC) were generated from human peripheral blood monocytes, as previously decribed.19 Briefly, adherent monocytes from PBMC were cultured for 6 days in flat-bottom 24-well plates in RPMI supplemented with 10% autologous serum, GM-CSF (1000 U/ml) and IL-4 (1000 U/ml). Fresh medium plus cytokines was added every 3 days. The resulting preparation routinely contained > 90% immature DCs, as assessed by morphology and flow cytometry.
Generation of γδ T-cell clones
To generate γδ T-cell clones, PBMC were depleted from αβ T cells (by anti-TCR αβ mAb) and from NK cells (by anti-CD16 mAb) through magnetic-activated cell sorting (MACS) (Miltenyi Biotec), as described previously.2 After depletion procedures, cells were seeded under limiting-dilution conditions (0·45 cells/well) and cultured with irradiated allogeneic PBMC and EBV-transformed B cells (3 × 104 cells/well) as feeder cells in 96-well round-bottom microtitre plates. The medium consisted of RPMI and antibiotics, 10% human AB serum, 200 U/ml IL-2 and PHA (1 µg/ml). Growing microcultures were periodically supplemented with 100 U/ml IL-2 and irradiated feeder cells. The phenotype of clones was assessed by flow cytometric analysis using anti-Vδ1–FITC (Biozol), anti-Vδ2-FITC or anti-Vγ9-FITC (both Beckman Coulter, Krefeld, Germany) mAbs.
Flow cytometric analysis and mAbs
Cells were harvested after the indicated culture periods and analysed using one- or two-colour flow cytometry (FACScan flow cytometer; Becton Dickinson), using the cellquest program. Cells were stained with the appropriate concentrations of the following mAbs: FITC-conjugated anti-pan γδ TCR (Beckman Coulter) or anti-TCR-αβ (T-Cell Diagnostics, Woburn, MA), PE-conjugated anti-CD3, anti-CD25 (α-chain IL-2 receptor), anti-CD69, anti-CD122 (β-chain IL-2 receptor; Beckman Coulter), anti-CD54, anti-CD95 or anti-HLA-DR mAb (all from Beckman Coulter), unconjugated anti-TLR3 mAb (Biocarta, Hamburg, Germany) or an appropriate isotype-control mAb. Lymphocytes were gated based on their forward (FSC) and side-scatter (SSC) profile for each experiment.
For cytokine-inhibition assays, PBMC were cultured in the presence or absence of neutralizing anti-cytokine immunoglobulin at the indicated concentrations. Monoclonal anti-human type I IFN receptor (IFN-α receptor chain 2; mouse) was purchased from DPC Biermann (Bad Nauheim, Germany), polyclonal anti-human IL-15 (rabbit) and monoclonal anti-human IL-12 (mouse) were purchased from Endogen (Woburn, MA). Polyclonal anti-human TNF-α (rabbit), polyclonal anti-human IFN-γ (rabbit) and monoclonal anti-human IL-2 (mouse) were from TEBU (Frankfurt, Germany). Isotype-matched control antibodies (mouse, rabbit) were purchased from Pharmingen (Hamburg, Germany).
Cytokine analysis
Quantification of IFN-γ in PBMC supernatants was performed by enzyme-linked immunosorbent assay (Endogen). Supernatants were collected after 24 or 64 hr and stored at −80°, after centrifugation, until analysis was performed according to the manufacturer's instructions. Samples were analysed in triplicate. The sensitivity of the assay used was < 2 pg/ml for IFN-γ. Intracellular cytokine staining was performed to determine the IFN-γ production of γδ T cells at a single-cell level. Monensin (5 µg/ml; Sigma) was added for 5 hr to the cells in culture to induce intracellular accumulation of newly synthesized proteins. Cells were harvested and stained for the surface expression of TCR-γδ by incubation with PE-conjugated anti-pan TCR γδ mAb for 15 min. After staining, the cells were fixed with a paraformaldehyde-containing solution for 10 min at room temperature in the dark, according to the manufacturer's instructions (DAKO IntraStain; DAKO, Hamburg, Germany). Cells were washed with phosphate-buffered saline (PBS) containing 1% fetal calf serum (FCS) and permeabilized with 0·5% saponin for 5 min at room temperature. FITC-conjugated anti-IFN-γ (Beckman Coulter) was added to permeabilized cells and incubated for 15–30 min at room temperature in the dark. Cells were washed with PBS/1% FCS and analysed on a FACScan flow cytometer. As a control, samples were incubated with an irrelevant isotype-matched mAb (Becton Dickinson).
Results
Activation of γδ T cells by poly(I:C)
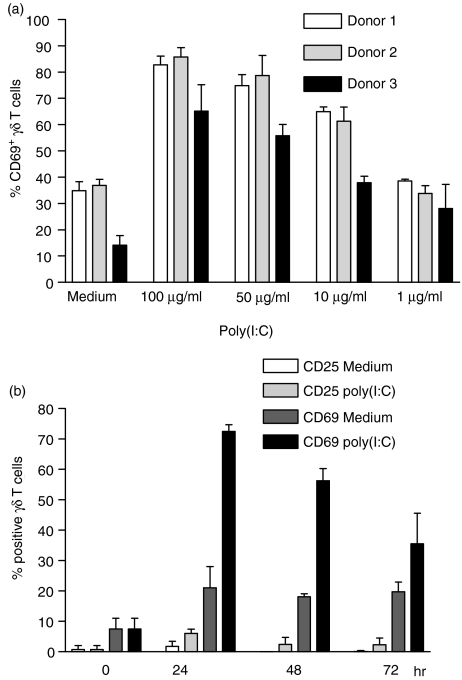
To investigate the activating properties of poly (I:C) on γδ T cells, PBMC from different donors were incubated with different concentrations of poly (I:C), without additional cytokines. After 24 hr, the surface expression of CD69 was measured on γδ T cells. As shown in Fig. 1(a), poly(I:C) induced a dose-dependent CD69 up-regulation on a large percentage of γδ T cells of all donors. In addition, the kinetics of γδ T-cell activation by poly(I:C) was followed by determining the expression of CD69 and CD25 on γδ T cells during a 72-hr culture period. CD69 was up-regulated to a maximum level on γδ T cells after 24 hr of incubation, and showed a subsequent decrease at 48- and 72-hr time-points (Fig. 1b). In contrast to known γδ T-cell ligands, such as the phosphoantigen IPP, poly(I:C) did not induce significant CD25 expression on γδ T cells during the culture period (Fig. 1b). Other activation markers, such as HLA-DR, CD122, CD54 or CD95, were also not expressed on γδ T cells in response to poly(I:C) (data not shown). Up-regulation of CD69 by poly(I:C) was not observed on CD4+ and CD8+αβ T cells, while NK cells showed a similar expression pattern of activation markers (only CD69, no CD25 or HLA-DR up-regulation) as γδ T cells (data not shown). Therefore, in PBMC cultures, both γδ T cells and NK cells exhibited signs of a partial activation status after exposure to poly(I:C).
Figure 1.
Partial activation of γδ T cells by polyinosinic-polycytidylic acid [poly(I:C)]. (a) Expression of CD69 on γδ T cells after stimulation of primary peripheral blood mononuclear cells (PBMC) with different concentrations of poly(I:C). Results represent mean values ± standard deviation (SD) of triplicate cultures from three different healthy donors. (b) Percentage of CD69+ and CD25+γδ T cells before (0 hr) and after 24, 48 and 72 hr of primary PBMC culture in the presence of poly(I:C) (100 µg/ml) or medium alone. Results are shown as mean ± SD of triplicate cultures from one representative donor. Similar activation profiles were observed in four additional donors.
Poly(I:C)-induced activation of γδ T cells is not associated with IFN-γ production
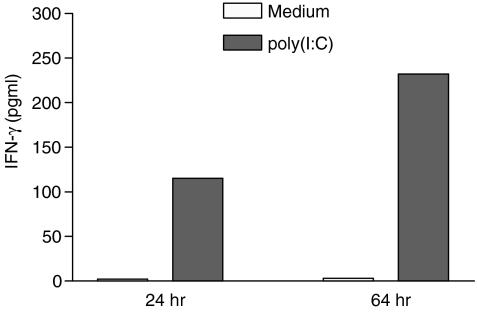
An effector function of γδ T cells is the release of cytokines after activation, particularly T helper 1 (Th1) cytokines (TNF-α, IFN-γ). We have previously reported that γδ T cells produce high levels of IFN-γ after specific stimulation with phosphoantigens, such as IPP or the aminobisphosphonate pamidronate.2 To test whether poly(I:C) can induce IFN-γ secretion, IFN-γ concentrations were measured in the supernatants of poly(I:C)-treated PBMC cultures. Incubation with poly(I:C) (100 µg/ml) induced the production of significant amounts of IFN-γ after 24- and 64-hr culture periods (Fig. 2). However, single-cell analysis revealed no significant increase of intracytoplasmatic IFN-γ concentrations in γδ T cells, despite a marked up-regulation of CD69. In contrast, non-specific (PMA/ionomycin) and specific (IPP, pamidronate) stimulation of γδ T cells induced a significant increase of intracellular IFN-γ levels (Table 1). These data indicate that cells other than γδ T cells contribute to poly(I:C)-mediated IFN-γ production in PBMC cultures.
Figure 2.
Interferon-γ (IFN-γ) production of peripheral blood mononuclear cells (PBMC) stimulated with polyinosinic-polycytidylic acid [poly(I:C)]. PBMC were cultured with poly(I:C) (100 µg/ml) or medium alone. Supernatants were assessed for IFN-γ content by enzyme-linked immunosorbent assay (ELISA) after 24 and 64 hr of culture. The mean values of three separate experiments are shown.
Table 1.
Polyinosinic-polycytidylic acid [poly(I:C)] stimulation does not induce interferon-γ (IFN-γ) production in γδ T cells
| % CD69+γδ T cells | % CD25+γδ T cells | % IFN-γ+γδ T cells | |
|---|---|---|---|
| Medium | 27 | 5 | 0 |
| PMA/ionomycin | 100 | ND | 83 |
| IPP | 93 | 88 | 41 |
| Pamidronate | 79 | 50 | 57 |
| poly(I:C) | 73 | ND | 9 |
Intracellular IFN-γexpression of γδT cells was measured by single-cell analysis after culture of peripheral blood mononuclear cells (PBMC) for 72 hr in medium alone or in the presence of phorbol 12-myristate 13-acetate (PMA) (25 ng/ml)/ionomycin (500 ng/ml), isopentenylpyrophosphate (IPP) (5 µg/ml), pamidronate (10 µg/ml) or poly(I:C) (50 µg/ml), as described in the Materials and methods. In addition, the expression of CD69 and CD25 on γδT cells was determined by standard two-colour fluorescence-activated cell sorter (FACS) analysis.
The data show the mean percentages of positive γδ T cells from one experiment that is representative of three separate experiments. ND, not determined.
Potent enhancement of the antigen-mediated γδ T-cell proliferative response by poly(I:C)
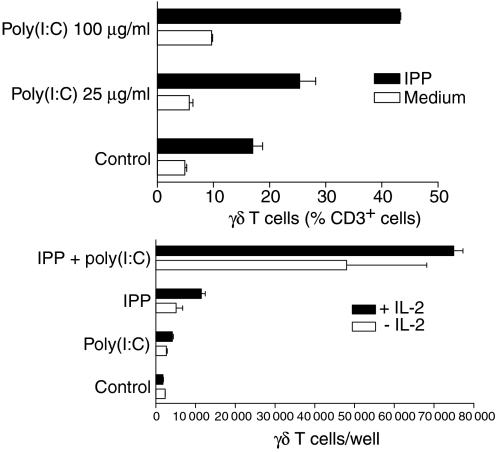
Next, we evaluated the effect of poly(I:C) on γδ T-cell expansion in PBMC cultures. Phosphoantigen-activated γδ T cells proliferate in the presence of exogenous growth factors, such as IL-2 and IL-15. Therefore, we analysed the percentage of γδ T cells in PBMC after 7 days of culture in the presence of a specific antigen (IPP 2·5 µg/ml) and low-dose IL-2 (10 U/ml). The results show that poly(I:C) enhanced the proliferation of γδ T cells in a dose-dependent manner, although poly(I:C) alone did not significantly alter the percentage of γδ T cells in PBMC cultures (Fig. 3a). This costimulatory effect of poly(I:C) was even more impressive when absolute γδ T-cell numbers were measured. As shown in Fig. 3(b), a combination of poly(I:C) and IPP induced a vigorous proliferative response of γδ T cells in the presence or absence of exogenous IL-2. This expansion of γδ T cells was clearly superior compared with the expansion induced by IPP and low-dose IL-2. In line with the results obtained by analysing the relative γδ T-cell fractions, γδ T cells did not significantly proliferate in the presence of poly(I:C) alone. These results demonstrate that poly(I:C) can provide potent costimulatory signals for antigen-mediated γδ T-cell proliferation.
Figure 3.
Enhancement of the γδ T-cell proliferative response by polyinosinic-polycytidylic acid [poly(I:C)]. (a) Peripheral blood mononuclear cells (PBMC) were stimulated with different concentrations of poly(I:C) in the presence (black bars) or absence (white bars) of isopentenylpyrophosphate (IPP) (2·5 µg/ml). Low doses of interleukin-2 (IL-2) (10 U/ml) were present in all cultures. After 7 days of culture, the percentage of γδ T cells was determined by two-colour fluorescence-activated cell sorter (FACS) analysis using anti-CD3 and anti-γδ T-cell receptor monoclonal antibody. Results represent mean values ± standard deviation (SD) of three separate experiments. (b) PBMC were incubated with poly(I:C) (100 µg/ml), IPP (2·5 µg/ml), or both, in the presence (closed bars) or absence (open bars) of low doses of IL-2 (10 U/ml). Absolute numbers of γδ T cells were determined on day 7 by counting the number of viable cells per well and measuring the percentage of γδ T cells by FACS analysis. The results are expressed as mean values ± SD of triplicate cultures of one representative donor. Similar results were obtained with three other donors.
Effect of poly(I:C) on γδ T-cell clones and purified γδ T cells
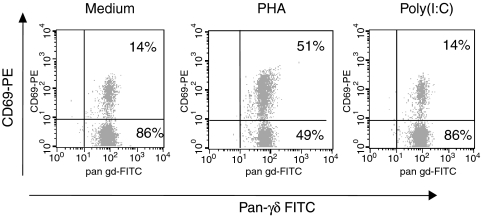
To examine the potential direct effects of poly(I:C) on γδ T cells, γδ T-cell clones (two Vγ9δ2+ clones and one Vδ1+ clone) were selected and cultured for 24 hr in medium, without mitogens or IL-2, before analysis. The expression of CD69 on these clones was measured before and after 24 hr of culture in the presence of PHA (1 µg/ml), poly(I:C) (100 µg/ml), or medium. While PHA up-regulated the activation marker on all tested clones, poly(I:C) had no influence on CD69 expression on γδ T-cell clones in comparison with the medium-only control (Fig. 4). Similar results were obtained when purified γδ T cells (> 98% purity after a positive selection procedure) were used instead of Vγ9Vδ2 T-cell clones (data not shown). In addition, we were unable to detect cell-surface or intracellular TLR3 expression in γδ T cells by flow cytometry; and were also unable to detect TLR3 expression in cell lysates of highly purified γδ T cells, as investigated by Western blot using a mAb against TLR3 (data not shown).
Figure 4.
Polyinosinic-polycytidylic acid [poly(I:C)] fails to activate γδ T-cell clones. The Vγ9Vδ2+γδ T-cell clone, K31, was cultured with phytohaemagglutinin (PHA) (1 µg/ml), poly(I:C) (100 µg/ml), or medium alone (i.e. without the addition of exogenous cytokines). After 24 hr, the expression of CD69 was determined by two-colour fluorescence-activated cell sorter (FACS) analysis. This analysis is representative for one γδ T-cell clone. Similar results were obtained with two additional Vγ9Vδ2+γδ T-cell clones. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Cytokine-mediated activation of γδ T cells
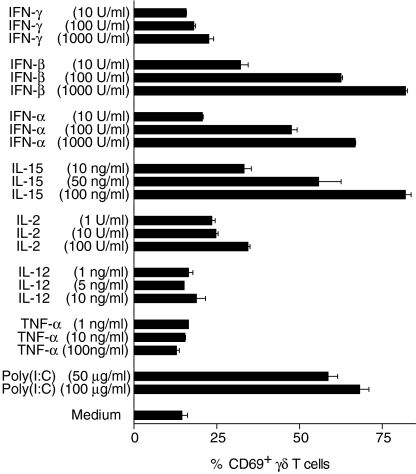
As poly(I:C) is a strong inducer of pro-inflammatory cytokines (especially type I IFNs and IL-12), we investigated whether poly(I:C)-induced partial activation of γδ T cells can be mimicked by recombinant cytokines. Therefore, PBMC were incubated with various concentrations of the following recombinant cytokines: IFN-α, IFN-β, IFN-γ, IL-12, TNF-α, IL-2 and IL-15. After 24 hr, the up-regulation of CD69 on γδ T cells was analysed. As shown in Fig. 5, treatment of PBMC with type I IFNs (IFN-α, IFN-β) strongly up-regulated CD69 expression on γδ T cells in a dose-dependent manner, comparable to that seen in poly(I:C)-treated PBMC cultures. IL-15 and, to a lesser extent, IL-2, also induced a dose-dependent activation of γδ T cells, as assessed by CD69 up-regulation. In contrast, TNF-α, IL-12 or IFN-γ did not induce a significant increase of CD69 expression on γδ T cells at any dose tested. In accordance with the results obtained using poly(I:C), type I IFNs and IL-15 did not induce significant up-regulation of other activation markers, such as CD25 or HLA-DR (data not shown). Therefore, type I IFNs and IL-15 are candidate cytokines for mediating non-specific γδ T-cell activation by poly(I:C).
Figure 5.
The effect of different recombinant cytokines on the activation of γδ T cells. The percentage of CD69+γδ T cells after 24 hr of culture of primary peripheral blood mononuclear cells (PBMC) are shown in the presence of different concentrations of various cytokines [interferon (IFN)-γ, -β, -α; interleukin (IL)-15, -2, -12; and tumour necrosis factor-α (TNF-α)] and polyinosinic-polycytidylic acid [poly(I:C)] in comparison to a medium-only control. Results are shown as mean values ± SD of triplicate cultures of one representative donor. Similar results were obtained with three other donors.
CD11c+ immature DC are responsible for poly(I:C)-mediated activation of γδ T cells
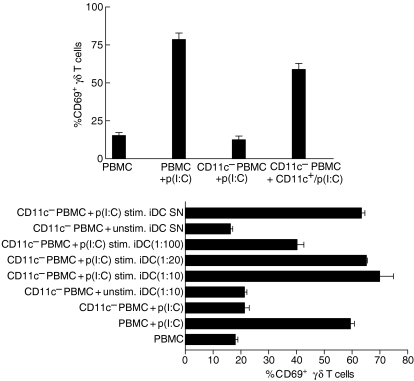
As CD11c+ DC express TLR3 as a known ligand for poly(I:C), CD11c+ cells were depleted from PBMC by MACS. After this depletion procedure, PBMC (the CD11c– fraction) failed to up-regulate CD69 on γδ T cells in the presence of poly(I:C) (Fig. 6a). The activation of γδ T cells within this CD11c– fraction could be restored by the addition of poly(I:C)-stimulated CD11c+ cells. From these results and data obtained with purified γδ T cells/γδ T-cell clones, we conclude that poly(I:C) activates γδ T cells indirectly via cells within the CD11c+ fraction of PBMC. To test whether CD11c+ DC can directly mediate γδ T-cell stimulation by poly(I:C), we analysed the effects of monocyte-derived immature CD11c+ DC (iDC) on γδ T cells. As shown in Fig. 6(b), the treatment of CD11c+ iDC with poly(I:C) induced CD69 up-regulation on γδ T cells, while unstimulated CD11c+ iDC failed to activate γδ T cells. Interestingly, low numbers of poly(I:C)-stimulated CD11c+ iDC within PBMC cultures are sufficient to mediate γδ T-cell activation, as significant up-regulation of CD69 on γδ T cells was observed until a PBMC/DC ratio of 1 : 100 was reached (Fig. 6b). In addition, the supernatant of poly(I:C)-stimulated CD11c+ iDC was as effective as CD11c+ iDC for stimulating γδ T cells, indicating that soluble factors, e.g. cytokines secreted by CD11c+ iDC, are responsible for the activation of γδ T cells by poly(I:C) (Fig. 6b). The separation of CD11c– PBMC from CD11c+ PBMC or CD11c+ iDC by a transwell system, confirmed that soluble factors contribute to the poly(I:C)-mediated stimulation of γδ T cells via CD11c+ iDC (data not shown).
Figure 6.
Soluble factors from CD11c+ dendritic cells (DC) are responsible for the polyinosinic-polycytidylic acid [poly(I:C)]-induced activation of γδ T cells. (a) Peripheral blood mononuclear cells (PBMC) were depleted from CD11c+ cells by magnetic-activated cell sorting (MACS). The negative selected cell population contained < 0·1% CD11c-expressing cells. Expression of CD69 on γδ T cells within undepleted PBMC (PBMC) or CD11c+-depleted (CD11c–) PBMC was determined after 24 hr of stimulation with 50 µg/ml poly(I:C) [p(I:C)] by two-colour fluorescence-activated cell sorter (FACS) analysis. The positive selected cell population (CD11c+) was incubated with 50 µg/ml poly(I:C) for 12 hr [CD11c+/p(I:C)], extensively washed and then incubated with CD11c– PBMC, for an additional 24 hr, at a ratio of 1 : 1. The results are shown as mean ± standard deviation (SD) of triplicate cultures from one representative donor. (b) Immature DC (iDC) were incubated with medium only (unstim. iDC) or with 50 µg/ml poly(I:C) [p(I:C) stim. iDC] for 12 hr. Afterwards, the supernatant (SN) from these iDC or extensively washed iDC were incubated with CD11c− PBMC, at the indicated ratios, for 24 hr, and expression of CD69 on γδ T cells was determined by two-colour FACS analysis. Results are shown as mean ± SD of triplicate cultures from one representative donor. Similar results were observed in two additional donors. Isopentenylpyrophosphate (IPP), phytohaemagglutinin (PHA) and phorbol 12-myristate 13-acetate (PMA).
Type I IFNs contribute to the poly(I:C)-mediated activation of γδ T cells
To investigate, in greater detail, the soluble factors responsible for the poly(I:C)-induced activation of γδ T cells, we used neutralizing antibodies against different cytokines and assessed their capacity to affect the poly(I:C)-mediated up-regulation of CD69 in PBMC cultures. Inhibition of type I IFNs (IFN-α, IFN-β) and IL-15 was reasonable, because both cytokines were shown to activate γδ T cells similarly to poly(I:C). Table 2 shows that the addition of a neutralizing Ab against type I IFN receptor markedly reduced the CD69 up-regulation of γδ T cells by poly(I:C). In contrast, the anti-IL-15 Ab had no influence on the poly(I:C)-mediated activation of γδ T cells. An activating effect of the neutralizing Abs used was excluded by culturing PBMC with the neutralizing Ab alone or with an irrelevant control Ab in the absence of poly(I:C). In addition, neutralizing antibodies against IL-2, IL-12, IFN-γ and TNF-α, were also not able to inhibit the poly(I:C)-mediated activation of γδ T cells in PBMC cultures (data not shown). Thus, poly(I:C)-induced IFN-αβ, produced by CD11c+ DC, is responsible for the activation of γδ T cells by poly(I:C).
Table 2.
Effect of neutralizing anti-type I interferon (IFN)-receptor and anti-interleukin-15 (anti-IL-15) immunoglobulin on the polyinosinic-polycytidylic acid [poly(I:C)]-induced activation of γδ T cells
| Medium | Anti-type I IFN-R | Anti-IL-15 | |
|---|---|---|---|
| Medium | 11·9 ± 0·9 | 9·5 ± 1·1 | 12·3 ± 0·4 |
| poly(I:C) | 52·1 ± 9·9 | 18·8 ± 2·6 | 59·0 ± 2·4 |
| IFN-α | 58·8 ± 1·9 | 27·9 ± 2·2 | – |
| IFN-β | 40·9 ± 2·4 | 19·7 ± 5·8 | – |
| IL-15 | 71·9 ± 2·7 | 18·3 ± 0·3 |
Primary peripheral blood mononuclear cells (PBMC) were cultured in medium alone, or in medium containing poly(I:C) (50 µg/ml) or the indicated cytokines (100 U/ml IFN-α, 100 U/ml IFN-β, 50 ng/ml IL-15), in the presence or absence of the following neutralizing antibodies: anti-type I IFN-receptor (anti IFN-α receptor chain 2; 5 µg/ml) or anti-IL-15 (5 µg/ml). After 24 hr of culture, the expression of CD69 on γδ T cells was assessed by two-colour fluorescence-activated cell sorter (FACS) analysis. Results represent the mean values ± standard deviation (SD), obtained in three separate experiments.
Discussion
Several previous studies have already shown that poly(I:C) has pleiotropic immunostimulatory effects on various types of immune cells.20–22 One of the main effects of viral dsRNA [mimicked by poly(I:C)] has been thought to be the induction of type I IFNs, which play an essential role in innate antiviral immunity. However, until recently it was controversial as to which immune cells can directly respond to poly(I:C). Receptors of the TLR family have been recently identified to recognize pathogen-associated molecular patterns (PAMPs), such as dsRNA.13 The expression of TLR3, which recognizes dsRNA and its synthetic mimetic poly(I:C), has been described to be restricted to DC subsets, fibroblasts and intestinal epithelial cells.14,16,17 Therefore, CD11c+ DC were initially reported to be the only cell population within PBMC to recognize poly(I:C).16,18 However, a recently published study demonstrates that human NK cells, which are known to play an important role in the first line of defence against viral infections, also express TLR3 and thus can directly recognize the TLR3 ligand, poly(I:C).23 We therefore investigated, in this study, whether human γδ T cells are also a target of poly(I:C)-mediated immunostimulatory effects.
We demonstrate here, for the first time, that poly(I:C) induces the activation of peripheral blood γδ T cells and has remarkable costimulatory effects on the γδ T-cell proliferative response in the presence of additional signals (such as phosphoantigens). However, several lines of evidence indicate that γδ T cells do not directly recognize poly(I:C). First, highly purified γδ T cells or γδ T-cell clones failed to up-regulate CD69 in response to poly(I:C). Second, TLR3 protein expression, permitting direct detection of poly(I:C), was absent in purified γδ T cells. Third, a similar pattern of activation (up-regulation of CD69, but not of CD25 or HLA-DR) was observed when γδ T cells were stimulated with recombinant type I IFNs (IFN-α and IFN-β), which are known to be secreted by CD11c+ DC in response to dsRNA. Fourth, depletion of IFN-producing CD11c+ cells from PBMC, or blocking the IFN type I receptor, abolished the poly(I:C)-induced activation of γδ T cells. In agreement with other studies we observed that poly(I:C) also induces activation of NK cells, but not of αβ T cells. These findings suggest that although γδ T cells and NK cells play an important part in mounting early effective antiviral responses, activation by viral dsRNA is differentially regulated in these cells. While human NK cells can directly recognize viral dsRNA via TLR3 receptor expression,23γδ T-cell activation by these immunostimulatory RNA compounds is indirectly mediated via type I IFNs from TLR3-expressing CD11c+ DC. This is consistent with previous studies demonstrating that TLR3 mRNA was not detectable at significant levels in purified T cells, as assessed by quantitative reverse transcription–polymerase chain reaction (RT–PCR).23,24 In addition, the Toll/IL-1R (TIR) containing adapter molecule 1, a recently described adaptor molecule that preferentially mediates TLR3 signalling, has been found exclusively in immature DC, macrophages and NK cells,25 supporting our findings that γδ T cells cannot directly recognize poly(I:C) via TLR3. In contrast to γδ T cells, poly(I:C) alone has been shown to induce effector function (secretion of pro-inflammatory cytokines, induction of cytotoxicity) in NK cells,23 while it induces only a partial activation status in γδ T cells (CD69 up-regulation without up-regulation of CD25 or HLA-DR and no IFN-γ production). However, our data demonstrate that poly(I:C) has remarkable costimulatory effects on the γδ T-cell proliferative response in the presence of additional signals for the γδ TCR. Although we did not analyse the effect of type I IFNs on the γδ T-cell proliferative response in detail, type I IFNs, which mediate the poly(I:C) effects on γδ T cells, have been proposed to promote and maintain antigen-specific T-cell responses, especially of memory-type cytotoxic murine αβ T cells, and to support proliferation of these cells in vivo, which is probably mediated by type I IFN-induced IL-15 production in bystander cells.26,27 Therefore, poly(I:C) may be useful for enhancing phosphoantigen-specific γδ T-cell responses, which are currently under development for cancer immunotherapy.28
Our results confirm and extend previous studies showing that γδ T cells can be activated by various cytokines (IL-2, IL-15, TNF-α, IL-12, IFN-α and IFN-β) in the absence of specific antigen stimulation.29–33 In contrast to previously reported data, we did not observe any up-regulation of CD69 on γδ T cells in human PBMC cultures by exogenous tumour necrosis factor-α (TNF-α) or IL-12. An obvious explanation for this discrepancy could be the different culture conditions or cytokine concentrations, as Lahn et al. used FCS instead of human serum to mediate γδ T-cell activation by TNF-α, and Ueta et al. used higher concentrations of IL-12 (300 ng/ml) to induce γδ T-cell stimulatory effects.31,32 Hence, by using FCS-containing serum we were able to confirm the activating effect of TNF-α on γδ T cells (data not shown).
Another class of immunostimulatory nucleic acids, bacterial DNA containing unmethylated CpG motifs (CpG ODN), has been shown to activate effectors of the innate immunity, including NK cells and γδ T cells.33 Similarly to poly(I:C), distinct CpG ODN induce partial activation of γδ T cells, which is also indirectly mediated by type I IFNs.33 CpG ODN-induced production of type I IFNs is mediated by a different subset of human DCs (plasmacyoid DC), which express TLR9 as a receptor for CpG ODN.18,34 In contrast to poly(I:C), CpG ODNs are capable of inducing IFN-γ in activated γδ T cells.33 This finding suggests that the mechanism of γδ T-cell activation by poly(I:C) and CpG ODN is different, although type I IFNs play an important role in γδ T-cell stimulation by both compounds. As the primary target of immunostimulatory nucleic acids are different DC subsets (CD11c+ iDC versus plamacytoid DC), the amount and type of cytokines produced by these DC subsets in response to poly(I:C) and CpG ODN, respectively, might explain the different quality of γδ T-cell activation.
Although several studies suggest an important role of γδ T cells in viral infections, the identity of specific viral antigens recognized by γδ T cells remains unknown. So far, a few naturally occurring Vγ9Vδ2 T-cell ligands have been isolated from different bacteria (Mycobacteria spp, Escherichia coli) and identified as structurally related phosphoantigens which have been linked to the non-classical mevalonate pathway of isoprenoid synthesis found in bacteria, algae and plants (Rohmer pathway).35–38 This supports the concept that γδ T cells discriminate between self and non-self through the recognition of specific biosynthetic routes. However, this metabolic pathway has not been demonstrated in viruses and there is no evidence, to date, for the presence of phosphoantigens in viruses. Our data suggest that the documented expansion of γδ T cells during several virus infections in vivo and in vitro might result, in part, from a bystander effect mediated through PAMPs such as dsRNS. Thus, our data indicate a potent adjuvant effect of dsRNA for γδ T-cell activation and proliferation during viral infections. However, the recognition of a specific antigen/cellular ligand seems to be crucial, because the remarkable enhancement of γδ T-cell proliferation by poly(I:C) in our study was dependent on the presence of phosphoantigens (e.g. IPP), and the recognition of virus-infected cells by γδ T cells has been shown to be TCR-γδ-mediated in other studies.10 As γδ T cells exhibit a significant crossreactivity between different viruses, their antiviral activity is probably not directed against a specific viral antigen, but rather against a putative cellular (self) ligand, induced or modified by viral infection.10,39
In conclusion, the ability of PAMPs, such as dsRNA or CpG OGN, to induce bystander activation of γδ T cells and function as a potent costimulatory proliferation signal, provides further evidence that human γδ T cells can collaborate with the innate immune system during the primary immune response to infectious agents.
Acknowledgments
This work was supported by grant 10-1897-Ku2, from Dr Mildred Scheel Stiftung für Krebsforschung and grant 01 KS 9603, from the Interdisziplinaeres Zentrum für Klinische Forschung, University of Würzburg. We thank Kerstin Otto and Juergen Becker for assistance in generating DCs, Doris Kraemer for assistance in Western blot analysis and Sibylle Schneider-Schaulies for helpful discussion.
References
- 1.Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 2.Kunzmann V, Bauer E, Feurle J, et al. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–92. [PubMed] [Google Scholar]
- 3.Barnes PF, Abrams JS, Lu S, et al. Patterns of cytokine production by mycobacterium-reactive human T-cell clones. Infect Immun. 1993;61:197–203. doi: 10.1128/iai.61.1.197-203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cipriani B, Borsellino G, Poccia F, et al. Activation of C-C beta-chemokines in human peripheral blood γδ T cells by isopentenyl pyrophosphate and regulation by cytokines. Blood. 2000;95:39–47. [PubMed] [Google Scholar]
- 5.De Libero G. Sentinel function of broadly reactive human γδ T cells. Immunol Today. 1997;18:22–6. doi: 10.1016/s0167-5699(97)80010-2. [DOI] [PubMed] [Google Scholar]
- 6.De Paoli P, Gennari D, Martelli P, et al. Gamma delta T cell receptor-bearing lymphocytes during Epstein–Barr virus infection. J Infect Dis. 1990;161:1013–6. doi: 10.1093/infdis/161.5.1013. [DOI] [PubMed] [Google Scholar]
- 7.Fisch P, Malkovsky M, Kovats S, et al. Recognition by human Vγ9/Vδ2 T cells of a GroEL homolog on Daudi Burkitt's lymphoma cells. Science. 1990;250:1269–73. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- 8.Sciammas R, Kodukula P, Tang Q, et al. T cell receptor-γ/δ cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J Exp Med. 1997;185:1969–75. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace M, Malkovsky M, Carding SR. γ/δ T-lymphocytes in viral infections. J Leukoc Biol. 1995;58:277–83. doi: 10.1002/jlb.58.3.277. [DOI] [PubMed] [Google Scholar]
- 10.Bukowski JF, Morita CT, Brenner MB. Recognition and destruction of virus-infected cells by human gamma delta CTL. J Immunol. 1994;153:5133–40. [PubMed] [Google Scholar]
- 11.Levy HB. Induction of interferon in vivo and in vitro by polynucleotides and derivatives, and preparation of derivatives. Methods Enzymol. 1981;78:242–51. [PubMed] [Google Scholar]
- 12.Meurs E, Chong K, Galabru J, et al. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–90. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- 13.Alexopoulou L, Holt AC, Medzhitov R, et al. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto M, Kikkawa S, Kohase M, et al. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem Biophys Res Commun. 2002;293:1364–9. doi: 10.1016/S0006-291X(02)00380-7. [DOI] [PubMed] [Google Scholar]
- 15.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 16.Muzio M, Bosisio D, Polentarutti N, et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 17.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadowaki N, Antonenko S, Liu YJ. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c– type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type I IFN. J Immunol. 2001;166:2291–5. doi: 10.4049/jimmunol.166.4.2291. [DOI] [PubMed] [Google Scholar]
- 19.Thurner B, Roder C, Dieckmann D, et al. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J Immunol Methods. 1999;223:1–15. doi: 10.1016/s0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- 20.Haines DS, Strauss KI, Gillespie DH. Cellular response to double-stranded RNA. J Cell Biochem. 1991;46:9–20. doi: 10.1002/jcb.240460104. [DOI] [PubMed] [Google Scholar]
- 21.Manetti R, Annunziato F, Tomasevic L, et al. Polyinosinic acid: polycytidylic acid promotes T helper type 1-specific immune responses by stimulating macrophage production of interferon-α and interleukin-12. Eur J Immunol. 1995;25:2656–60. doi: 10.1002/eji.1830250938. [DOI] [PubMed] [Google Scholar]
- 22.Cella M, Salio M, Sakakibara Y, et al. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med. 1999;189:821–9. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt KN, Leung B, Kwong M, et al. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J Immunol. 2004;172:138–43. doi: 10.4049/jimmunol.172.1.138. [DOI] [PubMed] [Google Scholar]
- 24.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 25.Oshiumi H, Matsumoto M, Funami K, et al. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–7. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 26.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–50. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Sun S, Hwang I, et al. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–9. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 28.Wilhelm M, Kunzmann V, Eckstein S, et al. γδ T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–6. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 29.Kjeldsen-Kragh J, Quayle AJ, Skalhegg BS, et al. Selective activation of resting human γδ T lymphocytes by interleukin-2. Eur J Immunol. 1993;23:2092–9. doi: 10.1002/eji.1830230908. [DOI] [PubMed] [Google Scholar]
- 30.Garcia VE, Jullien D, Song M, et al. IL-15 enhances the response of human γδ T cells to nonpeptide microbial antigens. J Immunol. 1998;160:4322–9. [PubMed] [Google Scholar]
- 31.Lahn M, Kalataradi H, Mittelstadt P, et al. Early preferential stimulation of γδ T cells by TNF-α. J Immunol. 1998;160:5221–30. [PubMed] [Google Scholar]
- 32.Ueta C, Kawasumi H, Fujiwara H, et al. Interleukin-12 activates human γδ T cells: synergistic effect of tumor necrosis factor-α. Eur J Immunol. 1996;26:3066–73. doi: 10.1002/eji.1830261237. [DOI] [PubMed] [Google Scholar]
- 33.Rothenfusser S, Hornung V, Krug A, et al. Distinct CpG oligonucleotide sequences activate human γδ T cells via interferon-α/β. Eur J Immunol. 2001;31:3525–34. doi: 10.1002/1521-4141(200112)31:12<3525::aid-immu3525>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 34.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 35.Jomaa H, Feurle J, Luhs K, et al. Vγ9/Vδ2 T cell activation induced by bacterial low molecular mass compounds depends on the 1-deoxy-d-xylulose 5-phosphate pathway of isoprenoid biosynthesis. FEMS Immunol Med Microbiol. 1999;25:371–8. doi: 10.1111/j.1574-695X.1999.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 36.Feurle J, Espinosa E, Eckstein S, et al. Escherichia coli produces phosphoantigens activating human γδ T cells. J Biol Chem. 2002;277:148–54. doi: 10.1074/jbc.M106443200. [DOI] [PubMed] [Google Scholar]
- 37.Hintz M, Reichenberg A, Altincicek B, et al. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 2001;509:317–22. doi: 10.1016/s0014-5793(01)03191-x. [DOI] [PubMed] [Google Scholar]
- 38.Belmant C, Espinosa E, Poupot R, et al. 3-Formyl-1-butyl pyrophosphate. A novel mycobacterial metabolite activating human γδ T cells. J Biol Chem. 1999;274:32079–84. doi: 10.1074/jbc.274.45.32079. [DOI] [PubMed] [Google Scholar]
- 39.Maccario R, Revello MG, Comoli P, et al. HLA-unrestricted killing of HSV-1-infected mononuclear cells. Involvement of either γ/δ+ or α/β+ human cytotoxic T lymphocytes. J Immunol. 1993;150:1437–45. [PubMed] [Google Scholar]