Abstract
To evaluate the T-cell large-scale differential gene expression in systemic lupus erythematosus (SLE) patients presenting with glomerulonephritis we studied SLE patients before and after immunosuppressive treatment. Large-scale gene expression of peripheral blood mononuclear T cells was evaluated using cDNA microarray nylon membranes containing 5184 cDNAs. Data were analysed using the SAM and Cluster and Treeview software. When untreated patients were compared to healthy individuals, 38 genes, most of them located on chromosomes 1, 3, 6, 17 and 19, were repressed, and when untreated patients were compared to treated ones, 154 genes, located on chromosomes 1, 6, 7, 12 and 17, were induced. In terms of biological function of coded proteins, the differentially expressed genes were associated with apoptosis, cell cycle, chromosomal scaffold, cytokine/chemokine, DNA repair/replication, Golgi/mitochondrial proteins, mRNA processing, signalling molecules and tumour suppressors. Two autoantigen genes related to RNA splicing (small nuclear riboprotein 70 000 MW-U1 SNR, and splicing factor 3a, 60 000 MW), and the tetranectin-plasminogen-binding protein were repressed. The Fc fragment of immunoglobulin G low affinity IIb, apoptotic protease activating factor-1, two subunits of cytochrome c, caspase 8, complement C5a, HLA-DRA, HLA-DQB1, transforming growth factor-β receptor II, small nuclear ribonucleoprotein polypeptide N (Sm protein N) genes, heterogeneous nuclear riboprotein-C, and argininosuccinate lyase genes, among others, were induced. A total of 10 genes were repressed in untreated patients and induced in treated ones, among them tumour necrosis factor (ligand) superfamily member 9, tumour protein p53, mannosidase alpha class IA, and CD22. Although some of these differentially expressed genes are typically expressed in B cells, CD22 and CD32 have also been reported in T cells and may provide regulatory signals to B cells. Assessment of differential gene expression may provide hybridization signatures that may identify susceptibility, diagnostic and prognostic markers of SLE.
Keywords: T cells, lupus, systemic lupus erythematosus, microassay, genomics, proteomics
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by B-cell hyperactivity, autoantibody production, and immune complex deposition in vital organs.1 Complex interactions between environmental agents and disease susceptibility genes have been suggested as triggering factors. Although many of these genes have not yet been identified, it is currently hypothesized that the expression of such genes may predispose to aberrant cellular and humoral immune responses. Altered T-cell immune responses to autoantigens may result in autoantibody production, formation and deposition of immune complexes, producing chronic inflammation that damages many tissues, particularly the kidney parenchyma, and that may lead to end-stage renal disease.2
Renal involvement in SLE is characterized by infiltration of mononuclear cells, mainly T CD4+ cells polarized to the T helper 1 (Th1) cytokine pattern, and by the deposition of pathogenic anti-DNA autoantibodies and autoantigen complexes within the glomeruli.3–7 Lupus nephritis presents heterogeneous histological features that are classified according to the World Health Organization as mesangial (class II), focal proliferative (class III), diffuse proliferative (class IV) and membranous glomerulonephritis (class V).8,9
The understanding of the mechanisms responsible for the initiation and maintenance of autoantibody formation in SLE has been limited by the availability of assays that can detect one or a few of these abnormalities. Recently, new investigative tools including cDNA microarrays have become available, simultaneously detecting the expression of thousand of genes in a single experiment.10 The microarray technology has been successfully used for the identification of gene expression signatures characteristic of cancer,11 chronic inflammatory diseases,12 autoimmune disorders,13 and others. Such approach has led to the identification of candidate genes that might be useful for diagnosis, follow-up, treatment, disease stratification, and prognosis. SLE produces immune abnormalities in a wide variety of cell populations including B and T lymphocytes, monocytes, and natural killer cells.14 Thus, an intended goal in SLE research would be the evaluation of the gene expression profile associated with specific cell subpopulations. However, no studies have been conducted regarding the gene profile of the cells involved in the pathogenesis of SLE, and only few studies have examined the gene expression in total peripheral blood mononuclear cells.14,15 The objective of the present study was to assess the large-scale differential gene expression in T lymphocytes of untreated SLE patients presenting with a characteristic form of immunecomplex disease such as lupus nephritis using cDNA microarrays. Furthermore, the hybridization signature of T lymphocytes from SLE patients under immunosuppressive treatment with cyclophosphamide and corticosteroids was also assessed.
Materials and methods
Subjects
Six women with lupus nephritis (three presenting with focal proliferative and three with membranous glomerulonephritis) aged 30–45 years (median 33) followed at the Division of Clinical Immunology, University Hospital of the School of Medicine of Ribeirão Preto, University of São Paulo, Brazil, were studied. Three untreated patients were evaluated during the acute phase of the disease before the administration of specific therapy, and three patients were evaluated after the administration of one to five (median four) intravenous pulses of methylprednisolone plus cyclophosphamide. The diagnosis of SLE was made according to the criteria defined by the American College of Rheumatology.16 The diagnosis of lupus nephritis was based on clinical, and laboratory data and on biopsy analysis.10
Three healthy women aged 26–45 years (median 35) presenting no previous family history of autoimmune disorders were also studied as controls.
Informed consent was obtained from all individuals, and the local Ethics Committee approved the protocol of the study (Protocol # 7946/99).
T lymphocyte isolation
Total peripheral blood mononuclear cells were separated on a Ficoll-Hypaque density gradient (Sigma, Saint Louis, MO) and T cells were isolated using magnetic beads (DYNAL, Norway) after depletion of non-T cells, according to manufacturer instructions. The recovery of T cells was always higher than 97% as determined by flow cytometry (FACSVantage Device, Becton-Dickinson, Foster City, CA) analysis using an anti-CD3 fluorescein isothiocyanate labelled antibody (Becton Dickinson).
Total RNA extraction
Total RNA was extracted using the Trizol® reagent (Invitrogen, Carlsbad, CA) according to manufacturer instructions. The integrity of RNA samples was evaluated by denaturing agarose gel electrophoresis under standard conditions and by Northern-blot analysis using an oligonucleotide probe recognizing the 28S rRNA fraction.
cDNA microarray method
Commercially acquired membranes (GF211, GeneFilter Microarrays-Research Genetics, Huntsville, AL) containing an array of 5184 known cDNAs were used. Each spot of the microarray contained 0·5 ng cDNA immobilized onto a positively charged nylon membrane. cDNAs were approximately 1 kb in length and contained the entire 3′ untranslated region (UTR), and all sequences were from the IMAGE/LLNL consortium (http://www.image.llnl.gov/image).17
Microarray prehybridization and hybridization procedures were performed according to the instructions of Research Genetics, using complex 33P-labelled cDNA probes prepared from total RNA of T lymphocytes. The hybridization signals were captured by a phosphor imager apparatus (Cyclone model, Packard Instruments, Meriden, CT) and quantified using the OptiQuant® software (Packard Instruments) with local background subtraction, whose spots were matched with a template grid.
Data regarding gene nomenclature and function were obtained from Research Genetics (http://www.resgen.com), SOURCE18 and ELOGE (Ipsogen, http://www.ipsogen.fr).
Data analysis
Raw data obtained using the OptiQuant software were transferred to Excel® software (Microsoft, Redmond, WA), and normalized to minimize differences among hybridizations on the basis of the genomic DNA spotted on the microarrays. In addition to normalization, whenever necessary, data were also centralized by subtracting the expression value of each gene from the average expression of the whole array.19
To detect the differentially and significantly expressed genes, the Significance Analysis of Microarray (SAM) algorithm was used.20 To construct dendrograms and heat maps, hierarchical clustering of gene expression was performed using algorithms which compare means of different genes whose standard deviations do not overlap, using the TreeView (heat maps) and Cluster (dendrograms) software programs (http://www.rana.stanford.edu/clustering).21 The Pearson correlation coefficient was used to cluster similarity distances.
Results
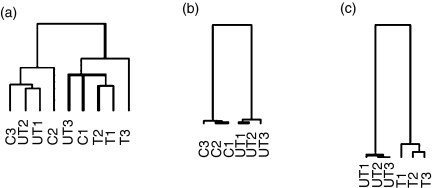
The heat map and dendrograms referring to the hybridization signatures of patients and normal individuals involving the 5184 genes whose expression levels were evaluated in this study can be observed on line. The hierarchical clustering using the whole set of genes was not able to discriminate groups of individuals, as shown in the upper part of the dendrogram; however, the whole set of genes was subgrouped into hundreds of clusters based on the similarity of the expression pattern among the different individuals (lateral dendrogram (http://www.rge.fmrp.usp.br/passos/sle/wholegeneset).
The comparisons of the gene expression between controls and untreated patients and between untreated and treated patients were performed using the SAM software. Untreated patients exhibited 38 genes whose expression was significantly decreased in relation to controls (repressed genes), and treated patients showed 154 genes whose expression was significantly increased compared to untreated patients (induced genes). The dendrograms referring to the whole set of genes, and those referring to the repressed and induced genes are shown in Fig. 1(a, b and c), respectively. The heat maps including the names of repressed or induced genes can be observed on line (http://www.rge.fmrp.usp.br/passos/sle/genelist), and an illustrative set of selected genes potentially related to the pathogenesis of SLE is shown in Table 1. When the significantly and differentially expressed genes were grouped using the Cluster and TreeView softwares, the three series of individuals were completely individualized (Fig. 1b,c).
Figure 1.
Dendrograms based exclusively on the differential gene expression profile of 5184 genes observed in patients and controls as analysed by the Cluster and Treeview softwares. Using the whole set of genes, hierarchical clustering did not discriminate between patients and healthy controls (dendrogram a). When differentially and significantly expressed genes, identified by the SAM program, were reclustered using the Cluster and Treeview softwares, the discrimination between each series of individuals was possible, permiting comparisons between untreated and healthy controls (dendrogram b), and comparisons between treated and untreated patients (dendrogram c).
Table 1.
Accession number, chromosome location and possible role in the pathogenesis of SLE of selected genes whose expression was repressed when untreated patients were compared to healthy individuals, and genes whose expression was induced when untreated patients were compared to treated ones
| (’000 MW) | Accession no. | Location | Associations with SLE pathogenesis | *Fold change | †q-value |
|---|---|---|---|---|---|
| Repressed genes | |||||
| Small nuclear ribonucleoprotein (70) | R02346 | 19q13.3 | SLE autoantigen | −7·812 | 23·91 |
| Splicing factor 3a, subunit 3 (60) | R43015 | 1p34.2 | SLE autoantigen | −2·153 | 23·91 |
| Nucleolar autoantigen (55) | W81191 | 17q21.2 | Autoantigen, interstitial cystitis | −3·175 | 23·91 |
| Tetranectin (plasminogen-binding protein) | W73889 | 3p21 | Plasminogen activation | −1·748 | 23·91 |
| TNF (ligand), member 9 | AA778663 | 19p13.3 | SLE nephritis development | −3·958 | 23·91 |
| CD22 antigen | N53534 | 19q13.1 | B-cell receptor regulation | −2·387 | 30·26 |
| Zinc finger protein 74 | AA629838 | 22q11.21 | Not yet associated to SLE | −3·149 | 23·91 |
| Phosphonoformate immuno-associated protein 5 | W80632 | 13 | Not yet associated to SLE | −1·912 | 30·26 |
| E1b (55) associated protein 5 | AA464198 | 19q13.13 | Not yet associated to SLE | −1·807 | 23·91 |
| Sema domain, Ig domain | AA454570 | 3p21.3 | Not yet associated to SLE | −8·387 | 23·91 |
| Induced genes | |||||
| Fc fragment of IgG, receptor for CD32 | R68106 | 1q23 | Antibody modulation | 7·876 | 38·31 |
| Apoptotic protease activating factor | N51014 | 12q21 | Apoptosis | 2·437 | 38·31 |
| Cytochrome-c oxidase subunity VIc | AA456931 | 8q22 | Apoptosis | 3·318 | 38·31 |
| Cytochrome-c oxidase subunity VIII | AA862813 | 11q13 | Apoptosis | 1·921 | 38·31 |
| Complement component 5 (C5) | N53664 | 9q32 | Complement system pathway | 2·591 | 38·31 |
| MHC, class II, DR α (HLA-DRA) | R47979 | 6p21.3 | Susceptibility to SLE | 148·387 | 38·31 |
| MHC, class II, DP β 1 (HLA-DPB1) | AA486532 | 6p21.3 | Susceptibility to SLE | 19·630 | 38·31 |
| TGF-β R II | AA487034 | 3p22 | Cell growth and differentiation | 62·562 | 38·31 |
| Thyroid autoantigen (70) (Ku antigen) | AA486207 | 22q13 | SLE autoantigen | 2·842 | 38·31 |
| Small nuclear ribonucleoprotein (SNRPN) | T54926 | 15q12 | SLE autoantigen | 3·369 | 38·31 |
| Heterogeneous nuclear RNP-C (HRNPC) | H05899 | 14q11.1 | mRNA processing and splicing | 1·523 | 38·31 |
| Splicing factor arg/ser rich 9 (SFRS9) | AA491213 | 12q24.31 | mRNA processing and splicing | 1·623 | 38·31 |
| Argininosuccinate lyase (ASL) | AA486741 | 7cen-q11.2 | NO production | 5·056 | 38·31 |
| Tumour protein p53 (TP53) | R39356 | 17p13.1 | Tumour suppressor | 4·676 | 38·31 |
| Mannosidase-α, class 1A, member 1 | T91261 | 6p22 | Mannose removal | 10·825 | 38·31 |
| TNF (ligand), member 9 | AA778663 | 19p13.3 | SLE nephritis development | 3·512 | 38·31 |
| CD22 antigen | N53534 | 19q13.1 | B-cell receptor regulation | 91·725 | 38·31 |
| Zinc finger protein 74 | AA629838 | 22q11.21 | Not yet associated to SLE | 3·872 | 38·31 |
| Phosphonoformate immuno-associated protein 5 | W80632 | 13 | Not yet associated to SLE | 2·878 | 38·31 |
| Elb (55) associated protein 5 | AA464198 | 19q13.13 | Not yet associated to SLE | 1·366 | 38·31 |
| Sema domain, Ig domain | AA454570 | 3p21.3 | Not yet associated to SLE | 8·764 | 38·31 |
Fold change is the variability in the gene expression between patient and normal individuals or treated and untreated patients (negative values correspond to repressed and positive values to induced genes).
q-value is a P-value adapted for multiple testing to measure the difference in the expression of each gene.
Ig, immunoglobulin; TNF, tumour necrosis factor.
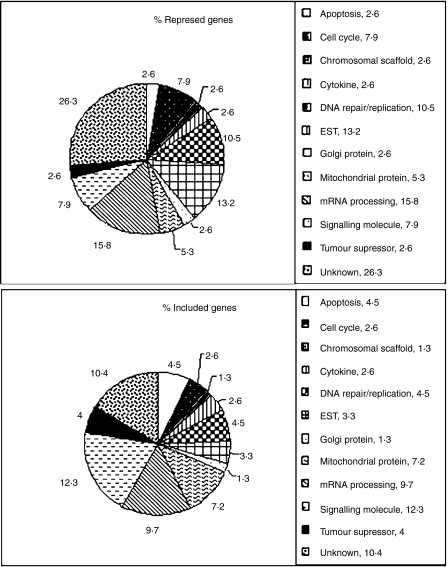
Most of the repressed genes were located on chromosome 1 (six genes 15·8%), followed by chromosomes 19 (five genes 13·2%), 3, 6 and 17 (three genes each of 7·8%), and 9, 12, 13, 14, 16, 20 and 22 (one gene each of 2·6%). The name and chromosomal location of four genes (10·5%) could not be assigned by the SOURCE18 databank. Most of the induced genes were located on chromosome 17 (12 genes 7·8%), followed by chromosomes 1 (11 genes 7,14%), 19 (nine genes 5·8%), 6 (eight genes 5·2%), 7 and 12 (seven genes each of 4·6%), 8, 11, 20, 21 and chromosome X (six genes each of 3·9%), 2, 9, 16 and 22 (five genes each of 3·24%), 15 and 10 (four genes each of 2,6%), 13 and 5 (three genes each of 2·0%), 3, 4 and 14 (two genes each of 1·3%). The name and chromosomal location of 19 genes (12·4%) could not be assigned using the SOURCE databank. Figure 2 illustrates the biological function of coded proteins of repressed and induced genes. A total of 10 genes were repressed in untreated patients and induced after treatment, located on chromosomes 3, 6, 13, 17, 19, 20 and 22 (Table 1).
Figure 2.
Biological function of differentially and significantly expressed genes. When untreated patients were compared to healthy controls, 38 genes were repressed and when untreated patients were compared to treated ones, 154 genes were induced.
Discussion
The speed, ease, and feasibility of simultaneously monitoring differential expression of thousands of genes with the cDNA microarray-based system are demonstrated here in the analysis of a complex disease such as SLE. The initial clustering of the whole set of genes of the present study was not able to distinguish SLE patients from controls; however, hundreds of gene clusters were observed. For example, tumour necrosis factor, splicing factors, alpha mannosidase, transcription factor related to RNA polymerase II and type XVI collagen genes presented closely similar expression and were grouped into one cluster, the major histocompatiblity complex DP alpha and DP beta genes into a second cluster, and RNP antigen, TNF receptor associated factor and apoptotic factor genes into a third cluster, and many other clusters. The SAM program permitted the identification of 38 repressed genes when patients were compared to controls, and 154 induced genes when patients were compared before and after treatment. Among these genes, we selected for discussion some that have been associated or might potentially be associated with the pathogenesis of the disease. In addition, with the use of SAM and Cluster softwares it was possible to distinguish patients from controls and untreated patients from treated ones.
Most of the repressed genes were located on chromosomes 19, 1, 3 and 17. Interestingly, three autoantigen genes, the small nuclear ribonucleoprotein involved in maturation, synthesis and splicing of RNA,22 the splicing factor 3a and the nucleolar autoantigen (55 000 MW) similar to rat synaptonemal complex protein, were identified; however, only the first two autoantigens have been described in association with SLE. Although repressed in T lymphocytes, these autoantigens may be induced in other cell populations or tissue types. The tetranectin-plasminogen-binding protein gene is a regulator of plasminogen activation. Considering that SLE patients are prone to developing thrombosis in the acute phase of the disease,23,24 the repression of this gene may contribute to the development of thrombosis, a common finding in SLE patients.
Most of the induced genes were located on chromosomes 17, 1, 19 and 6. The Fc fragment of immunoglobulin G (IgG) low affinity IIb gene, associated with CD32 receptor-mediated phagocytosis of immunecomplexes, is involved in the modulation of antibody production by B cells.18 Although the CD32 gene is considered to encode a B-cell specific molecule, mRNA for CD32 has also been detected in the cytoplasm of T cells.25 The apoptotic protease activating factor (APAF-1) gene activates procaspase-9 to caspase-9, which initiates the death by neglect apoptosis involving the downstream activation of caspases 3, 6, and 7·26 In addition, mitochondrial cytochrome-c functions as a cofactor with APAF-1 to activate caspase 9·18 The induction of APAF-1 and two genes coding for two subunits of cytochrome-c (cytochrome-c oxidase subunits VIc 8q22-q23 and VIII 11q13), as observed in this study, supports the idea of increased lymphocyte apoptosis in SLE patients, as previously reported.27 On the other hand, caspase-8 may induce apoptosis by the Fas–FasL pathway or may also be activated in a caspase-9-dependent manner.28 The induction of the caspase-8 gene, as shown in the present study, also indicates a pro-apoptotic state in SLE patients. The complement component 5 gene is responsible for the production of C5, and the activation of this protein leads to the production of C5a and activation of the terminal pathway. Because increased expression of C5a receptor mRNA is described in SLE patients with nephritis,29 and since C5a induces the expression of CR1 and CR3 on polymorphonuclear cells,30 the increased expression of this gene, as described here, may contribute both to clearance and deposition of immune complexes in glomeruli. Two MHC genes were up-regulated: the major histocompatibility complex (MHC) human leucocyte antigen (HLA)-DRA gene, which codes for the nonpolymorphic alpha-chain of several DR molecules, and the MHC HLA-DPB1 gene, which codes for the polymorphic beta-chain of DP molecules.18 An increased expression of the DR alpha-chain suggests an intense production of different types of DR molecules, and the polymorphism of DPB1 genes has been associated with susceptibility to SLE.31 The protein coded by the transforming growth factor-β (TGF-β) receptor II (TGFBR2) gene and its receptors are expressed ubiquitously, acting as key regulators of cell growth and differentiation.32 Increased levels and increased expression of TGF-β and TGFBR2 mRNA have been described in SLE patients with nephritis.33,34 The thyroid autoantigen (Ku antigen) gene codes for a group of DNA-associated antigens identified as targets of autoantibody production in patients with SLE and related disorders.35 The small nuclear ribonucleoprotein polypeptide N (SNRPN) gene is involved in pre-mRNA processing, possibly tissue-specific alternative splicing events. SLE patients have autoantibodies directed against some of the SNRNP polypeptides, including Sm protein N.18 The heterogeneous nuclear ribonucleoprotein-C gene and the splicing factor-arginine/serine-rich 9 gene are involved in mRNA processing and splicing events.22 The argininosuccinate lyase gene codes the enzyme that recycles coproducts of the nitric oxide synthetase to produce nitric oxide (NO).36 Considering that SLE patients with nephritis present increased plasma levels of NO,37 the induction of this gene may provide an increased availability of l-arginine and l-citrulline to produce NO.
Among genes that were repressed in untreated patients and induced during treatment, the tumour protein p53 (TP53) gene is a nuclear protein that acts as a tumour suppressor in many tumour types, induces growth arrest or apoptosis, and regulates the cell cycle. Alterations of the TP53 gene occur not only as a consequence of somatic mutations in human malignancies, but also as germline mutations.18 Increased expression of the protein products and mRNA of p53 are described in lupus nephritis, indicating that proto-oncogenes may be associated with mesangial proliferation and matrix expansion, particularly in proliferative types of glomerulonephritis and in glomerular sclerosis.38 The Homo sapiens mannosidase, alpha, class 1A, member 1 gene codes for the enzyme that catalyses the removal of three distinct mannose residues from hybrid N-linked oligosaccharides, being essential for complex N-glycan branching in glycoproteins.18,39 The null allele for α-mannosidase in the mouse is associated with incomplete complex-type N-glycan production, elevated IgA, IgG and IgM and C3 deposition in the kidney glomeruli, and increased mononuclear cell infiltrate in kidney, liver and lung tissue. The altered N-glycosylation of glycoproteins has been reported to be the cause of systemic autoimmune disease with symptoms of lupus nephritis in the absence of the enzyme.39 Although no previous human studies are available regarding the role of α-mannosidase I in SLE, more than 20 mutations of the α-mannosidase genes have been described.40 Thus, it is possible that SLE patients may have particular alleles associated with lupus nephritis. The tumour necrosis factor (ligand) superfamily, member 9 gene, codes for a protein that has been associated with the development of nephritis in SLE patients.41 The CD22 antigen gene codes for a B-cell transmembrane glycoprotein that acts as a negative regulator of the B-cell antigen receptor signal transduction;42 however, his B-cell antigen has also been identified in normal and activated T cells.25 A repression of this gene in untreated SLE patients may be associated with facilitation of B-cell hyperactivity, and the induction of the gene in treated patients may be associated with a control of B-cell hyperactivity. In addition, the polymorphism of this gene has been suggested as a potential candidate for susceptibility to SLE or other autoimmune disorders.42
Although the population of T lymphocytes obtained from SLE patients of this study was highly purified, the overexpression of mRNAs that codify surface markers considered to be characteristic of B cells was a surprising finding. The expression of mRNA for MHC class II molecules, CD22 and CD32 antigens is reported on the surface and in the cytoplasm of normal and activated T cells, as detected by flow cytometry and immunoelectron microscopy analyses. These antigens are reported to be located within cytoplasmic vesicles or within the rough endoplasmic reticulum in these T cells. Following in vitro T-lymphocyte activation, a distinct intracytoplasm increased expression of these CD molecules was observed, which was not accompanied by concomitant increase on cell surface expression. The up-regulation of these B-cell CD antigens within the cytoplasm of T cells suggest that these antigens may be synthesized and released into the fluid phase as soluble immunoregulatory molecules.25 The increased expression of these CD markers as observed in treated SLE patients may be related either to B-cell activation as well as to B-cell regulation, as the binding to CD32 is reported to modulate B- and T-cell receptors.18 Therefore, the increased mRNA expression does not mean that all synthesized protein will be expressed on the cell surface. The findings of the present study indicate that much has to be learned about the role of these CD molecules in T-cell activation and in SLE pathogenesis.
Other genes repressed in untreated patients and induced in treated patients, which have not been evaluated as contributors to the pathogenesis of SLE, include two genes associated with RNA metabolism/processing (zinc finger protein 74 and Elb 55 000 MW associated protein 5), one gene associated with cell motility and adhesion (sema domain semaphorin family), and one gene with unknown function (phosphonoformate immuno-associated protein 5).18
Recent advances in functional genomics have made possible new approaches to the diagnosis and management of a wide range of autoimmune diseases. Microarray experiments produce profiles of gene expression that may identify susceptibility, pathogenic, diagnostic and prognostic markers, and may also reflect the drug response profile, which may help clinicians in monitoring disease activity. Finally, the evaluation of differential gene expression may provide clues to detect previously unrecognized genes associated with the disease.
Acknowledgments
This research was supported by the Brazilian agencies Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP Proc. # 99/12135–9) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo ao Ensino, Pesquisa e Assistência (FAEPA) do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto, USP.
References
- 1.Dixon FJ. The pathogenesis of glomerulonephritis. Am J Med. 1968;44:493–8. doi: 10.1016/0002-9343(68)90050-8. [DOI] [PubMed] [Google Scholar]
- 2.Kammer GM, Perl A, Richardson BC, Tsokos C. Abnormal T cell signal transduction in systemic lupus erythematosus. Arthrtis Rheum. 2003;46:1139–54. doi: 10.1002/art.10192. [DOI] [PubMed] [Google Scholar]
- 3.Datta SK, Patel H, Berry D. Induction of a cationic shift in IgG anti-DNA auto antibodies. Role of T helper cells with classical and novel phenotypes in three murine models of lupus nephritis. J Exp Med. 1987;165:1252–68. doi: 10.1084/jem.165.5.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shivakumar S, Tsokos GC, Datta SK. T-cell receptor α/β expressing double-negative (CD4-/CD8-) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA auto antibodies associated with lupus nephritis. J Immunol. 1989;143:103–12. [PubMed] [Google Scholar]
- 5.Suzuki N, Harada T, Mizushima Y, Sakane T. Possible pathogenic role of cationic anti-DNA auto antibodies in the development of nephritis in patients with systemic lupus erythematosus. J Immunol. 1993;151:1128–36. [PubMed] [Google Scholar]
- 6.Rajagopalan S, Zordan T, Tsokos GC, Datta SK. Pathogenic anti-DNA autoantibody-inducing T helper cell lines from patients with active lupus nephritis. isolation of CD4-8- T helper cell lines that express the γδ T-cell antigen receptor. Proc Natl Acad Sci USA. 1990;87:7020–4. doi: 10.1073/pnas.87.18.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai-Mehta A, Mao C, Rajagopalan S, Robinson T, Datta SK. Structure and specificity of T cell receptors expressed by potentially pathogenic anti-DNA autoantibody-inducing T cells in human lupus. J Clin Invest. 1995;95:531–41. doi: 10.1172/JCI117695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace DJ, Podell TE, Weiner JM, Cox MB, Klinenberg JR, Forouzesh S, Dubois EL. Lupus nephritis. Experience with 230 patients in private practice from. J Medical. 1950;72:209–20. doi: 10.1016/0002-9343(82)90812-9. To 1980 Am. [DOI] [PubMed] [Google Scholar]
- 9.Appel GB, Silva FG, Pirani CL, Meltzer JL, Estes D. Renal involvement in systemic lupus erythematosus (SLE). A study of 56 patients emphasizing histologic classification. Medicine (Baltimore) 1978;57:371–410. doi: 10.1097/00005792-197809000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Kurella M, Hsiao L, Yoshida T, Randall DJ, Chow G, Sarang SS, Jensen VR, Gullans RS. DNA microarray analysis of complex biologic process. J Natl Soc Nephrol. 2001;12:72–8. doi: 10.1681/ASN.V1251072. [DOI] [PubMed] [Google Scholar]
- 11.Sgroi DC, Teng S, Robinson G, Le Vangie R, Hudson JR, Elkahloun AG. In vivo gene expression profile analysis of human breast cancer progression. Cancer Res. 1999;59:5656–61. [PubMed] [Google Scholar]
- 12.Heller RA, Schena M, Chai A, Shalon D, Bedilion T, Gilmore J, Woolley DE, Davis RW. Discovery and analysis of inflammatory disease-related genes using cDNA microarrays. Proc Natl Acad Sci USA. 1997;94:2150–5. doi: 10.1073/pnas.94.6.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanders ED, Goulden MG, Kennedy TC, Kempsell KE. Analysis of immune system gene expression in small rheumatoid arthritis biopsies using a combination of subtractive hybridization and high-density cDNA arrays. J Immunol Meth. 2000;233:131–40. doi: 10.1016/s0022-1759(99)00126-x. [DOI] [PubMed] [Google Scholar]
- 14.Rus V, Atamas SP, Shustova V, Luzina IG, Selaru F, Magder LS, Via CS. Expression of cytokine- and chemokine-related genes in peripheral blood mononuclear cells from lupus patients by cDNA array. Clin Immunol. 2002;102:283–90. doi: 10.1006/clim.2001.5182. [DOI] [PubMed] [Google Scholar]
- 15.Alcorta D, Preston G, Munger W, Sulivan P, Yang JJ, Wawa I. Microarray studies of gene expression in circulating leukocytes in kidney diseases. Exp Nephrol. 2002;10:139–49. doi: 10.1159/000049909. [DOI] [PubMed] [Google Scholar]
- 16.Tan EM, Cohen AS, Ries JF, et al. The revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 17. Image Consortium (Integrated Molecular Analysis of Genomes and their Expression) ( http://www.image.llnl.gov/image) [DOI] [PubMed]
- 18. SOURCE (Stanford Online Universal Resource for Clones and ESTs) ( http://genome-www5.stanford.edu/cgi-bin/source/sourcesearch)
- 19.Nguyen C, Rocha D, Granjeaud S, Baldit M, Bernard K, Naquet P, Jordan BR. Differential gene expression in murine thymus assayed by quantitative hybridization of arrayed cDNA clones. Genomics. 1995;29:207–16. doi: 10.1006/geno.1995.1233. [DOI] [PubMed] [Google Scholar]
- 20.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays apllied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisen M, Spellman P, Brown P, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–8. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinol-Romo S, Swanson MS, Gall JG, Dreyfuss G. A novel heterogeneous nuclear RNP protein with a unique distribution on nascent transcripts. J Cell Biol. 1989;109:2575–87. doi: 10.1083/jcb.109.6.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiraz S, Ertenli I, Bnekli M, Haznedaroglu IC, Calguneri M, Celik I, Apras S, Kirazli S. Clinical significance of hemostatic markers and thrombomodulin in systemic lupus erythematosus: evidence for a prothrombotic state. Lupus. 1999;8:737–41. doi: 10.1191/096120399678840918. [DOI] [PubMed] [Google Scholar]
- 24.Petri M. Clinical and management aspects of the antiphospholipid antibody syndrome. In: Wallace JD, Hahn BH, editors. Dubois' Lupus Erythematosus. Baltimore: Williams & Wilkins; 1996. pp. 1067–96. [Google Scholar]
- 25.Sandilands GP, Perry M, Wooton M, Hair J, More IA. B-cell antigens with normal and activated human T cells. Immunol. 1999;96:424–33. doi: 10.1046/j.1365-2567.1999.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Molec Cell. 1998;1:949–57. doi: 10.1016/s1097-2765(00)80095-7. [DOI] [PubMed] [Google Scholar]
- 27.Silva LM, Garcia AB, Donadi EA. Increased lymphocyte death by neglect-apoptosis is associated with lymphopenia and auto antibodies in lupus patients presenting with neuropsychiatric manifestations. J Neurol. 2002;249:1048–54. doi: 10.1007/s00415-002-0781-6. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari D, Stepczynska A, Los M, Wesselborg S, Schulze-Osthoff Differential regulation and ATP requirement for caspase-8 and caspase-3 activation during CD95- and anticancer drug-induced apoptosis. J Exp Med. 1998;0(188):979–84. doi: 10.1084/jem.188.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abe K, Miyazaki M, Koji T, et al. Enhanced expression of conplement C5a receptor mRNA in human diseased kidney assessed by in situ hybridization. Kidney Int. 2001;60:137–46. doi: 10.1046/j.1523-1755.2001.00780.x. [DOI] [PubMed] [Google Scholar]
- 30.Doi T, Takemura H, Onodera H, et al. Small increase of CR1 and CR3 by C5a-receptor on polymorphonuclear leukocytes in systemic lupus erythematosus. Arerugi. 1997;46:1108–13. [PubMed] [Google Scholar]
- 31.Yao Z, Hartung K, Deicher HD, Brunnler G. DNA typing for HLA- DPB-alleles in German patients with systemic lupus erythematosus using the polymerase chain reaction and DIG-ddUTP-labelled oligonucleotide probes. Eur J Immunogenet. 1996;20:259–66. doi: 10.1111/j.1744-313x.1993.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 32.Koli K, Keski-Oja J. Transforming growth factor-beta system and its regulation by members of the steroid-thyroid hormone superfamily. Adv Cancer Res. 1996;70:63–94. doi: 10.1016/s0065-230x(08)60872-6. [DOI] [PubMed] [Google Scholar]
- 33.Kuhara T, Kagami S, Kuroda Y. Expression of beta 1-integrins on activated mesangial cells in human glomerulonephritis. J Am Soc Nephrol. 1997;8:1679–87. doi: 10.1681/ASN.V8111679. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto T, Watanabe T, Ikegaya N, Fugigaki Y, Matsui K, Masaoka H, Nagasi M, Hishida A. Expression of types I, II, and III TGF–beta receptors in human glomerulonephritis. J Am Soc Nephrol. 1998. pp. 2253–61. 9. [DOI] [PubMed]
- 35.Reeves WH. Antibodies to the p70/p80 (Ku) antigens in systemic lupus erythematosus. Rheum Dis Clin North Am. 1992;18:391–414. [PubMed] [Google Scholar]
- 36.Wiesinger H. Arginine metabolism and the synthesis of nitric oxide in the nervous system. Prog Neurobiol. 2001;64:365–91. doi: 10.1016/s0301-0082(00)00056-3. [DOI] [PubMed] [Google Scholar]
- 37.Ho CY, Wong CK, Li EK, Tam LS, Lam CW. Elevated plasma concentration of nitric oxide, soluble thrombomodulin and soluble vascular cell adhesion molecule-1 in patients with systemic lupus erythematosus. Rheumatol. 2003;42:117–22. doi: 10.1093/rheumatology/keg045. [DOI] [PubMed] [Google Scholar]
- 38.Takemura T, Okada M, Akano N, Murakami K, Hino S, Yagi K, Takekoshi Y, Yoshioka K. Proto-oncogene expression in human glomerular diseases. J Pathol. 1996;178:343–51. doi: 10.1002/(SICI)1096-9896(199603)178:3<343::AID-PATH481>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 39.Chui D, Sellakumar G, Green R, et al. Genetic remodeling of protein glycosylation in vivo induces autoimmune disease. Proc Natl Acad Sci USA. 2001;98:1142–7. doi: 10.1073/pnas.98.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berg T, Riise HM, Hansen GM, Malm D. Tranebjaergl, Tollersrud OK, Nilssen O. Spectrum of mutations in alpha-mannosidosis. Am J Hum Genet. 1999;64:77–88. doi: 10.1086/302183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim T, Kim H, Lee S, Cho C, Park S, Choi H, Han H, Kim D. Systemic lupus erythematosus with nephritis is strongly associated with the TNFB*2 homozygote in the Korean population. Hum Immunol. 1996;46:10–7. doi: 10.1016/0198-8859(95)00170-0. [DOI] [PubMed] [Google Scholar]
- 42.Hatta Y, Tsuchiya N, Matsushita M, Shiota M, Hagiwara K, Tokunaga K. Identification of the gene variations in human CD22. Immunogenetics. 1999;49:280–6. doi: 10.1007/s002510050494. [DOI] [PubMed] [Google Scholar]