Abstract
Acute, lethal graft-versus-host disease (GvHD) develops in B6D2F1 hybrid recipients of wild-type, C57BL/6, parental strain grafts; however, when interferon-γ (IFN-γ) gene knockout (gko) donors are used, the disease is prolonged and associated with a higher level of engraftment, particularly of T cells. Lesions containing large, mixed cellular infiltrates develop in the skin, liver, pancreas, salivary gland, lung and kidney. In our current study, we wished to determine whether GvHD features a preponderance of T helper 2 (Th2) cytokines in the absence of donor-derived IFN-γ, and whether autoantibody production, commonly associated with chronic GvHD, also occurs. Because mitogen responsiveness is consistently suppressed in mice with acute GvHD, we wished to measure this response in recipients of IFN-γ gko grafts. Our findings indicate that spleen cells from the latter produce interleukin (IL)-4, IL-5 and IL-13 in culture, but respond poorly to concanavalin A (Con A) and lipopolysaccharide (LPS). Their sera contain anti-nuclear antibodies (ANA), some of which are specific for double-stranded (ds)DNA and are predominantly immunoglobulin (Ig)M and IgG1. We also noted the presence of numerous eosinophils in the infiltrates developing within the target organs. In some respects, this syndrome bears resemblance to both systemic lupus erythematosus (SLE) and chronic GvHD. However, histological evidence of glomerulonephritis is lacking and proteinuria fails to develop in recipients of IFN-γ gko grafts, suggesting that IFN-γ may be necessary for the development of lupus nephritis. On a broader scope, our findings underscore the importance of IFN-γ in the pathogenetic mechanism of GvHD, and demonstrate that the absence of this cytokine promotes the development of chronic GvHD and autoimmunity.
Introduction
Graft-versus-host disease (GvHD) is a serious complication of allogeneic bone marrow transplantation (BMT) and is a major impediment to its therapeutic success. Acute GvHD is a rapidly progressive syndrome characterized by profound wasting,1,2 immunosuppression3–5 and tissue injury in the skin, liver, intestinal mucosa6 and lung.7 Without major immunosuppressive therapy, it is invariably fatal. A significant feature of acute GvHD is a heightened sensitivity to endotoxin. This was first demonstrated in a study showing that mice with GvHD succumb to injections of endotoxin when given in doses too small to have any discernible effect in control mice. This lethal effect was associated with the appearance of high levels of tumour necrosis factor-α (TNF-α) in the serum and the development of fatal endotoxaemic shock.8 The authors of this study suggested that macrophage priming for TNF-α release by the T helper 1 (Th1) cytokine interferon-γ (IFN-γ) was the basis of this phenomenon. Later, other investigators showed that this sensitivity to endotoxin was abrogated by inhibiting IFN-γ production in GvHD mice with the use of polarized T helper 2 (Th2) cells.9 These studies clearly support the long-held idea that Th1 cytokines, especially IFN-γ, have a major role in promoting acute GvHD.
Chronic GvHD has a more indolent course, involves a wider range of organs and has more diverse clinical manifestations. It may present with features resembling systemic lupus erythematosus (SLE),10 such as immune complex disease, glomerulonephritis and autoantibody formation. Th2 cytokines are believed to be the principal mediators of this form of GvHD.11
It is thought that the predominance of either Th1 or Th2 cytokines in the initial stages of GvHD may be critical in determining whether the disease follows an acute or a chronic course. It has been suggested that all GvH reactions start with the production of Th2 cytokines and the activation of B cells. Both the production of IFN-γ by donor CD4+ cells and the engraftment of CD8+ cells favour the transition to acute GvHD.12 Without this, the disease continues to evolve into its chronic form. Some evidence suggests that donor-derived natural killer (NK) cells may be the source of IFN-γ that causes acute GvHD to develop.13
We have been investigating the role of IFN-γ in the immunopathogenesis of murine GvHD by using IFN-γ gene knockout (gko) donors to determine how the graft's inability to produce IFN-γ affects the natural history of the disease. We employed a parent→F1 hybrid model using either wild-type C57BL/6 or C57BL/6J-Ifgtm1Ts (IFN-γ gko) donors and (C57BL/6 × DBA/2)F1 hybrid recipients. With wild-type donors, the resulting GvHD is rapidly progressive and 100% lethal by day 30 postinduction. When IFN-γ gko donors are used, the disease is still fatal, but its course is greatly prolonged. Although the syndrome retains some features of acute GvHD, including wasting and macrophage priming for lipopolysaccharide (LPS)-induced TNF-α release, the lesions that develop are considerably different. They consist of focal alopecia, corneal dryness and clouding, and large mixed inflammatory infiltrates in the liver, pancreas, skin, kidney, salivary gland and lung. Some of these lesions resemble those observed in the sicca/Sjogren's-like syndrome sometimes seen in association with chronic GvHD. Interestingly, neither glomerulonephritis nor immune complex deposition in the glomerular basement membrane were observed in this model.14 The purpose of our present study was to characterize further the syndrome that develops in IFN-γ gko graft recipients. Specifically, we wished to determine whether this syndrome is associated with the predominance of Th2 cytokines, the development of GvHD-associated immunosuppression and the production of autoantibodies.
Materials and methods
Mice
Female C57BL/6J-Ifgtm1Ts (IFN-γ gko) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and used to start a breeding colony at The University of Manitoba. Offspring were used at 13–16 weeks of age. These mice were housed in filter-topped cages. Male and female C57BL/6J (H-2b, hereafter referred to as wild-type) donors and (C57BL/6J × DBA/2 J)F1 hybrid recipients (H-2b/d; hereafter referred to as B6D2F1) were obtained directly from The Jackson Laboratory. All animal experimentation was performed according to the guidelines outlined by the Canadian Council on Animal Care.
Induction of GvHD
GvH reactions were induced in 13–16-week-old B6D2F1 hybrid recipients using either wild-type or IFN-γ gko donors. Donors were age- and gender-matched to the recipients. The method used to induce GvHD has been described in detail previously.15 Briefly, lymph nodes and spleens were harvested from donors, pooled and dissociated into a cell suspension using a stainless steel wire mesh. The suspension was then washed, filtered through gauze and adjusted to a final concentration of 2 × 108 cells/ml in Hanks' balanced salt solution (HBSS). Recipients were injected via the tail vein with 60 × 106 donor cells suspended in 300 µl of HBSS.
Experimental design
GvHD in IFN-γ gko graft recipients was monitored by daily observation. The ability of spleen cells from IFN-γ gko graft recipients to proliferate in response to concanavalin A (Con A) and LPS was measured on day 15 postinduction. On days 1, 4, 8 and 22, the spontaneous production levels of interleukin (IL)-4, IL-5, IL-13 and IFN-γ were measured in spleen cell culture supernatants from IFN-γ gko graft recipients. These were compared with the levels observed in wild-type graft recipients. Any moribund, IFN-γ gko graft recipients in the agonal stages of GvHD (days 50–120) were killed and autopsied. The parameters used to identify moribund mice included weight loss (> 15% of the preinduction body weight), hunched posture, excoriating skin lesions and a marked reduction in activity level. Tissue samples were collected from these mice for histopathological study. Serum was collected and assayed for the presence of anti-nuclear antibodies (ANA). In addition, three IFN-γ gko graft recipients were tested for proteinuria on days 1, 3, 20, 50 and 80. In a separate experiment, sera collected from IFN-γ gko graft recipients on days 1, 3, 8, 15, 22, 35, 50, 75 and 80 postinduction was analysed for the presence of antibodies to double-stranded DNA (anti-dsDNA). The isotypes of anti-dsDNA present in sera collected on day 50 postinduction were then identified.
Mitogen responsiveness
Spleens were harvested from recipients of IFN-γ gko grafts, from recipients of wild-type grafts and from B6D2F1 control mice that had not received grafts. Cell suspensions were prepared by pressing the organs through a stainless-steel wire mesh. The suspension was divided into three parts: one was incubated with Con A (Pharmacia Inc., Dorval, Canada) at a concentration of 500 ng/ml; one was incubated with LPS (Sigma, St Louis, MO) at a concentration of 10 µg/ml; and one remained untreated. The three suspensions were adjusted to a final concentration of 2 × 106 cells/ml in complete RPMI containing 5% fetal calf serum (FCS), with or without the addition of mitogen. Two hundred microlitres of each suspension was added, in triplicate, to the wells of a 96-well, round-bottomed microtitre plate (Falcon; Becton-Dickinson, Lincoln Park, NJ) and incubated at 37° in 5% CO2. After 48 hr, 1 µCi of [3H]thymidine ([3H]TdR; Amersham, Oakville, Canada) was added to each well and the plates were incubated at 37° in 5% CO2 for an additional 18 hr. Cells were then harvested onto Filtermats (Skatron Inc., Sterling, VA) with a cell harvester, dried, placed in vials containing 3 ml of scintillation fluid and counted using a Beckman liquid scintillation counter (Beckman Instruments, Irvine, CA). Splenic Con A and LPS responses from individual mice were expressed as a stimulation index (SI), calculated from the disintegrations per minute (d.p.m.), as follows: SI = (d.p.m. with mitogen in culture ÷ d.p.m. without mitogen in culture)
Measurement of IL-4, IL-5, IL-13 and IFN-γ in spleen cell bulk cultures
Spontaneous release of cytokine was measured in bulk spleen cell cultures from recipients of IFN-γ gko grafts, recipients of wild-type grafts and B6D2F1 control mice. Spleens were harvested aseptically in HBSS from recipients on days 1, 4, 8 and 22 postinduction. A sterile cell suspension was prepared in 5% RPMI-1640 supplemented with HEPES (10 mm) and cultured as described previously.14 Supernatants were removed from each well 72 hr later and stored at −70° until enzyme-linked immunosorbent assays (ELISA) were performed. IL-4 was captured with 11B11 monoclonal antibody (mAb) (PharMingen, San Diego, CA) and detected using biotinylated BVD4-24G2 mAb (PharMingen). IL-5 was captured with 18051D mAb (PharMingen) and detected with biotinylated 18062D monoclonal antibody (mAb) (PharMingen). IL-13 was captured with MAB413 mAb (R & D Systems, Minneapolis, MN) and detected with biotinylated anti-mouse IL-13 polyclonal antibody (R & D Systems). IFN-γ was detected with XMG1.2 mAb [American Type Culture Collection (ATCC), Rockville, MD] and captured with biotinylated R46A2 (ATCC). ELISA analysis was performed using streptavidin-conjugated alkaline phosphatase (ALP), as previously described.14,16
Detection of ANA by indirect immunofluorescence
Kallestad™ slides coated with HEp-2 cell line substrate (Sanofi Diagnostics, Montreal, Quebec) were used to detect ANA in sera from IFN-γ gko graft recipients, wild-type graft recipients and B6D2F1 control mice. To perform this test, sera were diluted 1 : 2 in phosphate-buffered saline (PBS) and applied to slides at a volume of 25 µl/well. Slides were placed in a humidified chamber for 20 min and then into PBS that was stirred for 10 min. The slides were then removed from the PBS and blotted with paper. Twenty-five microlitres of a 1 : 50 dilution of fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 anti-mouse immunoglobulin (Ig)G + IgM (H + l; Caltag Laboratories, Burlingame, CA) was added to each well on the slide. Slides were then placed in a dark, humidified chamber for 20 min, washed and coverslipped. All incubations were performed at 25°. The slides were examined by fluorescence microscopy and photographed.
In a separate experiment, the titre of ANA was assayed as follows. Twofold serial dilutions were performed of sera from IFN-γ gko graft recipients and from MRL/lpr mice. The dilution at which nuclear staining was no longer detectable was then determined. This dilution was used as a measure of the titre of ANA in serum of an individual mouse.
Histopathology
Samples of skin, salivary gland, liver, lung and kidney were collected, fixed in 10% neutral-buffered formalin for 24 hr, machine processed through graded alcohol and then embedded in paraffin. Sections (4 µm) were cut, stained with haematoxylin and eosin, and examined by light microscopy. Samples of salivary gland and liver were also taken for electron microscopy. This tissue was fixed in 2% buffered gluteraldehyde for 2 hr, rinsed in phosphate buffer, postfixed in buffered osmic acid for 2 hr and stained for 20 min in 2% aqueous uranyl acetate. After dehydration in graded ethanol, the tissue was embedded in Spurr (J.B. EM Services, Dorval, Canada). Ultrathin sections were cut, stained with lead citrate for 5 min and examined in a Philips EM 201 (Philips, Mahwat, NJ).
Urinalysis
Urine from three IFN-γ gko graft recipients was tested for the presence of protein on days 1, 3, 20, 50 and 80 postinduction. This was undertaken by placing an Albustix® reagent strip (Bayer Inc., Etobicoke, Canada) in the urine stream and comparing the colour of the indicator with the scale provided.
Detection of anti-dsDNA using enzyme immunoassay (EIA)
To detect anti-dsDNA in sera from IFN-γ gko graft recipients, we modified a Kallestad EIA kit (Sanofi) intended for the detection of human anti-dsDNA by replacing the ALP-conjugated anti-human IgG/IgM with ALP-conjugated rat anti-mouse immunoglobulin, κ light chain (187.1; PharMingen). Standards consisted of several twofold serial dilutions of a 1 : 4000 dilution of pooled MRL/lpr sera (a gift kindly provided by Dr David Bradley, University of North Dakota). Test samples consisted of several twofold serial dilutions of a 1 : 50 dilution of sera from IFN-γ gko graft recipients. Negative controls consisted of several twofold serial dilutions of a 1 : 50 dilution of sera from both untreated B6D2F1 mice and wild-type graft recipients. Positive controls consisted of a 1 : 4000 dilution of pooled MRL/lpr sera from mice known to contain high concentrations of autoantibodies. Microtitre plates coated with dsDNA were incubated with sera for 1 hr, washed three times in the wash buffer supplied with the kit and aspirated. Plates were then incubated for 30 min with detection Ab, washed three times, and incubated with substrate for 30 min. The reaction was stopped using the Stop Reagent supplied with the kit. Absorbance values (A) at 550 nm were measured using a SpectraMax Plus plate reader (Molecular Devices, Sunnyvale, CA). The A550 for the highest concentration of MRL/lpr sera on the standard curve (between 1·0 and 1·5 for all assays performed) was given a value of 1 U of anti-dsDNA. We determined the number of units of anti-dsDNA in the test sera by interpolating a value directly from the standard curve.
Determining the isotype of anti-dsDNA by EIA
The EIA kit used for the detection of anti-dsDNA in sera from IFN-γ gko graft recipients was also used to determine the isotypes present. The following biotinylated antibodies were used: anti-mouse IgM (R6-60.6; PharMingen), anti-mouse IgG1 (A85-1; PharMingen), anti-mouse IgG2a (R19-15; PharMingen), anti-mouse IgG2b (R12-3; PharMingen), anti-mouse IgG3 (R40-82; PharMingen) and anti-IgA (C10; PharMingen). Test samples consisted of a 1 : 50 dilution of sera from IFN-γ gko graft recipients. For comparison, 1 : 50 dilutions of sera from untreated B6D2F1 mice and 1 : 4000 dilutions of sera from MRL/lpr sera were also tested. All samples were incubated on dsDNA-coated plates for 30 min. Subsequent incubation periods were as follows: biotinlyated antibody, 2 hr; streptavidin-ALP, 45 min; and substrate, 30 min. All wells were washed three times and aspirated after each step. The reaction was stopped 30 min after the addition of the substrate, and the A550 values were determined using the SpectraMax Plus plate reader.
Results
Mitogen responsiveness
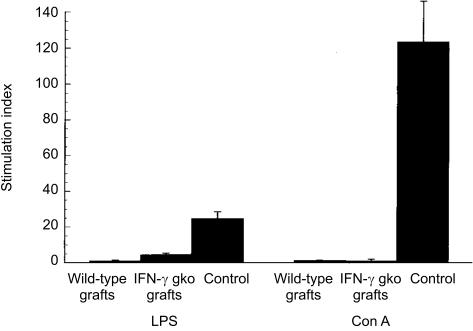
Suppression of mitogen responsiveness is a characteristic feature of murine GvHD and reflects the development of immunosuppression in the recipients.5 Figure 1 shows the level of proliferative responses to LPS and Con A seen in spleen cell cultures from IFN-γ gko graft recipients, wild-type graft recipients and B6D2F1 control mice. Our results indicate that the levels of LPS and Con A responsiveness in splenocytes are suppressed in both wild-type and IFN-γ gko graft recipients, when compared to those observed in B6D2F1 control mice (P < 0·01).
Figure 1.
The mean level of lipopolysaccharide (LPS) and concanavalin A (Con A) responsiveness observed for spleen cell cultures from recipients of wild-type grafts (day 15 postinduction), recipients of interferon-γ (IFN-γ) gene knockout (gko) grafts (day 15 postinduction) and B6D2F1 control mice. Error bars indicate the standard error (SE) of the mean disintegrations per minute (d.p.m.) for three individual mice on each day. The differences in the stimulation indices (SIs) for LPS and Con A responsiveness in recipients of IFN-γ gko grafts and B6D2F1 control mice were significant (Student's t-test: P < 0·01).
Comparison of Th1 and Th2 cytokine production by spleen cell cultures
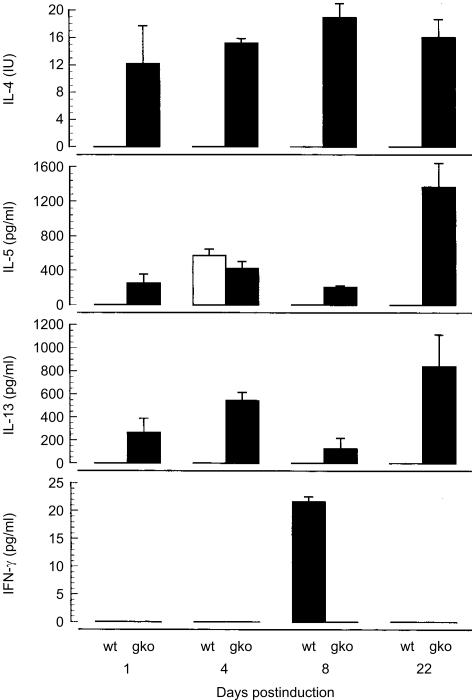
To determine whether Th1 or Th2 cytokines predominate in IFN-γ gko graft recipients, we compared levels of IL-4, IL-5, IL-13 and IFN-γ in splenocyte cultures from IFN-γ gko graft recipients and from wild-type graft recipients. The levels of cytokine were assayed in supernatants from spleen cell bulk cultures prepared from recipient mice on days 1, 4, 8 and 22 postinduction. Figure 2 shows that IL-4 was present in IFN-γ gko graft recipients on all of these days, but was undetectable in wild-type graft recipients. Similarly, IL-13 was detected in IFN-γ gko graft recipients on all of the days tested, but not in wild-type graft recipients. Another Th2 cytokine, IL-5, was present at all four time-points in IFN-γ gko graft recipients but only on day 4 in wild-type graft recipients. IFN-γ was detected in supernatants from wild-type graft recipients on day 8 postinduction, but was undetectable in IFN-γ gko graft recipients at all time-points. These cytokines were not detected in similar spleen cell cultures from B6D2F1 controls.
Figure 2.
Interleukin (IL)-4, IL-5, IL-13 and interferon-γ (IFN-γ) levels determined by enzyme-linked immunosorbent assay (ELISA) in spleen cell cultures prepared from recipients of IFN-γ gene knockout (gko) grafts and recipients of wild-type (wt) grafts. Spleens were harvested on days 1, 4, 8 and 22 postinduction. Error bars represent the standard error (SE) of the mean cytokine levels obtained from three individual mice in each group.
Characterization of cellular infiltrates in the salivary gland and liver
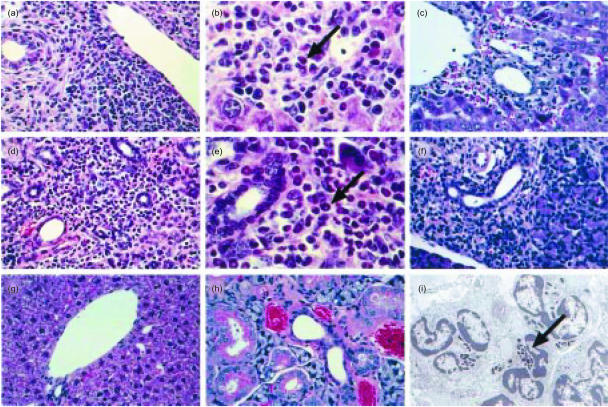
Starting at about day 50 postinduction, we observed large, mixed cellular infiltrates in the salivary gland, lung, liver, skin and pancreas of IFN-γ gko graft recipients. Although these contained both lymphocytes and some neutrophils, numerous eosinophils were also observed, particularly in the salivary gland and liver. In the salivary gland, the infiltrates were centred on the excretory ducts and in the liver they were most pronounced in portal areas and around the central veins. Figure 3 shows examples of the histopathology in the salivary gland and liver as seen by light microscopy. Electron microscopy confirmed the presence of numerous eosinophils within the infiltrates. Cellular infiltrates in the liver and salivary gland were also observed in recipients of wild-type grafts (Fig. 3c, 3f), but were not nearly as extensive as those seen in recipients of IFN-γ gko grafts, and did not contain eosinophils. We did not see infiltrates of any kind in sections of liver or salivary gland taken from B6D2F1 control mice.
Figure 3.
Photomicrographs showing the histopathology of graft-versus-host disease (GvHD) in the salivary gland and liver. (a) Liver from an interferon-γ (IFN-γ) gene knockout (gko) graft recipient (magnification ×340). (b) Liver from an IFN-γ gko graft recipient (magnification ×680). (c) Liver from a wild-type graft recipient (magnification ×340). (d) Salivary gland from an IFN-γ gko graft recipient (magnification ×340). (e) Salivary gland from an IFN-γ gko graft recipient (magnification ×680). (f) Salivary gland from a wild-type graft recipient (magnification ×340). (g) Liver from a B6D2F1 control mouse (magnification ×340). (h) Salivary gland from a B6D2F1 control mouse (magnification ×680). (i) Electronmicrograph showing eosinophils in the salivary gland of an IFN-γ gko graft recipient (magnification ×6000). Note the characteristic crystalloid cores within the granules (arrow). Arrows indicate eosinophils in (b) and (e). Sections from IFN-γ gko graft recipients were collected on day 22 postinduction. Those from wild-type graft recipients were collected on day 8 postinduction. B6D2F1 = (C57BL/6 × DBA/2)F1 hybrid.
Urinalysis
Table 1 shows that the concentrations of protein in urine from recipients of IFN-γ gko mice were low (< 0·3 g/l), and fell into the same range as those observed in B6D2F1 control mice.
Table 1.
Protein levels in urine from interferon-γ (IFN-γ) gene knockout (gko) graft recipients and B6D2F1 hybrid control mice
| Group | Day post-induction | Protein concentration (g/l) |
|---|---|---|
| IFN-γ gko | 1 | < 0·3 |
| graft recipients* | 3 | < 0·3 |
| 20 | < 0·3 | |
| 50 | < 0·3 | |
| 80 | < 0·3 | |
| B6D2F1 controls | – | < 0·3 |
Three mice were tested on each day and all produced the same result. B6D2F1 = (C57BL/6 × DBA/2)F1 hybrid.
Detection of ANA in sera
Sera from IFN-γ gko graft recipients were assayed for the presence of ANA by indirect immunofluorescence. The photomicrographs shown in Fig. 4 illustrate the patterns of fluorescence produced by sera collected from recipients in the later stages of the disease (days 50–120 postinduction). Two patterns of nuclear staining were observed (Fig. 4a, 4b). One was a homogeneous pattern, with fluorescence distributed evenly throughout the nucleii. The other was a ‘rim pattern’ in which the most intense staining was seen around the periphery of the nucleii. Both patterns are typically seen in SLE.17 As shown in Table 2, nine of 20 mice tested positively for ANA using this method. Two showed a rim pattern and the remainder showed a homogeneous pattern of nuclear staining. In 25% of the serum samples from IFN-γ gko graft recipients, nuclear staining was observed at dilutions of less than 1 : 64. The remaining 75% showed positive nuclear staining at dilutions of less than 1 : 32. Pooled sera from MRL/lpr mice stained nucleii of HEp-2 cells at dilutions of less than 1 : 2000.
Figure 4.
The pattern of nuclear fluorescence observed on slides coated with HEp-2 substrate. Slides shown in (a) and (b) were treated with sera from two individual interferon-γ (IFN-γ) gene knockout (gko) graft recipients killed on day 97 postinduction. Anti-nuclear antibodies (ANA) were visualized by indirect immunofluorescence.
Table 2.
Summary of anti-nuclear antibody (ANA) findings in recipients of interferon-γ (IFN-γ) gene knockout (gko) grafts
| No. of mice with fluorescent nucleii | No. of mice with a homogeneous nuclear staining pattern | No. of mice with a peripheral nuclear staining pattern | Serum dilution at which nuclear staining was no longer detectable* |
|---|---|---|---|
| 9/20 | 7/9 | 2/9 | 1 : 32 (75%) |
| 1 : 64 (25%) |
Pooled sera from MRL/lpr mice consistently stained nucleii of HeP-2 cells at dilutions of less than 1 : 2000.
Identification of anti-dsDNA in sera
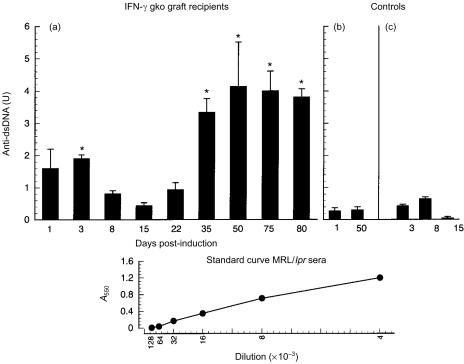
EIA analysis was performed to determine whether any of the ANA observed were specific for dsDNA. Figure 5 shows the mean level of anti-dsDNA in three recipients of IFN-γ gko grafts killed on several days ranging from days 1–80 postinduction. Sera from recipients of IFN-γ gko mice (killed as early as day 3 postinduction) contained levels of anti-dsDNA that were 4–5-fold higher than those seen in B6D2F1 control mice. Maximal levels of anti-dsDNA were seen between days 35 and 80 postinduction, and were in the range of 3·5–4 U. The levels of anti-dsDNA in the sera of normal B6D2F1 hybrid mice and recipients of wild-type grafts did not exceed 0·6 U. The differences in the levels observed in IFN-γ gko graft recipients and B6D2F1 hybrid control mice were significant on days 3, 35, 50, 75 and 80 (P < 0·05).
Figure 5.
Mean levels of antibodies to double-stranded DNA (anti-dsDNA) observed in interferon-γ (IFN-γ) gene knockout (gko) graft recipients on several days postinduction (a). Controls consisted of B6D2F1 mice (b) and recipients of wild-type grafts (c). Error bars represent the standard error (SE) of the mean anti-dsDNA levels obtained from three individual mice in each group. Significant differences [P < 0·05; analysis of variance (anova) followed by a Tukey's Multiple Comparison Test] between the levels observed for IFN-γ gko graft recipients and B6D2F1 control mice are indicated by an asterisk. Also shown is a representative standard curve, obtained by serial dilution of MRL/lpr sera, starting at a 1 : 4000 dilution. One unit of anti-dsDNA was defined in each experiment as the absorbance at 550 nm (A550) observed for MRL/lpr sera at a 1 : 4000 dilution. Sera from IFN-γ gko graft recipients produced A550 values in the linear portion of the standard curve at several dilutions of 1 : 50 or greater.
The standard curve, obtained using MRL/lpr sera, was linear for twofold serial dilutions ranging from 1 : 4000 to 1 : 128000. In these experiments, 1 U of anti-dsDNA was defined as the A550 value produced by the 1 : 4000 dilution of MRL/lpr sera on the standard curve. One in 50 dilutions of sera from IFN-γ gko graft recipients were found to contain 1–4 U of anti-dsDNA, indicating that the levels of anti-dsDNA were 20–80 times lower than those present in sera from MRL/lpr mice. They were, however, significantly greater (P < 0·05) than those seen in either B6D2F1 control mice or wild-type graft recipients.
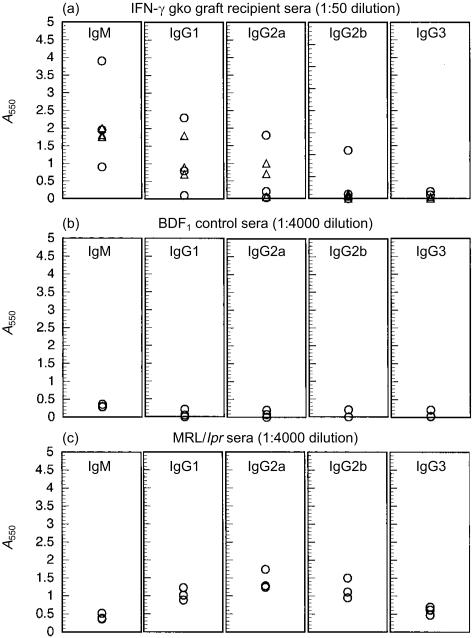
Isotype identification of anti-dsDNA
Figures 6(a), 6(b) and 6(c) show the isotypes of anti-dsDNA detected in recipients of IFN-γ gko grafts, B6D2F1 control mice and MRL/lpr mice, respectively. The levels of the different isotypes detected in IFN-γ gko graft recipients, in descending order, are as follows: IgM, IgG1, IgG2a, IgG2b, and IgG3. In MRL/lpr mice, the profile was different, with IgG1, IgG2a and IgG2b levels being similar to one another, and IgG3 and IgM levels being somewhat lower. In our experiments, we observed that sera from MRL/lpr mice had to be diluted 80-fold more than sera from IFN-γ gko graft recipients to produce similar A550 values. This is consistent with the results shown in Fig. 5, where the overall level of anti-dsDNA was also up to 80-fold higher in MRL/lpr mice when compared to recipients of IFN-γ gko grafts.
Figure 6.
Absorbance values at 550 nm (A550) determined for sera assayed by enzyme immunoassay (EIA) for the presence of antibodies to double-stranded DNA (anti-dsDNA) using isotype-specific antibody for detection. (a) A550 values determined for sera from recipients of interferon-γ (IFN-γ) gene knockout (gko) grafts killed on day 50 postinduction. Data was pooled from two separate assays. (b) A550 values determined for sera from three individual, age-matched, B6D2F1 control mice in three separate assays. (c) A550 values determined for pooled MRL/lpr sera in three separate assays. B6D2F1 = (C57BL/6 × DBA/2)F1 hybrid.
Discussion
These experiments were performed to further describe the evolution of GvHD in recipients of grafts from IFN-γ-deficient donors. The GvH reactions observed in IFN-γ gko graft recipients were associated with a predominance of Th2 cytokines and suppression of mitogen responsiveness. Antibodies specific for nuclear antigens (such as dsDNA) were present in the sera, and extensive infiltration of both lymphocytes and eosinophils was observed in the salivary gland, skin, lung, liver and pancreas. These findings clearly underscore the importance of IFN-γ in the pathogenesis of GvHD.
In our histopathological study of GvHD development in IFN-γ gko graft recipients, we found that the cellular infiltrates present in the salivary gland, lung, liver and skin are rich in eosinophils. This was first evident in specimens collected from day 22 onwards, and the proportion of eosinophils appeared to increase over time. Our cytokine measurements showed that the level of IL-5, a growth and activation factor for eosinophils, becomes markedly elevated in IFN-γ gko graft recipients at around day 22 postinduction. Although we did observe some IL-5 production in recipients of wild-type grafts on day 4, this occurred well before any lesions developed in the target tissues. Using a model for studying skin allograft rejection, Le Moine and colleagues showed recently that IL-5 was able to mediate rejection. The histopathology of the rejection reaction observed was also associated with the presence of a large number of eosinophils within the tissue infiltrates.18 Whether the presence of these cells is instrumental in the pathogenetic mechanism of GvHD in IFN-γ gko graft recipients is unknown.
In the present study ANA were identified in sera from IFN-γ gko graft recipients, including some that were specific for dsDNA. In SLE, anti-dsDNA are associated with the development of glomerulonephritis19 and it has been shown that this involves the deposition of immune complexes along the glomerular basement membrane.19 Recipients of IFN-γ gko grafts showed no significant proteinuria and we did not observe any evidence of glomerulonephritis in kidney sections examined by light microscopy in either this study (data not shown) or in our previous study. In our previous study, we were unable to identify electron-dense deposits within the glomeruli by electron microscopy.14 In the present study, we also found that anti-dsDNA of the IgG3 isotype were absent and titres of IgG2a were low in recipients of IFN-γ gko grafts. These isotypes have been associated with the development of glomerular lesions in lupus-prone mice.20,21 In these studies, MRL/lpr or (NZB × NZW)F1 hybrid mice deficient in IFN-γ receptors showed a dramatic reduction in the incidence of glomerulonephritis, which was associated with a reduction in the titres of IgG2a and IgG3 in the anti-dsDNA response. We therefore suggest that in our model, isotype switching of the anti-dsDNA response to glomerulonephritic IgG subclasses may be diminished because the graft cannot produce sufficient amounts of IFN-γ. Our observation that isotype switching to IgG2a still occurred in some recipient mice may be explained by reports showing that, although IFN-γ is necessary for the generation of a normal IgG2a response, it is not essential for switching to this isotype.22,23 Our data also suggest that IFN-γ may be involved in the development of glomerulonephritis by increasing the levels of nephritogenic autoantibodies in vivo, and that in the absence of this cytokine, the levels of anti-dsDNA may be insufficient to produce immune-complex glomerulonephritis.
It is well known that IL-12 stimulates IFN-γ production during the development of Th1 immune responses.24 Using the C57BL/6→B6D2F1 model of acute GvHD, Williamson and colleagues studied the role of IL-12 by treating recipient mice with anti-IL-12 in vivo.25,26 Their findings showed some similarities to the type of GvHD we observed in recipients of IFN-γ gko grafts in both of our studies.14 These include: a high level of engraftment of both CD4+ and CD8+ T cells; the predominance of Th2 cytokines; the presence of anti-dsDNA; and the absence of immune-complex glomerulonephritis. The major difference in these two models is in the outcome of the disease. The GvHD that develops in recipients of IFN-γ gko grafts generally produces 100% mortality within 90 days and is associated with the development of large lesions in a number of different tissues. Infiltrates of this type are not seen in anti-IL-12-treated recipients, and more than 75% survive until at least day 130 postinduction.26 It is possible that a difference in the amount of residual IFN-γ present may be responsible for these discrepancies. The level of spontaneous IFN-γ measured in spleen cell cultures from anti-IL-12-treated recipients on day 10 was half of that observed in similar cultures from untreated mice with acute GvHD.25 We did not detect IFN-γ in cultures from recipients of IFN-γ gko grafts, but recognize that although the graft could not produce this cytokine, recipient-derived IFN-γ may have been present, but at a level too low to be detectable by ELISA. If residual IFN-γ is present in vivo in the IL-12-depletion model, it could dampen the Th2 response, which may prevent the development of pathological changes associated with chronic GvHD. Another notable difference in the two models is the responsiveness of recipients to Con A. In IL-12-depleted recipients, this response was higher on day 20 than that seen in cultures from untreated mice with acute GvHD.25 In contrast, Con A responsiveness was virtually undetectable in IFN-γ gko graft recipients. The role of IFN-γ in the suppression of mitogen responsiveness in the development of GvHD has been investigated previously. One study showed that nitric oxide (NO) acts downstream to IFN-γ in the biochemical pathway, leading to diminished Con A responsiveness in GvHD.27 Another showed that suppression of LPS responsiveness in GvH mice is also mediated by a mechanism involving NO.28 It is possible that suppression of mitogen responsiveness is NO dependent in IFN-γ gko graft recipients, but that NO production is induced by cytokines other than IFN-γ. Indeed, IFN-γ-independent iNOS mRNA expression was shown to occur in a model of visceral leishmaniasis employing IFN-γ gko mice.29 The observation that Con A responsiveness persists in the IL-12-depletion model of GvHD suggests that suppression of this response may be IL-12 dependent.
In summary, it is known that IFN-γ is an important immunoregulatory cytokine in the development of GvHD; however, its precise role in the pathogenetic mechanism is not completely understood. The presence of IFN-γ in the early stages of GvHD leads to the development of a Th1-mediated form of GvHD that culminates in fatal endotoxaemic shock. Our results indicate that the absence of donor-derived IFN-γ leads to the development of a form of GvHD that follows a chronic course and is associated with the production of Th2 cytokines and ANA. Our data suggest that modulating IFN-γ production during the development of GvHD may have some therapeutic value. However, it is very clear from our findings that abrogating its production results in a severe form of chronic GvHD and would therefore be of no benefit to the patient.
Acknowledgments
We acknowledge the excellent technical assistance of Bill Stefura and Dr Edward Rector. This work was supported by a grant to J.G.G. from the Medical Research Council of Canada.
Abbreviations
- ANA
anti-nuclear antibody
- B6D2F1
(C57BL/6 × DBA/2)F1
- BMT
bone marrow transplantation
- d.p.m.
disintegrations per minute
- gko
gene knockout
- GvH
graft-versus-host
- GvHD
graft-versus-host disease
- SI
stimulation index
- SLE
systemic lupus erythematosus.
References
- 1.Piguet PF, Grau GE, Allet B, Vassalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs.-host disease. J Exp Med. 1987;166:1280–9. doi: 10.1084/jem.166.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holler E, Kolb HJ, Hintermeier-Knabe R, Mittermüller J, Thierfelder S, Kaul M, Wilmanns W. Role of tumor necrosis factor alpha in acute graft-versus-host disease and complications following allogeneic bone marrow transplantation. Transplant Proc. 1993;25:1234–6. [PubMed] [Google Scholar]
- 3.Howard JG, Woodruff MFA. Effect of the graft-versus-host reaction on the immunological responsiveness of the mouse. Proc R Soc Lond. 1961;154:532–9. [Google Scholar]
- 4.Blaese M, Martinez C, Good RA. Immunologic incompetence of immunologically runted animals. J Exp Med. 1964;119:211–24. doi: 10.1084/jem.119.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lapp WS, Ghayur T, Mendes M, Seddick M, Seemayer T. The functional and histological basis for graft-versus-host induced immunosuppression. Immunol Rev. 1985;88:107–33. doi: 10.1111/j.1600-065x.1985.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara JLM, Deeg HJ. Mechanisms of disease: graft-versus-host disease. N Engl J Med. 1991;324:667–74. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 7.Piguet PF, Grau GE, Collart MA, Vassalli P, Kapanci Y. Pneumopathies of the graft-versus-host reaction. Alveolitis associated with an increased level of tumor necrosis factor mRNA and chronic interstitial pneumonitis. Lab Invest. 1989;61:37–45. [PubMed] [Google Scholar]
- 8.Nestel FP, Price KS, Seemayer TA, Lapp WS. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor a during graft-versus-host disease. J Exp Med. 1992;175:405–13. doi: 10.1084/jem.175.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fowler DH, Kurasawa K, Husebekk A, Cohen PA, Gress RE. Cells of Th2 cytokine phenotype prevent LPS-induced lethality during murine graft versus-host-reaction. Regulation of cytokines and CD8+ lymphoid engraftment. J Immunol. 1994;152:1004–13. [PubMed] [Google Scholar]
- 10.Gleichmann E, Pals ST, Rolink AG, Radaszkiewicz T, Gleichmann H. Graft-versus-host reactions: clues to the etiopathology of a spectrum of immunological diseases. Immunol Today. 1984;5:324–32. doi: 10.1016/0167-5699(84)90126-9. [DOI] [PubMed] [Google Scholar]
- 11.Krenger W, Ferrara JLM. Graft-versus-host disease and the Th1/Th2 paradigm. Immunol Res. 1996;15:50–73. doi: 10.1007/BF02918284. [DOI] [PubMed] [Google Scholar]
- 12.Rus V, Svetic A, Nguyen P, Gause WC, Via CS. Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft-versus-host disease: regulatory role of donor CD8+ T cells. J Immunol. 1995;155:2396–406. [PubMed] [Google Scholar]
- 13.Ellison CA, HayGlass KT, Fischer JMM, Rector ES, MacDonald GC, Gartner JG. Depletion of natural killer cells from the graft reduces interferon-γ levels and lipopolysaccharide-induced tumor necrosis factor-a release in F1 hybrid mice with acute graft-versus-host disease. Transplantation. 1998;66:284–94. doi: 10.1097/00007890-199808150-00002. [DOI] [PubMed] [Google Scholar]
- 14.Ellison CA, Fischer JMM, HayGlass KT, Gartner JG. Murine graft-versus-host disease in an F1-hybrid model using IFN-γ gene knockout donors. J Immunol. 1998;161:631–40. [PubMed] [Google Scholar]
- 15.Gartner JG, Merry AC, Smith CI. An analysis of pulmonary natural killer-cell activity in F1-hybrid mice with acute graft-versus-host reactions. Transplantation. 1988;88:4606–879. doi: 10.1097/00007890-198812000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Giene RS, Mosmann TR, HayGlass KT. Chemically modified antigen preferentially elicits induction of Th1-like cytokine synthesis patterns in vivo. J Exp Med. 1993;178:349–53. doi: 10.1084/jem.178.1.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reichlin M, Harley JB. Antinuclear antibodies: an overview. In: Wallace DJ, editor. Dubois' Lupus Erythematosus. 4. Malvern, PA: Lea & Febiger; 1993. pp. 195–201. [Google Scholar]
- 18.Le Moine A, Surquin M, Demoor FX, et al. IL-5 mediates eosinophilic rejection of MHC class II-disparate skin allografts in mice. J Immunol. 1999;163:3778–84. [PubMed] [Google Scholar]
- 19.Foster MH, Kelley VR. Lupus nephritis: update on pathogenesis and disease mechanisms. Semin Nephrol. 1999;19:173–81. [PubMed] [Google Scholar]
- 20.Haas C, Ryffel B, Le Hir M. IFN-gamma is essential for the development of autoimmune glomerulonephritis in MRL/Ipr mice. J Immunol. 1997;158:5484–91. [PubMed] [Google Scholar]
- 21.Haas C, Ryffel B, Le Hir M. IFN-gamma receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone (NZB × NZW) F1 mice. J Immunol. 1998;160(8):3713–8. [PubMed] [Google Scholar]
- 22.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–7. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 23.Finkelman FD, Holmes J, Katona IM, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–33. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 24.Arase H, Arase N, Saito T. Interferon gamma production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J Exp Med. 1996;183:2391–6. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williamson E, Garside P, Bradley JA, Mowat AM. IL-12 is a central mediator of acute graft-versus-host disease in mice. J Immunol. 1996;157:689–99. [PubMed] [Google Scholar]
- 26.Williamson E, Garside P, Bradley JA, et al. Neutralizing IL-12 during induction of murine acute graft-versus-host disease polarizes the cytokine profile toward a Th2-type alloimmune response and confers long term protection from disease. J Immunol. 1997;159:1208–15. [PubMed] [Google Scholar]
- 27.Krenger W, Falzarano G, Delmonte J, Snyder KM, Byon JC, Ferrara JL. Interferon-gamma suppresses T-cell proliferation to mitogen via the nitric oxide pathway during experimental acute graft-versus-host disease. Blood. 1996;88:1113–21. [PubMed] [Google Scholar]
- 28.Falzarano G, Krenger W, Snyder KM, Delmonte J, Jr, Karandikar M, Ferrara JLM. Suppression of B-cell proliferation to lipopolysaccharide is mediated through induction of the nitric oxide pathway by tumor necrosis factor-α in mice with acute graft-versus-host disease. Blood. 1996;87:2853–60. [PubMed] [Google Scholar]
- 29.Taylor AP, Murray HW. Intracellular antimicrobial activity in the absence of interferon-gamma: effect of interleukin-12 in experimental visceral leishmaniasis in interferon-gamma gene-disrupted mice. J Exp Med. 1997;185:1231–9. doi: 10.1084/jem.185.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]