Abstract
The age-dependent variation in the proportion and number of lymphocyte subsets was examined at various extrathymic sites, including the liver, small intestine, colon and appendix in mice. In comparison with young mice (4 weeks of age), the number of total lymphocytes yielded by all tested organs was greater in adult (9 weeks) and old (40 weeks) mice. The major lymphocyte subset that expanded with age was interleukin-2 receptor (IL-2R) β+ CD3int cells (50% of them expressed NK1.1) in the liver, whereas it was CD3+ IL-2Rβ− NK1.1− cells at all intraepithelial sites in the intestine. Although NK1.1+ CD3+ cells were present at intraepithelial sites in the intestine, the proportion of this subset was rather low. The ratio of CD4 to CD8 tended to decrease among natural killer T (NKT) cells and T cells at all intraepithelial sites in the intestine with age. A unique population of double-positive CD4+ CD8+ cells in the small intestine increased in old mice. B220+ T cells were found mainly in the appendix and colon, and the proportion of these T cells decreased in old mice. Conventional NKT cells were very few in Jα281−/− and CD1d−/− mice in the liver, while NKT cells which existed in the appendix remained unchanged even in these mice. This was because unconventional CD8+ NKT cells were present in the intestine. The present results suggest that despite the fact that both the liver and intraepithelial sites in the intestine carry many extrathymic T cells, the distribution of lymphocyte subsets and their age-associated variation are site-specific.
Keywords: ageing, DP cells, extrathymic T cells, mucosal immunity
Introduction
Congenitally athymic nude mice carry only a small number of lymphocytes when young (i.e. up to 10 weeks), but acquire a considerable number of lymphocytes (i.e. extrathymic T cells) in the liver and at the intraepithelial site of the intestine when old.1–3 This is because of the phenomenon in which extrathymic T cells tend to increase as a function of age.4,5 In the case of euthymic mice, extrathymic T cells expand with ageing when thymic involution occurs. It is therefore recognized that the number of extrathymic T cells surpasses that of thymus-derived T cells in various immune organs of mice with ageing.6
To detect extrathymic T cells, two-colour staining is often used for CD3 and interleukin-2 receptor β (IL-2Rβ) and for CD3 and NK1.1 in the liver and intestine. This is because most extrathymic T cells, if not all, preferentially express IL-2Rβ and NK1.1 on their cell surface.1,2 We also stain for other extrathymic T cells, including γδT-cell receptor (TCR) or CD4+ CD8+ double-positive cells, especially in the intestine. Such staining was applied in the present study, mainly in C57BL/6 (B6) mice, which express NK1.1 antigens genetically. Double-positive cells which exist in the small intestine are unique because they are more mature than those in the thymus. Therefore, we focused on these cells.
In light of these facts, in the present study we examined how the distribution of lymphocyte subsets varied at the extrathymic sites of immune organs in mice with ageing. In parallel with lymphocytes from the liver, we isolated lymphocytes from intraepithelial sites of the intestine, including the small intestine, colon and appendix. Depending on the extrathymic sites it was found that unique lymphocyte subsets were present and that the proportion of such lymphocytes varied with ageing.7 In addition to euthymic mice, Jα281−/– and CD1d−/– mice were used which lack conventional natural killer T (NKT) cells (i.e. NK1.1+ TCRint cells) carrying an invariant chain of Vα14Jα281 for TCR-α.8–11 The finding of age-dependent variation in the proportion and number of intestinal lymphocytes was of interest in both normal mice and NKT cell-deficient mice.
Materials and methods
Mice
C57BL/6 (B6), Jα281−/− (B6 background)8 and CD1d−/− (B6 background) mice9,10 were used at the ages of 4, 9 and 40 weeks to examine age-associated changes of intraepithelial lymphocytes (IEL) in the intestine. All mice were maintained under specific pathogen-free conditions in the animal facility of Niigata University (Niigata, Japan).
Cell preparations
Mice were anaesthetized with ether and killed after complete exsanguination through incised axillary arteries and veins, and the liver, small intestine, colon and appendix were removed. The liver was pressed through 200-gauge stainless mesh and then suspended in Eagle's minimum essential medium (Nissui Pharmaceutical Co., Tokyo, Japan) supplemented with 5 mm HEPES and 2% heat-inactivated newborn calf serum. After washing, the cells were fractionated by centrifugation in 15 ml of 35% Percoll solution (Pharmacia Fine Chemicals, Piscataway, NJ) for 15 min at 420 g.12 The resulting pellet was resuspended in erythrocyte-lysing solution (155 mm NH4Cl, 10 mm KHCO3, 1 mm sodium ethylenediaminetetraacetate, and 170 mm Tris–HCl, pH 7·3). The appendix,13 small intestine and colon were removed and flushed with Dulbecco's phosphate-buffered saline (D-PBS, pH 7·6) to eliminate the luminal contents. The mesentery, Peyer's patches and appendix lymphoid follicles were then resected. To obtain IEL, the intestine was cut longitudinally and then into 1- to 2-cm pieces. These fragments were incubated for 15 min in 15 ml Ca2+-free and Mg2+-free PBS containing 55 mm ethylenediaminetetraacetic acid, in a 37° shaking-water bath. The supernatant was then collected. The cell suspension was collected and centrifuged in a discontinuous 40%/80% Percoll gradient solution at 830 g for 25 min. Cells from the 40%/80% interface were collected.
Since the number of cells yielded by one mouse was few, cells were pooled from two to six mice, the resulting number of cells then being divided by the number of mice used. The mean and one SD of the number of cells were produced by three or four separate experiments.
Flow cytometric analysis
The surface phenotype of cells was analysed using monoclonal antibodies (mAbs) in conjunction with two-colour or three-colour immunofluorescence.14 The mAbs used in this study included fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, biotin- and Cy-chrome-conjugated reagents of anti-CD3ɛ (145-2C11), anti-IL-2Rβ (IL-2Rβ-chain; TM-β1), anti-NK1.1 (PK136), anti-B220 (RA3-6B2), anti-CD4 (RM4-5), anti-CD8α (53-6·7) anti-TCR-β (H57-597), anti-TCR-γδ (GL3), and anti-CD45 (30F11) mAbs (PharMingen Co., San Diego, CA). Biotin-conjugated reagents were developed with PE- (PharMingen), or TRI colour- (Caltag Laboratory, San Francisco, CA) conjugated streptavidin. To prevent non-specific binding of mAbs, CD16/32 (2.4G2) was added before staining with labelled mAb. Dead cells were excluded by forward scatter and side scatter gatings. To analyse lymphocytes, CD45+ cells were gated. The fluorescence-positive cells were analysed by FACScan using lysis II software (Becton Dickinson Co., Mountain View, CA).
Statistical analysis
The difference between the values was determined by Student's t-test.
Results
Age-associated increase in the number of lymphocytes yielded by the liver and intestine
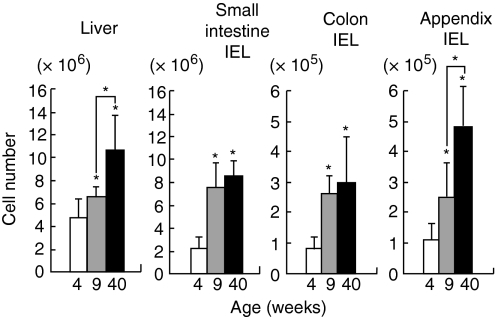
To examine the age-dependent variation of the number of lymphocytes at the extrathymic sites, lymphocytes were isolated from the liver and IEL in the small intestine, colon and appendix (Fig. 1). Mice were used at the ages of 4, 9 and 40 weeks. At all tested sites, the number of lymphocytes increased as a function of age (P<0·05). Such age-associated increase began at an early age (9 weeks) in all tested organs.
Figure 1.
Age-associated increase in the number of lymphocytes yielded by the liver and intraepithelial sites of the small intestine, colon and appendix. Mice were used at the ages of 4, 9 and 40 weeks. The mean and one SD were produced from four separate experiments. *, P<0·05.
Identification of lymphocyte subsets in immunofluorescence tests
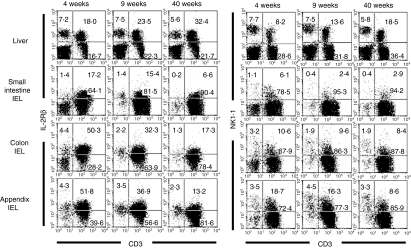
To identify the phenotype of lymphocytes, two-colour staining for CD3 and IL-2Rβ and that for CD3 and NK1.1 were conducted in various organs (Fig. 2). In the case of the liver, a prominent increase was seen in IL-2Rβ+ CD3int and its subset of NK1.1+ CD3int cells. In the small intestine, the major lymphocyte population was constituted of CD3+ cells with the phenotype of IL-2Rβ– NK1.1–. This pattern did not significantly change with ageing at this site.
Figure 2.
Phenotypic characterization of lymphocytes in immunofluorescence tests. Two-colour staining for CD3 and IL-2Rβ and that for CD3 and NK1.1 were conducted in mice at the ages of 4, 9 and 40 weeks. Numbers in the figure represent the percentages of fluorescence-positive cells in corresponding areas. Data representative of three experiments are depicted.
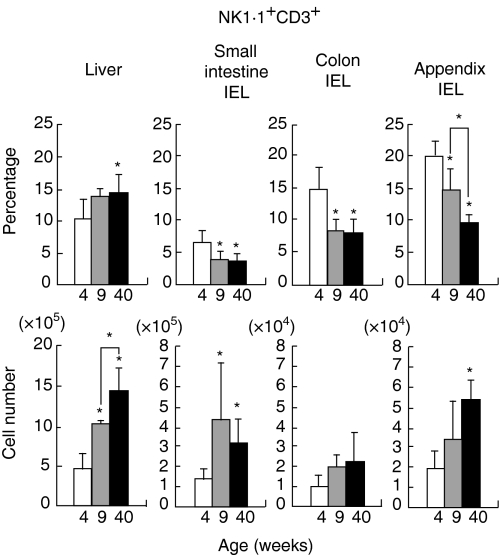
On the other hand, IEL of the colon and appendix carried both IL-2Rβ+ and IL-2Rβ– subsets of CD3+ T cells, especially in young mice (4 weeks). Among these T cells, a small population (10–20%) expressed NK1.1. With ageing, the proportion of IL-2Rβ+ CD3+ cells and that of NK1.1+ CD3+ cells tended to decrease. The actual percentage and the absolute cell number were found by repeated experiments (n=4) in mice at various ages (Fig. 3). The most striking age-associated changes were seen in the appendix, i.e. a decrease in the percentage of NK1.1+ CD3+ cells, and in the liver, i.e. an increase in the number of NK1.1+ CD3int cells. Although the percentage of NK1.1+ CD3+ cells decreased with ageing in the small intestine and colon, the absolute number of these cells did not decrease because of the increase in the total number of cells.
Figure 3.
Age-dependent variation of NKT cells in the percentage and cell number in various organs. The percentage of NK1.1+ CD3+ cells and the absolute number of NK1.1+ CD3+ cells were produced by repeated experiments (n=4). The mean and one SD were produced at each age. *, P<0·05.
Comparison of the distribution of lymphocyte subsets in various immune organs
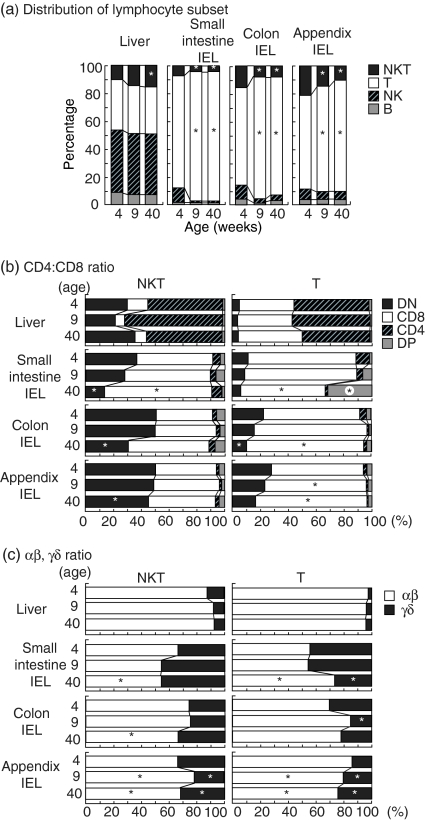
In the final portion of these immunofluorescence experiments, the distribution pattern of lymphocyte subsets (the mean value) was evaluated by repeated experiments (n=4) (Fig. 4). As shown in Fig. 4(a), the distribution pattern differed between the liver and intestine (including the small intestine, colon and appendix). T and B cells were abundant in the liver, whereas only T cells were abundant in the intestine. The percentage of NKT cells in the liver increased with ageing, while that in the intestine decreased with ageing.
Figure 4.
Distribution of immunoparameters at extrathymic sites of mice of various ages. (a)Distribution of lymphocyte subsets, (b)ratio of CD4 to CD8 among NKT and T cells; (c)ratio of TCR-αβ to TCR-γδ among NKT and T cells. The mean values in each column are for four experiments. *P<0·05.
The ratio of CD4+ and CD8+ among NKT cells and T cells was then estimated at various sites (Fig. 4b). In the case of the liver, NKT cells were a mixture of double-negative (CD4– CD8–) and CD4+. In sharp contrast, NKT cells in the intestine (including the small intestine, colon and appendix) were double-negative or CD8+. The percentage of such double-negative cells decreased with ageing. In the case of T cells, the liver contained both CD4+ and CD8+. However, the intestine contained mainly CD8+ cells. The percentage of these CD8+ cells in the intestine (at all sites) tended to increase with ageing. Only in the small intestine was a significant number of double-positive (CD4+ CD8+) cells found and this proportion increased with ageing.
The ratio of αβT and γδT cells among NKT and T cells was calculated (Fig. 4c). γδT cells were few in the liver but abundant in the intestine15 including the small intestine, colon and appendix. This tendency was seen for both NKT cells and T cells. The percentage of γδT cells among NKT cells in the intestine tended to increase with ageing while that among T cells in the intestine varied with ageing.
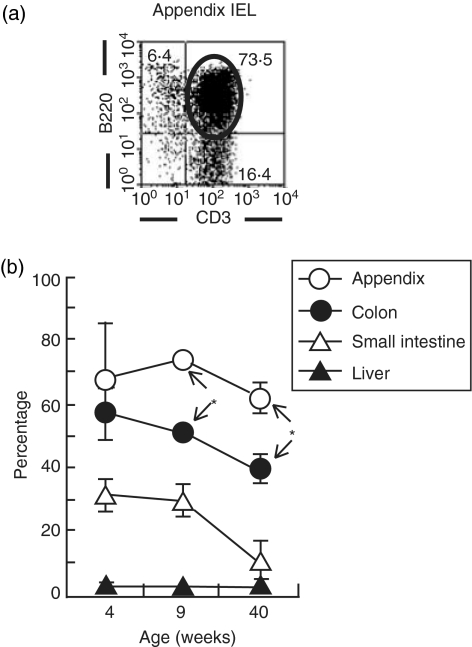
Variation of B220+ T cells in IEL of the appendix with ageing
B220+ T cells, namely, T cells with a B-cell marker, were few in the liver and small intestine (< 30%). In contrast, they were abundant in the appendix and colon. This result was confirmed by two-colour staining for CD3 and B220 (Fig. 5a). Age-associated variation was then examined by repeated experiments (n=4). Generally speaking, the proportion of B220+ T cells in all tested organs tended to decrease with ageing.
Figure 5.
Identification of B220+ CD3+ cells in the intestine and liver. Two-colour staining for CD3 and CD220 was conducted in IEL of the appendix and other sites. A representative profile of B220+ CD3+ cells in the appendix (a)and the age-dependent variation in the percentages of B220+ CD3+ cells (b) are depicted. *P<0·05.
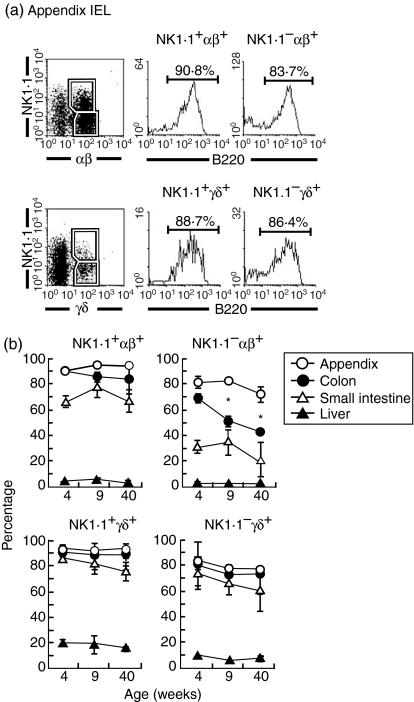
It is still unknown how B220+ T cells express NK1.1 antigens. This subject was first examined in the appendix (Fig. 6a). Three-colour staining of NK1.1, TCR-αβ (or TCR-γδ), and B220 was conducted for gated analysis of B220. In the case of the appendix, both NK1.1+ and NK1.1– subsets among TCR-αβ+ and TCR-γδ+ cells were mainly B220+.
Figure 6.
Further characterization of B220+ T cells. (a)Appendix IEL, (b)age-dependent variation of B220+ T cells in various organs. To characterize B220 expression on T-cell subsets, three-colour staining for TCR-αβ (or TCR-γδ), NK1.1 and B220 was conducted. Numbers in the figure represent the percentages of fluorescence-positive cells in corresponding areas. The experiments for age-dependent variation were repeated (n=4) and the mean and one SD are shown. *P<0·05.
Age-dependent variation in the expression of B220 on T cells was further examined in various organs (Fig. 6b). The majority of NK1.1+ subsets among TCR-αβ+ cells and TCR-γδ+ in the appendix, colon and small intestine was B220+, irrespective of age. On the other hand, NK1.1– TCR-αβ+ cells partially expressed B220 and tended to decrease its expression, especially in the colon. In the case of NK1.1– TCR-γδ+ cells, the expression level of B220 remained unchanged with ageing. Lymphocytes in the liver did not express B220 antigen.
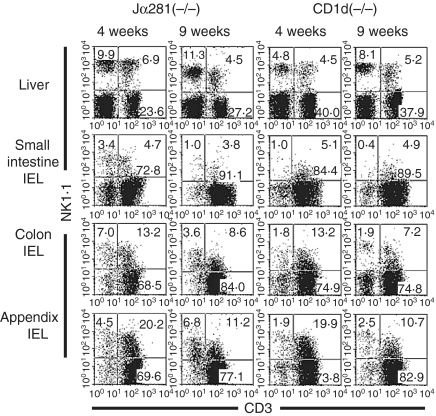
The existence of NK1.1+ T cells in the intestine, but not the liver, was independent of Jα281 and CD1d
NKT cells were found to be abundant in the liver and appendix. It was examined whether the existence of these NKT cells was influenced by the lack of Jα281 and CD1d (Fig. 7). In these experiments, Jα281−/− mice and CD1d−/− mice were used at the ages of 4 and 9 weeks. As expected, the proportion of NKT cells was very low in these mice, irrespective of age. On the other hand, a large proportion (up to 20%) of NKT cells was found in the appendix of both Jα281−/− and CD1d−/− mice. The majority of these NKT cells were CD8+ (data not shown).
Figure 7.
Phenotypic characterization of lymphocytes in Jα281−/− and CD1d−/− mice. NK-cell-deficient mice were used at the ages of 4 and 9 weeks. Two-colour staining for CD3 and NK1.1 was conducted to identify NKT cells. Representative data of three experiments are depicted.
Discussion
In the present study, we demonstrated that the number and proportion of lymphocyte subsets existing at extrathymic sites in the liver and intestine varied in specific manners depending on each subset as a function of age. The most striking change was an increase in the number of whole lymphocytes in the liver, small intestine, colon and appendix. This phenomenon is of interest because the number of lymphocytes in the thymus, spleen and lymph nodes (i.e. the sites for thymus-derived T cells) inversely decreases with ageing. In the case of the liver, the number and proportion of IL-2Rβ+ CD3int cells and its subset of NK1.1+ CD3int cells (i.e. NKT cells) increased with ageing. However, the proportion of IL-2Rβ+ CD3+ cells and NK1.1+ CD3+ cells in the intestine decreased with ageing. Since the total number of lymphocytes increased in the intestine, the number of IL-2Rβ+ CD3+ cells and NK1.1+ CD3+ cells tended to increase in this organ.
As is well established, Jα281−/− and CD1d−/− mice lack conventional NKT cells.8–11 The majority of NKT cells in the liver of normal mice were conventional NKT cells which carried an invariant chain of Vα14Jα281 and the phenotype of CD4+ or double-negative CD4– CD8–.6 These NKT cells eventually disappeared in Jα281−/− and CD1d−/− mice in this study. In contrast, NKT cells in the intestine did not decrease in proportion and number, even in Jα281−/− and CD1d−/− mice, and comprised TCR-αβ+ or TCR-γδ+, which expressed CD8 antigens. It was found that these unconventional NKT cells were most abundant in the appendix and decreased with ageing, especially in proportion. Although the proportion of NKT cells was smaller in the small intestine and colon, portions at these sites were also found to decrease with ageing.
Why are both the liver and intestine extrathymic sites? This is because the liver was primarily generated from the intestine in phylogeny.16,17 Indeed, both epithelia in the intestine and hepatocytes in the liver are regenerated spontaneously under normal conditions.18 Therefore, it is speculated that the liver and the intraepithelial sites in the intestine became sites for the generation of extrathymic T cells. However, the composition of lymphocyte subsets was found to be somewhat different between the liver and intestine, as summarized in Fig. 4(a). In the intestine, B cells were abundant in the liver but scarce in the intestine.
Another difference in the distribution of lymphocyte subsets between the liver and intestine was the presence of CD4+ cells in the liver but their almost complete absence from the intestine (see Fig. 4b). Instead, CD8+ cells and double-negative CD4– CD8– cells were abundant in the intestine. Such CD8+ T cells, including TCRαβ+ and TCRγδ+ cells, increased in proportion with ageing. The existence of double-positive CD4+ CD8+ cells is unique among T cells in the small intestine19,20 and this population increases prominently in old mice.21
CD3+ T cells with a B-cell number (i.e. B220+ T cells) were most abundant in the appendix11,22 and this population was also present in the colon and small intestine. It tended to decrease in proportion with ageing. These B220+ T cells were distributed in αβT cells, as well as in NK1.1+ and NK1.1– subsets. In the present study, we revealed the distribution of lymphocyte subsets at extrathymic sites of the liver and intestine and the age-dependent variation of these lymphocyte subsets in normal mice and mice lacking conventional NKT cells.
As previously mentioned, the most prominent increase of lymphocyte subsets with ageing was found in the number of NK1.1+ CD3 in the liver and appendix (see Fig. 3) and in the proportion of CD4+ CD8+ double-positive cells (among αβT cells) and γδT cells (among NKT cells) in the small intestine (see Fig. 4). Finally, we speculated as to why these subsets increased with aging. All these lymphocyte subsets are of extrathymic origin and carry autoreactivity.23–26 Since the increase in the number or proportion of these subsets occurred in parallel with thymic involution in this study, it is indicated that the immune system in aged mice requires autoreactivity against the altered self-cells that are generated with ageing. In contrast, the immune system in young mice requires reactivity against foreign antigens (i.e. thymus-derived T-cell clones eliminate autoreactivity and therefore carry the reactivity against foreign antigens). It is speculated that the present phenomenon seen in aged mice is beneficial for the health of aged animals.
Acknowledgments
The authors wish to thank Mrs Masako Watanabe for preparation of the manuscript. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture, Japan.
References
- 1.Iiai T, Watanabe H, Seki S, et al. Ontogeny and development of extrathymic T cells in mouse liver. Immunology. 1992;77:556–63. [PMC free article] [PubMed] [Google Scholar]
- 2.Halder RC, Kawamura T, Bannai M, et al. Intensive generation of NK1.1− extrathymic T cells in the liver by injection of bone marrow cells isolated from mice with a mutation of polymorphic major histocompatiblity complex antigens. Immunology. 2001;102:450–9. doi: 10.1046/j.1365-2567.2001.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin T, Matsuzaki G, Kenai H, Nomoto K. Extrathymic and thymic origin of murine IEL. Are most IEL in euthymic mice derived from the thymus? Immunol Cell Biol. 1995;73:469–73. doi: 10.1038/icb.1995.73. [DOI] [PubMed] [Google Scholar]
- 4.Tsukahara A, Seki S, Iiai T, et al. Mouse liver T cells: their change with aging and in comparison with peripheral T cells. Hepatology. 1997;26:301–9. doi: 10.1002/hep.510260208. [DOI] [PubMed] [Google Scholar]
- 5.Sagiyama K, Tsuchida M, Kawamura H, et al. Age-related bias in function of natural killer T cells and granulocytes after stress. Reciprocal association of steroid hormones and sympathetic nerves. Clin Exp Immunol. 2004;135:56–63. doi: 10.1111/j.1365-2249.2004.02340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe H, Miyaji C, Kawachi Y, Iiai T, Ohtsuka K, Iwanaga T, Takahashi-Iwanaga H, Abo T. Relationships between intermediate TCR cells and NK1.1—T cells in various immune organs. NK1.1+T cells are present within a population of intermediate TCR cells. J Immunol. 1995;155:2972–83. [PubMed] [Google Scholar]
- 7.Rozing J, de Geus B. Changes in the intestinal lymphoid compartment throughout life. implications for the local generation of intestinal T cells. Int Rev Immunol. 1995;12:13–25. doi: 10.3109/08830189509056699. [DOI] [PubMed] [Google Scholar]
- 8.Cui J, Shin T, Kawano T, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 9.Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–77. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 10.Smiley ST, Kaplan MH, Grusby JJ. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275:977–9. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 11.Bannai M, Kawamura T, Naito T, et al. Abundance of unconventional CD8+ natural killer T cells in the large intestine. Eur J Immunol. 2001;31:3361–9. doi: 10.1002/1521-4141(200111)31:11<3361::aid-immu3361>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsuka K, Iiai T, Watanabe H, Tanaka T, Miyasaka M, Sato K, Asakura H, Abo T. Similarities and differences between extrathymic T cells residing in mouse liver and intestine. Cell Immunol. 1994;153:52–66. doi: 10.1006/cimm.1994.1005. [DOI] [PubMed] [Google Scholar]
- 13.Krieglstein CF, Cerwinka WH, Laroux FS, Grisham MB, Schurmann G, Bruwer M, Granger DN. Role of appendix and spleen in experimental colitis. J Surg Res. 2001;101:166–75. doi: 10.1006/jsre.2001.6223. [DOI] [PubMed] [Google Scholar]
- 14.Ohtsuka K, Hasegawa K, Yamagiwa S, Sato K, Nakayama M, Watanabe H, Asakura H, Abo T. Intraepithelial lymphocytes in colon have similar properties to intraepithelial lymphocytes in small intestine and hepatic intermediate TCR cells. Digest Dis Sci. 1996;41:902–11. doi: 10.1007/BF02091529. [DOI] [PubMed] [Google Scholar]
- 15.Kagnoff MF. Current concepts in mucosal immunity III. Ontogeny and function of gamma delta T cells in the intestine. Am J Physiol. 1998;274:G455–8. doi: 10.1152/ajpgi.1998.274.3.G455. [DOI] [PubMed] [Google Scholar]
- 16.Abo T, Kawamura T, Watanabe H. Physiological responses of extrathymic T cells in the liver. Immunol Rev. 2000;174:135–49. doi: 10.1034/j.1600-0528.2002.017415.x. [DOI] [PubMed] [Google Scholar]
- 17.Seki S, Habu Y, Kawamura T, Takeda K, Dobashi H, Ohkawa T, Hiraide H. The liver as a crucial organ in the first line of host defense. The roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol Rev. 2000;174:35–46. doi: 10.1034/j.1600-0528.2002.017404.x. [DOI] [PubMed] [Google Scholar]
- 18.Abo T, Watanabe H, Sato K, Iiai T, Moroda T, Takeda K, Seki S. Extrathymic T cells stand at an intermediate phylogenetic position between natural killer cells and thymus-derived T cells. Nat Immun. 1995;14:173–87. [PubMed] [Google Scholar]
- 19.Tsukada C, Yokoyama H, Miyaji C, Ishimoto Y, Kawamura H, Abo T. Immunopotentiation of intraepithelial lymphocytes in the intestine by oral administrations of β-glucan. Cell Immunol. 2003;221:1–5. doi: 10.1016/s0008-8749(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 20.Sasahara T, Tamauchi H, Ikewaki H, Kubota K. Unique properties of a cytotoxic CD4+CD8+ intraepithelial T-cell line established from the mouse intestinal epithelium. Microbiol Immunol. 1994;38:191–9. doi: 10.1111/j.1348-0421.1994.tb01764.x. [DOI] [PubMed] [Google Scholar]
- 21.Takimoto H, Nakamura T, Takeuchi M, Sumi Y, Tanaka T, Nomoto K, Yoshikai Y. Age-associated increase in number of CD4+CD8+ intestinal intraepithelial lymphocytes in rat. Eur J Immunol. 1992;22:159–64. doi: 10.1002/eji.1830220124. [DOI] [PubMed] [Google Scholar]
- 22.Yamagiwa S, Sugahara S, Shimizu T, et al. The primary site of CD4−8–B220+αβT cells in lpr mice: the appendix in normal mice. J Immunol. 1998;160:2665–74. [PubMed] [Google Scholar]
- 23.Smith H, Chen I-M, Kubo R, Tung KS. Neonatal thymectomy results in a repertoire enriched in T cells deleted in adult thymus. Science. 1989;245:749–52. doi: 10.1126/science.2788921. [DOI] [PubMed] [Google Scholar]
- 24.Jones LA, Chin LT, Merriam GR, Nelson LM, Kruisbeck AM. Failure of clonal deletion in neonatally thymectomized mice: tolerance is preserved through clonal anergy. J Exp Med. 1990;172:1277–85. doi: 10.1084/jem.172.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moroda T, Iiai T, Kawachi Y, Kawamura T, Hatakeyama K, Abo T. Restricted appearance of self-reactive clones into intermediate T cell receptor cells in neonatally thymectomized mice with autoimmune disease. Eur J Immunol. 1996;26:3084–91. doi: 10.1002/eji.1830261239. [DOI] [PubMed] [Google Scholar]
- 26.Moroda T, Kawachi Y, Iiai T, et al. Self-reactive forbidden clones are confined to pathways of intermediate T cell receptor cell differentiation even under immunosuppressive conditions. Immunology. 1997;91:88–94. doi: 10.1046/j.1365-2567.1997.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]