Abstract
Poly(ADP-ribose) polymerase-1 (PARP-1) is activated in response to DNA injury in the nucleus of eukaryotic cells and has been implicated in intestinal barrier dysfunction during inflammatory bowel diseases. In this study we investigated whether PARP-1 may regulate the inflammatory response of experimental colitis at the level of signal transduction mechanisms. Mice genetically deficient of PARP-1 (PARP-1−/−) and wild-type littermates were subjected to rectal instillation of trinitrobenzene sulphonic acid (TNBS). Signs of inflammation were monitored for 14 days. In wild-type mice, TNBS treatment resulted in colonic ulceration and marked apoptosis, which was associated with decreased colon content of the antiapoptotic protein Bcl-2, whereas the proapoptotic Bax was unchanged. Elevated levels of plasma nitrate/nitrite, metabolites of nitric oxide (NO), were also found. These inflammatory events were associated with activation of c-Jun-NH2 terminal kinase (JNK), phosphorylation of c-Jun and activation of the nuclear transcription factor activator protein-1 (AP-1) in the colon. In contrast, PARP-1−/− mice exhibited a significant reduction of colon damage and apoptosis, which was associated with increased colonic expression of Bcl-2 and lower levels of plasma nitrate/nitrite when compared to wild-type mice. Amelioration of colon damage was associated with a significant reduction of the activation of JNK and reduction of the DNA binding of AP-1. The data indicate that PARP-1 exerts a pathological role in colitis possibly by regulating the early stress-related transcriptional response through a positive modulation of the AP-1 and JNK pathways.
Keywords: c-Jun-NH2-terminal kinase, c-Jun, Bcl-2, Bax, colitis
Introduction
In inflammatory bowel diseases (IBD), such as Crohn's disease and ulcerative colitis, alteration of epithelial function is associated with an aberrant production of reactive oxygen and nitrogen species, which can promote structural alteration of DNA.1–3 The role of oxidative DNA damage has been emphasized as a major pathogenetic event in eukaryotic cells through activation of the nuclear enzyme poly(ADP-ribose) polymerase-1 (PARP-1). PARP-1 is a chromatin-associated enzyme, which is activated by stranded DNA nicks and breaks in damaged cells and it modifies a wide variety of nuclear proteins through attachment of poly(ADP-ribose) units.4 Excessive activation of PARP-1 depletes the cellular pools of NAD+, ATP, and other high-energy phosphates leading to the loss of cell membrane integrity and viability. On the contrary, inhibition of poly(ADP-ribosyl)ation preserves the cellular energy pool, thus preventing metabolic failure and providing cytoprotection.5,6 In previous in vivo studies we have demonstrated that genetic ablation of PARP-1 ameliorates the pathophysiological changes of experimental colitis.7 The hypothesis that PARP-1 plays a detrimental role in the development and persistence of tissue damage has been further supported by several other studies reporting beneficial effects of inhibitors of this nuclear polymerase in experimental models of colitis.8–10
During inflammation, oxidative and nitrosative stress represents an important signal for the activation of c-Jun-NH2-terminal kinase (JNK). This stress-regulated protein relays signals from the oxidant extracellular stimuli to the cell nucleus leading to the activation of the transcription factor activator protein-1 (AP-1). Activation of AP-1 has been shown to results in adaptive modifications of the damaged cells such as the expression of genes for pro-inflammatory mediators and cellular death by apoptosis.11
It has been suggested that apoptotic cell death may also be controlled by the Bcl-2 family members. The Bcl-2 family of proteins includes both inhibitors, such as Bcl-2 itself, and inducers of apoptosis, such as Bax. The balance between antiapoptotic and pro-apoptotic members has been proposed to be critical to determining if a cell undergoes to apoptosis.12,13
To provide insight into the mechanism, by which inhibition of PARP-1 may afford protection in colon inflammation, we have compared the signal transduction profile of AP-1 of PARP-1-deficient (PARP-1−/−) and wild-type mice subjected to hapten-induced colitis. Furthermore, we determined the extent of apoptosis and we evaluated the expression of Bcl-2 and Bax proteins in the colon. The results indicate that PARP-1-induced changes of AP-1, JNK and apoptotic modulators may contribute to the intestinal derangement during inflammation.
Materials and methods
Animals
PARP-1−/− mice and their wild-type littermates PARP-1+/+ (129/SV × C57BL/6, 20–22 g) were housed in a room with controlled temperature (22°) and 12-hr light/dark cycle. The animals were food-fasted 24 hr before experimentation and allowed food and water ad libitum after the induction of colitis.
Induction of colitis
The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by US National Institutes of Health (NIH Publication no. 85–23 revised 1996) and commenced with the approval of the Institutional Animal Care and Use Committee. Colitis was induced by using a technique of hapten-induced colonic inflammation as previously described.7 A 3.5F catheter was inserted into the colon of fasted mice via the anus until approximately the splenic flexure (4 cm from the anus). 2,4,6-Trinitrobenzene sulphonic acid (TNBS, 1 mg/mouse) was dissolved in 50% ethanol (v/v) and injected (0·2 ml) into the colon via the rubber cannula. Animals were then kept in a vertical position for 30 s and returned to their cages. In a group of mice, occurrence of diarrhoea and survival was monitored for 14 days. In a second group, mice were killed at 1, 3, 7 and 14 days after TNBS administration and a segment of the colon 4 cm long was excised for the evaluation of macroscopic damage. Tissue segments 1 cm in length were then fixed in 10% buffered formalin or immediately frozen in liquid nitrogen and stored at −70° for the histological, immunohistochemical and biochemical studies described below.
Evaluation of colonic damage
After removal, the colon was gently rinsed with saline solution, opened by a longitudinal incision and immediately examined under microscope. The visible colonic damage was assessed by a semiquantitative scoring system.7 The following morphological criteria were taken under consideration: score 0, no damage; score 1, localized hyperaemia without ulcers; score 2, linear ulcers, with no significant inflammation; score 3, linear ulcers with inflammation at one site; score 4, two or more major sites of ulceration and/or inflammation; score 5, two or more sites of inflammation and ulceration extending >1 cm along the length of the colon; score 6–8, 1 point is added for each cm of ulceration beyond an initial 2 cm. All measurements of damage were performed by two observers blinded to the experimental protocol.
Determination of apoptosis
Cell death by apoptosis in the inflamed colon was evaluated by measurement of oligonucleosomal DNA fragments by a histochemical TdT ‘Tunel’-like staining (TdT-FragEL kit, Oncogene Research Products, Cambridge, MA). Briefly, paraffin-embedded sections, after deparaffination, were permeabilized with protease K (2 mg/ml) in 10 mm Tris (pH 8) at room temperature for 20 min. Endogenous peroxidase was quenched with 3% H2O2 in methanol for 5 min. Sections were incubated with a reaction buffer composed by biotin-dCTP and unlabeled dCTP and TdT enzyme (terminal deoxynucleotidyl transferase) in a humidified chamber at 37°. In this assay TdT binds to exposed 3′OH ends of DNA fragments and catalyses the addition of biotin-labelled and unlabelled deoxynucleotides. Biotinylated nucleotides were then detected using a streptavidin–horseradish peroxidase conjugate and diaminobenzidine.14
Measurement of nitrite/nitrate production
Nitrite/nitrate production, an indicator of NO synthesis, was measured in plasma samples as previously described.15 Nitrate in the plasma was reduced to nitrite by incubation for 3 hr with nitrate reductase (670 mU/ml) and NADPH (160 mm) at room temperature. Nitrite concentration in the samples was then measured by the Griess reaction, by adding 100 µl of Griess reagent (0·1% naphthalethylenediamine dihydrochloride in H2O and 1% sulphanilamide in 5% concentrated H3PO4; vol 1 : 1) to 100 µl samples. The optical density at 550 nm (OD550) was measured using a Spectramax 250 microplate reader (Molecular Devices, Sunnyvale, CA). Nitrate concentrations were calculated by comparison with OD550 of standard solutions of sodium nitrate prepared in saline solution.
Subcellular fractionation and nuclear protein extraction
Tissue samples from colons were homogenized with a Polytron homogenizer in a buffer containing 0·32 m sucrose, 10 mm tris-HCl, pH 7·4, 1 mm ethyleneglycoltetraacetic acid (EGTA), 2 mm ethylenediaminetetraacetic acid (EDTA), 5 mm NaN3, 10 mmβ-mercaptoethanol, 20 µm leupeptin, 0·15 µm pepstatin A, 0·2 mm phenylmethylsulphonyl fluoride (PMSF), 50 mm NaF, 1 mm sodium orthovanadate, 0·4 nm microcystin. The homogenates were centrifuged (1000 g, 10 min) and the supernatant (cytosol + membrane extract) was collected to evaluate contents of Bax, Bcl-2 and inducible nitric oxide synthase (iNOS). The pellets were solubilized in Triton buffer (1% Triton-X-100, 150 mm NaCl, 10 mm Tris-HCl, pH 7·4, 1 mm EGTA, 1 mm EDTA, 0·2 mm sodium orthovanadate, 20 µm leupeptin A, 0·2 mm PMSF). The lysates were centrifuged (15 000 g, 30 min, 4°), and the supernatant (nuclear extract) was collected to evaluate the content of phosphorylated c-Jun, and activity of JNK and AP-1.
Electrophoretic mobility shift assay
Electrophoretic mobility shift assays were performed as previously described.16 An oligonucleotide probe corresponding to AP-1 consensus sequence (5′-CGC TTG ATG ACT CAG CCG GAA-3′) was labelled with γ-[32P]ATP using T4 polynucleotide kinase and purified in Bio-Spin chromatography columns (Bio-Rad, Hercules, CA). Ten µg of nuclear protein were preincubated with EMSA buffer (12 mm HEPES pH 7·9, 4 mm Tris-HCl pH 7·9, 25 mm KCl, 5 mm MgCl2, 1 mm EDTA, 1 mm dithiothreitol, 50 ng/ml poly [d(I-C)], 12% glycerol v/v, and 0·2 mm PMSF) on ice for 10 min before addition of the radiolabelled oligonucleotide for an additional 10 min. Excess of unlabelled oligonucleotide was added in some samples for competition to verify the specificity of AP-1 binding (data not shown). Protein–nucleic acid complexes were resolved using a non-denaturing polyacrylamide gel consisting of 5% acrylamide (29 : 1 ratio of acrylamide : bisacrylamide) and run in 0·5× TBE (45 mm Tris-HCl, 45 mm boric acid, 1 mm EDTA) for 1 hr at constant current (30 mA). Gels were transferred to Whatman 3M paper, dried under a vacuum at 80° for 1 hr, and exposed to photographic film at −70° with an intensifying screen. Densitometric analysis was performed using ImageQuant (Molecular Dynamics, Sunnyvale, CA).
Assay of JNK activity
JNK activity was determined by immune complex kinase assay and was estimated as the ability to phosphorylate glutathione-S-transferase (GST)-c-Jun. 16 Tissue samples were homogenized with a Polytron homogenizer in a buffer containing 0·32 m sucrose, 10 mm Tris-HCl, pH 7·4, 1 mm EGTA, 2 mm EDTA, 5 mm NaN3, 10 mmβ-mercaptoethanol, 20 µm leupeptin, 0·15 µm pepstatin A, 0·2 mm PMSF, 50 mm NaF, 1 mm sodium orthovanadate, 0·4 nm microcystin. The homogenates were centrifuged (1000 g, 10 min) and the pellets were solubilized in Triton buffer (1% Triton-X-100, 150 mm NaCl, 10 mm Tris-HCl, pH 7·4, 1 mm EGTA, 1 mm EDTA, 0·2 mm sodium orthovanadate, 20 µm leupeptin A, 0·2 mm PMSF). The lysates were centrifuged (15 000 g, 30 min, 4°), and the supernatant (nuclear extract) was collected. After immunoprecipitation of proteins (500 µg) with specific antibody directed to JNK1, the immunoprecipitate was incubated for 30 min at 30° in 40 µl of reaction buffer containing 25 mm HEPES (pH 7·6), 20 mm MgCl2, 20 mm glycerolphosphate, 0·1 mm sodium orthovanadate, 2 mm dithiothreitol, 25 µm ATP, and 5 µCi of [γ-32P]ATP. GST-c-Jun(1–79) (4 µg) was used as substrate. Reaction products were separated by sodium dodecyl sulphate (SDS)–polyacrylamide gel electrophoresis and visualized by autoradiography. Densitometric analysis was performed using ImageQuant.
Immunohistochemical staining for phosphorylated JNK
Expression of phosporylated JNK was detected in colonic sections by immunohistochemistry.16 Frozen sections 5 µm thick were fixed in 4% paraformaldehyde and incubated for 2 hr with a blocking solution (0·1 m phosphate buffered saline containing 0·1% Triton-X-100 and 2% normal goat serum) in order to minimize non-specific adsorption. Sections were then incubated overnight with 1 : 1000 dilution of primary anti-JNK1 antibody or with control solutions. Controls included buffer alone or non-specific purified rabbit immunoglobulin G (IgG) to rule out any non-specific binding. Specific labelling was detected by incubating for 30 min with a biotin-conjugated goat anti-rabbit IgG and amplified with avidin–biotin peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA) after quenching endogenous peroxidase with 0·3% H2O2 in 100% methanol for 15 min. Diaminobenzidine was used as a chromogen.
Western blot analyses
Cytosol expression of Bax, Bcl-2 and iNOS, and nuclear expression of phosphorylated c-Jun were determined by immunoblot analyses. Cytosol and nuclear extracts were boiled in loading buffer (125 mm Tris-HCl, pH 6·8, 4% SDS, 20% glycerol, and 10% 2-mercaptoethanol) and 50 µg of protein were loaded per lane on an 8–16% Tris–glycine gradient gel. Proteins were separated electrophoretically and transferred to nitrocellulose membranes. For immunoblotting, membranes were blocked with 5% non-fat dried milk in Tris-buffered saline (TBS) for 1 hr and then incubated with primary antibodies against Bax, Bcl-2, iNOS, or phosphorylated c-Jun for 1 hr. The membrane were washed in TBS with 0·1% Tween 20 and incubated with secondary peroxidase-conjugated antibody. Immunoreaction was visualized by chemiluminescence. Densitometric analysis of blots was performed using ImageQuant.
Materials
The primary antibodies directed at Bcl-2, Bax, JNK1, iNOS and the oligonucleotide probe for AP-1 consensus were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The primary antibody directed at c-Jun phosphorylated at serine 63 and 73 was obtained from New England Biolabs, Inc. (Beverly, MA). All other chemicals were from Sigma/Aldrich (St. Louis, MO).
Data analysis
All values in the figures and text are expressed as mean ± standard error of the mean (SEM) of n observations, where n represents the number of mice (n = 4–12 animals for each group). Data sets were examined by one- and two-way analysis of variance followed by Bonferroni's correction post hoc t-test. Statistical analysis of scores was performed using the Mann–Whitney U-test. A P-value less than 0·05 was considered significant.
Results
Genetic deficiency of PARP-1 reduces severity of colitis and epithelial apoptosis in inflamed colon
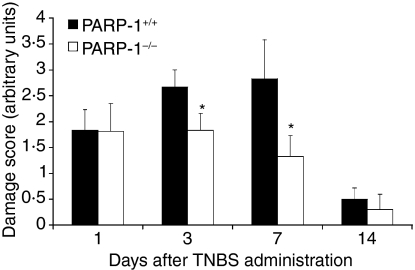
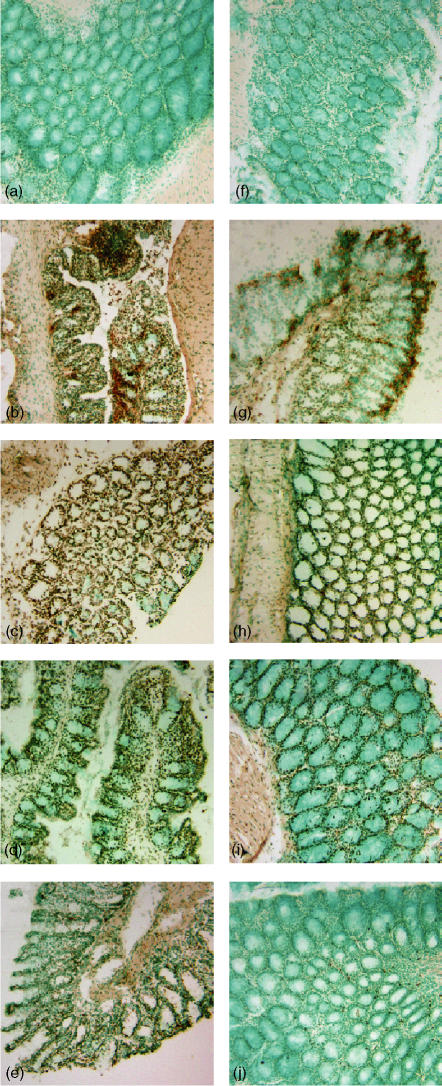
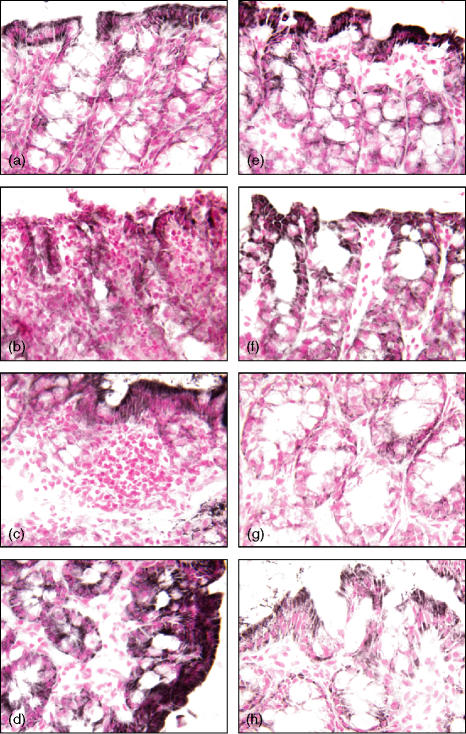
Wild-type mice exhibited serious bloody diarrhoea as early as 1 day after administration of TNBS. Macroscopic evaluation of the distal colon and rectum after TNBS treatment revealed the presence of mucosal oedema and haemorrhagic ulcerations up to 7–14 days. In contrast, PARP-1−/− mice appeared healthy and exhibited a very mild diarrhoea. The mucosal surface of the colon and rectum showed localized erythema and oedema up to 3–7 days and no damage at 14 days (see Fig. 1 for damage score). This result is consistent with our previous findings showing a faster resolution of colonic damage in the absence of the polymerase enzyme.7 To test whether tissue damage was associated with cell death by apoptosis, we measured oligonucleosomal DNA fragmentation in inflamed colon (Fig. 2). Almost no apoptotic cells were detectable in colon of control mice (i.e. not subjected to TNBS administration). At 1 and 3 days after TNBS administration, tissues obtained from wild-type mice demonstrated a marked appearance of dark brown apoptotic cells and intercellular apoptotic fragments mostly localized in the deranged epithelium and in infiltrated inflammatory cells. At 7 and 14 days, apoptosis persisted mainly in the healing epithelium. In contrast, tissues obtained from PARP-1−/− mice demonstrated a small number of apoptotic cells or fragments organized at the surface of the epithelial mucosa at 1 and 3 days after TNBS administration. At 7 and 14 days apoptosis persisted in some infiltrated inflammatory cells in the restored colonic architecture (Fig. 2).
Figure 1.
Damage score in PARP-1+/+ and PARP-1−/− mice after TNBS intracolonic administration. Each data point is the mean ± SEM of 6–12 animals for each group. *P < 0·05 versus wild-type (PARP-1+/+) mice.
Figure 2.
Effect of genetic deficiency of PARP-1 on time course of changes in colonic apoptosis after TNBS administration. Representative colonic sections from a wild-type sham (a) or PARP-1−/− (f) mouse showed negative staining (day 0). In wild-type mice, at day 1 (b), 3 (c) and 7 (d) after TNBS administration a brown staining revealed the presence of apoptosis in the epithelium and infiltrated inflammatory cells. At day 14 (e) apoptotic epithelial cells were still present in the healing mucosa. Epithelial apoptosis staining was reduced in PARP-1−/− at day 1 (g), 3 (h) and 7 (i); at day 14 (j) apoptosis staining was observed in some infiltrated inflammatory cells. Magnification ×100.
Genetic deficiency of PARP-1 reduces iNOS expression and NO production
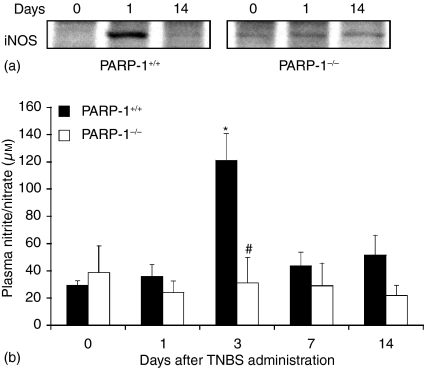
Production of NO from iNOS during colitis has been suggested to contribute significantly to tissue damage.15,17,18 Therefore, we next determined NO production and colonic expression of iNOS. In wild-type mice, an early increase of colonic expression of iNOS (Fig. 3a) was associated with elevated plasma levels of nitrite/nitrate, stable metabolites of NO (Fig. 3b), at 1 and 3 days after TNBS administration and declined thereafter. In PARP-1−/− mice colonic expression of iNOS and plasma levels of nitrite/nitrate were markedly reduced (Fig. 3).
Figure 3.
Effect of genetic deficiency of PARP-1 on NO production. (a)Representative Western blot analysis for iNOS. (b)Plasma levels of nitrite/nitrate. Each data point represents the mean ± SEM of four to six animals for each group. *P < 0·05 versus day 0 of the same genotype; #P < 0·05 versus wild-type (PARP-1+/+) mice.
Genetic deficiency of PARP-1 alters the ratio between Bcl-2 and Bax
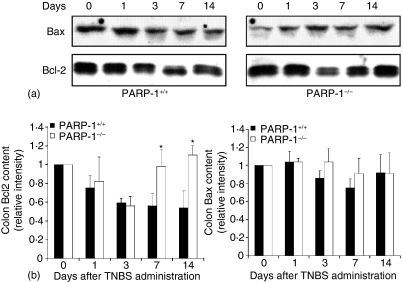
Since apoptosis is regulated at the expression of antiapoptotic proteins, such as Bcl-2, and pro-apoptotic proteins, such as Bax12,13 we next investigated colonic expression of Bcl-2 and Bax by Western blotting. The antiapoptotic protein Bcl-2 diminished as early as 1 and 3 days after TNBS administration in colons of wild-type mice, further declining thereafter. In PARP-1−/− mice expression of Bcl-2 exhibited a similar early decrease 1 and 3 days after TNBS administration; however, the expression of the antiapoptotic protein was significantly increased at 7 and 14 days when compared to wild-type mice (Fig. 4). Expression of the pro-apoptotic Bax remained unchanged after TNBS administration in mice of both genotypes (Fig. 4).
Figure 4.
Effect of genetic deficiency of PARP-1 on cytosol expression of Bcl-2 and Bax in the colon. (a) Representative Western blot analysis for Bcl-2 and Bax. (b) Image analysis of Bcl-2 and Bax content determined by densitometry. Fold increase was calculated versus respective sham value (time 0) set to 1·0. *P < 0·05 versus wild-type (PARP-1+/+) mice.
Genetic deficiency of PARP-1 reduces activation of AP-1 pathway
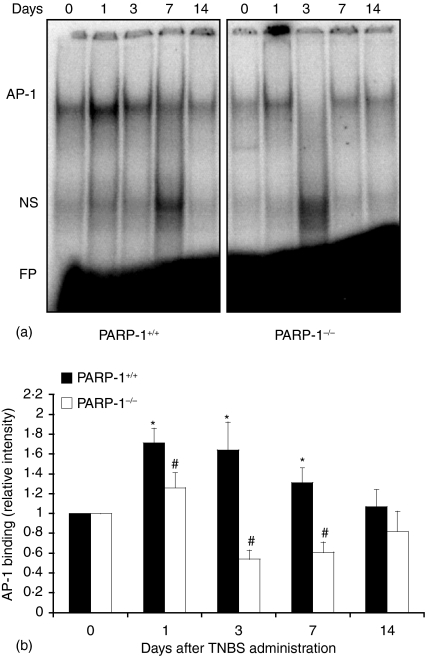
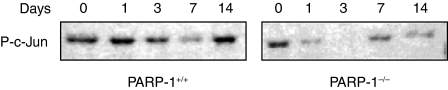
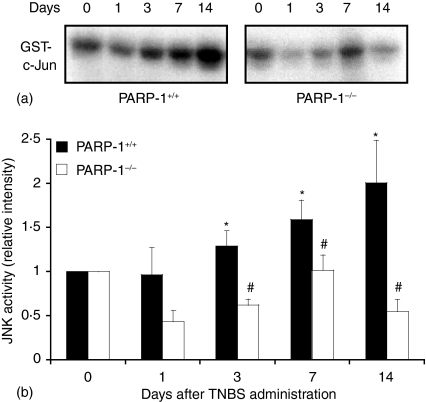
As activation of AP-1 has been implicated in inflammatory conditions and apoptosis11 we determined the nuclear activation of this factor. In wild-type mice DNA binding of AP-1 increased as early as 1–3 days after TNBS administration and declined thereafter. However, in PARP-1−/− mice the degree of activation AP-1 was significantly reduced when compared to wild-type mice (Fig. 5). Since the activity of AP-1 may be regulated at the availability of its subunits11 we determined the expression of phosphorylated c-Jun in nuclear extracts by Western blotting. The content of c-Jun, with phosphorylated sites at serine 63 and 73, increased in a biphasic manner with maximum expression at 1 and 14 days after TNBS administration in wild-type mice. In contrast, PARP-1−/− mice exhibited lower content of phosphorylated c-Jun in comparison to wild-type mice, thus suggesting that PARP-1 activation is required for the phosphorylation of this subunit (Fig. 6). Since phosphorylation of c-Jun is mediated by JNK11 we further determined the cellular localization and the nuclear activity of JNK. A time course analysis showed that JNK activity steadily increased after TNBS administration in wild-type mice (Fig. 7). Expression of the active phosphorylated form of JNK, evaluated by immunoreaction, was observed in epithelial cells of the mucosal brush in colonic sections from control wild-type and PARP-1−/− mice at day 0 (Fig. 8). However, in the area of ulcer formation in colonic sections from TNBS-treated wild-type mice, a marked positive immunostaining staining for the phosphorylated JNK was found mainly localized throughout the disrupted epithelium, whereas a weak or no staining was found in infiltrated inflammatory cells (Fig. 8). The degree of staining in the epithelium steadily increased in a time-dependent manner and well paralleled the increased phosphorylative catalytic activity as evaluated by the ‘in gel’ kinase assay (Fig. 7). In PARP-1−/− mice the expression and degree of activity of JNK was significantly reduced in comparison with wild-type mice (Figs 7 and 8).
Figure 5.
Effect of genetic deficiency of PARP-1 on the activation of AP-1 in the colon. (a) Representative autoradiograph of electrophoretic mobility shift assay for AP-1. (b) Image analysis of activation of AP-1 determined by densitometry from the autoradiograph. Results are representative of three separate time-course experiments. Fold increase was calculated versus respective sham value (time 0) set to 1·0. *P < 0·05 versus day 0 of the same genotype; #P < 0·05 versus wild-type (PARP-1+/+) mice. NS = non-specific binding; FP = free probe.
Figure 6.
Nuclear content of phosphorylated c-Jun as determined by Western blot analyses in wild-type (PARP-1+/+) and PARP-1−/− mice. Immunoreactive c-Jun was detected with a specific antibody against c-Jun phosphorylated at serine 63 and 73.
Figure 7.
Effect of genetic deficiency of PARP-1 on the activity of JNK in the colon. (a) Representative autoradiograph of analysis for JNK activity. (b) Image analyses of JNK activity determined by densitometry. Fold increase was calculated versus respective sham value (time 0) set to 1·0. Results are representative of three separate time-course experiments. *P < 0·05 versus day 0 of the same genotype; #P < 0·05 versus wild-type (PARP-1+/+) mice.
Figure 8.
Effect of genetic deficiency of PARP-1 on colonic expression of phosphorylated JNK after TNBS administration as evaluated by immunohistochemistry. Representative colonic sections from a wild-type sham (a) or PARP-1−/− (f) mouse showed positive staining (day 0) in epithelial cells of the mucosal surface. In wild-type mice, at day 1 (b), and 7 (c) after TNBS administration an intense brown staining revealed the increased expression of phosphorylated JNK in the deranged epithelium, but not in infiltrated inflammatory cells. At day 14 (d) a marked epithelial staining was still present in the healing mucosa. Epithelial staining of phosphorylated JNK was reduced in PARP-1−/− at day 1 (e), 7 (f) and 14 (g). Magnification ×400.
Discussion
It has been previously reported that genetic deficiency or pharmacological inhibition of PARP-1 confers resistance to colitis in rodents.7–9 The present study further demonstrates that deficiency of PARP-1 affords beneficial effects and that amelioration of damage is associated with reduction of apoptotic cell death. Furthermore, to our knowledge these are the first data demonstrating that the signal transduction pathway of AP-1 is activated during colitis and is regulated by PARP-1.
A hallmark of the pathophysiology of IBD is an exaggerated release of oxygen and nitrogen reactive species15,17–19 and an overproduction of pro-inflammatory mediators20 which are eventually responsible for cell dysfunction and death. A variety of these proinflammatory genes (i.e. cytokines and adhesion molecules) are regulated at the transcription level by AP-1.11 Several reports have documented a role for AP-1 activation in intestinal inflammation. Gonsky and colleagues21 have demonstrated that activation of lamina propria T cells from normal, ulcerative colitis, or Crohn's disease mucosa through the CD2 pathway leads to induction of AP-1 complexes that bind to the interleukin-2 promoter. Transcriptional activation of AP-1 is enhanced in immunostimulated human colonic epithelial cells.22 In addition, we have previously demonstrated that AP-1 DNA binding is increased during inflammation of small bowel induced by vascular impairment in IL-10 deficient mice (experimental animals prone to develop IBD).16 In our experimental model of colitis, we found that DNA binding activity of AP-1 is increased after TNBS administration. The increase in AP-1 DNA binding occurred at an earlier stage (i.e. 1–3 days after TNBS administration) and correlated with the severity of inflammation, while it declined at the initiation of the healing process.
AP-1 activity is regulated at two major levels: the abundance and stability of the AP-1 dimer components. The abundance of AP-1 proteins is regulated at the transcriptional level of the respective genes of its subunits such as c-Jun, c-Fos or activating transcription factors. In addition, the stability of c-Jun subunit can be modulated through phosphorylation on serine 63 and 73 by JNK.11 When examined at a more molecular level, the reduction of AP-1 activity observed in PARP-1−/− mice appears to be a downstream event of a reduced activity of JNK and, consequently of a reduced availability of c-Jun. Interestingly, the kinetics of JNK activation did not match the earlier activation of AP-1 and the biphasic occurrence of phosporylated c-Jun, as the enzyme activity steadily increased up to 14 days after TNBS administration in wild-type mice. These results suggest that JNK may also participate in the inflammatory process of colitis independently of its transcriptional effects on AP-1 and/or c-Jun phosporylation. In this regard, JNK activation has been proposed to be a proapoptotic event through direct phosphorylation of mitochondrial proteins, including Bcl-2 family members.23 Therefore, it is possible that the elevated and steady activity of JNK may be required for the activation of different signalling cascades, including apoptotic pathways at different stages of the inflammatory process and may differ from the temporal changes of c-Jun phosphorylation and/or AP-1 activation. Interestingly, in our study localization of phosphorylated JNK was predominant in disrupted epithelial cells, but not in inflammatory cells in wild-type mice. Although JNK and other stress-activated kinases are activated in macrophages and neutrophils during colitis24 enhanced expression and activation of JNK has also been observed in epithelial cells under inflammatory conditions.16,22 In a recent study enhanced activation of JNK has been observed predominantly in epithelial cells of inflamed mucosal biopsies of steroid-resistant patients with IBD and has been suggested to interfere with the anti-inflammatory effects of steroids. On the contrary, in steroid-sensitive patients, activation of p38, and JNK, along with nuclear factor-κB (NF-κB), AP-1, was mainly found in lamina propria macrophages and neutrophils.25 Therefore, it is possible that differential localization of JNK in epithelial or inflammatory cells may confer variability in susceptibility, resistance, immunological response and clinical course among different species of animals and patients. Nevertheless, our study demonstrates that PARP-1 is a requisite for a complete activation of the phosphorylative activity of this kinase in colitis. Similarly to these findings, we have also demonstrated that selective inhibitors of PARP-1 significantly decreased colon damage and cell apoptosis in a rat model of colitis. These protective effects were associated with inhibition of DNA binding of the transcription factors AP-1 and NF-κB in the inflamed colon.26
Our findings are in line with other reports demonstrating a role of poly(ADP-ribosyl)ation in signal transduction. It has been proposed that PARP-1 activation also plays a critical role in the regulation of transcription, possibly by participating in the repair of DNA strands breaks or by catalysing the poly(ADP-ribosyl)ation of transcription factors or post-translation proteins.27 For example, PARP-deficient cells are defective in NF-κB-dependent transcriptional activation and show a down-regulation of iNOS after genotoxic stress.28,29 We have also demonstrated that AP-1 DNA binding is completely abolished in PARP-1-deficient murine fibroblasts, most probably secondary to alterations of the AP-1 dimer phosphorylation.30 Similarly to our findings, Ha and collegueas31 have recently demonstrated that PARP-1-deficient glial cells lack their ability to induce AP-1 after endotoxin or cytokine challenge.
The reduction of the activation of the AP-1 pathway in PARP-1−/− may also explain the reduction of neutrophil recruitment into the site of inflammation. Accordingly, in our previous study we have demonstrated that in experimental colitis genetic ablation of PARP-1 completely abolished intercellular adhesion molecule-1 expression7 whose transcription is also dependent on AP-1 activation.30 In the current study, PARP-1 deficiency also abolished the colonic expression of iNOS and attenuated the increase in plasma NO metabolites. Our data are in agreement with previous findings demonstrating that abrogation of PARP activity leads to down-regulation of iNOS expression, most probably to alteration of transcription.32,33
The role of apoptotic cell death in the inflamed intestine has not yet been completely defined. In ulcerative colitis frequency of apoptosis is considerably increased34 and loss of epithelial cells appears to occur mainly by apoptosis.35 These findings have suggested that epithelial apoptosis may lead to a breakdown of the epithelial barrier function facilitating the invasion of pathogenic micro-organisms. On the other hand, cell death by apoptosis regulates the lymphocyte population and may terminate immune response at the inflammatory site. It has been proposed that an enhanced T-cell resistance against apoptosis may contribute to disease perpetuation and mucosal immune dysregulation in patients with Crohn's disease.36 T cells isolated from areas of inflammation in Crohn's disease and ulcerative colitis manifest decreased CD2 pathway-induced apoptosis.37 The resistance of T cells to apoptosis appears to be associated with a higher ratio of Bcl-2 to Bax.38 In our study, the amelioration of colon architecture observed in PARP-1−/− mice was associated with reduction of epithelial apoptotic death. The family of Bcl-2-related proteins constitutes the most relevant class of apoptotic regulators and, more specifically, the ratio of anti or proapoptotic proteins determines whether the cell will survive or die.12,13 An important observation of our study was that the expression of Bcl-2 was higher in PARP-1−/− mice when compared to wild-type mice, well paralleling the earlier repair of mucosal lesions. These data are in agreement with our recent observations that PARP-1 may regulate apoptotic regulators at the genetic level.39 However, the time-course of apoptotic cell death showed differences between wild-type and PARP-1−/− mice already at day 1, not paralleling the later changes in Bcl-2, NO production and JNK activity, thus suggesting that earlier changes in apoptosis may be regulated by other underlying signalling events. For example, many studies have shown that Fas/FasL is an important pathway of epithelial cell apoptosis in IBD. Fas, also named Apo-1 or CD95, has comprehensive expressions in normal colonic epithelium and can bind anti-Fas antibody or FasL to change the constitution of cell surface, leading to apoptosis of cells that express Fas.40 It has been demonstrated that high expression of FasL in patients with ulcerative colitis would accelerate migration and activity of neutrophils and lymphocytes and could cause apoptosis of epithelial cells expressing Fas.34,35,41 Furthermore, the expression of Fas and FasL, along with other proapoptotic proteins Bax and p53 has been shown to dramatically increase in rat model of chemical-induced colitis.42 Therefore, it is possible that altered regulation of other proapoptotic proteins may be different according to the stage of colonic injury. The changes in AP-1 and JNK activation and Bcl-2 expression may parallel later period of inflammation and the initiation of the healing process. Whether prolonged activation of JNK and epithelial apoptosis may also maintain the inflammatory process of chronic colitis needs further investigation.
In summary, our data demonstrated that in colitis epithelial apoptosis is due to imbalance between antiapoptotic and pro-apoptotic factors. In this regard, inhibition of PARP-1 may reduce ideal triggers for the apoptotic process by shifting the ratio of apoptotic regulators towards Bcl-2 along with reduction of JNK activity and the AP-1-dependent pro-inflammatory mediators. Thus, our data further support that specific targeting of PARP-1 pathway may be a new therapeutic approach to treat IBD.
Acknowledgments
Funding for this study was provided by the Crohn's & Colitis Foundation of America (First Award) Dr Basilia Zingarelli.
References
- 1.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species.role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lih-Brody L, Powell SR, Collier KP, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–86. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 3.D'Odorico A, Bortolan S, Cardin R, D'Inca′ R, Martines D, Ferronato A, Sturniolo GC. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand J Gastroenterol. 2001;36:1289–94. doi: 10.1080/003655201317097146. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard VJ, Rouleau M, Poirier GG. PARP-1, a determinant of cell survival in response to DNA damage. Exp Hematol. 2003;31:446–54. doi: 10.1016/s0301-472x(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Dawson VL, Dawson TM, Snyder SH. Nitric oxide activation of poly (ADP-ribose) synthetase in neurotoxicity. Science. 1994;263:687–9. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- 6.Zingarelli B, O'Connor M, Wong H, Salzman AL, Szabó C. Peroxynitrite-mediated DNA strand breakage activates poly-adenosine diphosphate ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipopolysaccharide. J Immunol. 1996;156:350–8. [PubMed] [Google Scholar]
- 7.Zingarelli B, Szabó C, Salzman AL. Blockade of poly (ADP-ribose) synthetase inhibits neutrophil recruitment, oxidant generation, and mucosal injury in murine colitis. Gastroenterology. 1999;116:335–45. doi: 10.1016/s0016-5085(99)70130-7. [DOI] [PubMed] [Google Scholar]
- 8.Mabley JG, Jagtap P, Perretti M, et al. Anti-inflammatory effects of a novel, potent inhibitor of poly (ADP-ribose) polymerase. Inflamm Res. 2001;50:561–9. doi: 10.1007/PL00000234. [DOI] [PubMed] [Google Scholar]
- 9.Mazzon E, Dugo L, Li JH, Di Paola R, Genovese T, Caputi AP, Zhang J, Cuzzocrea S. GPI 6150, a PARP inhibitor, reduces the colon injury caused by dinitrobenzene sulfonic acid in the rat. Biochem Pharmacol. 2002;64:327–37. doi: 10.1016/s0006-2952(02)01075-4. [DOI] [PubMed] [Google Scholar]
- 10.Jijon HB, Churchill T, Malfair D, Wessler A, Jewell LD, Parsons HG, Madsen KL. Inhibition of poly (ADP-ribose) polymerase attenuates inflammation in a model of chronic colitis. Am J Physiol. 2000;279:G641–51. doi: 10.1152/ajpgi.2000.279.3.G641. [DOI] [PubMed] [Google Scholar]
- 11.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–6. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 12.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–20. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 13.Sartorius U, Schmitz I, Krammer PH. Molecular mechanisms of death-receptor-mediated apoptosis. Chem Biochem. 2001;2:20–9. doi: 10.1002/1439-7633(20010105)2:1<20::AID-CBIC20>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 14.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zingarelli B, Szabó C, Salzman AL. Reduced oxidative and nitrosative damage in murine experimental colitis in the absence of inducible nitric oxide synthase. Gut. 1999;45:199–209. doi: 10.1136/gut.45.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zingarelli B, Yang Z, Hake PW, Denenberg A, Wong HR. Absence of endogenous interleukin-10 enhances early stress response during postischemic injury in mice intestine. Gut. 2001;5:610–22. doi: 10.1136/gut.48.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grisham MB, Pavlick KP, Laroux FS, Hoffman J, Bharwani S, Wolf RE. Nitric oxide and chronic gut inflammation: controversies in inflammatory bowel disease. J Invest Med. 2002;50:272–83. doi: 10.2310/6650.2002.33281. [DOI] [PubMed] [Google Scholar]
- 18.Pavlick KP, Laroux FS, Fuseler J, Wolf RE, Gray L, Hoffman J, Grisham MB. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med. 2002;33:311–22. doi: 10.1016/s0891-5849(02)00853-5. [DOI] [PubMed] [Google Scholar]
- 19.Simmonds NJ, Allen RE, Stevens TRJ, Van Someren RNM, Blake DR, Rampton DS. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology. 1992;103:186–96. doi: 10.1016/0016-5085(92)91112-h. [DOI] [PubMed] [Google Scholar]
- 20.Fiocchi C. Inflammatory bowel disease. Ethiol Pathogenesis Gastroenterol. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 21.Gonsky R, Deem RL, Hughes CC, Targan SR. Activation of the CD2 pathway in lamina propria T cells up-regulates functionally active AP-1 binding to the IL-2 promoter, resulting in messenger RNA transcription and IL-2 secretion. J Immunol. 1998;160:4914–22. [PubMed] [Google Scholar]
- 22.Abreu-Martin MT, Palladino AA, Faris M, Carramanzana NM, Nel AE, Targan SR. Fas activates the JNK pathway in human colonic epithelial cells: lack of a direct role in apoptosis. Am J Physiol. 1999;276:G599–605. doi: 10.1152/ajpgi.1999.276.3.G599. [DOI] [PubMed] [Google Scholar]
- 23.Schroeter H, Boyd CS, Ahmed R, Spencer JP, Duncan RF, Rice-Evans C, Cadenas E. c-Jun N-terminal kinase (JNK)-mediated modulation of brain mitochondria function: new target proteins for JNK signalling in mitochondrion-dependent apoptosis. Biochem J. 2003;372:359–69. doi: 10.1042/BJ20030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waetzig GH, Seegert D, Rosenstiel P, Nikolaus S, Schreiber S. p38 mitogen-activated protein kinase is activated and linked to TNF-α signaling in inflammatory bowel disease. J Immunol. 2002;168:5342–51. doi: 10.4049/jimmunol.168.10.5342. [DOI] [PubMed] [Google Scholar]
- 25.Bantel H, Schmitz ML, Raible A, Gregor M, Schulze-Osthoff K. Critical role of NF-κB and stress-activated protein kinases in steroid unresponsiveness. FASEB J. 2002;16:1832–4. doi: 10.1096/fj.02-0223fje. [DOI] [PubMed] [Google Scholar]
- 26.Zingarelli B, O'Connor M, Hake PW. Inhibitors of poly (ADP-ribose) polymerase modulate signal transduction pathways in colitis. Eur J Pharmacol. 2003;469:183–94. doi: 10.1016/s0014-2999(03)01726-6. [DOI] [PubMed] [Google Scholar]
- 27.Vispé S, Yung TM, Richot J, Serizawa H, Satoh MS. A cellular defense pathway regulating transcription through poly (ADP-ribosyl) ation in response to DNA damage. Proc Natl Acad Sci U S A. 2000;97:9886–91. doi: 10.1073/pnas.170280397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassa PO, Hottiger MO. A role of poly (ADP-ribose) polymerase in NF-κB transcriptional activation. Biol Chem. 1999;380:953–9. doi: 10.1515/BC.1999.118. [DOI] [PubMed] [Google Scholar]
- 29.Oliver FJ, Menissier-de Murcia J, Nacci C, et al. Resistance to endotoxic shock as a consequence of defective NF-κB activation in poly (ADP-ribose) polymerase-1 deficient mice. EMBO J. 1999;18:4446–54. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andreone TL, Denenberg A, O'Connor M, Hake PW, Zingarelli B. Poly (ADP-ribose) polymerase regulates activation of c-Jun-NH2-terminal kinase and activator protein-1 in murine fibroblasts. J Immunol. 2003;170:2113–20. doi: 10.4049/jimmunol.170.4.2113. [DOI] [PubMed] [Google Scholar]
- 31.Ha HC, Hester LD, Snyder SH. Poly (ADP-ribose) polymerase-1 dependence of stress-induced transcription factors and associated gene expression in glia. Proc Natl Acad Sci U S A. 2002;99:3270–5. doi: 10.1073/pnas.052712399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roebuck KA, Rahman A, Lakshminarayanan V, Janakidevi K, Malik AB. H2O2 and tumor necrosis factor-α activate intercellular adhesion molecule 1 (ICAM-1) gene transcription through distinct cis-regulatory elements within the ICAM-1 promoter. J Biol Chem. 1995;270:18966–74. doi: 10.1074/jbc.270.32.18966. [DOI] [PubMed] [Google Scholar]
- 33.Hauschildt S, Scheipers P, Bessler W, Schwarz K, Ullmer A, Flad HD, Heine H. Role of ADP-ribosylation in activated monocytes/macrophages. Adv Exp Med Biol. 1997;419:249–52. doi: 10.1007/978-1-4419-8632-0_31. [DOI] [PubMed] [Google Scholar]
- 34.Strater J, Wellisch I, Riedl S, Walczak H, Koretz K, Tandara A, Krammer PH, Moller P. CD95 (APO-1/Fas) -mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis. Gastroenterology. 1997;113:160–7. doi: 10.1016/s0016-5085(97)70091-x. [DOI] [PubMed] [Google Scholar]
- 35.Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK. Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol. 1996;180:152–9. doi: 10.1002/(SICI)1096-9896(199610)180:2<152::AID-PATH649>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 36.Bu P, Keshavarzian A, Stone DD, Liu J, Le PT, Fisher S, Qiao L. Apoptosis: one of the mechanisms that maintains unresponsiveness of the intestinal mucosal immune system. J Immunol. 2001;166:6399–403. doi: 10.4049/jimmunol.166.10.6399. [DOI] [PubMed] [Google Scholar]
- 37.Boirivant M, Marini M, Di Felice G, Pronio AM, Montesani C, Tersigni R, Strober W. Lamina propria T cells in Crohn's disease and other gastrointestinal inflammation show defective CD2 pathway-induced apoptosis. Gastroenterology. 1999;116:557–65. doi: 10.1016/s0016-5085(99)70177-0. [DOI] [PubMed] [Google Scholar]
- 38.Ina K, Itoh J, Fukushima K, et al. Resistance of Crohn's disease T cells to multiple apoptotic signals is associated with a Bcl-2/Bax mucosal imbalance. J Immunol. 1999;163:1081–90. [PubMed] [Google Scholar]
- 39.Zingarelli B, Hake PW, O'Connor M, Denenberg A, Kong S, Aronow BJ. Absence of poly (ADP-ribose) polymerase-1 alters nuclear factor-κB activation and gene expression of apoptosis regulators after reperfusion injury. Mol Med. 2003;9:143–53. doi: 10.2119/2003-00011.zingarelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–56. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 41.Yukawa M, Iizuka M, Horie Y, et al. Systemic and local evidence of increased Fas-mediated apoptosis in ulcerative colitis. Int J Colorectal Dis. 2002;17:70–66. doi: 10.1007/s003840100340. [DOI] [PubMed] [Google Scholar]
- 42.Vetuschi A, Latella G, Sferra R, Caprilli R, Gaudio E. Increased proliferation and apoptosis of colonic epithelial cells in dextran sulfate sodium-induced colitis in rats. Dig Dis Sci. 2002;47:1447–57. doi: 10.1023/a:1015931128583. [DOI] [PubMed] [Google Scholar]