Abstract
Previous studies in mice have reported a decrease in epidermal Langerhans cell (LC) density in aged skin, however, the impact of this reduction on LC function and cutaneous immune responses is unclear. In the present series of experiments, the frequency of major histocompatibility complex class II+ LC in the epidermis of older (6-month-old) mice was found to be reduced significantly compared with that observed for young (6–8-week-old) mice. LC mobilization and the subsequent accumulation of dendritic cells (DC) in regional lymph nodes in response to topical challenge with a chemical allergen were found to be less vigorous in older mice. Flow cytometric analyses of DC derived from the draining lymph nodes of fluorescein isothiocyanate (FITC)-sensitized mice revealed that the frequency of FITC+-DC arriving in draining lymph nodes was also reduced in older mice but that the fluorescence intensity was comparable. Control and allergen-treated-older mice also displayed decreased total lymph node cellularity. Contact hypersensitivity responses were found not to be compromised in older mice. However, the cytokine regulation of LC migration in the two age groups of mice did differ. LC migration provoked by intradermal injection of tumour necrosis factor-α (TNF-α) was reduced in older animals, whereas, the percentage of LC that migrated in response to exogenous interleukin-1β (IL-1β) was comparable for both young and aged mice. Since both allergen- and TNF-α-induced LC responses are known to require receipt by LC of a signal from IL-1β for effective migration, the suggestion is that impaired LC migration in older mice may be due to a reduced availability of epidermal IL-1β.
Introduction
There has been considerable interest in the relationships between ageing, senescence of the immune system and disorders of the elderly, including the recrudescence of certain infectious diseases.1–6 Associated with a generalized impairment of immune function it is known that elderly subjects are increasingly susceptible to cutaneous viral and fungal infections and to skin cancers that are associated with immunological deficiencies.7–9 Although age-related changes in immune competence are associated with systemic decrements in both T- and B-lymphocyte function,4,10–12 consideration of cutaneous immunity has focused largely on cytokine production and the frequency of resident Langerhans cell (LC) and dendritic cell (DC) populations. There have been several reports that, compared with their younger counterparts, ageing mice and hamsters have decreased numbers of epidermal LC, and of Thy-1+ DC.13–17 Despite the evidence for age-related reductions in the density of resident LC, there is little information available on associated changes in the function of these cells, or of the influence of age on their ability to migrate from the epidermis in the context of antigen delivery to skin-draining lymph nodes.
Bone-marrow-derived LC represent part of the larger family of DC. They form a semicontiguous network in the epidermis, where they are believed to act as sentinels of the adaptive immune system. Encounter with antigen in the skin, or other forms of local trauma, induces the migration of LC, including those bearing antigen, from the epidermis to draining lymph nodes via the afferent lymphatics.18–20 During migration LC are subject to functional maturation and acquire the characteristics of immunostimulatory DC.21 The mobilization and migration of LC and their subsequent localization within the paracortical regions of skin-draining lymph nodes are processes known to be induced and regulated by cytokines and chemokines.22 Of particular importance in stimulating LC migration are tumour necrosis factor-α (TNF-α), an inducible product of keratinocytes, and interleukin-1β (IL-1β), which in murine epidermis is expressed exclusively by LC. These two epidermal cytokines each deliver a mandatory signal for LC migration. The available evidence indicates that LC-derived IL-1β acts in an autocrine fashion, via type I IL-1 receptors (IL-1RI), to provide one stimulus for migration, and also provokes the production by adjacent keratinocytes of TNF-α. The second signal for mobilization is provided to LC by TNF-α acting through type 2 TNF receptors (TNF-R2).23–29 It has been shown also that the intradermal administration of either IL-1β or TNF-α alone is able to provoke migration. In the former case, exogenous IL-1β is able to induce the production of TNF-α, while in the latter instance there is sufficient constitutive IL-1β available to act in concert with exogenous TNF-α to support migration.23,27
Here we have investigated the influence of age on the integrity of LC migration and on their responsiveness to the cytokine signals that induce this process. For this purpose we have examined in young adult (6–8 weeks) and older (6 months) BALB/c strain mice LC migration and the accumulation of DC in draining lymph nodes induced by topical exposure of animals to oxazolone, a potent contact allergen. In addition, the ability of DC from each age group to transport allergen to draining lymph nodes and the impact on contact hypersensitivity responses have been investigated. In parallel studies we have determined also the ability of LC to respond to the stimuli provided by IL-1β and TNF-α.
Materials and methods
Animals
Young adult (6- to 8-week-old) and aged (6-month-old) BALB/c strain mice, obtained from the Specific Pathogen-Free Breeding Unit (Alderley Park, Cheshire, UK), were used throughout these studies.
Chemicals and exposure
The skin-sensitizing chemicals 4-ethoxy-2-phenyloxazol-5-one [oxazolone (Ox); Sigma Chemical Co., St Louis, MO] and fluorescein isothiocyanate [(FITC) type I; Sigma Chemical Co.] were dissolved in 4 : 1 acetone : olive oil (AOO) or 1 : 1 acetone : dibutylphthalate, respectively. For analysis of LC migration and DC accumulation, groups of mice received 25 µl of test chemical (0·5% Ox or 1% FITC) on the dorsum of both ears. For sensitization and elicitation of contact hypersensitivity, groups of mice were treated topically with 50 µl of 1% Ox in AOO on both shaved flanks. Control mice received an equivalent volume of AOO alone. Five days later, the ear thickness of each mouse was measured using an engineers' micrometer (Moore and Wright, Sheffield, UK). The dorsum of both ears was then treated immediately with 25 µl of the challenge concentration (0·25%) of Ox. Elicitation reactions were measured 24, 48 and 72 hr later and results were displayed as the mean percentage increase in ear thickness relative to prechallenge values. The significance of differences in contact hypersensitivity reactions was evaluated using the paired Student's t-test.
Cytokines and injection
Recombinant murine TNF-α (specific activity 2 × 108 U/mg by L929 cytotoxicity assay; endotoxin level: 0·009 ng/μg) was obtained from Genzyme (West Malling, Kent, UK). Recombinant murine IL-1β (endotoxin content: < 0·1 ng/μg of IL-1β) was purchased from R & D Systems (Oxon, UK). Cytokines were diluted in sterile phosphate-buffered saline (PBS) containing 0·1% bovine serum albumin (BSA) as carrier protein and were administered locally by intradermal injection into both ear pinnae (30 µl) using 1 ml syringes with 30-gauge stainless steel needles. Control mice received an equivalent volume of carrier protein alone or were untreated.
Preparation and analysis of epidermal sheets
Ears were split with the aid of forceps into dorsal and ventral halves. Dorsal ear halves were incubated for 90 min at 37° with 0·02 m ethylenediaminetetraacetic acid (EDTA; Sigma Chemical Co.) dissolved in PBS. The epidermis was separated from the dermis using forceps and was washed in PBS. Epidermal sheets were fixed in acetone for 20 min at −20°. Following fixation, epidermal sheets were washed in PBS and then incubated at room temperature for 30 min with rat anti-mouse I-Ad/I-Ed monoclonal antibody [clone 2G9, rat immunoglobulin (IgG)2aκ; Pharmingen, San Diego, CA], or purified rat IgG2aκ (Pharmingen), each diluted to 5 µg/ml in 0·1% BSA/PBS. Sheets were washed in PBS prior to incubation for a further 30 min with FITC-conjugated F(ab)2 goat anti-rat IgG (Serotec, Kidlington, Oxford, UK) diluted 1 : 100 in 0·1% BSA/PBS. Finally, sheets were washed in PBS and mounted on microscope slides in Citifluor (Citifluor Ltd, London, UK) and sealed with nail varnish. Samples were examined in a blinded fashion by fluorescence microscopy and the frequency of stained cells was assessed using an eye-piece with a calibrated grid (0·32 × 0·213 mm at ×40 magnification). Four epidermal sheets from each experimental group were incubated with anti-I-Ad/I-Ed antibody and two epidermal sheets from each experimental group were treated with isotype-matched control antibody. In no instance was any fluorescence detected following treatment with isotype-matched control antibody. For each sample 10 consecutive fields in the central portion of the ear were examined. Results are expressed as the mean number of cells/mm2 (± SE) derived from examination of 10 fields/sample for each of four samples. The statistical significance of differences between experimental groups was calculated using the Student's two-sided t-test.
Isolation and enumeration of lymph node dendritic cells
DC were analysed either following enrichment from lymph node cells (LNC) by density gradient centrifugation or within whole LNC suspensions prepared following enzyme digestion of lymph nodes. In each case, draining (auricular) lymph nodes were excised and pooled for each experimental group. To isolate DC-enriched fractions, single-cell suspensions of LNC were prepared by mechanical disaggregation through 200-mesh stainless steel gauze. LNC were washed with, and suspended in, RPMI-1640 growth medium (Gibco, Paisley, UK) supplemented with 25 mm HEPES, 400 µg/ml streptomycin, 400 µg/ml ampicillin and 10% heat-inactivated fetal calf serum (RPMI-FCS). Viable cell counts were performed by exclusion of 0·5% trypan blue and the cell concentration was adjusted to 5 × 106 cells/ml in RPMI-FCS. DC-enriched populations were prepared as described previously.20 Briefly, 2 ml of Metrizamide (Sigma Chemical Co.; 14·5% in RPMI-FCS) was layered gently under 8 ml of the cell suspension, and tubes were centrifuged for 15 min (600 g) at room temperature. Cells accumulating at the interface were collected, washed once, and resuspended in RPMI-FCS. The frequency of DC in such low buoyant density fractions was assessed routinely by direct morphological examination using phase contrast microscopy. Results are expressed as DC/node. Alternatively, the frequency and antigen-bearing characteristics of DC within whole LNC suspensions were examined by flow cytometry following enzyme digestion of lymph nodes.30,31 Briefly, lymph nodes were disrupted gently in medium containing 0·5 mg/ml collagenase IV and 0·02 mg/ml DNase I (both from Sigma Chemical Co.) followed by incubation in the enzyme solution for 25 min at room temperature. The resulting cell suspension was filtered through 200-mesh stainless steel gauze. Cells were washed and resuspended in medium.
Flow cytometric analysis of DC
For enumeration of DC by flow cytometry, LNC suspensions (5 × 105/well) were incubated with mouse Fc block (rat anti-mouse CD16/CD32; clone 2·4G2, rat IgG2bκ from Pharmingen) for 10 min on ice followed by incubation for 30–45 min on ice with R-phycoerythrin (R-PE) -conjugated hamster anti-mouse CD11c (clone HL3 from Pharmingen) or R-PE-conjugated hamster IgG (clone G235-2356 from Pharmingen) each diluted to 10 µg/ml in medium. Cells were then exposed to rat IgG (5 µg/ml from Serotec) for 10 min to block non-specific rat IgG-binding sites prior to incubation for a further 30–45 min on ice with FITC-conjugated rat anti-mouse I-A/I-E (clone 2G9; rat IgG2aκ from Pharmingen) or FITC-conjugated rat IgG2aκ (clone R35-95 from Pharmingen), both diluted to 2 µg/ml in medium.
To examine DC isolated from FITC-sensitized mice, LNC suspensions (5 × 105/well) or DC-enriched fractions (2·5 × 105/well) or were incubated on ice for 30–45 min with R-PE-conjugated rat anti-mouse I-A antibody (clone M5/114.15.2, rat IgG2bκ from Pharmingen) diluted to 2 µg/ml or R-PE-conjugated hamster anti-mouse CD11c. Control cell populations were incubated either with R-PE-conjugated rat IgG2bκ isotype control antibody (Pharmingen) or R-PE-conjugated hamster IgG. Cells were preblocked with mouse Fc block where necessary.
Cell suspensions were washed and resuspended in cold 0·5% BSA/0·05% azide/PBS prior to analysis either by single or two-colour flow cytometry using a FACSCalibre flow cytometer (BD Biosciences, Oxford, UK).
Epidermal cell suspensions and analysis by flow cytometry
Dorsal ear halves were incubated with 0·25% trypsin (Gibco BRL, Paisley, UK) in Hanks' balanced salt solution (HBSS, Gibco) for 20 min at 37°. Epidermal sheets were removed, washed in RPMI containing 20% FCS and a single cell suspension was prepared by gentle mechanical disaggregation through sterile 200-mesh stainless steel gauze. Epidermal cell suspensions were washed twice in RPMI containing 20% FCS and fixed for 20 min on ice in 2% formaldehyde (4% stock: 1·4 ml 10× PBS/7·6 ml water/1 ml 40% formaldehyde). All buffers used during trypsin digestion and cell preparation for staining contained 10 µm monensin and 100 µm glyburide (Sigma Chemical Co.). To detect IL-1β expression, epidermal cell suspensions (5 × 105/well) were incubated on ice with rat anti-mouse IL-1β (10 µg/ml, clone 30311.11, rat IgG1, R & D Systems) or purified rat IgG1 (10 µg/ml, clone R3-34, Pharmingen), followed by incubation with R-PE-conjugated goat F(ab′)2 anti-rat IgG (1 : 100, Serotec). Non-specific rat IgG-binding sites were blocked by treatment of cells with rat IgG (5 µg/ml) for 10 min prior to staining with FITC-conjugated rat anti-mouse I-A/I-E (5 µg/ml) or FITC-conjugated rat IgG2a,κ isotype control (5 µg/ml). Incubations were performed in 5% FCS/PBS either in the presence or absence of 0·1% saponin. Cells were washed in 0·5% BSA/0·05% azide/PBS prior to analysis of 50 000 cells using a FACSCalibre flow cytometer and CellQuest software (BD Biosciences).
Results
The influence of ageing on allergen-induced LC migration and draining lymph node DC accumulation
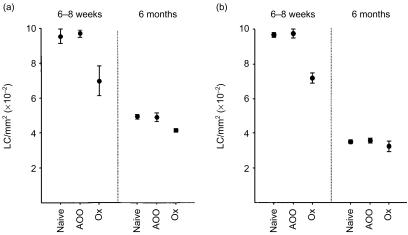
In the first series of experiments, groups of BALB/c strain mice, aged either between 6 and 8 weeks old or approximately 6 months old, were exposed topically on the dorsum of both ears to the contact allergen oxazolone (Ox; 0·5%). Control mice received vehicle (AOO) in place of Ox or were left untreated (naive). Four hours later, ears were removed and epidermal sheets prepared for analysis of epidermal major histocompatibility complex (MHC) class II+ LC. As illustrated in Fig. 1, a substantial reduction in baseline epidermal LC numbers was observed for older naive mice compared with young naive mice, with the mean LC densities for the two independent experiments shown in Fig. 1 dropping from 935·1 ± 6·3 cells/mm2 for 6–8-week-old mice to 421·9 ± 29·9 cells/mm2 for older mice (mean ± SE of counts derived from eight ears, four ears/experiment). Exposure to vehicle (AOO) was without effect on baseline epidermal LC frequencies in either age group. However, a significant decrease in LC numbers was observed following exposure of young mice to Ox compared with vehicle treatment (Fig. 1a: 27·8% reduction; P < 0·05, Fig. 1b: 26·4% reduction; P < 0·001). Although application of Ox was associated also with a decrease in MHC class II+ LC numbers in older mice the vigour of this response was considerably less marked (Fig. 1a: 15·7% reduction; P < 0·05, Fig. 1b: 9·9%; not significant).
Figure 1.
Reduced LC migration in aged mice in response to allergen. Groups of 6–8-week-old and 6-month-old mice (n = 2/group) received 25 µl of 0·5% Ox in AOO, or AOO alone, on the dorsum of both ears. Control mice were untreated (naive). Ears (n = 4/group) were removed 4 hr later and epidermal sheets were prepared for analysis of MHC class II+ LC frequency. Two independent experiments are shown.
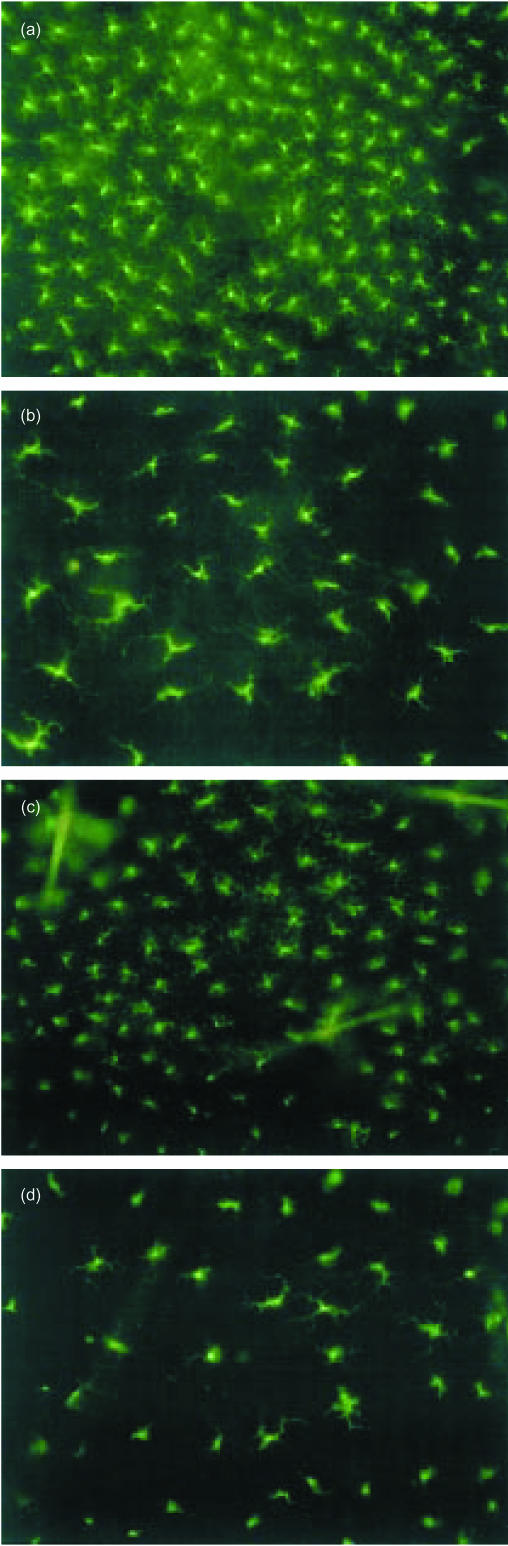
Marked differences in LC morphology were apparent between young and older mice. Resting LC appeared larger with longer dendritic processes in epidermal sheets prepared from older naive mice than those observed in epidermal sheets derived from younger mice (Fig. 2a,b). The difference in resting LC numbers between young and older mice is also readily apparent. During the short (4 hr) exposure to Ox, LC remained largely unchanged in appearance or staining intensity for MHC class II (Fig. 2c,d). Treatment of mice with AOO was also without effect on LC morphology (not shown).
Figure 2.
Changes in MHC class II+ LC morphology associated with ageing. Groups of young and aged mice were treated and epidermal sheets were prepared as described in the legend to Fig. 1. Samples were examined by fluorescence microscopy. Photos show LC morphology in samples prepared from 6–8-week-old mice: naive and 4 hr following exposure to Ox, and in 6-month-old mice: naive and 4 hr following exposure to Ox. All magnifications ×50.
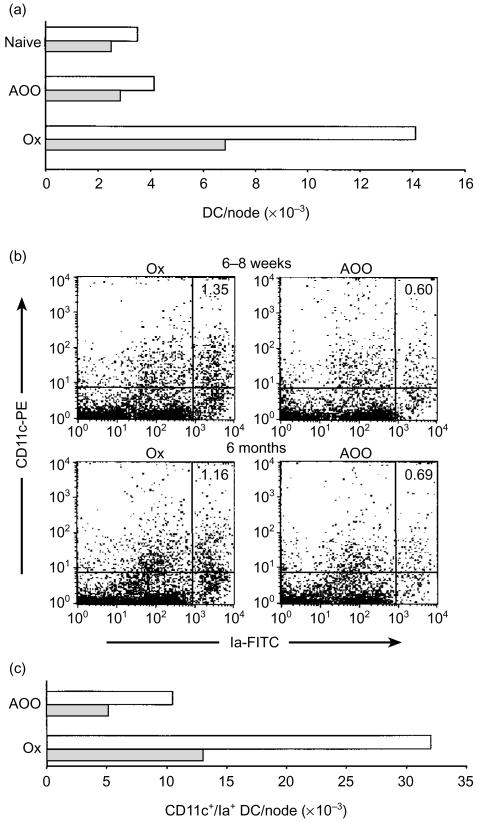
In parallel experiments, the extent of draining lymph node DC accumulation induced following exposure of young and aged mice to Ox was investigated (Fig. 3). Draining (auricular) lymph nodes were excised 18 hr following treatment of mice with Ox or AOO and the frequency of DC/node was determined either in DC-enriched fractions by direct morphological analysis (Fig. 3a) or in LNC suspensions by flow cytometry (Fig. 3b,c). In each case, total DC numbers per node were calculated using LNC counts derived from each treatment group (Table 1). As illustrated in Fig. 3(a), the frequency of DC/node assessed in DC-enriched fractions was lower in the older age group of mice for all treatment groups; an observation that was associated with lower LNC counts derived from these mice (Table 1). Following exposure to Ox, the number of DC accumulating in draining lymph nodes increased 3·4-fold in young mice (relative to the AOO-treated group) compared with a 2·4-fold increase for older animals. Flow cytometric analysis of DC in LNC suspensions stained for CD11c and Ia expression revealed a similar pattern of results (Fig. 3b,c). Using the percentage of CD11c+/Ia+ cells for each group (shown in the upper right quadrants in Fig. 3b) together with LNC counts (Table 1), the frequency of DC/node was determined. In agreement with the data presented in Fig. 3(a), lower DC yields were obtained for older animals for both treatment groups, associated also with lower LNC counts in these mice. Following exposure to Ox, CD11c+/Ia+ DC numbers increased 3·1-fold in younger mice compared with 2·5-fold for older animals. Although lower DC counts in resting lymph nodes were associated generally with reduced lymph node cellularity in older mice, less vigorous DC accumulation in these mice in response to allergen did not correlate directly with changes in LNC numbers (see Table 1). It is notable that although DC yields were substantially higher in the experiment displayed in Fig. 3(b,c), where DC were enumerated in whole LNC suspensions by flow cytometry, both approaches used to quantify lymph node DC numbers generated the same pattern of results. Thus, older animals apparently possess lower lymph node DC numbers (associated with reduced lymph node cellularity) and mount a less vigorous response to allergen with respect to induced increases in lymph node DC accumulation.
Figure 3.
Reduced lymph node DC accumulation in aged mice in response to allergen. (a) Groups of mice (n = 10/group) received 25 µl of 0·5% Ox in AOO, or AOO alone, on the dorsum of both ears. Control mice were untreated (naive). Draining (auricular) lymph nodes were excised 18 hr later and the frequency of DC/node was assessed. Open bars, 6–8-week-old mice; shaded bars, 6-month-old mice. (b) Groups of mice (n = 2/group) were exposed to Ox or AOO and lymph nodes were excised as described above. LNC suspensions were dual stained for CD11c/MHC class II (Ia) expression and analysed by flow cytometry. Quadrant statistics were set on control lymph node cell suspensions incubated with IgG-PE/Ia-FITC with the percentage of CD11c+/Ia+ cells displayed in the upper right quadrants. Results are derived from analysis of 5 × 105 LNC. (c) The frequency of CD11c+/Ia+ DC/node derived from flow cytometric data shown in (b). Open bars, 6–8-week-old mice; shaded bars, 6-month-old mice.
Table 1.
Lymph node cellularity in 6–8-week-old and 6-month-old mice
| Treatment group | Naive | AOO | Ox |
|---|---|---|---|
| I | |||
| Young | 2.5×106 | 2.8×106 | 4.7×106 |
| Old | 1.7×106 | 1.5×106 | 3.0×106 |
| II | |||
| Young | nd | 1.7×106 | 2.4×106 |
| Old | nd | 0.7×106 | 1.1×106 |
LNC counts/node for experiments displayed in (a) Fig. 3a and (b) Fig. 3b-c, nd=not determined. Lymph node cellularity increased 1.7-fold and 2.0-fold for young and older mice, respectively, in experiment I (Ox versus AOO), and 1.4-fold and 1.6-fold for young and older mice, respectively, in experiment II (Ox versus AOO).
The influence of ageing on delivery of allergen to draining lymph nodes
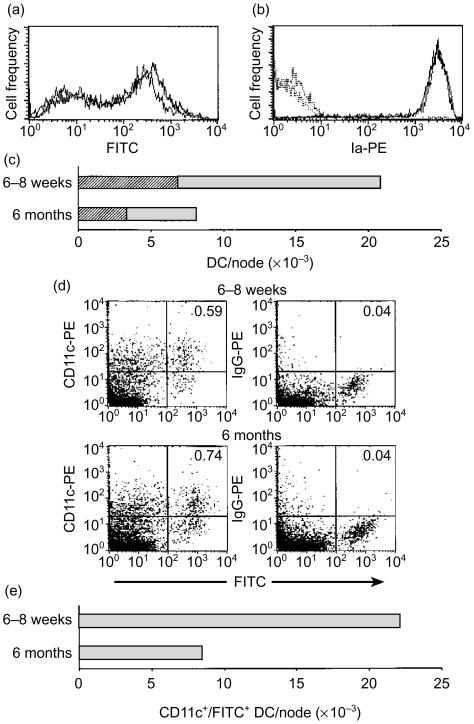
Groups of mice were exposed topically to 1% FITC (a sensitizing fluorochrome) and the arrival of antigen (FITC)-bearing DC in draining lymph nodes was examined either in DC-enriched fractions stained for MHC class II (Ia) expression (Fig. 4a-c) or in unenriched LNC suspensions stained for CD11c (Fig. 4d,e). Firstly, the frequency and intensity of FITC+ DC arriving in draining lymph nodes 22 hr following exposure of mice to FITC was examined in DC-enriched fractions prepared from LNC suspensions. As illustrated in Fig. 4(a), no substantial differences between young and older mice were apparent either with respect to the proportion of FITC+ DC in DC-enriched fractions or the intensity of FITC expressed by these cells, although slightly higher levels of FITC were associated with DC from older mice. Corresponding DC-depleted fractions were negative for FITC (not shown). Furthermore, the level of MHC class II (Ia) determinants exhibited by DC prepared from young and older mice did not differ (Fig. 4b). Although the amounts of FITC associated with DC arriving in draining lymph nodes and the level of MHC class II expressed by these cells were very similar for both young and older mice, the extent of DC accumulation, and therefore the total number of antigen-bearing cells arriving in the lymph node, did vary between groups (Fig. 4c). In young mice the frequency of DC/node in the experiment shown in Fig. 4(c) was 20 814 DC/node (6869 FITC+ DC/node) compared with 8143 DC/node (3339 FITC+ DC/node) for older mice. Corresponding total LNC counts for these mice were 5·6 × 106 and 4·3 × 106 cells/node for 6–8-week-old and 6-month-old mice, respectively. Flow cytometric analysis of LNC prepared 18 hr following exposure of mice to FITC and stained for CD11c expression revealed a similar pattern of results (Fig. 4d,e). Using the percentage CD11c+/FITC+ cells for each group (shown in the upper right quadrants; Fig. 4d) and LNC counts for this experiment (3·7 × 106 and 1·1 × 106 cells/node for young and older mice, respectively), a reduced frequency of DC/node was observed for older mice compared with younger animals. The level of CD11c expressed by DC isolated from each age group was similar, with again possibly increased amounts of FITC associated with the DC from older mice. Taken together these data suggest that the main difference between young and older mice regarding delivery of allergen to draining lymph nodes resides at the level of the absolute numbers of antigen-bearing DC arriving in lymph nodes in response to allergen exposure.
Figure 4.
Delivery of allergen to draining lymph nodes by DC remains intact in aged mice. (a–c) Groups of 6–8-week-old and 6-month-old mice received 25 µl of 1% FITC on the dorsum of both ears. Draining (auricular) lymph nodes were excised 22 hr later and DC-enriched fractions of lymph node cell suspensions were prepared and stained for MHC class II (Ia) expression using an R-PE-conjugated antibody. Results derive from analysis of 104 cells. (a) The level of FITC associated with DC accumulating in the lymph nodes of 6–8-week-old (grey line) and 6-month-old (black line) mice. (b) Ia expression by DC arriving in the lymph nodes of 6–8-week-old (grey line) and 6-month-old (black line) mice. No specific staining was observed for isotype control antibody (young mice, grey dotted line; and older mice, black dotted line). (c) Total DC/node (shaded fill) incorporating those associated with allergen (FITC; diagonal fill) in DC-enriched fractions determined by direct morphological examination. (d) Draining auricular lymph nodes were excised 18 hr following exposure of mice to 1% FITC (n = 2 mice/group) and LNC stained for CD11c. Quadrant statistics were set on control LNC suspensions incubated with IgG-PE and the percentage CD11c+/FITC+ is displayed in the upper right quadrants. Results are derived from analysis of 5 × 105 LNC. (e) The frequency of CD11c+/FITC+ DC/node derived from flow cytometric data shown in (d).
The influence of ageing on contact hypersensitivity responses
To investigate whether the vigour of LC migration and subsequent influx of antigen-bearing DC into draining lymph nodes has an impact upon the development of contact hypersensitivity responses (CHS), groups of young and older mice were sensitized on both flanks with 1% Ox. Vehicle (AOO) was applied to the flanks of control mice. All mice were challenged 5 days later on the dorsum of both ears with 0·25% Ox and CHS responses were measured as a function of challenge-induced increases in ear thickness 24, 48 and 72 hr later (Fig. 5). Overall, the ability of older mice to mount CHS responses was not compromised, although the pattern of these responses over the 72-hr period was subtly and consistently different. In the two independent experiments shown in Fig. 5, significantly more vigorous CHS responses were induced 24 hr following challenge of young mice with Ox in comparison with older mice (Fig. 5a: P < 0·05, Fig. 5b: P < 0·05). In contrast, challenge-induced increases in ear thickness appeared to be more persistent in the older mice, although these differences only reached significance in the experiment displayed in Fig. 5(b) (P < 0·005 for both 48 and 72 hr). Levels of irritation due to the challenge dose of Ox used (mice sensitized with AOO and challenged with 0·25% Ox) were low and were not significantly different between the two age groups.
Figure 5.
Influence of ageing on contact hypersensitivity responses. Groups of young (•, ▪) and aged (○, □) mice (n = 5/group) received 50 µl of 1% Ox (○, •), or AOO (□, ▪), on both shaved flanks. Five days later the ear thickness of each mouse was measured immediately prior to challenge on the dorsum of both ears with 0·25% Ox. Elicitation reactions were measured 24, 48 and 72 hr later and recorded as the mean percentage increase in ear thickness (± SE) relative to prechallenge values. Two independent experiments are shown.
The influence of ageing on cytokine regulation of LC migration
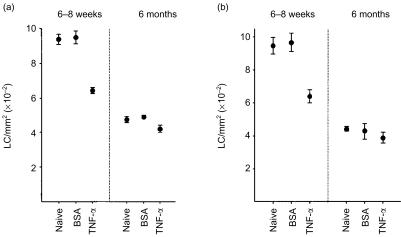
To explore the potential mechanism of reduced responsiveness of LC in older mice to migration induced by allergen, groups of young and older mice were exposed intradermally to cytokines known to be involved in the regulation of LC migration. Initially, the influence of TNF-α on epidermal LC frequency was investigated. We have reported previously that intradermal administration to young adult BALB/c strain mice of homologous recombinant TNF-α induces a significant reduction in epidermal LC frequency within 30 min of exposure.23 Consistent with these observations, intradermal administration of TNF-α to 6–8-week-old mice stimulated a significant reduction in LC numbers of 32·4% (P < 0·001; Fig. 6a) and 33·7% (P < 0·005; Fig. 6b), compared with mice that received injections of control protein (BSA) alone. In contrast, much weaker responses were observed following injection of older mice with TNF-α. Thus, reductions of 14·3% (P < 0·05) and 9·4% (not significant) were recorded for TNF-α in the experiments shown in Fig. 6(a,b), respectively. Control injections of BSA did not alter epidermal LC frequencies in either age group compared with relevant untreated (naive) LC numbers. In agreement with data shown in Fig. 1, baseline LC density in older naive mice was found to be approximately 50% of that recorded for younger mice, with mean LC frequencies for this series of experiments being 942·7 ± 24·6 cells/mm2 for 6–8-week-old mice and 458·4 ± 31·2 cells/mm2 for 6-month-old mice (mean ± SE for counts derived from eight ears, four ears/experiment; Fig. 6).
Figure 6.
Reduced LC migration in aged mice following injection of TNF-α. Groups of mice (n = 2/group) received 30 µl intradermal injections into both ear pinnae of 50 ng of TNF-α suspended in BSA, or of BSA alone. Control mice were untreated (naive). Ears (n = 4/group) were removed 30 min later and epidermal sheets were prepared for analysis of MHC class II+ LC frequency. Two independent experiments are shown.
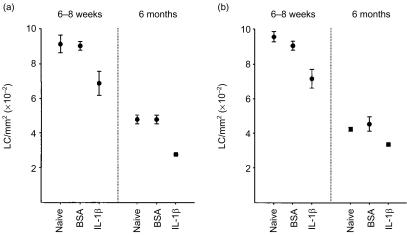
In subsequent experiments, the ability of LC to respond to intradermally administered IL-1β was investigated. We have reported previously that injection of young adult BALB/c strain mice with murine recombinant IL-1β, like TNF-α, induces significant changes in LC densities, with decreases of similar magnitude being apparent within 4 hr of exposure.27 In the present investigations, IL-1β provoked significant decreases in epidermal LC frequencies in young mice of 23·9% (P < 0·005; Fig. 7a) and 21·1% (P < 0·05; Fig. 7b) measured 4 hr following exposure, compared with control-treated mice that received injections of BSA alone. However, unlike treatment with either Ox or TNF-α, injection of older mice with IL-1β resulted in vigorous LC migration with substantial decreases of 41·8% (P < 0·005; Fig. 7a) and 26·0% (P < 0·05; Fig. 7b) being recorded, compared with BSA-treated mice. Consistent with data reported in Figs 1 and 6, LC counts remained high at 957·4 ± 31·3 cells/mm2 for young naive mice compared with much lower values of 451·8 ± 15·9 cells/mm2 for older mice (mean ± SE for counts derived from eight ears, four ears/experiment; Fig. 7).
Figure 7.
IL-1β-induced LC migration proceeds normally in aged mice. Groups of mice (n = 2/group) received 30 µl intradermal injections into both ear pinnae of 50 ng of IL-1β suspended in BSA, or of BSA alone. Control mice were untreated (naive). Ears (n = 4/group) were removed 4 hr later and epidermal sheets were prepared for analysis of MHC class II+ LC frequency. Two independent experiments are shown.
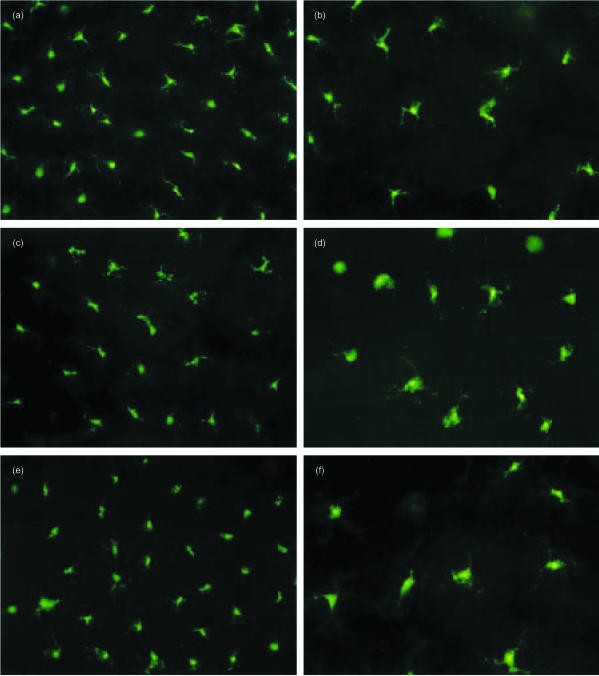
Figure 8 shows the morphology of LC recorded following cytokine treatment. Overall, LC remained larger with longer dendritic processes in epidermal sheets prepared from older mice. However, no significant differences between the 6- and 8-week-old mice and the 6-month-old mice were apparent in the morphology of LC following administration of TNF-α or IL-1β other than those described previously for 8–12-week-old mice.23,27
Figure 8.
LC morphology in epidermal sheets prepared from 6–8-week-old and 6-month-old mice following administration of TNF-α and IL-1β. Mice were treated and epidermal sheets prepared as described in the legends to Figs 6 and 7. Samples were examined by fluorescence microscopy. Photos show LC morphology in young mice: (a) naïve, (c) 30 min following exposure to TNF-α, and (e) 4 hr after IL-1β treatment, and 6-month-old mice: (b) naïve, (d) 30 min following injection of TNF-α, and (f) 4 hr after administration on IL-1β. All magnifications ×100.
Expression by epidermal cell suspensions of IL-1β
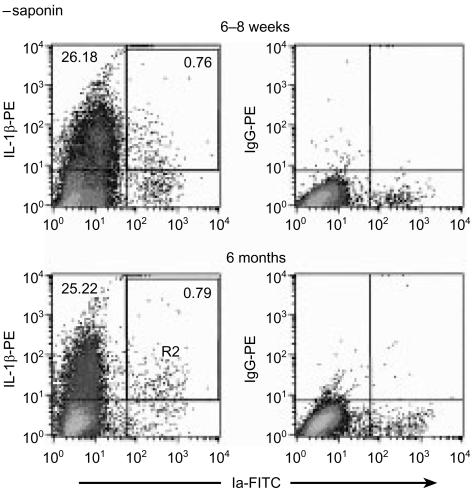
Epidermal cell suspensions were prepared from the ears of naïve mice and analysed by flow cytometry for the expression of IL-1β and MHC class II (Fig. 9). Cells were isolated and prepared for staining in the presence of monensin, an inhibitor of cytokines released through the classical exocytic route,32 and glyburide, an ATP-binding cassette transporter (ABC1) inhibitor reported to prevent IL-1β secretion.33 In the absence of saponin (data displayed in Fig. 9), approximately 25–26% of MHC class II– cells (keratinocytes) were positive for IL-1β in both the young and older age groups of mice. Similar percentages (0·76% and 0·79% for young and older mice, respectively) of MHC class II+ cells (LC) in epidermal cell suspensions dual stained for IL-1β, were also observed for both groups of mice. Following permeabilization of cell suspensions in 0·1% saponin, the percentages of IL-1β+ cells remained unchanged (data not shown). These results demonstrate that under the conditions employed, IL-1β release from LC was not inhibited and that all of the IL-1β detected was located at the cell surface. The inclusion of brefeldin (10 µg/ml), an inhibitor of conventional cytokine release, or YVAD-134 or oxidized ATP,35 inhibitors of caspase-1 and purinoreceptors, respectively, failed also to prevent IL-1β secretion from epidermal cell suspensions prepared in this way (not shown). In additional experiments, mice from both age groups were exposed to Ox for various periods up to 1 hr prior to analysis of IL-1β expression. However, no further differences in the levels of IL-1β displayed by epidermal cell suspensions prepared from either young or older mice could be detected. Although, in the current series of experiments, secretion of LC-derived IL-1β from epidermal cell suspensions prepared ex vivo was not preventable, the interpretation is that apparently no difference exists between the two age groups of mice with respect to the level of detectable IL-1β protein.
Figure 9.
IL-1β expression in epidermal cell suspensions. Epidermal cell suspensions prepared from the ears of naive mice were fixed and dual stained for IL-1β and MHC class II (Ia) expression in the absence of saponin. Numbers indicate the proportion of cells in each quadrant following analysis of 50 000 cells. R2=gate set to upper right hand quadrant to exclude debris strongly fluorescent for PE. The data are representative of duplicate experiments.
Discussion
The data presented here demonstrate clearly that, compared with their younger counterparts, older mice (6-month-old) display a reduced frequency of MHC class II+ LC in the epidermis. This observation is in agreement with the results of similar previous investigations in which it was shown that ageing mice and hamsters both have reduced numbers of epidermal LC (and Thy-1+ DC) compared with younger animals.13–17 One possible explanation for a decline in epidermal LC numbers with age, and possibly in DC populations generally, is the observation that granulocyte–macrophage colony-stimulating factor expression declines with age;36 a cytokine known to be required for the development of DC from bone marrow progenitors. Importantly, our investigations have revealed that, in addition, LC mobilization in response to topical challenge with a chemical allergen is less vigorous in older mice, with a reduced percentage of cells migrating from the epidermis. Associated with this, the accumulation of DC in draining lymph nodes was also considerably less marked than in younger animals and total lymph node cellularity was also somewhat reduced.
It is interesting that, notwithstanding the clear age-related differences in LC migration and DC accumulation reported here, the extent to which skin sensitization was acquired (measured as a function of challenge-induced increases in ear thickness in previously sensitized animals) varied little between the two age groups. In fact, if anything, the challenge-induced contact hypersensitivity reactions elicited in the older mice were somewhat more persistent than those seen in younger animals. These data are again consistent with the results of previous investigations. Thus, Choi and Sauder,15 found that despite the fact that aged (18-month-old) BALB/c strain mice had significantly fewer ATPase+ and MHC class II+ epidermal LC than did younger (10–12-week-old) animals, the groups exhibited comparable contact hypersensitivity reactions following sensitization and challenge with the skin allergen trinitrochlorobenzene. Similar conclusions were drawn also by Walters and Claman37 using young and old BALB/c strain mice sensitized and challenged with another contact allergen, dinitrofluorobenzene. It must be noted, however, that in all the studies described, only very potent contact allergens were investigated. It is possible that differences in contact hypersensitivity responses between the two age groups may emerge with less potent allergens.
Another possibility is that the maintenance of normal contact hypersensitivity reactions in older mice in the face of decreased numbers of epidermal LC, and a reduction in the mobilization of these cells following skin sensitization, is explained by the qualitatively normal delivery of antigen to draining lymph nodes. As described here, in both age groups a similar proportion of DC accumulating in draining lymph nodes was shown to bear antigen. Moreover, the amount of allergen (skin sensitizing fluorochrome, FITC) detected on antigen-bearing DC in lymph nodes was comparable in young and older mice. On this basis it could be argued that despite an overall reduction in antigen delivery to draining lymph nodes, associated with less vigorous LC migration and DC accumulation in older mice, there is nevertheless sufficient antigen, in an appropriate form, arriving in the paracortical regions of draining lymph nodes to permit the acquisition of skin sensitization. In this context it is possible that the total amount of antigen carried to draining lymph nodes by migrating LC is of lesser importance than the efficiency with which this antigen is transferred to resident paracortical DC for presentation to responsive T lymphocytes.38
Irrespective of the integrity of skin sensitization there is no doubt that LC mobilization and migration are less vigorous in older mice. It is relevant therefore to consider possible age-related changes in the cytokine signals that are known to be required for the movement of LC from the epidermis to draining lymph nodes. Our results reveal that in common with responses induced by contact allergen, the percentage of epidermal LC that migrated in response to intradermal injection of TNF-α was reduced in older animals. In contrast, however, the efficiency of LC migration in young and older mice (in terms of the percentage reduction in epidermal LC numbers) was comparable in response to exogenous IL-1β. Collectively these data demonstrate that there is not a generalized impairment of LC motility per se in aged mice. Thus, when an appropriate stimulus (IL-1β) was supplied then the percentage of epidermal cells induced to migrate away from the epidermis was normal in aged mice. These results indicate that the expression by LC of IL-1RI and TNF-R2 are probably not altered in older mice. However, these data suggest also that the primary lesion that impairs the efficiency of induced LC migration in aged mice is the reduced availability of IL-1β, one of the two cytokine signals known to be required for the normal mobilization of LC in response to topically applied antigen.23–28 Although it was possible to demonstrate that IL-1β was readily detectable in epidermal cell suspensions prepared from both young and older naïve mice, all of this was found to be located at the cell surface. Furthermore, no age-related or treatment-related differences in IL-1β expression were apparent in these experiments. It is concluded therefore that the putative reduction in availability of IL-1β may in fact be due to one or more of several factors, including, for example, reduced overall transcription or translation of IL-1β, secondary to a reduced number of LC in the epidermis; reduced transcription or translation of IL-1β on a per cell basis; compromised secretion of IL-1β; reduced conversion of pro-IL-1β to the bioactive cytokine; and/or increased activity of the receptor antagonist (IL-1Ra).
The view that reduced IL-1β is an important feature of age-related changes in cutaneous immune function is consistent with the results of previous independent investigations which have described age-related differences in the production of bioactive IL-1 by cultured human epidermal cells.8 Furthermore, in a study incorporating various strains of mice, reduced levels of mRNA for IL-1 were found in the epidermis of older animals compared with their younger counterparts.39 However, since LC are now known to represent the major source of IL-1β in murine epidermis, it is likely that the observed reduction in epidermal mRNA for IL-1β may actually reflect fewer epidermal LC in the older mice. Overall, the conclusion drawn is that there exists an impairment in LC with respect to their ability to produce IL-1β that occurs as a result of the ageing process.
Taken together the studies reported here demonstrate that the induction of epidermal LC migration and the accumulation of DC in skin draining lymph nodes are impaired in older mice compared with younger animals. The available evidence suggests that this age-related decline in the vigour of LC mobilization is secondary to a reduced availability within the epidermis of bioactive IL-1β. It is not clear, however, whether the reduction in IL-1β implicated here is secondary to changes in gene transcription and/or the processing of pro-IL-1β and whether these observations extend to other populations of DC.
Abbreviations
- AOO
acetone : olive oil
- BSA
bovine serum albumin
- CHS
contact hypersensitivity
- DC
dendritic cells
- FITC
fluorescein isothiocyanate
- IL-1β
interleukin-1β
- LC
Langerhans cells
- LNC
lymph node cells
- Ox
oxazolone
- TNF-α
tumour necrosis factor-α
References
- 1.Goodwin JS, Searles RP, Tung KSK. Immunological responses of a healthy elderly population. Clin Exp Immunol. 1982;48:403–10. [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez S, Shell C, Berk SL. Pulmonary tuberculosis in elderly men. Am J Med. 1987;82:602–6. doi: 10.1016/0002-9343(87)90106-9. [DOI] [PubMed] [Google Scholar]
- 3.Nagami P, Yoshikawa TT. Ageing and tuberculosis. Gerontology. 1984;30:308–15. doi: 10.1159/000212650. [DOI] [PubMed] [Google Scholar]
- 4.Miller RA. Aging and immune function. Int Rev Cytol. 1991;124:187–215. doi: 10.1016/s0074-7696(08)61527-2. [DOI] [PubMed] [Google Scholar]
- 5.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–4. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 6.Orme I. Mechanisms underlying the increased susceptibility of aged mice to tuberculosis. Nutr Rev. 1995;53:S35–8. doi: 10.1111/j.1753-4887.1995.tb01514.x. [DOI] [PubMed] [Google Scholar]
- 7.Tindall JP, Smith JG. Skin lesions in the aged. J Am Med Assoc. 1963;186:1039. doi: 10.1001/jama.1963.03710120021004. [DOI] [PubMed] [Google Scholar]
- 8.Sauder DN. Effect of age on epidermal immune function. Dermatol Clin. 1986;4:447–54. [PubMed] [Google Scholar]
- 9.Thivolet J, Nicolas JF. Skin ageing and immune competence. Br J Dermatol. 1980;122(Suppl.35):77. doi: 10.1111/j.1365-2133.1990.tb16129.x. [DOI] [PubMed] [Google Scholar]
- 10.Ford PM. The immunology of ageing. Clin Rheum Dis. 1986;12:1–10. [PubMed] [Google Scholar]
- 11.Menon M, Jaroslav BN, Koesterer R. The decline of cell-mediated immunity in aging mice. J Gerontol. 1974;29:499–505. doi: 10.1093/geronj/29.5.499. [DOI] [PubMed] [Google Scholar]
- 12.Makinodan T, Kay MMB. Age influence on the immune system. Adv Immunol. 1980;29:287–330. doi: 10.1016/s0065-2776(08)60047-4. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz JL, Weichselbaum R, Frim SR. The effect of aging on the density and distribution of oral mucosal Langerhans cells. Exp Gerontol. 1983;18:65–71. doi: 10.1016/0531-5565(83)90052-9. [DOI] [PubMed] [Google Scholar]
- 14.Rittman BRJ, Hill MW, Rittman GA, Mackenzie IC. Age-associated changes in Langerhans cells of murine oral epithelium and epidermis. Arch Oral Biol. 1987;320:885–9. doi: 10.1016/0003-9969(87)90102-6. [DOI] [PubMed] [Google Scholar]
- 15.Choi KL, Sauder DN. Epidermal Langerhans cell density and contact sensitivity in young and aged BALB/c mice. Mech Ageing Develop. 1987;39:69–79. doi: 10.1016/0047-6374(87)90087-x. [DOI] [PubMed] [Google Scholar]
- 16.Belsito DV, Epstein SP, Schultz JM, Baer RL, Thorbecke GJ. Enhancement by various cytokines or 2-β-mercaptoethanol of Ia antigen expression on Langerhans cells in skin from normal aged and young mice. J Immunol. 1989;143:1530–6. [PubMed] [Google Scholar]
- 17.Sprecher E, Becker Y, Kraal G, Hall E, Harrison D, Shultz LD. Effects of aging on epidermal dendritic cell populations in C57BL/6J mice. J Invest Dermatol. 1990;94:247–53. doi: 10.1111/1523-1747.ep12874586. [DOI] [PubMed] [Google Scholar]
- 18.Knight SC, Krejci J, Malkovsky M, Collizzi V, Gautam A, Asherson GL. The role of dendritic cells in the initiation of immune responses to contact sensitizers. 1. In vivo exposure to antigen. Cell Immunol. 1985;94:427–34. doi: 10.1016/0008-8749(85)90266-7. [DOI] [PubMed] [Google Scholar]
- 19.Macatonia SE, Knight SC, Edwards AJ, Griffiths S, Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987;166:1654–67. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cumberbatch M, Kimber I. Phenotypic characteristics of antigen-bearing cells in the draining lymph nodes of contact sensitized mice. Immunology. 1990;71:404–10. [PMC free article] [PubMed] [Google Scholar]
- 21.Kimber I, Cumberbatch M, Dearman RJ, Knight SC. Langerhans cell migration and cellular interactions. In: Lotze MT, Thomson AW, editors. Dendritic Cells, Biology and Clinical Applications. San Diego: Academic Press; 1999. pp. 295–310. [Google Scholar]
- 22.Kimber I, Cumberbatch M, Dearman RJ, Bhushan M, Griffiths CEM. Cytokines and chemokines in the initiation and regulation of epidermal Langerhans cell mobilization. Br J Dermatol. 2000;142:401–12. doi: 10.1046/j.1365-2133.2000.03349.x. 10.1046/j.1365-2133.2000.03349.x. [DOI] [PubMed] [Google Scholar]
- 23.Cumberbatch M, Fielding I, Kimber I. Modulation of epidermal Langerhans cell frequency by tumour necrosis factor-α. Immunology. 1994;81:395–401. [PMC free article] [PubMed] [Google Scholar]
- 24.Cumberbatch M, Kimber I. Tumour necrosis factor-α is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology. 1995;84:31–5. [PMC free article] [PubMed] [Google Scholar]
- 25.Wang B, Kondo S, Shivji GM, Fujisawa H, Mak TW, Sauder DN. Tumour necrosis factor receptor II (p75) signalling is required for the migration of Langerhans cells. Immunology. 1996;88:284–8. doi: 10.1111/j.1365-2567.1996.tb00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cumberbatch M, Dearman RJ, Kimber I. Langerhans cells require signals from both tumour necrosis factor-α and interleukin-1β for migration. Immunology. 1997;92:388–95. doi: 10.1046/j.1365-2567.1997.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cumberbatch M, Dearman RJ, Kimber I. Interleukin 1β and the stimulation of Langerhans cell migration: comparisons with tumour necrosis factor α. Arch Derm Res. 1997;289:277–84. doi: 10.1007/s004030050193. [DOI] [PubMed] [Google Scholar]
- 28.Cumberbatch M, Dearman RJ, Kimber I. Langerhans cell migration in mice requires intact type I interleukin 1 receptor (IL-1RI) function. Arch Dermatol Res. 1999;291:357–61. doi: 10.1007/s004030050422. 10.1007/s004030050422. [DOI] [PubMed] [Google Scholar]
- 29.Cumberbatch M, Griffiths CEM, Tucker SC, Dearman RJ, Kimber I. Tumour necrosis factor-α induces Langerhans cell migration in humans. Br J Dermatol. 1999;141:192–200. doi: 10.1046/j.1365-2133.1999.02964.x. 10.1046/j.1365-2133.1999.02964.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang B, Zhuang L, Fujisawa H, et al. Enhanced epidermal Langerhans cell migration in IL-10 knockout mice. J Immunol. 1999;162:277–83. [PubMed] [Google Scholar]
- 31.Solomon B, Cohen JL, Masurier C, Klatzmann D. Three populations of mouse lymph node dendritic cells with different origins and dynamics. J Immunol. 1998;160:708–17. [PubMed] [Google Scholar]
- 32.Pala P, Hussell T, Openshaw PJM. Flow cytometric measurement of intracellular cytokines. J Immunol Methods. 2000;243:107–24. doi: 10.1016/s0022-1759(00)00230-1. [DOI] [PubMed] [Google Scholar]
- 33.Hamon Y, Luciani M-F, Becq F, Verrier B, Rubartelli A, Chimini G. Interleukin-1β secretion is impaired by inhibitors of the Atp binding cassette transporter, ABC1. Blood. 1997;90:2911–15. [PubMed] [Google Scholar]
- 34.Schumann RR, Belka C, Reuter D, Lamping N, Kirscning CJ, Weber JR, Pfeil D. Lipopolysaccharide activates caspase-1 (interleukin 1-converting enzyme) in cultured monocytic and endothelial cells. Blood. 1998;91:577–84. [PubMed] [Google Scholar]
- 35.Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1β and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820–6. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- 36.Buchanan JP, Peters CA, Rasmussen CJ, Rothstein G. Impaired expression of hemopoietic growth factors: a candidate mechanism for the hemopoietic defect on aging. Exp Gerontol. 1996;1:135–44. doi: 10.1016/0531-5565(95)02016-0. [DOI] [PubMed] [Google Scholar]
- 37.Walters CS, Claman HN. Age-related changes in cell-mediated immunity in Balb/c mice. J Immunol. 1975;115:1438–43. [PubMed] [Google Scholar]
- 38.Knight SC, Iqball S, Roberts MS, Macatonia S, Bedford PA. Transfer of antigen between dendritic cells in the stimulation of primary T cell proliferation. Eur J Immunol. 1998;28:1636–44. doi: 10.1002/(SICI)1521-4141(199805)28:05<1636::AID-IMMU1636>3.0.CO;2-9. 10.1002/(sici)1521-4141(199805)28:05<1636::aid-immu1636>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 39.Sauder DN, Ponnappan U, Cinader B. Effect of age on cutaneous interleukin 1 expression. Immunol Lett. 1989;20:111–14. doi: 10.1016/0165-2478(89)90094-1. [DOI] [PubMed] [Google Scholar]