Abstract
This study evaluated whether different bacillus Calmette–Gue´rin (BCG) strains, routes of administration, vaccination age and percutaneous tools influenced immune responses to BCG vaccination in infants. Proliferative responses, cytokine production and cell-mediated cytotoxicity obtained in post-vaccinated children were compared to baseline cord bloods and unvaccinated 10-week-old infants. BCG vaccination generally induced strong lymphoproliferative and T helper type 1 (Th1)-type cytokine responses. There was a trend for greater responsiveness following the intradermal route of vaccination, with Japanese-172 strain and with delaying vaccination until 10 weeks. Cord mononuclear cells differentially stimulated the Th2-type cytokines interleukin-5 (IL-5) and IL-10 selectively in response to BCG, as compared to H37Rv or purified protein derivative stimulation. We document for the first time the generation of mycobacterium-specific cytotoxic T lymphocytes in neonates, following BCG vaccination. Cytotoxic activity correlated with the ratio of interferon-γ to IL-5, aside from a single instance where use of the Biovac® tool resulted in a striking dissociation selectively against H37Rv targets. These data have implications for correlates of protective immunity in design of vaccine studies.
Introduction
Developing countries have witnessed an escalation of the tuberculosis epidemic, particularly in sub-Saharan Africa.1 Tuberculosis has also re-emerged in industrialized countries due to a number of factors, including the spread of human immunodeficiency virus (HIV).2 This has led to renewed interest in vaccines aimed at preventing tuberculosis. While current Bacillus Calmette–Guérin (BCG) vaccines prevent the invasive complications of childhood tuberculosis, such as meningitis and miliary disease, they only provide variable protection against adult pulmonary disease.3,4 Variable BCG vaccine efficacy has been attributed to variation in BCG vaccine strain, host genetic factors, genetic variation in strains of Mycobacterium tuberculosis, or a masking effect due to environmental mycobacteria.4 While research efforts are currently directed at new vaccine candidates,5 it may be possible to improve the immunogenicity of the existing BCG vaccine. Factors such as vaccine dose, age at vaccination, and route of administration may also impact on the immune response, but these have not been systematically studied.
T helper type 1 (Th1)-type responses, characterized by the production of interferon γ (IFN-γ), have been considered to be required for protection against mycobacterial disease.6 Obstacles to successful neonatal vaccination include immaturity of T-cell immunity resulting in difficulty in priming neonatal T cells and bias of neonatal immunity towards a Th2-type response.7–9 Immunological responses are known to be governed by factors such as the dose and route of vaccine administration. Low-dose BCG vaccination in experimental animal models has been associated with a Th1-type response while higher doses have been associated with a mixed Th1- and Th2-type response.10 In adults, high doses of percutaneous BCG were required for an appropriate Th1 response.11 There is some evidence to suggest that intradermal BCG vaccination, the route recommended by the World Health Organization, may be a more efficient method of vaccine delivery (as measured by tuberculin skin tests) than percutaneous BCG vaccination.12 A recent study demonstrated for the first time Th1-type responses in human neonates receiving BCG vaccination at birth or at 2 months of age.13 However, knowledge of the correlates of protective immunity against the development of human tuberculosis is still incomplete and is the subject of ongoing discussion and research.14 Th1-type responses (exemplified by T-cell proliferation and IFN-γ production) may be necessary but not sufficient for protective immunity. Cytotoxic T lymphocytes (CTL) that recognize and lyse infected macrophages have also been considered important in protective immunity against tuberculosis.15,16 CTL have been isolated from patients with active tuberculosis.17,18 There are reports of CTL induction in humans following BCG vaccination, but to date these have only involved adult subjects.19,20
The aims of this study were therefore threefold. First, to determine whether cellular immune responses vary according to BCG strain (Japanese 172 or Danish 1331), route of administration [percutaneous (p.c.) or intradermal (i.d.)], type of tool used for p.c. vaccination (Bignell® or Biovac®), or age at vaccination (birth or 10 weeks) in a group of infants. Second, to ascertain the balance of Th1-type and Th2-type cytokines under these distinct vaccination conditions and third, to ascertain if BCG vaccination primes for CTL response in human neonates.
Materials and Methods
Study design
Infants/subjects
This study was conducted at a community-based, primary-care service. Healthy new-borns were recruited. Exclusion criteria for enrolment were birth weight below 2500 g, a family history of tuberculosis or known HIV infection. Five groups of children were immunized: 11 new-borns were given the Danish BCG strain i.d. at birth (DID); 11 new-borns were given the Danish strain i.d. at 10 weeks of age (del DID); 10 new-borns were given the Japanese BCG strain i.d. at birth (JID); 10 new-borns were given the Japanese strain by p.c. at birth using the Bignell® tool (JPC Big); 20 new-borns were given the Japanese strain by p.c. vaccination at birth using the Biovac® tool (JPC Bio). The Bignell® tool, manufactured in the UK, is a spring-loaded, multi-puncture device with 18 steel needles. The Biovac® tool is a locally manufactured multi-puncture device with nine needles and its skin penetration is determined by manual pressure. Peripheral blood (10 ml) was taken from all infants 10 weeks after BCG immunization and also prior to BCG immunization in the group immunized at 10 weeks (unv 10 week). After delivery of the infants (except for the JPC Bio group) 20 ml of cord blood was collected from the umbilical vein of the placenta. As there was no difference between the groups, all the results of the cord bloods were pooled (cords) in all instances except where the effect of maturation was to be investigated using cytokine responses from the same group of 10 infants (del DID) examined at three time points. These were cord blood, pre-BCG immunization (unv 10 week), and 20 weeks of age (10 weeks post BCG immunization, del DID). Written informed consent was obtained from the mothers of the infants enrolled into the study. The study was approved by the Ethics and Research Committee, University of Cape Town and their guidelines for clinical research were adhered to.
Vaccines and immunization tools
BCG vaccine (0·05 ml) Danish strain 1331 or Japanese 172 was injected intradermally via a 26-gauge needle into the lower deltoid region of the right arm at the insertion of the muscle by a trained nursing sister. For percutaneous vaccination a drop of vaccine was placed three-quarters of the way up the right arm in the deltoid region. The BCG vaccination tool was placed vertically above the drop of vaccine and the plunger pressed down for approximately 2 seconds. With the Biovac® tool, the plunger was released, the tool was rotated through 90° and the plunger was depressed again in order to make 18 needle holes.
Investigations
Peripheral blood mononuclear cell (PBMNC) and cord blood mononuclear cell (cord MNC) isolation
PBMNC and cord MNC were isolated by Ficoll–Hypaque (Histopaque-1077; Sigma Diagnostics, Inc., St Louis, MO) density gradient separation, cells were then washed in phosphate-buffered saline (PBS; Oxoid, Unipath Ltd, Hampshire, UK), and viable cells were adjusted to the optimal concentration for the specified tests. Cord blood and PBMNC reactivity from unvaccinated infants at 10 weeks served as a baseline response, for comparison to the vaccinated groups.
Lymphocyte proliferation and cytokine anlaysis
Lymphocyte proliferation was evaluated as previously described.21 Briefly, 1 × 105 cells per well were plated in microculture and proliferation was measured in response to each of the following stimulants: 5·75 × 10−3 mitogenic units/ml phytohaemagglutinin (PHA; Wellcome Research Laboratories, Beckenham, UK); tetanus toxoid (TT) antigen (formol toxoid – 10 Lf/0·5 ml; Lederle Laboratory, Wayne, NJ); purified protein derivative of Mycobacterium tuberculosis (PPD; Central Veterinary Laboratory, Weybridge, UK) at 3 µg/ml; Mycobacterium tuberculosis H37Rv strain (H37Rv) and Mycobacterium bovis BCG Japanese strain 172, each at a final concentration of approximately 10 organisms per macrophage [equivalent to 106 colony-forming units/ml (CFU/ml)]. Both of these organisms were heat killed. Duration of mitogen stimulation was 3 days and of antigen stimulation 6 days. Results were expressed as the difference in [3H]thymidine incorporation (delta counts per minute) after subtraction of background unstimulated counts. Prior to addition of [3H]thymidine, 100 µl of supernatant fluid was removed from each well and stored at −20° for cytokine evaluation. Commercially available antibody pairs were used for IFN-γ, interleukin-10 (IL-10) and IL-5 enzyme-linked immunosorbent assay (ELISA; PharMingen, San Diego, CA) following optimization. Recombinant cytokines were used as standards. Results are expressed in pg/ml after the deduction of unstimulated samples. The lower limit of detection for the cytokines was 15 pg/ml.
CD4+ CTL activity measured by adherent macrophage 16-hr sodium chromate 51Cr-release assay
The cytotoxic capacity of effector cells was evaluated as previously described.21 BCG-specific effector cells were generated by stimulating 1 × 106/ml PBMNC with heat-killed BCG Japanese strain (106 CFU/ml) in bulk culture for 6 days. Mycobacterial antigens H37Rv and BCG Japanese strain 172 (both at 5 × 105 CFU/ml and both heat killed) were added to monocyte-derived macrophage (MDM) target cells. TT was added as a control antigen. The effector to target ratios for each antigen were 10:1, 3:1 and 0·3:1. The mean percentage killing was calculated as follows: % specific lysis=[(mean test c.p.m.)/(mean test c.p.m. + mean c.p.m. after Triton-X treatment of same wells)] × 100% − % spontaneous release; where c.p.m. are counts per minute. The % spontaneous release was calculated as follows: [(mean c.p.m. in spontaneous release wells)/(mean c.p.m. in spontaneous release wells + mean c.p.m. after Triton-X treatment of same wells)] × 100%. To obtain antigen-specific cytotoxicity, the percentage kill obtained in unpulsed targets was subtracted from percentage kill of mycobacterial antigen-pulsed targets.
Statistical analysis
Data were analysed using Microsoft Excel 97 and Statistica 5.5 (Statsoft, Tulsa, OK). Proliferation, cytotoxicity and cytokine results obtained in children post-vaccination were compared to those obtained pre-vaccination at 10 weeks and to cord blood results. Comparisons were also made between different groups to ascertain whether a statistical difference was obtained between strain of BCG, route of administration, age of vaccination and different percutaneous vaccination tools.
Non-parametric techniques were used as the underlying measurements could not be assumed to be normally distributed. Cytokine responses that were not detectable in the assays were assigned lowest rank in these tests.
As sample sizes are small, and multiple comparisons have been performed, all P-values should be interpreted as an indication of strengths of association between the variables.
The levels of significance shown in the figures result from application of the Mann–Whitney U-test in the comparison of unvaccinated and post-vaccinated infants.
Box and whisker plots indicate the minimum, maximum, median and interquartile range. Outliers are indicated by circles. In the median plots, medians are indicated as short lines. In Figs 4(a) 5(c) and 6(d) single outliers have been removed from the graphs in order to facilitate display, but they were included in all the statistical analyses.
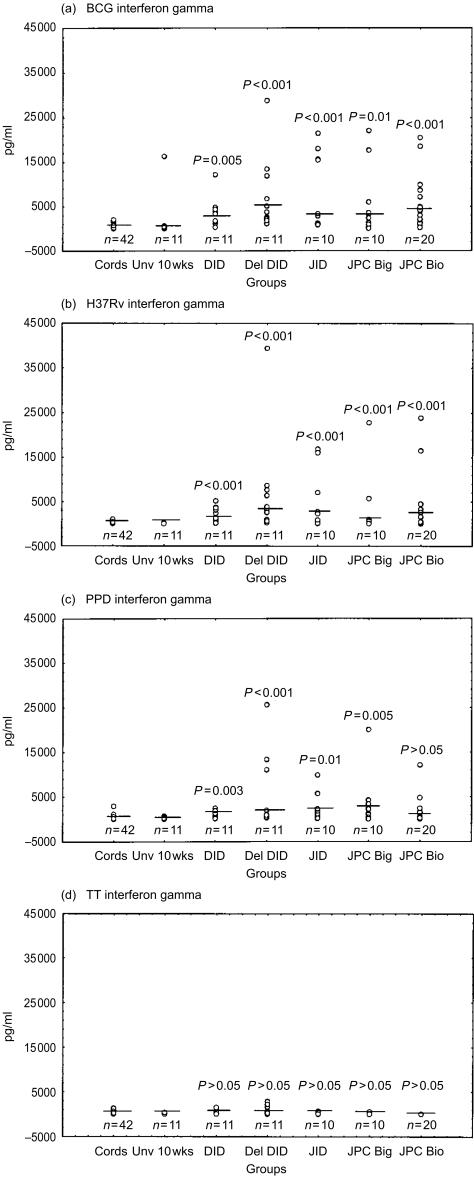
Figure 4.
Interferon-γ responses following stimulation with the mycobacterial antigens BCG (a), H37Rv (b), PPD (c) and TT (d) are indicated as pg/ml. Medians are indicated as short lines. A single outlier (61940 pg/ml) has been omitted from the del DID group in (a) to allow consistent scales and to facilitate comparison. The medians for the unstimulated samples are as follows; cords, 50 pg/ml; Unv 10 week, 82 pg/ml; DID, 37 pg/ml; del DID, 41 pg/ml; JID, 28 pg/ml; JPC Big, undetectable; and JPC Bio, undetectable.
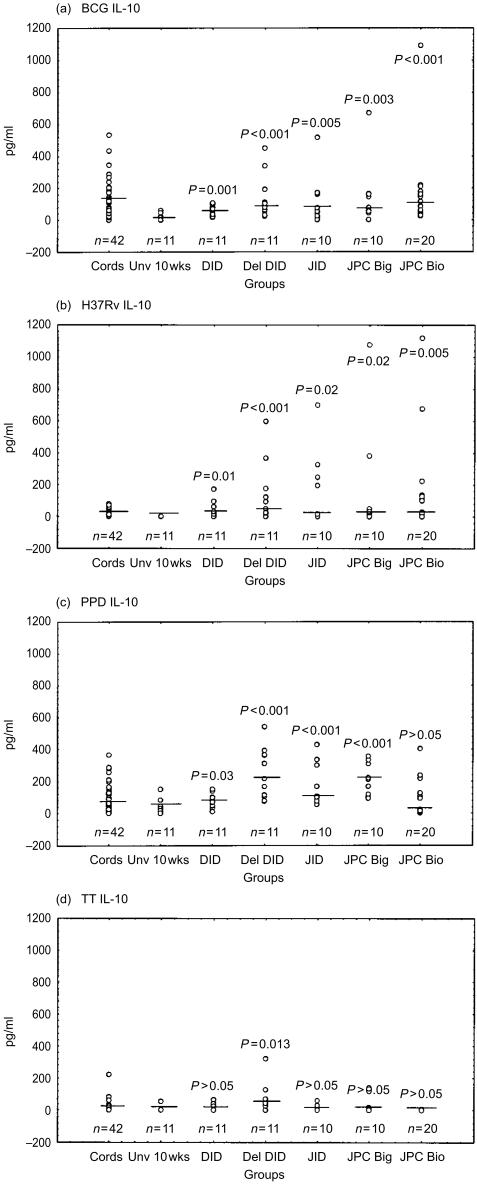
Figure 5.
IL-10 responses following stimulation with the mycobacterial antigens BCG (a), H37Rv (b), PPD (c) and TT (d) are indicated as pg/ml. Medians are indicated as short lines. A single outlier (3661 pg/ml) has been omitted from the JPC Bio group in (c) to facilitate display. The medians for the background unstimulated samples are as follows; cords, 62 pg/ml; Unv 10 week, undetectable; DID, undetectable; del DID, undetectable; JID, 9 pg/ml; JPC Big, undetectable; and JPC Bio, 13 pg/ml.
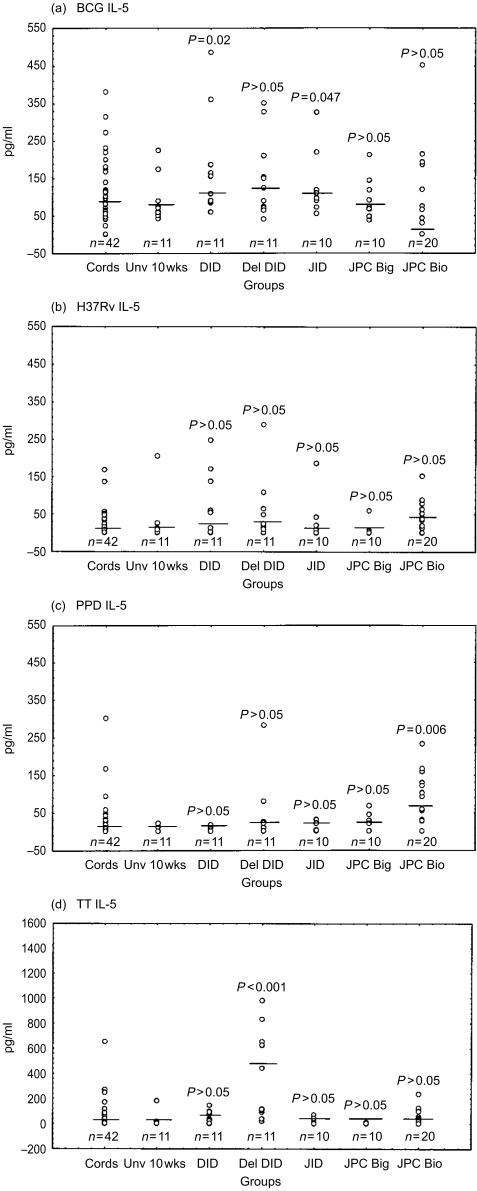
Figure 6.
IL-5 responses following stimulation with the mycobacterial antigens BCG (a), H37Rv (b), PPD (c) and TT (d) are indicated as pg/ml. Medicians are indicated as short lines. A single outlier (13385 pg/ml) has been omitted from the del DID group in (d) to facilitate display. The medians for the background unstimulated samples are as follows; cords, 59 pg/ml; Unv 10 week, 67 pg/ml; DID, 83 pg/ml; del DID, 50 pg/ml; JID, 63 pg/ml; JPC Big, 80 pg/ml; JPC Bio, 60 pg/ml.)
Results
In order to evaluate BCG immunogenicity in neonates, a comprehensive evaluation of cell-mediated immunity was performed. This comprised assessment of T-cell proliferation following in vitro mitogenic and mycobacterial antigenic stimulation, evaluation of antigen-specific cytotoxic capacity, and the balance of Th1-type (IFN-γ) and Th2-type (IL-4, IL-5 and IL-10) cytokines generated in response to BCG vaccination. Cord MNC and PBMNC reactivity in unvaccinated infants at 10 weeks served as a baseline response, for comparison to the vaccinated groups.
Proliferation responses
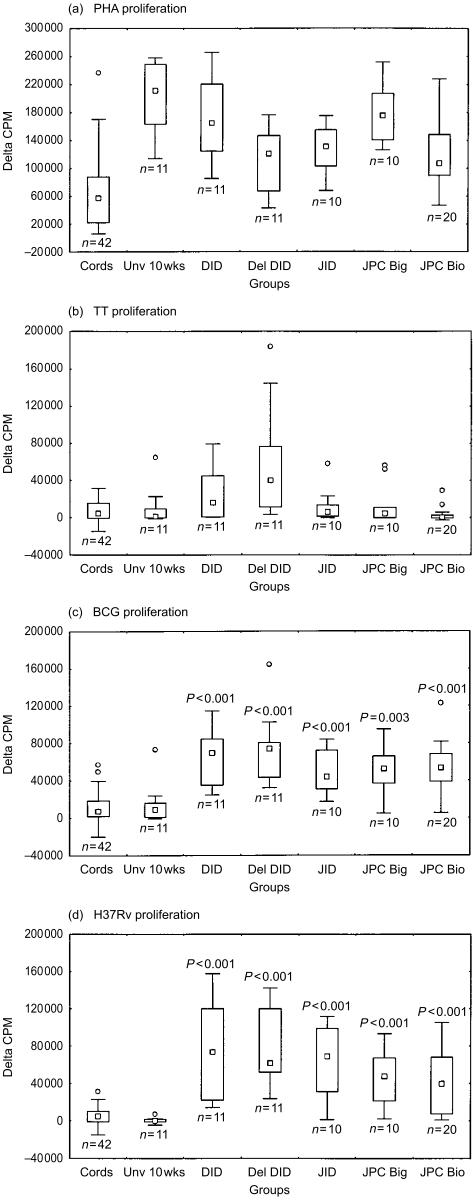
Responses to mycobacterial antigens, heat-killed BCG and H37Rv were measured in the five groups of infants. Responses to non-specific stimulation with the mitogen PHA, and the non-mycobacterial antigen TT served as controls (Fig. 1a–d). Similar PHA responses were observed indicating comparable T-cell reactivity in immune cells obtained from infants in all five vaccinated groups (Fig. 1a). These were significantly higher (P < 0·001) than pooled cord responses, which probably represents a maturational effect. Proliferative responses to heat-killed BCG and H37Rv (Fig. 1c, d) observed 10 weeks post-vaccination in the five groups of infants were significantly greater than baseline unvaccinated infants aged 10 weeks (P < 0·003). There was no statistical difference between the different infant groups. PPD responses showed a similar trend (data not shown). By contrast, proliferative responses to TT (Fig. 1b) were similar for baseline cords, unvaccinated infants, and post-vaccination responses in groups DID, JID, JPC Big and JPC Bio. Only the group who received Danish ID BCG delayed vaccination at 10 weeks of age (del DID) had significantly greater proliferative responses to TT, in comparison to the unvaccinated 10-week-old infant group (P < 0·01). This was to be expected as they had received more routine TT immunizations than the other infants.
Figure 1.
T-cell proliferative responses indicated as Δc.p.m. following stimulation with PHA (a), TT (b), BCG (c) and H37Rv (d). The five groups of neonates; Danish ID (DID), Danish ID administered at 10 weeks of age (del DID), Japanese ID (JID), Japanese PC via the Bignell® tool (JPC Big) and Japanese PC via the Biovac® tool (JPC Bio). The medians for the day 3 background unstimulated c.p.m. are as follows; cords; 4996; Unv 10 week, 862; DID, 1132; del DID, 565; JID, 479; JPC Big, 677; and JPC Bio, 5446. The medians for the day 6 background unstimulated c.p.m. are as follows; cords 23135; Unv 10 week, 1799; DID, 2036;, del DID, 1034; JID, 896; JPC Big, 1533; and JPC Bio, 641.
Cytotoxicity
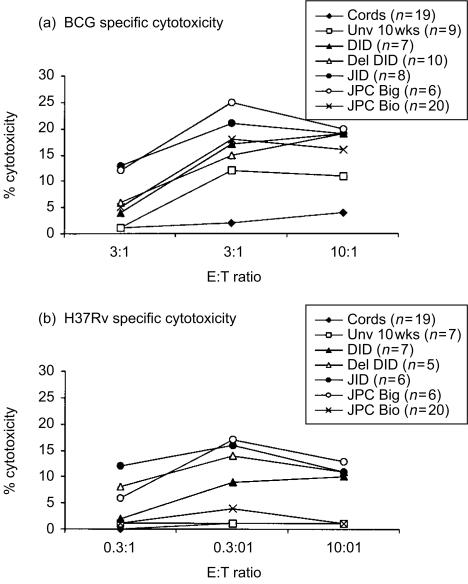
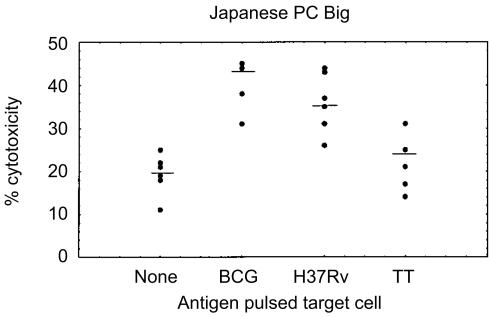
The results of antigen-specific cytotoxicity for all three effector to target (E:T) ratios are shown in Fig. 2. BCG vaccination induced higher levels of antigen-specific cytotoxicity against BCG and H37Rv pulsed autologous MDM in all five infant groups, as compared to control BCG-stimulated MNC cord effectors and the unvaccinated 10-week-old group. Highest antigen-specific kill was observed at a E:T ratio of 3:1, as increasing CTL effectors resulted in higher levels of non-specific killing of unpulsed target cells. Figure 3 illustrates the non-specific or anomalous cytotoxicity observed with unpulsed and TT-pulsed MDM in the JPC Big infant group, this pattern was representative for all groups. Unexpectedly, BCG-stimulated effectors from the 10-week unvaccinated infants killed BCG-infected macrophage target cells (Fig. 2a); but this was less than in the vaccinated groups. Table 1 indicates the rank order of cytotoxic activity for the different groups. The table lists both the percentage of responders (defined as ≥5% antigen-specific kill) and the relative strength of CTL generated (percentage of the group with ≥10% antigen-specific kill) for all infant groups. Infants receiving JPC via the Bignell® tool showed the highest CTL activity on both these criteria, whilst the JPC Bio group had the lowest CTL activity. The other three vaccinated infant groups occupied an intermediate position between JPC Big and JPC Bio. It is particularly notable that JPC Bio infants had the lowest response rate of 55% against virulent H37Rv-infected targets, and the weakest level of cytotoxicity (30% of donors with ≥10% antigen-specific kill). According to these criteria, this group may be expected to be the most susceptible to pathogenic mycobacteria if CTL are an important correlate of protective immunity, as has been suggested.15,16
Figure 2.
CTL activity of BCG-stimulated effectors against BCG-infected (a) and H37Rv-infected (b) targets is indicated as mean percentage specific cytotoxicity for three effector to target ratios 10:1, 3:1 and 0.3:1. Standard error of the mean was <5%for all groups. Note that values of the cord group in (b) are superimposed on the Unv 10 week group.
Figure 3.
CTL activity of PBMNC obtained from the group of infants receiving Japanese PC via the Bignell® tool is indicated as percentage cytotoxicity at an effector to target ratio of 3:1. Medians are indicated as short lines.
Table 1. Percentage of infants with cytotoxic responses in vaccinated groups.
| Responders* | High Kill† | |
|---|---|---|
| ≥ 5% | ≥ 10% | |
| BCG-specific cytotoxicity | ||
| Cords (n = 19) | 32% | 11% |
| Unv 10 week (n = 9) | 67% | 56% |
| DID (n = 7) | 86% | 86% |
| Del DID (n = 10) | 80% | 80% |
| JID (n = 8) | 100% | 100% |
| JPC Big (n = 6) | 100% | 100% |
| JPC Bio (n = 20) | 80% | 75% |
| H37Rv-specific cytotoxicity | ||
| Cords (n = 19) | 21% | 11% |
| Unv 10 week (n = 7) | 29% | 14% |
| DID (n = 7) | 71% | 57% |
| Del DID (n = 6) | 83% | 83% |
| JID (n = 6) | 83% | 67% |
| JPC Big (n = 6) | 100% | 83% |
| JPC Bio (n = 20) | 55% | 30% |
Responders are arbitrarily defined as ≥5% antigen-specific cytotoxicity.
Strength of killing is indicated by percentage of infants ≥ 10% antigen-specific cytotoxicity.
Data represents E:T ratio of 3:1.
Cytokines
Th1-type and Th2-type cytokines were evaluated by measurement of IFN-γ, IL-5 and IL-10 secreted proteins in supernatant samples produced in response to stimulation by mycobacterial antigens (heat-killed BCG, H37Rv and PPD). In preliminary experiments IL-4 could not be measured in standard ELISA under conditions where IL-5 was readily detectable (data not shown), confirming the inability of even sensitive ELISA methodology to detect labile cytokines such as IL-4.22 Cytokine results are shown in Figs 4, 5 and 6.
Response to BCG vaccination
For the mycobacterial antigens BCG and H37Rv, both IFN-γ and IL-10 production were significantly greater in all vaccinated groups as compared to the unvaccinated 10-week-old controls (Figs 4 and 5). Similar significant differences were obtained with vaccinated as compared to cord responses for IFN-γ (P < 0·001). Between immunized groups there was no significant difference in IFN-γ and IL-10 production in response to mycobacterial antigens. There was a trend for higher responses in delayed vaccination (del DID) as compared to the same vaccine given at birth for IFN-γ (P = 0·065) and IL-10 (P = 0·055) production. With regard to mycobacteria antigenic stimulation of IL-5 production, significantly higher levels of BCG-induced IL-5 were obtained in infants receiving DID (P = 0·016) and JID (P = 0·047) than in the unvaccinated control group. PPD-induced IL-5 was significantly higher in infants that received BCG using the Biovac® tool than in the unvaccinated group (P = 0·006).
By comparison to mycobacterial antigen responses, the antigen TT elicited significantly greater IL-5 (P < 0·001) and IL-10 (P = 0·013) in the del DID group that had received more TT vaccination, as compared to the unvaccinated group (Figs 5d and 6d).
Cord blood responses
With regard to cord MNC reactivity, differential induction of Th-2-type cytokines preferentially occurred in response to BCG, as compared to H37Rv stimulation. As shown in Fig. 5, BCG and PPD both induced higher IL-10 than did H37Rv (P < 0·001). IL-10 was detectable in cord MNC supernatants of 41/42 donors stimulated by BCG and 40/42 with PPD. Higher levels were obtained for BCG (median 127, range 16–534 pg/ml) as compared to PPD (median 83, range 8–366 pg/ml). By contrast, a striking finding was that IL-10 was undetectable in 43% of cord MNC donors following H37Rv stimulation. In those donors that did respond, lower levels of IL-10 were obtained (median 19, range 2–81 pg/ml) in comparison to BCG and PPD stimulation. Production of IL-5 by cord MNC was higher in response to BCG than H37Rv or PPD stimulation (P < 0·001). The proportion of donors with undetectable IL-5 was 62% in response to stimulation by H37Rv, as compared to 57% for PPD and 10% for BCG.
There were no statistically significant differences in cord IFN-γ responses to the three mycobacterial antigenic preparations. However, H37Rv was the weakest antigen with undetectable IFN-γ in 57% of cord MNC responses, as compared to 31% undetectable for PPD and 19% undetectable for BCG.
Cord blood responses were thus biased towards the Th2-type cytokines IL-10 and IL-5 specifically in response to BCG, but not H37Rv. However, by 10 weeks, PBMNC from the BCG unvaccinated infants produced significantly less IL-10 than cord MNC in response to BCG (P < 0·001), H37Rv (P < 0·003) and PPD (P = 0·025). This may indicate a maturational response unique to IL-10 (rather than exposure to other vaccines or infections), since IL-5 and IFN-γ production did not significantly differ between cord and unvaccinated infants, in response to the same three mycobacterial antigenic preparations.
Th1-type versus Th2-type cytokine responses
Since Th1-type and Th2-type cytokines are cross-regulatory and mutually inhibitory, their net biological effect may best be assessed by comparison of cytokine ratios, rather than evaluation of individual cytokine levels. According to this hypothesis, BCG vaccination-induced protective immunity would be expected to correlate best with a significant augmentation in Th1/Th2-type cytokine ratio, over the cord and unvaccinated 10-week values (Table 2). The effect of delayed BCG vaccination on the immune response is evident from comparison of cytokine responses to BCG and H37Rv stimulation in the same group of infants. Table 2 compares cord MNC reactivity with that of PBMNC at 10 weeks (unv 10 week) and post-vaccination at 20 weeks of age (del DID). A striking finding was that the unvaccinated 10-week PBMNC IFN-γ:IL-10 ratio was significantly increased over the cord blood value selectively in response to BCG stimulation (P = 0·0078). This was due to both increased IFN-γ and reduced IL-10 production at this time-point. In comparing the effect of BCG vaccination in the different infant groups at 10 weeks of age (Table 2), there was no significant increase in the IFN-γ:IL-10 ratio above the 10-week unvaccinated value, following BCG stimulation in vitro. This was due to the high unvaccinated 10-week value. IFN-γ:IL-10 ratios were significantly increased above the unvaccinated 10-week baseline values in response to H37Rv stimulation (P < 0·02). By contrast, a significant increase in IFN-γ:IL-5 ratio occurred in response to both BCG and H37Rv (P < 0·001) stimulation in all 10-week BCG-vaccinated groups as compared to unvaccinated 10-week baseline values (P < 0·001) (Table 2). It is also instructive to ask how variation in BCG vaccination at birth involving strain, route or tool specifically affects the increase in ratio of Th1-type:Th2-type cytokines (Table 2). Here, a significant increased IFN-γ:IL-5 ratio was observed only in response to BCG stimulation in comparison of the effect of tool (JPC Bio versus JPC Big) (P < 0·01) and age (del DID versus DID) (P = 0·02).
Table 2. Comparison of Th1-type:Th2-type cytokine ratios in cord blood and infants vaccinated at birth or at 10 weeks.
| IFN-γ:IL-10 | IFN-γ:IL-5 | |||
|---|---|---|---|---|
| BCG | H37Rv | BCG | H37Rv | |
| Delayed vaccination group | ||||
| Cords | 0·7 (0·0–3·2) | 1·0 (1·0–1·0) | 1·0 (0·0–2·3) | 1·0 (0·1–1·0) |
| Unv 10 weeks | 62·0 (1·2–366·0) | 1·0 (1·0–95·0) | 1·9 (0·5–7·1) | 1·0 (0·1–20·0) |
| Del DID | 61·8 (39·4–121·7) | 51·3 (20·8–92·2) | 33·6 (28·6–178·7) | 133·3 (20·8–3817·0) |
| Route (JPC, JID), PC tool (JPC Big, JPC Bio), Strain (JID, DID) comparison | ||||
| JPC Big | 39·1 (8·8–75·2) | 58·4 (18·3–71·0) | 45·0 (6·0–74·3) | 288·3 (95·5–894·0) |
| JPC Bio | 34·1 (22·6–78·0) | 39·7 (23·8–116·6) | 695·8 (36·3–4450·0) | 40·0 (2·0–2915·0) |
| DID | 49·0 (20·6–59·8) | 96·0 (38·2–145·0) | 18·3 (3·6–29·4) | 93·5 (6·9–579·8) |
| JID | 60·6 (31·5–105·0) | 129·3 (48·9–343·0) | 15·8 (13·2–47·3) | 837·4 (58·9–7062·0) |
Data expressed as median values, with 25th – 75th interquartile range in brackets.
A correlation between a type 1 cytokine secretion profile (IFN-γ:IL-4 ratio) and cytotoxic activity has previously been noted at the clonal level.23 We therefore evaluated whether this association held for bulk culture. Cord MNC and unvaccinated 10-week PBMNC had low median IFN-γ:IL-5 cytokine ratio values (< 2) following BCG stimulation. As expected, this correlated with low cytotoxicity against H37Rv- and BCG-infected target cells. As shown in Table 3, a positive relationship was noted between IFN-γ:IL-5 ratio and cytotoxic activity against BCG targets for all five BCG-vaccinated groups. A single exception occurs when the comparison is extended to cross-reactivity against H37Rv-infected targets, where the vaccination with the Biovac® tool resulted in dissociation between cytokine production and cytotoxic activity. This group exhibited the highest IFN-γ:IL-5 ratio (median 695) following BCG stimulation but minimal level of H37Rv antigen-specific kill (Table 3). Therefore, the general correlation between IFN-γ:IL-5 cytokine ratio and kill holds in nine of 10 cases overall. However, no correlation was found between IFN-γ:IL-10 cytokine ratios and cytotoxicity.
Table 3. Relationship between cytokine production and cytotoxicity.
| BCG strain | Variable | In vitro stimulation with BCG Japanese 172 IFN-γ:IL-5 ratio | BCG-specific kill percentage* | H37Rv-specific kill percentage* |
|---|---|---|---|---|
| JPC | TOOL-Big | 45 | 25 | 17 |
| JPC | TOOL-Bio | 695 | 18 | 4 |
| Del DID | Age | 34 | 15 | 14 |
| DID | Age/Strain | 18 | 17 | 9 |
| JID | Strain | 16 | 21 | 16 |
Antigen-specific kill for 3:1 E:T ratio.
Discussion
Despite available control measures and routine BCG vaccination, tuberculosis remains epidemic in South Africa and many other developing countries. While several hypotheses have been proposed for variable protection following BCG vaccination,4,24,25 generation of a suboptimum protective immune response is likely to be a contributory factor. Therefore, identification of any factors affecting the magnitude of immune response to BCG vaccination could potentially have an impact on vaccination practice.
Given the requirement for a Th1-type immune response in protective immunity against mycobacteria, we reasoned that vaccine efficacy would best be characterized by mycobacterial antigen-specific lymphoproliferative responses, IFN-γ production, high Th1-type/Th2-type cytokine ratios, and induction of CTL. These parameters were thus characterized in a group of infants from Cape Town, South Africa receiving BCG vaccine, with variation with regard to BCG strain, route, age and tool of administration. There were four main findings from this study. Comparable 10-week post-BCG vaccination in vitro cytokine and proliferative immune responses were found, with a trend for higher responses when BCG vaccination was delayed until 10 weeks and the use of Japanese strain via the intradermal route. Second, IL-10 levels in the supernatants from cord MNCs stimulated with BCG were higher than those in unvaccinated infants aged 10 weeks; a novel finding. Third, differential production of Th2-type cytokines IL-5 and IL-10 by cord MNC preferentially occurred in response to BCG (rather than H37Rv or PPD) stimulation and finally, we found evidence that BCG can prime for the generation of neonatal CTL but that the specificity of CTL activity was strongly influenced by the tool utilized in the vaccination procedure.
The ratio of IFN-γ:IL-10 cytokines produced by CD4+ Th1 clones has been found to be of critical importance in determining their pro- or anti-inflammatory functional bias.26 The ratio of Th1-type to Th2-type cytokines produced by PBMNC ex vivo has also indicated significant systemic bias in cytokine production towards either Th1 or Th2 dominance in autoimmune disease.27 The IFN-γ:IL-10 ratio has proved useful in documenting diurnal variation in the balance of Th1- and Th2-type cytokines.28 These considerations are also important in clinical tuberculosis where a Th1-type cytokine response is considered essential for protective immunity,6 while significant Th2-type cytokine production correlated with adverse clinical outcome.29 In animal experiments, there was a strong correlation with vaccine protective efficacy and Th1/Th2 balance.30 We therefore compared the ratios of Th1-type to Th2-type cytokines following BCG vaccination. A striking finding was high IL-10 production by cord MNC preferentially in response to BCG stimulation. BCG-induced IL-10 secretion had diminished by 10 weeks of age. This was associated with an increased IFN-γ:IL-10 ratio that may be the result of maturation of the immune response or may represent the effect of other vaccines received by neonates in the first 10 weeks of life. However, this was a selective effect and was not accompanied by significant changes in IFN-γ:IL-5 ratio in the unvaccinated 10-week-old group.
Considerable data exist on cord blood response to mitogen and allogeneic cell stimulation.31,32 Cord blood displays an altered cytokine profile in response to mitogen stimulation with defective IFN-γ and IL-10 responses; this progressively increases in the first year of life.31 Developmental delay in neonatal IL-10 production has been attributed to decreased expression of tumour necrosis factor-α and its receptors.33 However, IL-10 production in response to BCG stimulation by human cord MNC has not been well studied, although murine studies have previously documented a neonatal Th2 bias.7–9 The current study made use of heat-killed BCG and H37Rv antigens that probably enhanced the expression of heat-shock proteins, these may have represented a ‘danger’ signal to Th2 anti-inflammatory cells.34 It is possible that heat-shock protein or related antigens may be more immunogenic in BCG than H37Rv to account for our findings. Whilst BCG and H37Rv were grown in identical media and CFUs were enumerated, we have no data on viability of the preparations prior to heat killing. It cannot therefore be excluded that the preheat-treated BCG may have contained greater numbers of dead organisms as a trivial explanation for induction of higher Th2-type cytokine levels. However, this explanation would not account for the reduction in BCG-stimulated IL-10 by 10 weeks. Finally, helminth infection may have been a factor in our study population.35,36 Cord blood T cells have been shown to be hyporesponsive to IL-12 but respond to allergens and helminth infections with production predominantly of the Th2-type cytokines IL-4, IL-10, IL-13.37
The implication of IL-10 production in response to BCG stimulation for host protective immunity remains to be determined. IL-10 is conventionally considered an immunosuppressive, Th2-type cytokine in view of its inhibition of macrophage pro-inflammatory cytokine production and Th1-type responses.38 In keeping with this, IL-10 has been shown to antagonize macrophage function in mycobacterial infection39 and IL-10-deficient mice exhibit increased antimycobacterial immunity.40 It is also of interest that prenatal sensitization to helminths has been shown to be associated with decreased Th1-type immunity induced by BCG vaccination,41 although IL-10 was not measured in that study. On the other hand, there is also evidence that IL-10 plays an important role in protective immunity in several infections including Candida, trypanosomiasis and tuberculosis. Thus, IL-10 has been shown to be required for optimal development of CD4+ Th1 cells in mice infected with Candida albicans.42 Recent experimental evidence indicates an important role for IL-10 in survival and resistance of mice infected with African trypanosomes.43 CD4+ T-cell clones isolated from bronchoalveolar lavage of patients with active pulmonary tuberculosis produced both IFN-γ and IL-10.44 While IL-10 production may represent an attempt to limit local tissue damage, a role for IL-10 in protective immunity cannot be excluded. Thus IL-10 treatment in vitro endows human dendritic cells with the ability to inhibit intracellular growth of virulent mycobacteria.45 Reduced stimulation of IL-10 production by virulent H37Rv in the unvaccinated neonate could thus favour intracellular survival of the organism within parasitized dendritic cells. Further work is needed to establish the biological outcome of opposing IL-10 activity in mycobacterial infection, as in the case of Aspergillus infection.46
While CTL have been considered an important component of protective immunity against mycobacteria,15,16 previous studies have been limited to adults.19,47,48 To the best of our knowledge, this is the first study to measure cytolytic activity in neonates. Our hypotheses were that failure to prime for CTL activity could be a factor contributing to inadequate protective immunity following BCG vaccination, and that the ability of BCG to prime for CTL activity against H37Rv would represent the best measure of protective cytolytic capacity. In this context, it is interesting to note that the lowest cytolytic activity was observed against H37Rv-infected macrophages in neonates vaccinated with the Biovac® tool. Importantly, this represented a selective effect on CTL activity and did not affect cytokine production, nor result in any skewing of Th1:Th2 ratio (Table 2). Indeed, the Biovac® tool gave the highest IFN-γ:IL-5 ratio following BCG stimulation (Table 2). Aside from this exception, a correlation was found between increased IFN-γ:IL-5 ratio and cytotoxic activity. Increased IFN-γ:IL-5 ratio was also observed for an age-related maturational vaccine response (higher with both BCG and H37Rv in del DID than DID). These data suggest that IFN-γ:IL-5 ratio may provide a useful general indicator of the Th1-type response and vaccine efficacy. These findings, including an opposite trend in CTL activity and cytokine production dependent on the vaccine tool employed, may have important implications for correlates of protective immunity in mycobacterial vaccine studies. Previously, adjuvant-induced IFN-γ levels did not clearly correlate with protective immunity,30 suggesting the need for additional correlates of protection. The non-specific or anomalous killing described here has also previously been reported19,47,48 and may be due to 65000 MW heat-shock protein.49 The relevance of generation of CTL activity following BCG vaccination for protective immunity will need to be addressed by additional prospective studies. These will also need to evaluate the relative contribution of distinct CTL subsets (γδ+, CD4+, CD8+) and cytolytic mediator molecules such as perforin, granzymes and granulysin.14,18
Tuberculosis remains widespread in many developing countries 5 years after the World Health Organization declared it a global emergency, despite a concerted effort to combat the disease. The best hope of stemming the epidemic lies in the development of a more effective vaccine, since prospects for new drug development are limited by cost, while their efficacy would be limited by increasing multidrug resistance. Current efforts are directed at improved definition of immunological correlates of protective immunity,14,50 improved DNA-based modes of vaccination51 and molecular analysis of the mycobacterial genome.52,53 However, successful implementation of novel vaccine candidates in the future will need to take several additional factors into account. These include the impact of variation in vaccination strategy on immune parameters as documented in this study, host population genetic factors,54,55 and environmental influences including parasitic infections.36,37,41 We noted considerable donor variation in the immune response to BCG vaccination and larger studies will be required to confirm the trends reported here and to develop improved BCG vaccination strategies.
Acknowledgments
This work was presented at the Meeting of the WHO Working Group on Correlates of Protection Against Tuberculosis, 19th May 2000, Dulles Airport Marriot Hotel, Washington, D.C.; at the Keystone symposia Molecular and Cellular Aspects of Tuberculosis Research in the Post Genome Era (B1), January 25–30 January, 2001, Sagebrush Inn, Taos, New Mexico; Poster Number 253 in Poster Session 2. Funding was received from Sequella Global Tuberculosis Foundation and Medical Research Council of South Africa. We are grateful to Sr H. Malan and Sr W. Dollie for specimen collection, to Mrs L Bryce for sample processing and to Dr W Katz of the State Vaccine Institute for providing Japanese-172 percutaneous BCG vaccine.
References
- 1.Dolin PJ, Raviglione MC, Kochi A. Global tuberculosis incidence and mortality during 1990–2000. Bull WHO. 1994;72:213–20. [PMC free article] [PubMed] [Google Scholar]
- 2.Husson RN. Tuberculosis. In: Pizzo PA, Wilfert CM, editors. Pediatric AIDS. 2. Baltimore: Williams & Wilkins; 1994. pp. 289–307. [Google Scholar]
- 3.Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis – meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 4.Fine PEM. Variation in protection by BCG implications of and for heterologous immunity. Lancet. 1995;346:1339–45. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 5.Collins DM. New tuberculosis vaccines based on attenuated strains of the Mycobacterium tuberculosis complex. Immunol-Cell-Biol. 2000;78(4):342–8. doi: 10.1046/j.1440-1711.2000.00937.x. 10.1046/j.1440-1711.2000.00937.x. [DOI] [PubMed] [Google Scholar]
- 6.Schluger NW, Rom WN. The host immune response to tuberculosis. Am J Respir Crit Care Med. 1998;157:679–91. doi: 10.1164/ajrccm.157.3.9708002. [DOI] [PubMed] [Google Scholar]
- 7.Bona C, Bot A. Neonatal immunoresponsiveness. The immunologist. 1997;5:5–9. [Google Scholar]
- 8.Pennisi E. Teetering on the brink of danger. Science. 1996;271:1665–7. doi: 10.1126/science.271.5256.1665. [DOI] [PubMed] [Google Scholar]
- 9.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–6. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 10.Power CA, Wei G, Bretscher PA. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect Immun. 1998;66:5743–50. doi: 10.1128/iai.66.12.5743-5750.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowry PW, Ludwig TS, Adams JA, et al. Cellular immune responses to four doses of percutaneous Bacille Calmette–Guérin in healthy adults. J Infect Dis. 1998;178:138–46. doi: 10.1086/515614. [DOI] [PubMed] [Google Scholar]
- 12.Jarad NA, Empey DW, Duckworth G. Administration of the BCG vaccination using the multipuncture method in schoolchildren: a comparison with the intradermal method. Thorax. 1999;54:762–4. doi: 10.1136/thx.54.9.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchant A, Goetghebuer T, Ota MO, et al. Newborns develop a Th1-Type immune response to Mycobacterium bovis Bacillus Calmette–Guérin vaccination. J Immunol. 1999;163:2249–55. [PubMed] [Google Scholar]
- 14.Ellner JJ, Hirsch CS, Whalen CC. Correlates of protective immunity to Mycobacterium tuberculosis in humans. Clin Infect Dis. 2000;30:S279–82. doi: 10.1086/313874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serbina NV, Liu C-C, Scanga CA, Flynn JL. CD8+ CTL from lungs of Mycobacterium tuberculosis-infected mice express perforin in vivo and lyse infected macrophages. J Immunol. 2000;165:353–63. doi: 10.4049/jimmunol.165.1.353. [DOI] [PubMed] [Google Scholar]
- 16.Stenger S, Modlin RL. Cytotoxic T cell responses to intracellular pathogens. Curr Opin Immunol. 1998;10:471–7. doi: 10.1016/s0952-7915(98)80123-4. [DOI] [PubMed] [Google Scholar]
- 17.Lalvani A, Brookes R, Wilkinson RJ, et al. Human cytolytic and interferon-γ-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1998;95:270–5. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stenger S, Mazzaccaro RJ, Uyemura K, et al. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–7. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 19.Ravn P, Boesen H, Pedersen BK, Andersen P. Human T cell responses induced by vaccination with Mycobacterium bovis Bacillus Calmette–Guérin. J Immunol. 1997;158:1949–55. [PubMed] [Google Scholar]
- 20.Smith SM, Malin AS, Lukey PT, et al. Characterization of human Mycobacterium bovis Bacillus Calmette–Guérin-reactive CD8+ T cells. Infect Immun. 1999;67:5223–30. doi: 10.1128/iai.67.10.5223-5230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorgat F, Keraan MM, Lukey PT, Ress SR. Evidence for in vivo generation of cytotoxic T cells. PPD-stimulated lymphocytes from tuberculous pleural effusions demonstrate enhanced cytotoxicity with accelerated kinetics of induction. Am Rev Respir Dis. 1992;145:418–23. doi: 10.1164/ajrccm/145.2_Pt_1.418. [DOI] [PubMed] [Google Scholar]
- 22.Petrovsky N, Harrison LC. Cytokine-based human whole blood assay for the detection of antigen-reactive T cells. J Immunol Meth. 1995;186:37–46. doi: 10.1016/0022-1759(95)00127-v. [DOI] [PubMed] [Google Scholar]
- 23.Mutis T, Cornelisse YE, Ottenhoff THM. Mycobacteria induced CD4+ T cells that are cytotoxic and display Th1-like cytokine secretion profile: heterogeneity in cytotoxic activity and cytokine secretion levels. Eur J Immunol. 1993;23:2189–95. doi: 10.1002/eji.1830230921. [DOI] [PubMed] [Google Scholar]
- 24.Fine PEM. Vaccines, genes and trials. Novartis Found Symp. 1998;217:57–69. doi: 10.1002/0470846526.ch5. discussion 6972. [DOI] [PubMed] [Google Scholar]
- 25.Bannon MJ. BCG and tuberculosis. Arch Dis Child. 1999;80:80–3. doi: 10.1136/adc.80.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsikis PD, Cohen SB, Londei M, Feldmann M. Are CD4+ Th1 cells pro-inflammatory or anti-inflammatory? The ratio of IL-10 to IFN-gamma or IL-2 determines their function. Int Immunol. 1995;7:1287–94. doi: 10.1093/intimm/7.8.1287. [DOI] [PubMed] [Google Scholar]
- 27.Kallmann BA, Hüther M, Tubes M, et al. Systemic bias of cytokine production toward cell-mediated immune regulation in IDDM and toward humoral immunity in Graves' Disease. Diabetes. 1997;46:237–43. doi: 10.2337/diab.46.2.237. [DOI] [PubMed] [Google Scholar]
- 28.Petrovsky N, Harrison LC. Diurnal rhythmicity of human cytokine production. A dynamic disequilibrium in T helper cell type 1/ T helper cell type 2 balance? J Immunol. 1997;158:5163–8. [PubMed] [Google Scholar]
- 29.Seah GT, Scott GM, Rook GA. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J Infect Dis. 2000;181:385–9. doi: 10.1086/315200. [DOI] [PubMed] [Google Scholar]
- 30.Lindblad EB, Elhay MJ, Silva R, Appelberg R, Andersen P. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect Immun. 1997;65:623–9. doi: 10.1128/iai.65.2.623-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vigano A, Esposito S, Arienti D, et al. Differential development of type 1 and type 2 cytokines and b-chemokines in the ontogeny of healthy newborns. Biol Neonate. 1999;75:1–8. doi: 10.1159/000014071. [DOI] [PubMed] [Google Scholar]
- 32.Cohen SBA. Can cord blood cells support the cytokine stormin GvHD? Cytokine Growth Factor Rev. 2000;11:185–97. doi: 10.1016/s1359-6101(00)00004-6. [DOI] [PubMed] [Google Scholar]
- 33.Chheda S, Palkowetz KH, Garofalo R, Rassin DK, Goldman AS. Decreased interleukin-10 production by neonatal monocytes and T cells: relationship to decreased production and expression of tumor necrosis factor-alpha and its receptors. Pediatr Res. 1996;40:475–83. doi: 10.1203/00006450-199609000-00018. [DOI] [PubMed] [Google Scholar]
- 34.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 35.Adams JFA, Schölvinck Gie RP, Potter PC, Beyers N, Beyers AD. Decline in total serum IgE after treatment for tuberculosis. Lancet. 1999;353:2030–2. doi: 10.1016/s0140-6736(98)08510-9. [DOI] [PubMed] [Google Scholar]
- 36.Bentwich Z, Kalinkovich A, Weisman Z, Borkow G, Beyers N, Beyers AD. Can eradication of helminthic infections change the face of AIDS and tuberculosis? Immunol Today. 1999;20:485–7. doi: 10.1016/s0167-5699(99)01499-1. [DOI] [PubMed] [Google Scholar]
- 37.Beverly PCL. Vaccine immunity. Immunol Today. 1997;18:413–15. doi: 10.1016/s0167-5699(97)01120-1. [DOI] [PubMed] [Google Scholar]
- 38.de Waal Malefyt R, Yssel H, Roncarolo MG, Spits H, de Vries JE. Interleukin-10. Curr Opin Immunol. 1992;4:314–20. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]
- 39.Murray PJ, Wang L, Onufryk C, Tepper RI, Young RA. T Cell-Derived IL-10 antagonizes macrophage function in mycobacterial infection. J Immunol. 1997;158:315–21. [PubMed] [Google Scholar]
- 40.Murray PJ, Young RA. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect Immun. 1999;67:3087–95. doi: 10.1128/iai.67.6.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malhotra I, Mungai P, Wamachi A, et al. Helminth- and Bacillus Calmette–Guérin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol. 1999;162:6843–8. [PubMed] [Google Scholar]
- 42.Mencacci A, Cenci E, Del Sero G, et al. IL-10 is required for development of protective Th1 responses in IL-12-deficient mice upon Candida albicans infection. J Immunol. 1998;161:6228–37. [PubMed] [Google Scholar]
- 43.Namangala B, Noel W, De Baetselier P, Brys L, Beschin A. Relative contribution of interferon-gamma and interleukin-10 to resistance to murine African trypanosomosis. J Infect Dis. 2001;183:1794–800. doi: 10.1086/320731. [DOI] [PubMed] [Google Scholar]
- 44.Gerosa F, Nisii C, Righetti S, et al. CD4+ T cell clones producing both interferon-γ and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin Immunol. 1999;92:224–34. doi: 10.1006/clim.1999.4752. [DOI] [PubMed] [Google Scholar]
- 45.Förtsch D, Röllinghof M, Stenger S. IL-10 converts human dendritic cells into macrophage-like cells with increased antibacterial activity against virulent Mycobacterium tuberculosis. J Immunol. 2000;165:978–87. doi: 10.4049/jimmunol.165.2.978. [DOI] [PubMed] [Google Scholar]
- 46.Roilides E, Dimitriadou A, Kadiltsoglou I, et al. IL-10 exerts suppressive and enhancing effects on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. J Immunol. 1997;158:322–9. [PubMed] [Google Scholar]
- 47.Pithie AD, Rahelu M, Kumararatne DS, et al. Generation of cytolytic T cells in individuals infected by Mycobacterium tuberculosis and vaccinated with BCG. Thorax. 1992;47:695–701. doi: 10.1136/thx.47.9.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner J, Corrah T, Sabbally S, Whittle H, Dockrell HM. A Longitudinal study of in vitro IFN-γ production and cytotoxic T cell responses of tuberculosis patients in the Gambia. Tubercle Lung Dis. 2000;80:161–9. doi: 10.1054/tuld.2000.0241. [DOI] [PubMed] [Google Scholar]
- 49.Kale Ab B, Kiessling R, Van Embden JDA, et al. Induction of antigen-specific CD4+ HLA-DR-restricted cytotoxic T lymphocytes as well as nonspecific nonrestricted killer cells by recombinant mycobacterial 65-kDa heat shock protein. Eur J Immunol. 1990;20:369–77. doi: 10.1002/eji.1830200221. [DOI] [PubMed] [Google Scholar]
- 50.Murray PJ. Defining the requirements for immunological control of mycobacterial infections. Trends Microbiol. 1999;7:366–72. doi: 10.1016/s0966-842x(99)01567-x. [DOI] [PubMed] [Google Scholar]
- 51.Lowrie DB, Tascon RE, Bonato VLD, et al. Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400:269–71. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- 52.Behr MA, Wilson MA, Gill WP, et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–3. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 53.Brosch R, Gordon SV, Pym A, Eiglmeier K, Garnier T, Cole ST. Comparative genomics of the mycobacteria. Int J Med Microbiol. 2000;290:143–52. doi: 10.1016/S1438-4221(00)80083-1. [DOI] [PubMed] [Google Scholar]
- 54.Bellamy RJ, Hill AV. Host genetic susceptibility to human tuberculosis. Novartis Found Symp. 1998;217:3–13. doi: 10.1002/0470846526.ch2. discussion 1323. [DOI] [PubMed] [Google Scholar]
- 55.Qureshi ST, Skamene E, Malo D. Comparative Genomics and host resistance against infectious diseases. Emerging Infectious Diseases. 1999;5:36–47. doi: 10.3201/eid0501.990105. [DOI] [PMC free article] [PubMed] [Google Scholar]