Abstract
In recent years, nitric oxide (NO), a gas previously considered to be a potentially toxic chemical, has been established as a diffusible universal messenger that mediates cell–cell communication throughout the body. Constitutive and inducible NO production regulate numerous essential functions of the gastrointestinal mucosa, such as maintenance of adequate perfusion, regulation of microvascular and epithelial permeability, and regulation of the immune response. Up-regulation of the production of NO via expression of inducible nitric oxide synthase (iNOS) represents part of a prompt intestinal antibacterial response; however, NO has also been associated with the initiation and maintenance of inflammation in human inflammatory bowel disease (IBD). Recent studies on animal models of experimental IBD have shown that constitutive and inducible NO production seems to be beneficial during acute colitis, but sustained up-regulation of NO is detrimental. This fact is also supported by studies on mice genetically deficient in various NOS isoforms. However, the mechanism by which NO proceeds from being an indispensable homeostatic regulator to a harmful destructor remains unknown. Furthermore, extrapolation of data from animal colitis models to human IBD is questionable. The purpose of this review is to update our knowledge about the role of this universal mediator and the enzymes that generate it in the pathogenesis of IBD.
Keywords: gut, inflammatory bowel diseases, nitric oxide synthase, nitric oxide
Nitric oxide synthesis and chemistry
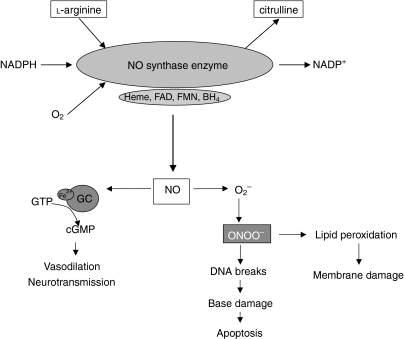
Nitric oxide (NO) is a free radical with moderate reactivity compared to other species, which gives rise to a multitude of organ-specific regulatory functions. NO is synthesized from the amino acid l-arginine (Fig. 1) by a family of enzymes generally referred to as the nitric oxide synthases (NOSs) (Fig. 2). The oxidation of a terminal nitrogen of the amino acid l-arginine produces NO and l-citrulline. Three isoforms have been identified: two are constitutively present in either neuronal (nNOS) or endothelial (eNOS) tissue and are termed constitutive NOS (cNOS), while a third isoform is expressed after induction by certain cytokines, microbes and bacterial products, and is thus called inducible nitric oxide synthase (iNOS).1 NO production by cNOS is low (nanomolar quantities) and short-lasting, being controlled by Ca2+-mobilizing agents in a very transient and highly controlled manner, and fully inhibited by calmodulin antagonists.2 In marked contrast, iNOS synthesizes NO in high (micromolar) amounts, it is regulated at the transcriptional level and is sensitive to inhibitors of DNA transcription and protein synthesis, such as actinomycin D and cycloheximide.3 NO production by iNOS is delayed by several hours following stimulation, but once induced is active for periods as long as 5 days. The delay between stimulation and enzyme generation suggests the requirement of de novo synthesis of a cofactor, e.g. tetrahydrobiopterin,2 for maximal activity.
Figure 1.
Production, metabolism and functional targets of nitric oxide (NO).
Figure 2.
Schematic alignment of the deduced amino acid sequences of nitric oxide synthases (NOSs). Depicted are consensus binding sites for haem, l-arginine, calmodulin (CaM), flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD) and NADPH. An NH2-terminal myristoylation site (myr) is present only on the endothelial constitutive NOS (NOS3).
The chemistry of NO provides a valuable blueprint that helps in recognizing the type of effects that this pluripotent molecule may have in various biological systems. In order to provide a guide through the maze of possible reactions of NO, NO effects can be separated into direct and indirect actions.4 Direct effects are those reactions in which NO interacts directly with a biological molecule or target, whereas indirect reactions occur when the final effector molecule is generated by the interaction of NO with reactive oxygen species.
Characteristic of the former are the direct interaction of NO with metal-containing proteins or with organic free radicals. Direct interaction of NO with metals occurs in vivo primarily with iron-containing proteins such as the haem moiety, leading to the formation of stable nitrosyl adducts.5 The most notable of these is the reaction of NO and guanylate cyclase, which leads to the formation of cGMP from GTP.6 cGMP has significant regulatory and anti-inflammatory effects, such as the regulation of vascular tone and the inhibition of platelet aggregation and leucocyte adhesion. The superoxide (O2–) scavenging is another direct action of NO serving to protect haem-containing enzymes involved in prostaglandin synthesis (e.g. cyclooxygenase) from reduction to their inactive forms.7
Several studies suggest that NO may also modulate iron-catalysed oxidation reactions by acting as an iron chelator. In vitro, NO can dramatically inhibit the O2–-driven Fenton reaction [an most important iron-catalysed oxidation reaction that produces powerful oxidants such as the hydroxy radical (OH˙)], suggesting that it may have remarkable antioxidant capabilities.7 Taken together, the above observations suggest that the direct effects of NO would be involved primarily, but not exclusively, in regulatory, protective and/or anti-inflammatory processes in vivo.
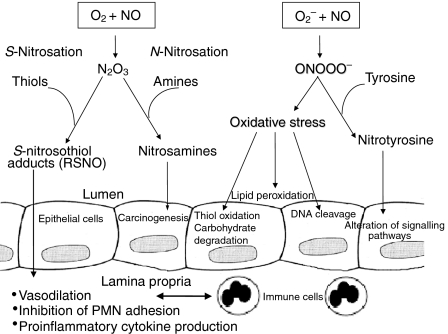
In contrast, the indirect effects of NO are mediated by intermediate reactive nitrogen oxide species derived from the interactions of NO with O2 or O2–, which give rise to two types of chemical stress: the nitrosative and the oxidative (Fig. 3). Both types of chemical stress are generally thought to be associated with certain pathophysiological situations, such as inflammation, where de novo expression of iNOS occurs.8 The interaction of NO with O2 leads to the autoxidation of NO and the formation of dinitrogen trioxide (N2O3), which is a potent nitrosating agent. N2O3 has been shown to N-nitrosate a variety of biological targets, such as amino compounds, to yield potentially carcinogenic nitrosamines. This might contribute to the known association between chronic inflammation and malignant transformation.4,9 On the other hand, S-nitrosation of certain thiols by N2O3 gives rise to nitrosothiol adducts.4,5 These adducts have been suggested to play a key role in various physiological and inflammatory processes, such as the regulation of blood flow, inhibition of neutophil adhesion and modulation of cytokine production (Fig. 3).10,11 S-nitrothiols may also provide a slow-releasing storage depot for NO, hence considerably extending its biological half-life. Lacking particular toxicity or oxidative potential when alone, O2– and NO have been suggested to interact rapidly with each other to produce the potent cytotoxic oxidant, peroxynitrite (ONOO–).12
Figure 3.
The role of nitric oxide chemistry in gut immunology.
Role of no in intestinal homeostasis
Initial studies conducted to examine the direct effects of NO on epithelial cell integrity have shown that NO per se is not cytotoxic for intestinal tissue.13 On the contrary, eNOS-derived NO appears to be a homeostatic regulator of numerous essential functions of the gastrointestinal mucosa, such as maintenance of adequate perfusion,14 and regulation of microvascular and epithelial permeability.15,16 The latter strongly reflects the functional integrity of the gastrointestinal mucosa barrier, and its disturbance is considered to be a quantitive index of injury or dysfunction.
Inhibition of NO production has been found to increase the epithelial permeability to substances of low molecular weight, as measured by the transepithelial movement of 51Cr-EDTA, and this effect was reversed using NO donors.16 This function of NO has been attributed to both an increase of cGMP content of intestinal epithelia and to the NO suppressive effects on platelet-activation factor (PAF) and histamine secretion by mucosal mast cells.17 Specifically, following the suppression of NO by treatment with NG-nitro-l-arginine methyl ester (L-NAME), the reduced availability of cGMP has been suggested to cause epithelial cell contraction and to increase the size of the interepithelial junctions, resulting in a leakier mucosal barrier.15 Furthermore, NO regulates major epithelial functions involved in host defence, such as mucus production and epithelial fluid secretion. NO induces gastric mucus secretion via the activation of soluble guanylate cyclase,18 and this NO production appears to be caused by the activation of cholinergic receptors. NO-donating compounds have been found to stimulate electrolyte secretion in colonic mucosa, and this effect may be mediated via enhancement of the local production of prostaglandin E2 (PGE2).19
The effects of NO on immune cells highlights its protective role in immune-mediated tissue injury. Constitutive production of NO inhibits neutrophil β2 integrin function, decreases endothelial P-selectin expression and reduces chemotactic responses to various chemokines, such as interleukin (IL)-8 and monocyte chemotactic protein-1 (MCP-1). Therefore, it reduces leucocyte chemotaxis, adhesion and recruitment in tissues.20–23 Additionally, the constitutive production of NO exerts its anti-inflammatory effects via modulation of platelet homotypic aggregation and platelet adhesion to vessel walls.24 NO has also been found to alter the cytokine profile released by macrophages, so that following a T helper 1 (Th1) response, Th1-associated cytokines are down-regulated and T helper 2 (Th2) cytokines are favoured.25
Production of large quantities of NO via the up-regulation of iNOS can have a variety of effects, which may be detrimental or beneficial depending on the amount, duration and anatomical site of synthesis. Production of large quantities of NO can inhibit key enzymes in the mitochondrial electron transport chain and citric acid cycle by nitrosylation of reactive groups, which are essential for enzyme catalytic function.26,27 NO can also have antiproliferative properties by inhibiting DNA synthesis via inactivation of the ribonucleotide reductase enzyme. These mechanisms may account for the cytotoxic and cytostatic effects of macrophage-derived NO on tumour cells and micro-organisms.28,29 Indeed, iNOS-induced NO has been found to exert a direct antimicrobial effect,30 and enteroinvasive bacteria, such as Escherichia coli, Salmonella and Shigella can directly induce iNOS expression, suggesting an important role of iNOS in the intestinal antibacterial response.31,32 Apart from being an important component of the host defence system, iNOS-mediated NO production may occasionally become part of a dysregulated immune response, resulting in chronic inflammatory disorders. One of the settings where this hypothesis has been most vigorously tested is in inflammatory bowel disease (IBD), where NO produced following the up-regulation of iNOS in epithelial cells has been closely associated with the initiation and maintenance of intestinal inflammation.
No production in ibd: the role of colonic epithelial cells
Early in the 1990s, various studies based on animal models as well as in humans, indicated that NO may be involved in gastrointestinal inflammation and that it may have a pathogenetic role in IBD.33 The concentrations of citrulline, the co-product of NO synthase, were found to be higher in rectal biopsy specimens from patients with active ulcerative colitis (UC) than in those from patients with quiescent disease or a normal histology, while incubation with Nω-monomethyl-l-arginine (L-NMMA), an effective inhibitor of all types of NOS, significantly reduced the concentration of citrulline in colonic biopsies, suggesting that the increased biosynthesis of citrulline must be a consequence of NO synthase activity, which simultaneously produces NO.34 Studies in patients with active UC and Crohn's disease (CD), compared to controls, revealed a substantial increase of Ca2+-independent NO synthase activity in UC, characteristic of the inducible isoform of NOS. iNOS activity in the colonic mucosa of patients with UC was about eightfold higher than in control mucosa.35 Luminal gas sampled from the colons of patients with active UC and controls was analysed by using a chemiluminescence technique, and NO concentrations were found to be more than 100 times higher in the patients than in the controls.36 It was suggested that increased values of luminal NO reflect NO production only in the superficial mucosal layers, and that as NO production in deeper mucosal layers is bound (e.g. by haemoglobulin in blood vessels) it therefore will not reach levels as high as those found in the lumen.36 Despite the evidence suggesting NO overproduction in gut inflammation, the cellular source of NO and iNOS in models of IBD has received little attention.
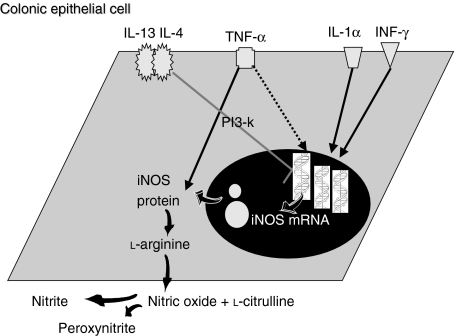
In 1995, we first demonstrated that human HT-29 colonic epithelial cells, in response to the combinations of IL-1α and interferon-γ (IFN-γ) express iNOS mRNA and produce large quantities of nitrite, while the addition of tumour necrosis factor-α (TNF-α) caused a significant up-regulation of this production (Fig. 4).37 Our results from the in vitro studies support the suggestions, mentioned above, that superficial mucosal layers might be responsible for increased luminal NO in IBD. In support of our in vitro findings, iNOS protein expression was demonstrated by immunohistochemistry in the epithelial cells of the colonic mucosa of patients with active UC and infectious colitis, but not in the mucosa of biopsies derived from patients in remission or from healthy individuals.32 Similarly, other studies, using in situ hybridization and immunohistochemistry, have demonstrated high expression of iNOS, which was localized to the surface epithelium and crypts in the mucosa from patients with UC.38,39 These results strongly suggest that colonic epithelial cells are the major source of NO production and NOS activity which has been reported to be increased in the mucosa of patients with UC.34–36 In addition, Singer et al. observed iNOS expression in the inflamed mucosa of patients with diverticulitis,38 which, compared with our results from infectious colitis,32 suggests that iNOS expression from colonic epithelial cells is more a feature of intestinal inflammation than of the IBD.
Figure 4.
Nitric oxide synthesis and regulation in colonic epithelial cells.
IL-4 and IL-13, but not IL-10, were found to produce a marked inhibition of nitrite generation, iNOS protein expression and iNOS mRNA in a human colonic cell line induced by the optimal cytokine cocktail of IL-1α/IFN-γ/TNF-α.32 Further investigation of the inhibitory effect of IL-13 on the expression and activation of iNOS in HT-29 cells revealed that this molecule exerts its effect via the activation of PtdIns3-kinases (Fig. 4).40 Although these results were obtained using a colonic epithelial cell line, the findings provide strong evidence that T cells and T-cell-derived cytokines, detected in the mucosa of patients with IBD,41,42 modulate the pro-inflammatory cytokine-derived iNOS expression and activity in colonic epithelium and might play a homeostatic role in gut inflammation. The observation that the anti-inflammatory cytokine, IL-13, potently inhibits the cytokine-induced nitrite generation by colonic mucosa in a similar manner to nitrite generation by the colonic epithelial cell line, HT-29, supports this hypothesis.43
The observation that proinflammatory cytokines induce NO production and iNOS activity in colonic epithelial cells, while Th2 and other immune cell-derived mediators regulate this process, suggests an involvement of colonic epithelial cells in NO overproduction and cell communication during the intestinal inflammation. However, the following question, ‘Is NO overproduction detrimental to the intestinal tissue?’, still remains unanswered. Although guilty by association, the exact role of NO overproduction in intestinal inflammation remains obscure.
Nitric oxide and intestinal inflammation
Several investigators have shown that the inhibition of NO causes many of the hallmark features of intestinal inflammation, whereas the delivery of exogenous NO reduces the sequelae of acute inflammation. Inhibition of NO synthesis has been found to increase acute damage of the intestinal mucosal from immune-mediated stress, such as ischemia–reperfusion and septic injury.44,45 Administration of exogenous NO protects the mucosa against the aforementioned models, and this protective effect may be exerted at different levels, including maintenance of blood flow, inhibition of platelet and leucocyte adhesion and/or aggregation within the vasculature, down-regulation of mast cell reactivity, and modulation of oxidative stress and stabilization of IκB, resulting in the inhibition of nuclear factor-κB (NF-κB) translocation.15,46–48 In addition, NO can reduce superoxide-induced damage either by inhibiting NADPH oxidase and superoxide release from neutrophils, or by scavenging neutrophil-derived superoxide.15,49 Accordingly, NO donors have been found to double the plasma antioxidant capacity of animals subjected to reperfusion-induced mucosal injury.
Considering the above observations, it would seem logical that the production of large quantities of NO, even iNOS derived, would improve blood flow, reduce leucocyte and platelet recruitment and oxidative stress, and hence reduce inflammation. On the contrary, the up-regulation of iNOS has been shown to correlate well with prolonged colonic inflammation, especially within epithelial cells around inflammatory foci.32 Excess NO produced by the iNOS may theoretically exacerbate the clinicopathological features of UC by direct cytotoxicity, activation of neutrophils,50 vasodilatation, reduced smooth muscle tone,51 increased production of nitrosamines (to cause cancer),52 and interaction with superoxide to form the highly toxic peroxynitrite radical.38
Peroxinitrite is considered a potent oxidant that reacts with proteins, lipids and DNA, and it is a potent initiator of DNA strand leakage. The latter is an obligatory stimulus for activation of the nuclear enzyme poly-ADP ribosyl synthetase (PARS), which can lead to increased permeability in epithelial cells.53 The peroxynitrite–PARS pathway may contribute to cellular injury in a number of pathophysiological conditions and has been implicated in the pathogenesis of gut inflammation as a result of causing apoptosis in the epithelial cell.54 The whole issue of in vivo peroxynitrite formation following iNOS up-regulation remains a matter of active debate.55 Furthermore, it is doubtful whether the nitration of tyrosine by peroxynitrite (if it occurs), is associated with functional impairment of the colonic epithelium. However, direct administration of peroxynitrite to colonic tissue has been reported to result in colitis,56 whereas mesalamine, currently used for the treatment of IBD, has been found to degrade peroxynitrite and reduce the peroxynitrite-induced apoptosis in vitro.57
The occurrence of toxic megacolon, a severe complication of UC, has also been associated with the appearance of large amounts of iNOS in the colonic muscularis propria.58 In addition, methylprednisolone was found to decrease NO generation by cultured colonic mucosa and it has been suggested that NO synthase activity is induced during the culture, and this steroid effect may relate to its therapeutic effect.59 Although iNOS expression is most certainly a key element, it remains extremely difficult to reconcile that NO varies from being helpful to harmful with a relatively minor change in production.
Modification of no production in experimental models of ibd: an unsolved puzzle
The potential role of NO as a mediator of inflammation in the gut has fuelled many studies in animal models of IBD, as summarized in Table 1. NO and its toxic derivative, peroxynitrite, have been implicated in the pathophysiology of experimental IBD by the demonstration of increased iNOS expression and citrulline production, together with iNOS and nitrotyrosine immunohistochemical co-localization in specimens of experimental ileocolitis.50,74,75
Table 1.
Effect of various nitric oxide synthase (NOS) inhibitors in experimental colitis models
| Model | NOS inhibitor | Effect on inflammation | Reference |
|---|---|---|---|
| TNBS ileitis | l-NAME | Attenuation | 60 |
| TNBS colitis | l-NAME | Attenuation | 61 |
| TNBS colitis | l-NAME | Attenuation | 62 |
| Acetic colitis | l-NAME | Attenuation | 62 |
| TNBS colitis | l-NAME (pretreatment) | Exacerbation | 63 |
| TNBS colitis | l-NAME | Attenuation (late treatment) | 63 |
| TNBS colitis | l-NAME | No effect | 64 |
| DSS colitis | l-NAME | Exacerbation | 65 |
| TNBS colitis | l-NMMA (early administration) | Exacerbation | 66 |
| TNBS colitis | l-NMMA (late administration) | No effect | 66 |
| TNBS colitis | l-NNA | Exacerbation | 67 |
| TNBS colitis | MEG | Attenuation | 68 |
| TNBS colitis | AG | Attenuation | 69 |
| DSS colitis | AG | Attenuation | 70 |
| TNBS colitis | AG | No effect | 64 |
| DSS colitis | AG | Exacerbation | 65 |
| TNBS colitis | AG | Attenuation | 67 |
| TNBS colitis | 1400W | Attenuation | 71 |
| TNBS colitis | 1400W | Attenuation | 72 |
| DSS colitis | 1400W | Attenuation | 73 |
AG, aminoguanidine; DSS, dextran sulphate sodium; L-NAME: NG-nitro-l-arginine methyl ester, L-NMMA, Nω-monomethyl-l-arginine; L-NNA, NG-nitro-l-arginine; MEG, mercaptoethylguanidine; TNBS, trinitrobenzene sulphonic acid; 1400 W, aminomethyl benzyl acetamidine.
Numerous investigations have been conducted by using NOS inhibitors to address the role of iNOS-derived NO in these experimental settings. Early studies, which used non-specific NOS isoform inhibitors such as L-NAME, have produced equivocal results. Trinitrobenzene sulfonic acid (TNBS) and dextran sulphate sodium (DSS) colitis was either aggravated or ameliorated, although NO synthesis was lowered (Table 1).60–62,64–66 Following these initial controversial reports, the scientific interest focused on the selective modification of iNOS activity rather than a general NOS inhibition, and in the reduction of relative amounts of colonic NO rather than elimination of its production. Relatively selective iNOS inhibitors became available, but these also gave conflicting results. Aminoguanidine (AG) and mercaptoethylguanidine (MEG) have been found to inhibit inflammation activity markers in TNBS and DSS models of colitis when compared to controls or animals treated with non-specific NOS inhibitors.67–70 However, these results were not confirmed by other investigators.64,65
NO supplementation represents another experimental approach to examine the role of NO in experimental IBD. Controversy also exists in this type of investigation. So, although initial studies report exacerbation of DSS-induced colitis following the administration of NO donors,65 subsequent studies showed that NO supplementation ameliorates DSS colitis partly via the down-regulation of endothelial intercellular adhesion molecule-1 and P-selectin, and of IL-12 and IFN-γ mRNA expression in colonic tissue.76 Furthermore, NO-releasing derivatives of prednisolone have been found to increase survival rates, improve macroscopic and histological scores, decrease the mucosal content of Th1-type cytokines, and diminish myeloperoxidase activity.77
The perplexity of the experimental models used to evaluate the effects of NOS inhibitors is well demonstrated by studies on pre-, early or late treatment. Elegant time studies by Laszlo et al., on the endotoxin-induced vascular damage in the intestine, have shown that concurrent administration of NOS inhibitors and endotoxin significantly increased vascular albumin leakage and intestinal damage. In marked contrast, the late administration of NOS inhibitors produced a dose-dependent reduction in the lipopolysaccharide (LPS)-provoked vascular albumin leakage.78 Similar results have been reported by many investigators who used different agents of variable selectivity for NOS isoforms to treat experimental colitis. In these studies, pretreatment or early administration of NOS inhibitors was found to exacerbate, whereas delayed treatment (i.e. at the time of expression of iNOS) was found to ameliorate, tissue injury.63,66
These observations led some investigators to suggest that early treatment with NOS inhibitors results in an inhibition of the constitutive isoforms of NOS, i.e. eNOS and nNOS, which play an essential role in mucosal homeostasis, and this may have deleterious effects on the host response to injury and the host mucosal integrity following challenge. Another possible explanation may be that iNOS could also play a protective role in the early phase of acute intestinal inflammation, whereas continuous overproduction of NO following the sustained up-regulation of iNOS, in the settings of chronic intestinal inflammation, may be detrimental. These suggestions may explain part of the controversy observed in the aforementioned studies concerning the questionable isoform-selectivity of agents used to date.79,80 Indeed, AG is only approximately fivefold more selective for iNOS than for nNOS.80 Both AG and MEG have also been reported to exhibit other actions, such as being radical and peroxynitrite scavengers.81
Furthermore, most of the aforementioned models of TNBS, AA or DSS colitis represent models of acute mucosal injury that are very different from human IBD.82 On the contrary, more recently applied models, with gradually or chronically developed colitis following the administration of smaller doses and more prolonged, continuous application of DSS, may be more relevant to the chronic inflammation observed in human IBD. Using such a model of chronic DSS colitis, both Obermeier et al.70 and Krieglstein et al.73 have shown that systemic administration of AG or N-(3-(Aminomethyl)benzyl) acetamidine (1400W), a highly selective inhibitor of iNOS,83 reduced colonic NO activity and attenuated colonic injury, whereas the same treatment aggravated acute DSS colitis induced by a single dose of DDS.83
In conclusion, studies of NOS inhibition in experimental animal colitis performed during the last 10 years seem to reach to a more or less definite assumption, that is that selective inhibition of iNOS may reduce, to some extent, the tissue damage observed following chronic up-regulation of this isoform in the settings of chronic colitis. On the contrary, the early inhibition of NO production during acute colitis produces equivocal results, indicating a protective role of either constitutive or inducible isoforms in the settings of an acute mucosal insult.
Studies on genetically modified animals
The substantial progress made in the field of gene-targeting technology has turned the investigational interest to focus on NOS knockout models in an attempt to further clarify the role of the NOS isoforms in IBD (summarized in Table 2).
Table 2.
Effect of nitric oxide synthase (NOS) gene deletion in experimental colitis models
| Model | Gene deletion | Effect to injury | Reference |
|---|---|---|---|
| TNBS | iNOS | Reduces susceptibility | 84 |
| Acetic acid | iNOS | Exacerbates acute inflammation | 85 |
| TNBS | iNOS | Increases susceptibility to acute injury | 86 |
| TNBS | iNOS | No effect on chronic injury | 86 |
| IL-10 (−/−) | iNOS | No effect | 87 |
| DSS | iNOS | Reduces susceptibility | 88 |
| DSS | iNOS | Reduces susceptibility | 73 |
| DSS | iNOS | Reduces susceptibility | 89 |
| DSS | eNOS | Increases susceptibility | 90 |
| DSS | eNOS | Reduces susceptibility | 89 |
| DSS | nNOS | Increases susceptibility | 89 |
| DSS | nNOS & eNOS | Reduces susceptibility | 89 |
| TNBS | eNOS | Increases susceptibility | 91 |
| TNBS | iNOS | Increases susceptibility | 91 |
DSS, dextran sulphate sodium; eNOS, endothelial nitric oxide synthase; IL-10, interleukin-10; iNOS, inducible nitric oxide synthase; nNOS, neuronal nitric oxide synthase; TNBS, trinitrobenzene sulphonic acid.
McCafferty et al. have consistently shown that acute mucosal damage, which occurs early before iNOS up-regulation, is aggravated in iNOS-deficient mice in a TNBS–hapten colitis model, but observed no difference from wild-type mice in the chronic phase of colitis.87 This indicates that iNOS, expressed in small amounts in the mucosa of untreated wild-type mice in that study, may offer some protection in the early phase of the acute, non-specific chemically induced colitis. However, epithelial cells did not show iNOS expression in that study, which is a major difference from human IBD32 and other experimental colitis models.84 In marked contrast, in the study of Zingarelli et al., genetic ablation of iNOS suppressed nitrosative and oxidative damage and resulted in a significant resolution of mice with TNBS colitis in comparison to controls.84
Much more consistency is observed regarding studies on models of chronic DSS colitis. Both Krieglstein et al.73 and Hokari et al.88 have reported that clinical, macroscopic and microscopic DSS colitis was significantly attenuated in the iNOS-deficient animals compared to controls, and that this result was not affected by the housing conditions of the mutant mice. Furthermore, Hokari et al. have shown that infiltration with β7-positive lymphocytes and expression of mucosal adressin cell adhesion molecule-1 expression were significantly attenuated in iNOS−/− animals compared to wild-type animals.88
Expression of iNOS has been suggested to play a beneficial role in the recovery phase of tissue injury.92 Further supporting evidence comes from a study of iNOS-deficient mice in the experimental acetic acid colitis setting. In that study, a twofold increase in macroscopic damage was observed during the progression of mucosal injury in iNOS-deficient mice, whereas neutrophil infiltration and tissue oedema were similar to those in wild-type animals. The interesting finding was that at the time of resolution of the acute inflammation in the wild-type mice, damage score and myeloperoxidase levels were still elevated in iNOS-deficient animals.85
There are substantial data to suggest that the process of tissue healing and regeneration following damage is affected by both eNOS and iNOS. l-Arginine, the substrate of NOS, was first noted to enhance wound healing in 1978.93 In gastric ulcers, both iNOS and eNOS are up-regulated on days 3–6, i.e. at the initiation of ulcer healing.94 Ulcer healing has been reported to accelerate with the administration of NO donors, whereas it is retarded by treatment with NO inhibitors.95,96 Studies in the eNOS- and iNOS-deficient mice have shown that healing is impaired in both models, and the administration of l-arginine does not improve collagen deposition, whereas adenoviral replacement of the deficient gene does.97–99 This may be a result of the fact that chemoattractant cytokines primarily involved in tissue healing, such as IL-8 and transforming growth factor-β1 (TGF-β1), and collagen production by fibroblasts, are regulated by NO.100–102 In models of arterial injury, NO donors promote re-endothelialization, an effect which may be related to the angiogenetic properties of NO arising from the regulation of vascular endothelial growth factor (VEGF) angiogenetic activity.103,104
Initial studies have suggested that constitutive production of NO is essential for the regulation of epithelial permeability.16,18,105 Vallance et al. has recently confirmed the above observations in a model of TNBS colitis and has highlighted the importance of constitutive NO production for mucosal homeostasis. In his study, eNOS-deficient animals exhibited increased susceptibility to TNBS-induced injury, a reduction in the number of goblet cells, impaired mucin production and increased bacterial translocation, indicative of mucosal barrier dysfunction.91 Constitutive endothelial NOS has also been suggested to play a role in leucocyte–endothelial interactions.20–23 However, two studies in the eNOS-deficient mice have reported controversial results concerning the role of eNOS in DSS colitis experimental settings. Sasaki et al. reported increased disease activity and degree of leucocyte infiltration accompanied by higher levels of expression of the mucosal addressin, MAdCAM-1, in eNOS−/− mice compared to wild-type mice.90 On the other hand, Beck et al. reported that loss of either eNOS or iNOS was protective.89
In conclusion, it appears that NO production by either eNOS or iNOS plays a beneficial role in the acute non-specific colitis settings. However, in models of chronic colitis in the settings of a dysregulated immune response, where iNOS is persistently up-regulated, NO production seems to play a detrimental role on mucosal integrity. The role of constitutive and inducible NO production during healing is currently under investigation, while the reliability of existing models for the experimental evaluation of healing processes is still questionable.
Limitations of currently available models
There is an obvious contradiction arising through the study of findings which occurred during the past 10 years, or extensive and reliable research on the issue of nitric oxide in IBD. However, when trying to address these discrepancies, it must be taken into account that studies differ in species, strains, housing conditions, models and execution (reviewed extensively by Grisham et al.106 and Kubes et al.55). Moreover, the end result of pharmacological interventions in NO production during the course of colitis depends strongly on the time-points of intervention, the combination of pharmacological agents used, as well as the bioavailability of these agents.
Another major concern in the interpretation of the findings in iNOS- and eNOS-deficient mice is that gene deletion is not confined to the mucosa of the genetically modified animal and generally affects all cell units participating in tissue injury and repair. There is recent evidence to suggest that cellular source strongly modulates the beneficial versus the detrimental effect of NOS.107 Therefore, when choosing to therapeutically manipulate NO, one has to consider not only the NOS isoform involved in the functional response in question, but also the cell unit where this modification takes place.
Finally, extrapolation of data from animal colitis models to human IBD is questionable. The role of NO in the pathophysiology of IBD has been mostly studied using the traditional animal models based on chemical irritation, such as the TNBS and DSS colitis models. The TNBS model represents a Th1-like model of transmural inflammation and has been more closely associated with human Crohn's disease, whereas the DSS colitis model represents a Th1/Th2-like model of superficial inflammation, resembling UC, which has been more closely associated with iNOS up-regulation.108,109 Although these models recapitulate the events that lead to acute mucosal injury and can be useful in investigating epithelial response to injury, they do not adequately address those events that occur during the chronic phase of gut inflammation. For example, DSS colitis can be generated in the absence of lymphocytes.110 Furthermore, the fact that different initiating factors, such as TNBS, DDS or acetic acid, give similar results, suggests that the chemical colitis may be an unspecific, stereotype response.111 Therefore, these models may be useful for studying events that occur at the time of inflammation and repair, but they have significant limitations in facilitating the understanding of events that initiate and maintain inflammation in human IBD.79 Spontaneous models, such as the cotton-top tamarin colitis,111 the C3H/HeJBir colitis112 and the SAMP1/Yit ileitis,113 seem more attractive because, similarly to human disease, inflammation occurs without any apparent exogenous manipulations.114 Future studies on these model systems may facilitate our understanding of the complex pathogenetic role of NO in human IBD.
Conclusions and future perspectives
NO overproduction via iNOS up-regulation by intestinal epithelium has been consistently associated with IBD (especially UC and its severe complications such as toxic megacolon). However, the mechanism by which NO proceeds from being an indispensable homeostatic regulator to a harmful destructor remains unknown. Extensive work on experimental models of chemically induced colitis and NOS isoform knockout animals has reached some more or less definite conclusions, namely that some constitutive eNOS- or nNOS-derived NO production is beneficial in settings of acute mucosal injury, but chronic overproduction via sustained overexpression of iNOS may be detrimental. However, the specificity of pharmacological agents used, to date, to manipulate NO production is questionable and the extrapolation of data from animal colitis models to human IBD is problematic. It also has to be taken into consideration that NO production does not only represent the up-regulation of a single molecule but rather a family of species that react differently in different environmental conditions. Therefore, the observed discrepancies in the experimental evaluation of the importance of NO in IBD may not reflect the irrelevance of NO in the pathogenesis and evolution of human IBD, but rather the difficulties that occur while attempting to investigate the role of multieffector molecules in complex and multivariable disease models.
Thus, we believe that further studies, especially in experimental models of spontaneous colitis, which will examine both reactive oxygen and nitrogen metabolite pathways, may provide more definite information about the role of NO in human IBD. Until then we can be sure of the following – that NO overproduction in the settings of local or systemic inflammatory responses has been selected to occur because it provides the host with an overall survival advantage.
References
- 1.Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298:249–58. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stuehr DJ, Griffith OW. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- 3.Morris SM, Billiar TR. New insights into the regulation of inducible nitric oxide synthesis. Am J Physiol. 1994;266:E829–39. doi: 10.1152/ajpendo.1994.266.6.E829. [DOI] [PubMed] [Google Scholar]
- 4.Wink DA, Mitchell JB. Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med. 1998;25:434–56. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 5.Wink DA, Cook JA, Kim SY, et al. Superoxide modulates the oxidation and nitrosation of thiols by nitric oxide-derived reactive intermediates. Chemical aspects involved in the balance between oxidative and nitrosative stress. J Biol Chem. 1997;272:11147–51. doi: 10.1074/jbc.272.17.11147. [DOI] [PubMed] [Google Scholar]
- 6.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 7.Rubbo H, Radi R, Trujillo M, et al. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–75. [PubMed] [Google Scholar]
- 8.Grisham MB, Jourd'Heuil D, Wink DA. Nitric oxide – I. Physiological chemistry of nitric oxide and its metabolites: implications in inflammation. Am J Physiol Gastrointest Liver Physiol. 1999;39:G315–21. doi: 10.1152/ajpgi.1999.276.2.G315. [DOI] [PubMed] [Google Scholar]
- 9.Tamir S, Tannenbaum SR. The role of nitric oxide (NO) in the carcinogenic process. Biochim Biophys Acta. 1996;1288:F31–6. doi: 10.1016/0304-419x(96)00021-2. [DOI] [PubMed] [Google Scholar]
- 10.Stamler JS, Jia L, Eu JP, et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–7. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 11.Lander HM, Milbank AJ, Tauras JM, et al. Redox regulation of cell signalling. Nature. 1996;381:380–1. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- 12.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 13.Kubes P, Reinhardt PH, Payne D, Woodman RC. Excess nitric oxide does not cause cellular, vascular, or mucosal dysfunction in the cat small intestine. Am J Physiol. 1995;269:G34–41. doi: 10.1152/ajpgi.1995.269.1.G34. [DOI] [PubMed] [Google Scholar]
- 14.Moncada S. The 1991 Ulf von Euler Lecture. The l-arginine : nitric oxide pathway. Acta Physiol Scand. 1992;145:201–27. doi: 10.1111/j.1748-1716.1992.tb09359.x. [DOI] [PubMed] [Google Scholar]
- 15.Alican I, Kubes P. A critical role for nitric oxide in intestinal barrier function and disfunction. Am J Physiol. 1996;33:G225–37. doi: 10.1152/ajpgi.1996.270.2.G225. [DOI] [PubMed] [Google Scholar]
- 16.Kubes P. Nitric oxide modulates epithelial permeability in the feline small intestine. Am J Physiol. 1992;262:G1138–42. doi: 10.1152/ajpgi.1992.262.6.G1138. [DOI] [PubMed] [Google Scholar]
- 17.Kanwar S, Wallace JL, Befus D, Kubes P. Nitric oxide synthesis inhibition increases epithelial permeability via mast cells. Am J Physiol. 1994;266:G222–9. doi: 10.1152/ajpgi.1994.266.2.G222. [DOI] [PubMed] [Google Scholar]
- 18.Brown JF, Keates AC, Hanson PJ, Whittle BJ. Nitric oxide generators and cGMP stimulate mucus secretion by rat gastric mucosal cells. Am J Physiol. 1993;265:G418–22. doi: 10.1152/ajpgi.1993.265.3.G418. [DOI] [PubMed] [Google Scholar]
- 19.Wilson KT, Vaandrager AB, De Vente J, Musch MW, de Jonge HR, Chang EB. Production and localization of cGMP and PGE2 in nitroprusside-stimulated rat colonic ion transport. Am J Physiol. 1996;270:C832–40. doi: 10.1152/ajpcell.1996.270.3.C832. [DOI] [PubMed] [Google Scholar]
- 20.Banick PD, Chen Q, Xu YA, Thom SR. Nitric oxide inhibits neutrophil beta 2 integrin function by inhibiting membrane-associated cyclic GMP synthesis. J Cell Physiol. 1997;172:12–24. doi: 10.1002/(SICI)1097-4652(199707)172:1<12::AID-JCP2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 21.Davenpeck KL, Gauthier TW, Lefer AM. Inhibition of endothelial-derived nitric oxide promotes P-selectin expression and actions in the rat microcirculation. Gastroenterology. 1994;107:1050–8. doi: 10.1016/0016-5085(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 22.Sato E, Simpson KL, Grisham MB, Koyama S, Robbins RA. Effects of reactive oxygen and nitrogen metabolites on MCP-1-induced monocyte chemotactic activity in vitro. Am J Physiol. 1999;277:L543–9. doi: 10.1152/ajplung.1999.277.3.L543. [DOI] [PubMed] [Google Scholar]
- 23.Sato E, Simpson KL, Grisham MB, Koyama S, Robbins RA. Reactive nitrogen and oxygen species attenuate interleukin-8-induced neutrophil chemotactic activity in vitro. J Biol Chem. 2000;275:10826–30. doi: 10.1074/jbc.275.15.10826. [DOI] [PubMed] [Google Scholar]
- 24.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987;2:1057–8. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 25.Huang FP, Niedbala W, Wei XQ, et al. Nitric oxide regulates Th1 cell development through the inhibition of IL-12 synthesis by macrophages. Eur J Immunol. 1998;28:4062–70. doi: 10.1002/(SICI)1521-4141(199812)28:12<4062::AID-IMMU4062>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 26.Forstermann U, Closs EI, Pollock JS, et al. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension. 1994;23:1121–31. doi: 10.1161/01.hyp.23.6.1121. [DOI] [PubMed] [Google Scholar]
- 27.Kurose I, Ebinuma H, Higuchi H, et al. Nitric oxide mediates mitochondrial dysfunction in hepatoma cells induced by non-activated Kupffer cells: evidence implicating ICAM-1-dependent process. J Gastroenterol Hepatol. 1995;10(Suppl. 1):S68–71. doi: 10.1111/j.1440-1746.1995.tb01803.x. [DOI] [PubMed] [Google Scholar]
- 28.Tepperman BL, Brown JF, Whittle BJ. Nitric oxide synthase induction and intestinal epithelial cell viability in rats. Am J Physiol. 1993;265:G214–G218. doi: 10.1152/ajpgi.1993.265.2.G214. [DOI] [PubMed] [Google Scholar]
- 29.Fukuo K, Inoue T, Morimoto S, et al. Nitric oxide mediates cytotoxicity and basic fibroblast growth factor release in cultured vascular smooth muscle cells. A possible mechanism of neovascularization in atherosclerotic plaques. J Clin Invest. 1995;95:669–76. doi: 10.1172/JCI117712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest. 1997;99:2818–25. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witthoft T, Eckmann L, Kim JM, Kagnoff MF. Enteroinvasive bacteria directly activate expression of iNOS and NO production in human colon epithelial cells. Am J Physiol. 1998;275:G564–71. doi: 10.1152/ajpgi.1998.275.3.G564. [DOI] [PubMed] [Google Scholar]
- 32.Kolios G, Rooney N, Murphy CT, Robertson DAF, Westwick J. Expression of inducible nitric oxide synthase activity in human colon epithelial cells: modulation by T-lymphocyte derived cytokines. Gut. 1998;43:56–63. doi: 10.1136/gut.43.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boughton-Smith NK. Pathological and therapeutic implications for nitric oxide in inflammatory bowel disease. J R Soc Med. 1994;87:312–4. doi: 10.1177/014107689408700602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Middleton SJ, Shorthouse M, Hunter JO. Increased nitric oxide synthesis in ulcerative colitis. Lancet. 1993;341:465–6. doi: 10.1016/0140-6736(93)90211-x. [DOI] [PubMed] [Google Scholar]
- 35.Boughton-Smith NK, Evans SM, Hawkey CJ, et al. Nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Lancet. 1993;342:338–40. doi: 10.1016/0140-6736(93)91476-3. [DOI] [PubMed] [Google Scholar]
- 36.Lundberg JO, Hellstrom PM, Lundberg JM, Alving K. Greatly increased luminal nitric oxide in ulcerative colitis. Lancet. 1994;344:1673–4. doi: 10.1016/s0140-6736(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 37.Kolios G, Brown Z, Robson RL, Robertson DAF, Westwick J. Inducible nitric oxide synthase activity and expression in a human colonic epithelial cell line, HT-29. Br J Pharmacol. 1995;116:2866–72. doi: 10.1111/j.1476-5381.1995.tb15938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singer II, Kawka DW, Scott S, et al. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996;111:871–85. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- 39.Godkin AJ, De Belder AJ, Villa L, et al. Expression of nitric oxide synthase in ulcerative colitis. Eur J Clin Invest. 1996;26:867–72. doi: 10.1111/j.1365-2362.1996.tb02131.x. [DOI] [PubMed] [Google Scholar]
- 40.Wright KL, Ward SG, Kolios G, Westwick J. Activation of phosphatidylinositol 3-kinase by interleukin-13. An inhibitory signal for inducible nitric-oxide synthase expression in epithelial cell line HT-29. J Biol Chem. 1997;272:12626–33. doi: 10.1074/jbc.272.19.12626. [DOI] [PubMed] [Google Scholar]
- 41.Sartor RB. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994;106:533–9. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- 42.Kolios G, Petoumenos C, Nakos A. Mediators of inflammation: production and implication in inflammatory bowel disease. Hepatogastroenterology. 1998;45:1601–9. [PubMed] [Google Scholar]
- 43.Kolios G, Wright KL, Linehan JD, Robertson DA, Westwick J. Interleukin-13 inhibits nitric oxide production in human colonic mucosa. Hepatogastroenterology. 2000;47:714–7. [PubMed] [Google Scholar]
- 44.Kubes P. Ischemia-reperfusion in feline small intestine: a role for nitric oxide. Am J Physiol. 1993;264:G143–9. doi: 10.1152/ajpgi.1993.264.1.G143. [DOI] [PubMed] [Google Scholar]
- 45.Hutcheson IR, Whittle BJ, Boughton-Smith NK. Role of nitric oxide in maintaining vascular integrity in endotoxin-induced acute intestinal damage in the rat. Br J Pharmacol. 1990;101:815–20. doi: 10.1111/j.1476-5381.1990.tb14163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng HB, Libby P, Liao JK. Induction and stabilization of I kappa B alpha by nitric oxide mediates inhibition of NF-kappa B. J Biol Chem. 1995;270:14214–9. doi: 10.1074/jbc.270.23.14214. [DOI] [PubMed] [Google Scholar]
- 47.MacNaughton WK, Cirino G, Wallace JL. Endothelium-derived relaxing factor (nitric oxide) has protective actions in the stomach. Life Sci. 1989;45:1869–76. doi: 10.1016/0024-3205(89)90540-7. [DOI] [PubMed] [Google Scholar]
- 48.Payne D, Kubes P. Nitric oxide donors reduce the rise in reperfusion-induced intestinal mucosal permeability. Am J Physiol. 1993;265:G189–95. doi: 10.1152/ajpgi.1993.265.1.G189. [DOI] [PubMed] [Google Scholar]
- 49.Clancy RM, Leszczynska-Piziak J, Abramson SB. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest. 1992;90:1116–21. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ribbons KA, Zhang X-J, Thompson JH, et al. Potential role of nitric oxide in a model of chronic colitis in rhesus macaques. Gastroenterology. 1995;108:705–11. doi: 10.1016/0016-5085(95)90442-5. [DOI] [PubMed] [Google Scholar]
- 51.Middleton SJ, Shorthouse M, Hunter JO. Relaxation of distal colonic circular smooth muscle by nitric oxide derived from human leukocytes. Gut. 1993;34:814–7. doi: 10.1136/gut.34.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res. 1994;305:253–64. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 53.Kennedy M, Denenberg AG, Szabo C, Salzman AL. Poly (ADP-ribose) synthetase activation mediates increased permeability induced by peroxynitrite in Caco-2BBe cells. Gastroenterology. 1998;114:510–8. doi: 10.1016/s0016-5085(98)70534-7. [DOI] [PubMed] [Google Scholar]
- 54.Sandoval M, Ronzio RA, Muanza DN, Clark DA, Miller MJ. Peroxynitrite-induced apoptosis in epithelial (T84) and macrophage (RAW 264.7) cell lines: effect of legume-derived polyphenols (phytolens) Nitric Oxide. 1997;1:476–83. doi: 10.1006/niox.1997.0160. [DOI] [PubMed] [Google Scholar]
- 55.Kubes P, McCafferty DM. Nitric oxide and intestinal inflammation. Am J Med. 2000;109:150–8. doi: 10.1016/s0002-9343(00)00480-0. [DOI] [PubMed] [Google Scholar]
- 56.Rachmilewitz D, Stamler JS, Karmeli F, et al. Peroxynitrite-induced rat colitis: a new model of colonic inflammation. Gastroenterology. 1993;105:1681–8. doi: 10.1016/0016-5085(93)91063-n. [DOI] [PubMed] [Google Scholar]
- 57.Sandoval M, Liu X, Mannick EE, Clark DA, Miller MJ. Peroxynitrite-induced apoptosis in human intestinal epithelial cells is attenuated by mesalamine. Gastroenterology. 1997;113:1480–8. doi: 10.1053/gast.1997.v113.pm9352850. [DOI] [PubMed] [Google Scholar]
- 58.Mourelle M, Casellas F, Guarner F, et al. Induction of nitric oxide synthase in colonic smooth muscle from patients with toxic megacolon. Gastroenterology. 1995;109:1497–502. doi: 10.1016/0016-5085(95)90636-3. [DOI] [PubMed] [Google Scholar]
- 59.Rachmilewitz D, Stamler JS, Bachwich D, Karmeli F, Ackerman Z, Podolsky DK. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Gut. 1995;36:718–23. doi: 10.1136/gut.36.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller MJ, Sadowska-Krowicka H, Chotinaruemol S, Kakkis JL, Clark DA. Amelioration of chronic ileitis by nitric oxide synthase inhibition. J Pharmacol Exp Ther. 1993;264:11–6. [PubMed] [Google Scholar]
- 61.Hogaboam CM, Jacobson K, Collins SM, Blennerhassett MG. The selective beneficial effects of nitric oxide inhibition in experimental colitis. Am J Physiol. 1995;268:G673–G684. doi: 10.1152/ajpgi.1995.268.4.G673. [DOI] [PubMed] [Google Scholar]
- 62.Rachmilewitz D, Karmeli F, Okon E, Bursztyn M. Experimental colitis is ameliorated by inhibition of nitric oxide synthase activity. Gut. 1995;37:247–55. doi: 10.1136/gut.37.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kiss J, Lamarque D, Delchier JC, Whittle BJ. Time-dependent actions of nitric oxide synthase inhibition on colonic inflammation induced by trinitrobenzene sulphonic acid in rats. Eur J Pharmacol. 1997;336:219–24. doi: 10.1016/s0014-2999(97)01246-6. [DOI] [PubMed] [Google Scholar]
- 64.Armstrong AM, Campbell GR, Gannon C, Kirk SJ, Gardiner KR. Oral administration of inducible nitric oxide synthase inhibitors reduces nitric oxide synthesis but has no effect on the severity of experimental colitis. Scand J Gastroenterol. 2000;35:832–8. doi: 10.1080/003655200750023200. [DOI] [PubMed] [Google Scholar]
- 65.Yoshida Y, Iwai A, Itoh K, et al. Role of inducible nitric oxide synthase in dextran sulphate sodium-induced colitis. Aliment Pharmacol Ther. 2000;14(Suppl. 1):26–32. doi: 10.1046/j.1365-2036.2000.014s1026.x. [DOI] [PubMed] [Google Scholar]
- 66.Hosoi T, Goto H, Arisawa T, et al. Role of nitric oxide synthase inhibitor in experimental colitis induced by 2,4,6-trinitrobenzene sulphonic acid in rats. Clin Exp Pharmacol Physiol. 2001;28:9–12. doi: 10.1046/j.1440-1681.2001.03388.x. [DOI] [PubMed] [Google Scholar]
- 67.Yamaguchi T, Yoshida N, Ichiishi E, Sugimoto N, Naito Y, Yoshikawa T. Differing effects of two nitric oxide synthase inhibitors on experimental colitis. Hepatogastroenterology. 2001;48:118–22. [PubMed] [Google Scholar]
- 68.Zingarelli B, Cuzzocrea S, Szabo C, Salzman AL. Mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, reduces trinitrobenzene sulfonic acid-induced colonic damage in rats. J Pharmacol Exp Ther. 1998;287:1048–55. [PubMed] [Google Scholar]
- 69.Nakamura H, Tsukada H, Oya M, et al. Aminoguanidine has both an anti-inflammatory effect on experimental colitis and a proliferative effect on colonic mucosal cells. Scand J Gastroenterol. 1999;34:1117–22. doi: 10.1080/003655299750024922. [DOI] [PubMed] [Google Scholar]
- 70.Obermeier F, Kojouharoff G, Hans W, Scholmerich J, Gross V, Falk W. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238–45. doi: 10.1046/j.1365-2249.1999.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kankuri E, Vaali K, Knowles RG, et al. Suppression of acute experimental colitis by a highly selective inducible nitric-oxide synthase inhibitor, N-[3-(aminomethyl) benzyl]acetamidine. J Pharmacol Exp Ther. 2001;298:1128–32. [PubMed] [Google Scholar]
- 72.Menchen LA, Colon AL, Moro MA, et al. N-(3-(aminomethyl)benzyl) acetamidine, an inducible nitric oxide synthase inhibitor, decreases colonic inflammation induced by trinitrobenzene sulphonic acid in rats. Life Sci. 2001;69:479–91. doi: 10.1016/s0024-3205(01)01139-0. [DOI] [PubMed] [Google Scholar]
- 73.Krieglstein CF, Cerwinka WH, Laroux FS, et al. Regulation of murine intestinal inflammation by reactive metabolites of oxygen and nitrogen: divergent roles of superoxide and nitric oxide. J Exp Med. 2001;194:1207–18. doi: 10.1084/jem.194.9.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller MJS, Thompson JH, Zhang X-J, et al. Role of inducible nitric oxide synthase expression and peroxynatrite formation in guinea pig ileitis. Gastroenterology. 1995;109:1475–83. doi: 10.1016/0016-5085(95)90633-9. [DOI] [PubMed] [Google Scholar]
- 75.Kankuri E, Asmawi MZ, Korpela R, Vapaatalo H, Moilanen E. Induction of iNOS in a rat model of acute colitis. Inflammation. 1999;23:141–52. doi: 10.1023/a:1020241028723. [DOI] [PubMed] [Google Scholar]
- 76.Salas A, Gironella M, Salas A, et al. Nitric oxide supplementation ameliorates dextran sulfate sodium-induced colitis in mice. Lab Invest. 2002;82:597–607. doi: 10.1038/labinvest.3780454. [DOI] [PubMed] [Google Scholar]
- 77.Fiorucci S, Antonelli E, Distrutti E, et al. NCX-1015a nitric-oxide derivative of prednisolone, enhances regulatory T cells in the lamina propria and protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice. Proc Natl Acad Sci USA. 2002;99:15770–5. doi: 10.1073/pnas.232583599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laszlo F, Whittle BJ, Moncada S. Time-dependent enhancement or inhibition of endotoxin-induced vascular injury in rat intestine by nitric oxide synthase inhibitors. Br J Pharmacol. 1994;111:1309–15. doi: 10.1111/j.1476-5381.1994.tb14887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laszlo F, Evans SM, Whittle BJ. Aminoguanidine inhibits both constitutive and inducible nitric oxide synthase isoforms in rat intestinal microvasculature in vivo. Eur J Pharmacol. 1995;272:169–75. doi: 10.1016/0014-2999(94)00637-m. [DOI] [PubMed] [Google Scholar]
- 80.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Szabo C, Ferrer-Sueta G, Zingarelli B, Southan GJ, Salzman AL, Radi R. Mercaptoethylguanidine and guanidine inhibitors of nitric-oxide synthase react with peroxynitrite and protect against peroxynitrite-induced oxidative damage. J Biol Chem. 1997;272:9030–6. doi: 10.1074/jbc.272.14.9030. [DOI] [PubMed] [Google Scholar]
- 82.Yamada Y, Marshall S, Specian RD, Grisham MB. A comparative analysis of two models of colitis in rats. Gastroenterology. 1992;102:1524–34. doi: 10.1016/0016-5085(92)91710-l. [DOI] [PubMed] [Google Scholar]
- 83.Garvey EP, Oplinger JA, Furfine ES, et al. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem. 1997;272:4959–63. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- 84.Zingarelli B, Szabo C, Salzman AL. Reduced oxidative and nitrosative damage in murine experimental colitis in the absence of inducible nitric oxide synthase. Gut. 1999;45:199–209. doi: 10.1136/gut.45.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCafferty DM, Mudgett JS, Swain MG, Kubes P. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology. 1997;112:1022–7. doi: 10.1053/gast.1997.v112.pm9041266. [DOI] [PubMed] [Google Scholar]
- 86.McCafferty DM, Miampamba M, Sihota E, Sharkey KA, Kubes P. Role of inducible nitric oxide synthase in trinitrobenzene sulphonic acid induced colitis in mice. Gut. 1999;45:864–73. doi: 10.1136/gut.45.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCafferty DM, Sihota E, Muscara M, Wallace JL, Sharkey KA, Kubes P. Spontaneously developing chronic colitis in IL-10/iNOS double-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2000;279:G90–G99. doi: 10.1152/ajpgi.2000.279.1.G90. [DOI] [PubMed] [Google Scholar]
- 88.Hokari R, Kato S, Matsuzaki K, et al. Reduced sensitivity of inducible nitric oxide synthase-deficient mice to chronic colitis. Free Radic Biol Med. 2001;31:153–63. doi: 10.1016/s0891-5849(01)00565-2. [DOI] [PubMed] [Google Scholar]
- 89.Beck PL, Xavier R, Wong J, et al. Paradoxical roles of different nitric oxide synthase isoforms in colonic injury. Am J Physiol Gastrointest Liver Physiol. 2004;286:G137–47. doi: 10.1152/ajpgi.00309.2003. [DOI] [PubMed] [Google Scholar]
- 90.Sasaki M, Bharwani S, Jordan P, et al. Increased disease activity in eNOS-deficient mice in experimental colitis. Free Radic Biol Med. 2003;35:1679–87. doi: 10.1016/j.freeradbiomed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 91.Vallance BA, Dijkstra G, Qiu B, et al. Relative contributions of nitric oxide synthase (NOS) isoforms during experimental colitis: endothelial derived NOS maintains mucosal integrity. Am J Physiol Gastrointest Liver Physiol. 2004;287:6865–79. doi: 10.1152/ajpgi.00187.2004. [DOI] [PubMed] [Google Scholar]
- 92.Miampamba M, Sharkey KA. Temporal distribution of neuronal and inducible nitric oxide synthase and nitrotyrosine during colitis in rats. Neurogastroenterol Motil. 1999;11:193–206. doi: 10.1046/j.1365-2982.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- 93.Seifter E, Rettura G, Barbul A, Levenson SM. Arginine: an essential amino acid for injured rats. Surgery. 1978;84:224–30. [PubMed] [Google Scholar]
- 94.Ma L, Wallace JL. Endothelial nitric oxide synthase modulates gastric ulcer healing in rats. Am J Physiol Gastrointest Liver Physiol. 2000;279:G341–G346. doi: 10.1152/ajpgi.2000.279.2.G341. [DOI] [PubMed] [Google Scholar]
- 95.Konturek SJ, Brzozowski T, Majka J, Pytko-Polonczyk J, Stachura J. Inhibition of nitric oxide synthase delays healing of chronic gastric ulcers. Eur J Pharmacol. 1993;239:215–7. doi: 10.1016/0014-2999(93)90997-v. [DOI] [PubMed] [Google Scholar]
- 96.Elliott SN, McKnight W, Cirino G, Wallace JL. A nitric oxide-releasing nonsteroidal anti-inflammatory drug accelerates gastric ulcer healing in rats. Gastroenterology. 1995;109:524–30. doi: 10.1016/0016-5085(95)90341-0. [DOI] [PubMed] [Google Scholar]
- 97.Shi HP, Efron DT, Most D, Tantry US, Barbul A. Supplemental dietary arginine enhances wound healing in normal but not inducible nitric oxide synthase knockout mice. Surgery. 2000;128:374–8. doi: 10.1067/msy.2000.107372. [DOI] [PubMed] [Google Scholar]
- 98.Yamasaki K, Edington HD, McClosky C, et al. Reversal of impaired wound repair in iNOS-deficient mice by topical adenoviral-mediated iNOS gene transfer. J Clin Invest. 1998;101:967–71. doi: 10.1172/JCI2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee PC, Salyapongse AN, Bragdon GA, et al. Impaired wound healing and angiogenesis in eNOS-deficient mice. Am J Physiol. 1999;277:H1600–H1608. doi: 10.1152/ajpheart.1999.277.4.H1600. [DOI] [PubMed] [Google Scholar]
- 100.Villarete LH, Remick DG. Nitric oxide regulation of IL-8 expression in human endothelial cells. Biochem Biophys Res Commun. 1995;211:671–6. doi: 10.1006/bbrc.1995.1864. [DOI] [PubMed] [Google Scholar]
- 101.Vodovotz Y, Chesler L, Chong HY, et al. Regulation of transforming growth factor beta 1 by nitric oxide. Cancer Res. 1999;59:2142–9. [PubMed] [Google Scholar]
- 102.Witte MB, Thornton FJ, Efron DT, Barbul A. Enhancement of fibroblast collagen synthesis by nitric oxide. Nitric Oxide. 2000;4:572–82. doi: 10.1006/niox.2000.0307. [DOI] [PubMed] [Google Scholar]
- 103.Ziche M, Morbidelli L, Masini E, Granger H, Geppetti P, Ledda F. Nitric oxide promotes DNA synthesis and cyclic GMP formation in endothelial cells from postcapillary venules. Biochem Biophys Res Commun. 1993;192:1198–203. doi: 10.1006/bbrc.1993.1543. [DOI] [PubMed] [Google Scholar]
- 104.Xiong M, Elson G, Legarda D, Leibovich SJ. Production of vascular endothelial growth factor by murine macrophages: regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway. Am J Pathol. 1998;153:587–98. doi: 10.1016/S0002-9440(10)65601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alican I, Kubes P. A critical role for nitric oxide in intestinal barrier function and dysfunction. Am J Physiol. 1996;270:G225–37. doi: 10.1152/ajpgi.1996.270.2.G225. [DOI] [PubMed] [Google Scholar]
- 106.Grisham MB, Pavlick KP, Laroux FS, Hoffman J, Bharwani S, Wolf RE. Nitric oxide and chronic gut inflammation: controversies in inflammatory bowel disease. J Invest Med. 2002;50:272–83. doi: 10.2310/6650.2002.33281. [DOI] [PubMed] [Google Scholar]
- 107.Poon BY, Raharjo E, Patel KD, Tavener S, Kubes P. Complexity of inducible nitric oxide synthase: cellular source determines benefit versus toxicity. Circulation. 2003;108:1107–12. doi: 10.1161/01.CIR.0000086321.04702.AC. [DOI] [PubMed] [Google Scholar]
- 108.Shibata Y, Taruishi M, Ashida T. Experimental ileitis in dogs and colitis in rats with trinitrobenzene sulfonic acid – colonoscopic and histopathologic studies. Gastroenterol Jpn. 1993;28:518–27. doi: 10.1007/BF02776950. [DOI] [PubMed] [Google Scholar]
- 109.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 110.Dieleman LA, Palmen MJ, Akol H, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–91. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim HS, Berstad A. Experimental colitis in animal models. Scand J Gastroenterol. 1992;27:529–37. doi: 10.3109/00365529209000116. [DOI] [PubMed] [Google Scholar]
- 112.Sundberg JP, Elson CO, Bedigian H, Birkenmeier EH. Spontaneous, heritable colitis in a new substrain of C3H/HeJ mice. Gastroenterology. 1994;107:1726–35. doi: 10.1016/0016-5085(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 113.Matsumoto S, Okabe Y, Setoyama H, et al. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43:71–8. doi: 10.1136/gut.43.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pizarro TT, Arseneau KO, Bamias G, Cominelli F. Mouse models for the study of Crohn's disease. Trends Mol Med. 2003;9:218–22. doi: 10.1016/s1471-4914(03)00052-2. [DOI] [PubMed] [Google Scholar]