Abstract
The pentameric B-subunit of cholera toxin (CTB) can be used as an efficient mucosal carrier of either immunogenic or tolerogenic T-cell epitopes. In this study a series of fusions was constructed between the genes encoding CTB and the B-chain of human insulin (InsB). The resulting fusion proteins were expressed in Escherichia coli and isolated as cytoplasmic inclusion bodies that were then dissolved and assembled in vitro. GM1 enzyme-linked immunosorbent assay (ELISA), sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and Western blot analyses showed that the protein construct in which InsB was fused to the C-terminus of a CTB monomer (CI) assembled into structures that both bound to the receptor GM1 ganglioside and reacted with monoclonal antibodies to CTB and insulin. Fusion of InsB to the N-terminus of CTB resulted in protein that could not assemble into pentameric CTB. In vitro assays showed that the CI fusion protein was 300-fold more potent than native insulin at inducing interleukin-2 (IL-2) production by an insulin-specific T-cell hybridoma. When administered orally, the CI fusion protein induced efficient immunological suppression of ovalbumin-specific T-cell responses in mice co-immunized parenterally with insulin and ovalbumin. These results demonstrate the stability, GM1 receptor-binding activity and antigenic authenticity of the CI fusion protein as well as its ability to elicit insulin-specific T-cell responses in vitro. In addition, we demonstrate that the CI fusion protein induces efficient immunosuppression after oral administration, raising the possibility of using such constructs in the treatment of type-1 diabetes.
Introduction
Cholera toxin (CT), the potent enterotoxin produced by pathogenic serogroups of Vibrio cholerae, is composed of A- and B-subunits, each with different properties. The B-subunit is organized into circular homopentamers that are responsible for binding of CT to cells via cell-surface ganglioside GM1 receptors. The A subunit is the toxic moiety which, after translocation through the cell membrane, enzymatically ADP-ribosylates the Gs molecule of adenylate cyclase, leading to increased formation of cyclic AMP.1,2
While CT is too toxic to be used for vaccination or other applications in humans, the non-toxic cholera toxin B (CTB) pentamer has attracted much attention as an efficient mucosal immunogen and, as such, is a component of a widely licensed oral cholera vaccine.3 Other applications for CTB include use as a receptor-blocking agent4 and as a carrier molecule, particularly for the delivery of foreign antigens to mucosal surfaces. The mucosal delivery of foreign antigens has two functions. One is the induction of positive immunity with the generation of local antibody responses to the antigen carried.5 More recently, however, it has been demonstrated that CTB can also greatly enhance the efficiency with which selected mucosally administered antigens can induce peripheral-systemic immunological suppression or non-responsiveness to the same antigens administered by a parenteral route (‘oral tolerance’).6 Based on this principle, mucosally administered conjugates between CTB and various tissue antigens have been shown to suppress the development or progress of a number of autoimmune diseases in animal models.7–9 The mucosal carrier and associated immunological properties of CTB are thought to be critically dependent on its pentameric structure and its ability to bind to GM1 receptors on the surface of cells. These features facilitate the uptake of coupled antigens across the mucosal barrier and have also been found to lead to a greatly enhanced presentation of carried antigens by all antigen-presenting cells tested.10
In the present article we describe the construction, expression and testing of two CTB-based fusion proteins carrying the B-chain of human insulin. The construction of fusion proteins between CTB and the whole or part of insulin is of special biotechnological interest as we have demonstrated previously that insulin, when conjugated chemically to CTB and administered by a mucosal route, can suppress the development of autoimmune diabetes in mice.8 We show that it is possible to produce gene-fusion proteins that demonstrate both enhanced presentation of the insulin antigen in vitro and the induction of bystander suppression of responses to co-administered antigens in vivo.
Materials and methods
Mice, cell lines and insulin
Female BALB/c and non-obese diabetic (NOD) mice, 6–8 weeks of age, were purchased from M & B (Ry, Denmark) and housed at the central animal facility at Novo Nordisk (Bagsveard, Denmark). The T-cell hybridoma, H11, specific for insulin B-chain residues 9–23, was maintained in culture medium (RPMI-1640 supplemented with 10% fetal calf serum, glutamine, penicillin and streptomycin) at 37° in a humid atmosphere containing 5% CO2. Human insulin was used as pure crystals taken from the last production step at Novo Nordisk, immediately prior to its use in drug formulations.
Bacterial strains, plasmids and CTB genes
Escherichia coli B strain BL2111 and the E. coli K12 strain DH5α12 were used, respectively, as recipients for recombinant plasmids and to express the different gene-fusion proteins. Bacterial strains were grown in Luria–Bertani (LB) broth and maintained on LB plates. When appropriate, ampicillin was added to growth medium and plates (100 µg/ml) in order to maintain plasmids. For long-term storage, strains were resuspended in LB broth containing glycerol (17% vol/vol) and stored at −70°.
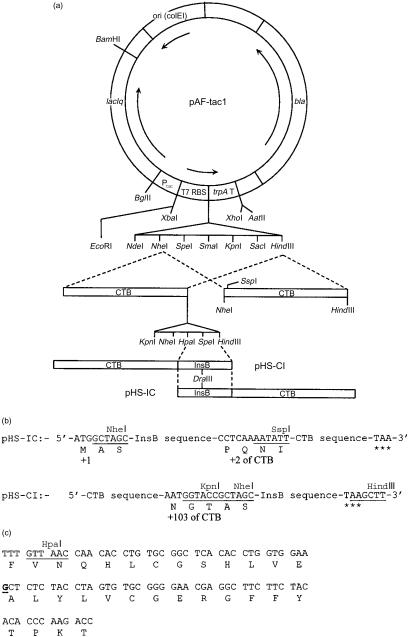
The general purpose plasmid expression vector, pAF-tac1, used for construction and expression of the recombinant genes, was constructed in this laboratory and has been described previously.13 The CTB genes used in the present work were inserted between NheI and HindIII sites in pAF-tac1 and encoded the mature CTB protein lacking the signal peptide that directs synthesized protein into the periplasmic space. In addition, these genes had been manipulated to carry unique in-frame restriction enzyme recognition sites at either the 5′ or 3′ end of the gene to facilitate the insertion of DNA fragments encoding foreign proteins or peptides (Fig. 1a).
Figure 1.
Construction of N- and C-terminal fusion proteins between the B-chain of human insulin (InsB) and the B-subunit of cholera toxin (CTB). (a) The expression plasmid pAFtac1 was used to construct plasmids carrying the gene fusions containing CTB and InsB. The different CTB genes were inserted between NheI and HindIII sites in the vector, allowing the insertion of in-frame InsB sequences, as indicated. (b) The sequences of the linkers in the plasmids pHS-IC and pHS-CI in which the InsB sequence was linked to the 3′ and 5′ end of the CTB gene, respectively. In pHS-IC the InsB sequence was placed between NheI and SspI sites with additional synthetic sequence replacing the CTB gene lost by digestion. As threonine was the final residue of the InsB protein and also the first residue of mature CTB, this residue was not repeated. In pHS-CI the sequence was inserted between HpaI and HindIII sites. Asterisks denote translation stop signals. (c) The DNA sequence of the InsB gene. The G residue underlined in bold was changed to T in pHS-CI(m), resulting in a change from alanine to serine at position 14.
DNA manipulation
Plasmids were prepared using kits from Qiagen GmbH (Hilden, Germany) or Promega (Madison, WI). Digestion with restriction enzymes, DNA ligation and agarose-gel electrophoresis were performed according to standard procedures14 using buffers and conditions recommended by the manufacturers of the reagents (Roche Dignostics Scandinavia AB, Bromma, Sweden and Fermentas MBI, Vilnius, Lithuania).
Synthetic oligonucleotides for insertion of DNA fragments into plasmids and for sequencing primers were purchased from Innovagen AB (Lund, Sweden). Synthetic oligonucleotides encoding the human insulin B-chain (InsB) were designed such that they would be in-frame with genes encoding mature CTB and varied according to whether they were to be inserted at the 5′ or 3′ end. The sequence of the region encoding InsB, however, was constant unless altered by spontaneous mutation (Fig. 1c). The resulting plasmids were pHS-IC (InsB inserted at the 5′ end) and pHS-CI (InsB inserted at the 3′ end) (Fig. 1a).
Bacteria were transformed by electroporation using a gene pulser (Bio-Rad Laboratories, Hercules, CA). Cells were made competent and pulsed according to protocols supplied by the manufacturer of the gene pulser.
DNA sequencing was used to confirm the sequence of oligonucleotides inserted into the plasmid vectors carrying the different CTB genes. Sequencing reactions were performed using the Big Dye cycle-sequencing kit according to the suppliers' instructions (Applied Biosystems, Foster City, CA). Cycle sequencing reactions was performed using the PCR System 2400 (Applied Biosystems). Reactions were processed and analysed using an A310 Genetic Analyser according to the manufacturer's instructions (Applied Biosystems).
Expression and in vitro assembly of fusion proteins
Genes encoding fusion proteins were expressed in E. coli strain BL21. Ehrlenmeyer flasks (2 l) containing 500 ml of LB broth supplemented with ampicillin were inoculated with a 1% (vol/vol) inoculum from an overnight starter culture of the strain carrying the appropriate plasmid. Cultures were grown with shaking (200 r.p.m.) at 37° for 2 hr, to reach an optical density at 600 nm (OD600) of 0·5–0·8, when expression was induced by the addition of IPTG (Saveen AB, Falkenberg, Sweden) to a final concentration of 1 mm. The cultures were then incubated for a further 3 hr (to reach an OD600 of 3·0–4·0) before the cells were harvested by centrifugation (11 500 g for 20 min).
As all recombinant constructs lacked a signal peptide, they were expressed as cytoplasmic proteins and accumulated as inclusion bodies. These could be easily isolated, resulting in relatively pure proteins. Cells were resuspended in an appropriate volume (30–40 mg dry weight of cells/ml assuming that 1 ml of culture with an OD600 of 1·0 is equivalent to 370-µg dry weight of cells) of buffer (200 mm Tris–HCl, pH 8·0) and treated with lysozyme and EDTA (1 mg/ml and 10 mm final concentrations, respectively) at room temperature for 10 min. MgCl2 (2 m) was added to a final concentration of 40 mm and the suspension was treated with DNAse I (10 µg/ml final concentration; Roche Biochemicals, Bromma, Sweden) for 10 min at room temperature. Finally, the cells were disrupted by sonication (Sonics and Materials Inc., Danburg, CT) until no intact cells could be observed by phase-contrast microscopy. The resulting suspension was centrifuged at 4500 g for 10 min to sediment inclusion bodies. The supernatant was discarded. The resulting inclusion body preparation was washed several times in phosphate-buffered saline (PBS) and finally dissolved in 6·5 m urea. The resulting solution was then subjected to dialysis against 0·1-m glycine buffer, pH 9·0, containing 0·5 m NaCl (GS buffer) and 3·5 m urea. In order to maximize assembly, the urea was gradually removed by sequentially reducing the urea concentration in the dialysis buffer until the protein solution was eventually dialysed against the GS buffer containing no urea. Precipitates were removed by centrifugation and the protein solution was concentrated by ultrafiltration using an Amicon filtration cell (Danvers, MA) with a 5000 molecular-weight (MW) cut-off filter (Millipore Corp., Bedford, MA).
Analytical methods
GM1 binding was detected and preliminary concentration estimations of the gene-fusion proteins were made using GM1 enzyme-linked immunosorbent assay (ELISA), according to previously described procedures.15 The primary antibody used in these assays was either the CTB pentamer-specific monoclonal antibody (mAb) LT39,16 or a commercially obtained insulin-specific mAb (Sigma Chemicals, St. Louis, MO). In ELISA assays in which plates were coated directly with the proteins without first coating with GM1, plates (Nunc, Roskilde, Denmark) were incubated overnight with protein solutions in PBS.
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) was carried out using a discontinuous gel system. Commercially produced precast gels (14%) and the electrophoresis apparatus were obtained from Novex (San Diego, CA). The same apparatus was used for transfer of proteins to nylon membranes (Hybond-C extra; Amersham Pharmacia, Freiburg, Germany) for Western blot analysis using buffers and procedures recommended by the manufacturer.
Generation of an insulin B-chain 9–23-specific T-cell hybridoma
Female NOD mice (6 weeks of age) were immunized with 50 µg of insulin peptide B:9–23 emulsified in complete Freund's adjuvant (total volume: 100 µl/mouse) at the base of the tail. Seven days later the inguinal and periaortic lymph nodes were removed. Cell suspensions were prepared and the resulting lymph node cells were cultured in 24-well culture plates (Nunc) at 10 cells/well in 2 ml of Clicks medium supplemented with 0·5% normal mouse serum and a final concentration of 25 µg/ml porcine insulin (Sigma). The cultures were incubated for 4 days at 37° and then harvested. The resultant cells were cultured in Dulbecco's modified Eagle's minimal essential medium (DMEM) (Gibco, Groningen, the Netherlands) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT) and 50 U/ml recombinant murine interleukin-2 (rmIL-2) (PharMingen, San Diego, CA) for 3 days. The cells derived from this second culture were fused, utilizing PEG 1500 (Sigma), to the T-cell receptor-αβ (TCR-αβ)-negative variant of the thymoma BW 5147.17 The fusion mixture was cultured in 96-well plates (Nunc) and, as growth became apparent, the contents of individual wells were transferred to a larger volume of medium (DMEM containing 10% FBS). The resultant hybrids were tested for function by antigen-stimulated IL-2 production, which was measured using a commercial sandwich ELISA kit (PharMingen) according to the manufacturer's instructions. Cell lines that exhibited high responses to insulin were cloned by limiting dilution. Clone H11 was selected for these experiments.
T-cell hybridoma assays
NOD mice were killed and the spleens removed aseptically. Single-cell suspensions were generated by teasing the spleen through a nylon mesh followed by washing and erythrocyte lysis. Subsequently, splenocytes were washed, counted and adjusted to the required cell number. Quadruplicate cultures containing 5×105 NOD splenocytes and 105 H11 T-cell hybridoma cells were established in 200 µl of culture medium in 96-well round-bottom tissue-culture plates (Nunc) and incubated for 16 hr at 37° together with graded concentrations of human insulin, InsB fused to the C-terminus of a CTB monomer (CI), CI protein carrying a single spontaneous mutation within InsB [CI(m)], or CTB. Following incubation, the culture supernatants were harvested and the concentration of IL-2 was determined by ELISA, as described above.
Immunization experiments
To assess the ability of the CI fusion protein to induce oral tolerance in vivo, we used a bystander immunization model.18 Briefly, groups of three female BALB/c mice were treated with CTB (300 pmol/dose), insulin (150 nmol/dose) or CI fusion protein (three groups fed with 30 pmol, 300 pmol or 3 nmol/dose, respectively) by oral gavage five times per week for a total of 4 weeks. After 2 weeks, mice were co-immunized in the footpad with 50 µg of insulin and 100 µg of ovalbumin (OVA) (grade V; Sigma) in complete Freund's adjuvant (Sigma). After a further 2 weeks, the mice were killed and the popliteal lymph nodes (PLN) were removed aseptically. Single-cell suspensions were generated as described above. To assess the OVA-specific proliferation, quadruplicate cultures of 2×105 PLN cells were established with graded concentrations of OVA in 200 µl of culture medium in 96-well round-bottomed tissue culture plates (Nunc) and incubated for 72 hr at 37°. Six hours before the end of the culture period, 0·5 µCi [3H]thymidine ([3H]TdR) (Amersham Pharmacia) was added to the cultures. Standard procedures for harvesting the cultures and measuring incorporated radioactivity were used. In separate cultures lymph node cells were stimulated with 300 µg/ml OVA, as described above. After 72 hr of incubation, supernatants were harvested and the concentration of interferon-γ (IFN-γ), IL-4, IL-10 and transforming growth factor-β (TGF-β) were assessed by ELISA.
Cytokine assays
Assays were performed using commercially available ELISAs (IFN-γ, IL-4 and IL-10 from PharMingen; and TGF-β from RandD Diagnostics, Minneapolis, MN), according to the manufacturers' instructions.
Results
Construction and biochemical characterization of CTB-based gene-fusion proteins carrying the insulin B-chain
Three types of gene-fusion proteins were constructed in which InsB was linked to CTB. In two of these InsB was linked to the N-terminus (IC) or C-terminus (CI) of CTB, respectively. An additional CI protein CI(m), carrying a single spontaneous mutation within the insulin B-chain (A14S; see Fig. 1c) was found as a result of sequencing recombinants obtained in the construction of the plasmid carrying CI. It was found that whereas the IC fusion protein gave no detectable assembly, as judged by GM1 ELISA or Western blot analysis, the CI and CI(m) fusion proteins gave rise to products that did assemble. These two latter proteins were used for further study.
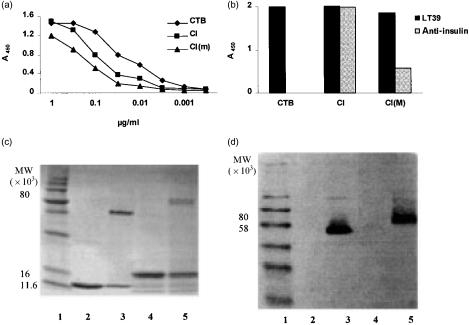
The two protein fusions that gave assembled product [CI and CI(m)] were characterized by GM1 ELISA and by SDS–PAGE. Both proteins bound to GM1-coated plates, giving similar results in a standard GM1 ELISA (Fig. 2a), indicating that significant assembly of monomers had occurred. In assays in which the fusion protein preparations were used to coat plates directly (without GM1) the reactivity with mAb LT39 was also found to be similar. However, an insulin-specific mAb that bound strongly to CI bound only weakly to CI(m), suggesting that the mutation affected the affinity of the antibody for the insulin B-chain (Fig. 2b).
Figure 2.
Characterization of CI and CI(m) fusion proteins by enzyme-linked immunosorbent assay (ELISA) and sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). (a) Reactivity of CI and CI(m) proteins with GM1 ganglioside and antibody to the B-subunit of cholera toxin (CTB) in GM1-ELISA in comparison with a native CTB control. The concentrations of the CI and CI(m) samples were initially adjusted as described in the text. The samples were serially diluted using threefold dilutions and incubated for 1 hr prior to addition of the CTB-specific monoclonal antibody (mAb) LT39. (b) Reactivity of CTB and the CI and CI(m) proteins, with respect to LT39 and anti-insulin mAbs, was tested by ELISA. The protein concentrations were adjusted to ≈ 1 µg/ml and used to coat microtitre plates directly (without prior coating with GM1), otherwise treatment with the primary and secondary antibodies and development were as described in the Materials and methods. (c) SDS–PAGE (15%) analysis of CI protein compared with native CTB. The concentration of SDS used in the gel and the sample preparation buffers was reduced to 50% of the concentrations normally used. Both boiled and non-boiled samples of each protein were loaded. Lanes: 1, molecular weight standard; 2, CTB (5 µg), boiled; 3, CTB, non-boiled; 4, CI protein (≈ 5 µg), boiled; 5, CI protein (≈5 µg), non-boiled. (d) Western blot analysis. Proteins from a similar gel as in (c) were reacted in Western blots with the CTB-specific mAb, LT39, which reacts with CTB pentamer. The lane numbering is the same as for the SDS–PAGE gel in (c).
SDS–PAGE of the unboiled CI protein showed a band of ≈ 80 000 MW that reacted with LT39 in Western blot and thus represented assembled pentamers; this band disappeared when samples were boiled (Fig. 2c,d). Similar results were obtained with CI(m) (results not shown).
Effective antigen presentation to an insulin-specific T-cell hybridoma
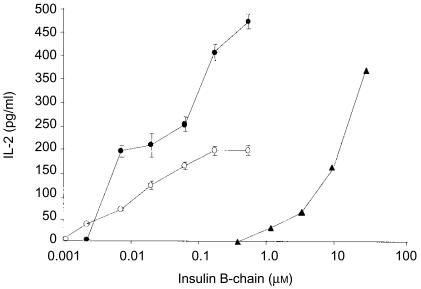
Previous work has shown that genetic fusion of peptides to CTB results in a dramatically increased ability of the fusion protein to stimulate antigen-presenting cells to activate the cognate T cells.10 In order to determine whether this was the case with the CTB fusion proteins carrying the insulin B-chain, we tested the ability of CI and CI(m) proteins to stimulate an insulin-specific T-cell hybridoma to produce IL-2. We found that the CI protein stimulated significant IL-2 production at a concentration of 3 µg/ml (≈ 0·18 µm with respect to the insulin B-chain) versus 300 µg/ml (≈ 50 µm with respect to the insulin B-chain) of native insulin required to give a similar level of IL-2 production (Fig. 3). In contrast, stimulation with the CI(m) protein resulted in low levels of IL-2 production, suggesting that the mutation in this protein affects recognition of the antigen by the T-cell receptor. No IL-2 production was seen when hybridoma cells were cultured with native CTB or in medium alone (data not shown). These data show that the CI fusion protein is, in molar terms, ≈ 280-fold more potent than native insulin at inducing insulin-specific T-cell responses.
Figure 3.
Induction of interleukin-2 (IL-2) production by the CI protein in an insulin-specific T-cell hybridoma. Quadruplicate cultures containing 5×105 splenocytes and 1×105 H-11 hybridoma cells in 200 µl of culture medium were established in round-bottomed 96-well tissue culture plates and incubated for 16 hr in the presence of human insulin (triangles), CI (closed circles) or CI(m) (open circles) fusion proteins. Following incubation the supernatants were harvested and the amount of IL-2 was determined in an IL-2-specific sandwich enzyme-linked immunosorbent assay (ELISA). Data represent the mean of quadruplicate cultures (±1 SD) for one of four experiments performed with similar results.
CI protein treatment induces bystander suppression to a co-administered antigen in mice
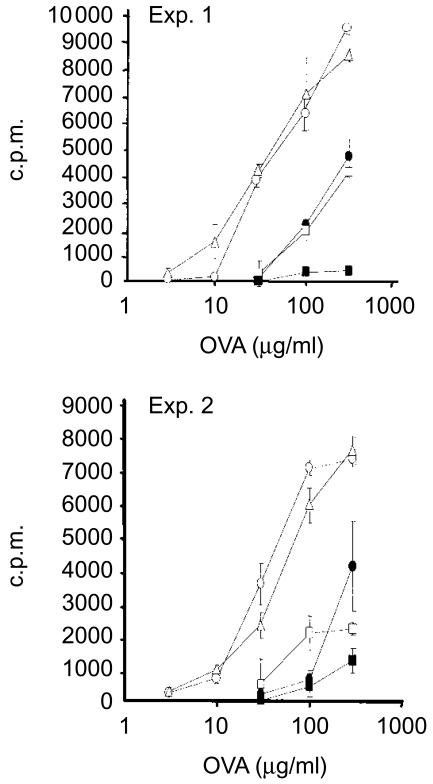
We then tested whether the CI fusion protein could induce suppression of T-cell responses in vivo. To achieve this we used a bystander immunization model in which mice were fed with CI fusion protein or insulin and subsequently immunized in a footpad with a mixture of OVA and insulin in Freund's complete adjuvant, after which the reactivity of T cells to OVA was tested in vitro.18 Figure 4 shows the results from two separate experiments. When PLN cells from insulin-fed mice were rechallenged with OVA in vitro 2 weeks after immunization with insulin and OVA, a significant reduction in the proliferation of T cells was observed when compared to controls. Feeding mice with 0·5 µg of the CI fusion protein did not influence the OVA-specific ex vivo proliferation. However, feeding with 5 µg of the CI fusion protein (equivalent to 0·3 nmol of insulin B-chain) mediated suppression of OVA-specific proliferation, comparable to feeding with 1 mg of native insulin (equivalent to 150 nmol of insulin B-chain). Further increasing the dose of CI protein resulted in a decrease in its suppressive potential, as reflected in the higher OVA-specific proliferation in cells from mice fed with 50 µg (equivalent to 3·0 nmol of insulin B-chain). Thus, genetically fusing the B-chain of insulin to CTB increases its immunosuppressive potential ≈ 550-fold when fed at low doses. When the dose of native insulin was reduced to 100 µg (15 nmol), no suppression was observed.18
Figure 4.
Induction of oral tolerance immunosuppression by the CI fusion protein in vivo in a bystander antigen system. In two separate experiments female BALB/c mice were fed with cholera toxin B (CTB), 300 pmol (open circles); human insulin, 150 nmol (closed circles); or CI fusion protein, 30 pmol (open triangles), 300 pmol (closed squares), or 3 nmol (open squares); by oral gavage, as described in the Materials and methods, for a total of 4 weeks. After 2 weeks the mice were immunized in the footpad with a mixture of insulin and ovalbumin (OVA), together with Freund's complete adjuvant. After a further 2 weeks the mice were killed and the popliteal lymph nodes (PLNs) from groups of three mice were pooled and stimulated with OVA in vitro. Data shown represent the mean value obtained from quadruplicate cultures (±1 SD). c.p.m., counts per minute.
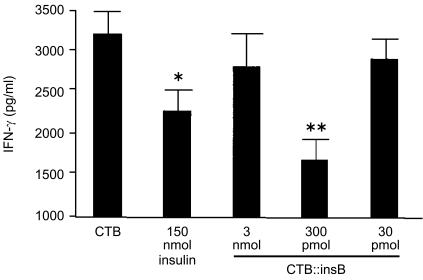
TGF-β, IL-4 and IL-10 were not detected in culture supernatants (data not shown), but there was a significant reduction in IFN-γ in animals treated with insulin alone (150 nmol/dose) or with CI protein (300 pmol/dose) (Fig. 5). The reduction in IFN-γ was more pronounced in animals treated with CI protein than in those treated with insulin.
Figure 5.
Suppression of interferon-γ (IFN-γ) in mice immunized with CI fusion protein. Lymph node cells from mice (the same animals as in Exp. 2 in Fig. 4) treated with 30 pmol, 300 pmol or 3 nmol of the CI fusion protein, or with 150 nmol of insulin (animals from Exp. 2 in Fig. 4) were stimulated with ovalbumin (OVA) for 72 hr and the supernatant was harvested and analysed for the level of IFN-γ production by sandwich enzyme-linked immunosorbent assay (ELISA). Asterisks denote a statistically significant difference from cholera toxin B (CTB)-treated mice, *P<0·05, **P<0·01. Data represent the mean value obtained from quadruplicate cultures (±1 SD).
Discussion
Prevention of autoimmune diabetes by oral insulin treatment (inducing immunological or ‘oral tolerance’) has been demonstrated in a number of animal models.19–22 Furthermore, the effectiveness of conjugating insulin to CTB in increasing efficiency and reducing effective doses has also been amply demonstrated in similar systems.8,23 For eventual clinical application of CTB in the enhancement of oral tolerance, genetically engineered fusion proteins have clear advantages over chemical conjugates. For example, the molecular composition of the protein will not vary, and processing a single protein is much easier than producing multiple biological components that must subsequently be chemically linked under conditions that will allow the products to be used for human consumption.
The present report describes the genetic construction of fusion proteins that combine CTB with the B-chain of human insulin. The B-chain was chosen as it has been shown to contain the predominant epitope of insulin that is implicated in the development of autoimmune diabetes in animal models, and feeding of epitopes from the B-chain can be used to prevent the development of disease in a manner similar to the whole protein.19,20 Success in the construction of CTB gene-fusion proteins has generally been found to be dependent upon the length of the carried peptide.24 Thus, an additional reason for choosing to work with the B-chain of insulin was to reduce the size of the carried antigen without compromising its potential biological activity. Also of importance is the site of insertion in CTB;24,25 in the present case the expression system produced large quantities of three fusion proteins, only two of which [CI and CI(m)] gave rise to assembled product, as judged by their ability to bind to GM1; the third (IC) failed to assemble.
Most importantly, the results show that the CI fusion protein was, on a molar basis, nearly 300-fold more efficient than native insulin at inducing IL-2 production in an insulin-specific T-cell hybridoma. The observed T-cell-mediated IL-2 production was insulin-specific, as demonstrated by the absence of IL-2 production by the T-cell hybridoma when incubated with CTB alone. These results demonstrate that linkage of CTB to the insulin B-chain dramatically enhances presentation of the insulin peptide to specific T cells and is in agreement with our previous findings in which different peptide antigens were linked to CTB.10 A fusion protein carrying an insulin B-chain with a single mutation (A14S) in the epitope recognized by the hybridoma's T-cell receptor induced only marginal IL-2 production. This confirmed the observed importance of this region of the peptide in recognition by the T-cell receptor.26 Previous work in our laboratory has demonstrated, using the NOD mouse model, the principle of using conjugation to CTB to enhance the effect of orally administered insulin in preventing progression to diabetes.8,23 In this study we chose to use an alternative model based on bystander suppression of responses to an unrelated antigen to determine whether the feeding of CI fusion protein could induce insulin-specific suppressive T cells. Normal mice were fed with the candidate tolerizing antigen preparation. After 2 weeks the mice were challenged in a footpad with the fed antigen together with another unrelated antigen and responses to the unrelated antigen were then measured and compared with appropriate controls.18 This model had the following advantages:
The use of mice that are not predisposed to diabetes better reflects the human situation in which not all cases are thought to be the result of genetic predisposition.27
The experiments take weeks rather than months.
The number of animals required to obtain statistically significant results is much reduced as it is not dependent upon colonies of mice that vary in the frequency of disease incidence.
In addition, von Herrath and co-workers have recently demonstrated that insulin-induced bystander suppression of auto-aggressive T cells was crucial for the prevention of diabetes by oral insulin treatment.28,29 In this respect, suppressive responses that are induced would be of benefit in the event of the development of an immune response to unidentified antigens that could contribute to insulin-dependent diabetes. That the read-out suppression in these experiments is caused by the recruitment of insulin-specific cells is confirmed by the lack of suppression if the insulin and OVA are injected into different footpads in the same individuals.18
Using this bystander model we showed that the CI fusion protein had much greater tolerizing efficiency than native insulin. Oral administration of 5 µg of CI fusion protein five times a week for 4 weeks induced potent bystander suppression of responses to the unrelated antigen (OVA) in mice co-immunized with insulin and OVA. For a corresponding effect with native insulin, 1 mg/dose was needed. Lower doses of native insulin did not produce suppressive effects in the bystander model used here18 or in the suppression of diabetes in NOD mice in a previous study.30 Interestingly, further increasing the amount of CI fusion protein to 50 µg/dose resulted in a slight decrease in its suppressive potential. Similar findings have been made in other models of oral tolerance, including the suppression of spontaneous diabetes in NOD mice by feeding insulin chemically conjugated to CTB.31 This reversal of the dose–response curve could be the result of a shift from so-called low-dose tolerance to high-dose tolerance, resulting in clonal anergy or deletion of regulatory T cells and consequently less efficient suppression.32 Alternatively, it has been shown recently that the relatively high concentration of CTB used in the latter experiments can induce apoptosis of regulatory T cells residing in the gut.33
It has been suggested that specific regulatory T cells which suppress the activation of bystander cells may mediate such active suppression by the secretion of T helper 2 (Th2)/T helper 3 (Th3) cytokines such as IL-4, IL-10 and TGF-β.32,34 In the present work, however, these cytokines were not detected. It may be that the mechanisms of tolerance induction associated with the linkage of antigen to CTB do not obligatorily induce production of these cytokines. On the other hand, there was a significant suppression of IFN-γ production in treated animals compared with controls, which was more marked in animals treated with 300 pmol CI protein.
Despite the success in preventing diabetes by oral administration of insulin in a number of animal systems,20,22,29,35 little success has, to date, been recorded in human trials.36,37 One possible reason for this might be the use of too-low doses of insulin.38 For insulin therapy to be manageable, adjuvant strategies must be identified to make it possible to reduce the amount of insulin needed to induce tolerance.39 Chemical conjugation of insulin to CTB has proved to be a feasible approach, increasing the tolerogenic potential of insulin by at least 100-fold in animal models.23 Here we demonstrate that an efficiently produced genetic fusion protein linking the B-chain of insulin to CTB is at least as efficient as the chemical conjugates at inducing immunological suppression in vivo and, furthermore, that this correlates with an increased level of antigen-specific stimulation of specific T cells in an in vitro assay. These results demonstrate the potential of such fusion proteins as important tools in future therapeutic strategies for type I diabetes.
Acknowledgments
We would like to thank Marianne Lindblad and Gun Wallerström for technical assistance with the preparation of recombinant proteins. This work was supported by the Swedish Technical Research Council (grant no. 97-296) and the Swedish Medical Research Council (grant no. 2001/Z0223).
References
- 1.Mekalanos JJ. Cholera toxin: genetic analysis, regulation, and role in pathogenesis. Curr Top Microbiol Immunol. 1985;118:97–118. doi: 10.1007/978-3-642-70586-1_6. [DOI] [PubMed] [Google Scholar]
- 2.Holmgren J. Receptors for cholera toxin and Escherichia coli heat-labile enterotoxin revisited. Prog Brain Res. 1994;101:163–77. doi: 10.1016/s0079-6123(08)61947-0. [DOI] [PubMed] [Google Scholar]
- 3.Holmgren J, Svennerholm AM. Development of oral vaccines against cholera and enterotoxinogenic Escherichia coli diarrhea. Scand J Infect Dis Suppl. 1990;76:47–53. [PubMed] [Google Scholar]
- 4.Glass RI, Holmgren J, Khan MR, Hossain KM, Huq MI, Greenough WB. A randomized, controlled trial of the toxin-blocking effects of B subunit in family members of patients with cholera. J Infect Dis. 1984;149:495–500. doi: 10.1093/infdis/149.4.495. [DOI] [PubMed] [Google Scholar]
- 5.Czerkinsky C, Russell MW, Lycke N, Lindblad M, Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989;57:1072–7. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun JB, Holmgren J, Czerkinsky C. Cholera toxin B subunit: an efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc Natl Acad Sci USA. 1994;91:10795–9. doi: 10.1073/pnas.91.23.10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun JB, Rask C, Olsson T, Holmgren J, Czerkinsky C. Treatment of experimental autoimmune encephalomyelitis by feeding myelin basic protein conjugated to cholera toxin B subunit. Proc Natl Acad Sci USA. 1996;93:7196–201. doi: 10.1073/pnas.93.14.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergerot I, Ploix C, Petersen J, et al. A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc Natl Acad Sci USA. 1997;94:4610–4. doi: 10.1073/pnas.94.9.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarkowski A, Sun JB, Holmdahl R, Holmgren J, Czerkinsky C. Treatment of experimental autoimmune arthritis by nasal administration of a type II collagen-cholera toxoid conjugate vaccine. Arthritis Rheum. 1999;42:1628–34. doi: 10.1002/1529-0131(199908)42:8<1628::AID-ANR10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 10.George Chandy A, Eriksson E, Lebens M, Nordström I, Schön E, Holmgren J. Cholera toxin B subunit as a carrier molecule promotes antigen presentation and increases CD40 and CD86 expression on antigen presenting cells. Infect Immun. 2001;69:5716–25. doi: 10.1128/IAI.69.9.5716-5725.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grodberg J, Dunn J. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J Bacteriol. 1988;170:1245–53. doi: 10.1128/jb.170.3.1245-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodcock DM, Crowther PJ, Doherty J, et al. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucl Acids Res. 1989;17:3469–78. doi: 10.1093/nar/17.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frisk A, Lebens M, Johansson C, Ahmed H, Svensson L, Ahlman K, Lagergard T. The role of different protein components from the Haemophilus ducreyi cytolethal distending toxin in the generation of cell toxicity. Microb Pathog. 2001;30:313–24. doi: 10.1006/mpat.2000.0436. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J, Fitsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 15.Svennerholm AM, Holmgren J. Identification of Escherichia coli heat-labile enterotoxin by means of ganglioside immunosorbent assay (GM1-ELISA) procedure. Curr Microbiol. 1978;1:19–23. [Google Scholar]
- 16.Svennerholm A-M, Wikström M, Lindblad M, Holmgren J. Monoclonal antibodies to Escherichia coli heat-labile enterotoxins. Neutralizing activity and differentiation of human and porcine LTs and cholera toxin. Med Biol. 1986;64:23–30. [PubMed] [Google Scholar]
- 17.Shimamura M, Oku M, Ohta S, Yamagata T. Haematopoietic cell lines capable of colonizing the thymus following in vivo transfer of expressed T-cell receptor gamma-gene immature mRNA. Immunology. 1992;77:369–76. [PMC free article] [PubMed] [Google Scholar]
- 18.Bregenholt S, Wang M, Zdravkovic M, Dyrberg T, Petersen JS. A new model for analysing immune-modulation of T cell responses induced by oral administration of islet cells antigens. Ann NY Acad Sci. 2002;958:179–81. doi: 10.1111/j.1749-6632.2002.tb02965.x. [DOI] [PubMed] [Google Scholar]
- 19.Song HY, Abad MM, Mahoney CP, McEvoy RC. Human insulin B chain but not A chain decreases the rate of diabetes in BB rats. Diabetes Res Clin Pract. 1999;46:109–14. doi: 10.1016/s0168-8227(99)00080-7. [DOI] [PubMed] [Google Scholar]
- 20.Polanski M, Melican NS, Zhang J, Weiner HL. Oral administration of the immunodominant B-chain of insulin reduces diabetes in a co-transfer model of diabetes in the NOD mouse and is associated with a switch from Th1 to Th2 cytokines. J Autoimmun. 1997;10:339–46. doi: 10.1006/jaut.1997.0148. [DOI] [PubMed] [Google Scholar]
- 21.Ramiya VK, Shang XZ, Pharis PG, Wasserfall CH, Stabler TV, Muir AB, Schatz DA, Maclaren NK. Antigen based therapies to prevent diabetes in NOD mice. J Autoimmun. 1996;9:349–56. doi: 10.1006/jaut.1996.0047. [DOI] [PubMed] [Google Scholar]
- 22.Ramiya VK, Maclaren NK. Insulin in diabetes prevention. Horm Res. 1997;48:67–70. doi: 10.1159/000191318. [DOI] [PubMed] [Google Scholar]
- 23.Arakawa T, Chong DK, Merritt JL, Langridge WH. Expression of cholera toxin B subunit oligomers in transgenic potato plants. Transgenic Res. 1997;6:403–13. doi: 10.1023/a:1018487401810. [DOI] [PubMed] [Google Scholar]
- 24.Backstrom M, Lebens M, Schodel F, Holmgren J. Insertion of a HIV-1-neutralizing epitope in a surface-exposed internal region of the cholera toxin B-subunit. Gene. 1994;149:211–7. doi: 10.1016/0378-1119(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 25.Liljeqvist S, Stahl S, Andreoni C, Binz H, Uhlen M, Murby M. Fusions to the cholera toxin B subunit. Influence on pentamerization and GM1 binding. J Immunol Methods. 1997;210:125–35. doi: 10.1016/s0022-1759(97)00170-1. [DOI] [PubMed] [Google Scholar]
- 26.Abiru N, Wegmann D, Kawasaki E, Gottlieb P, Simone E, Eisenbarth GS. Dual overlapping peptides recognized by insulin peptide B:9-23 T cell receptor AV13S3 T cell clones of the NOD mouse. J Autoimmun. 2000;14:231–7. doi: 10.1006/jaut.2000.0369. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Bravo F, Carrasco E, Gutierrez-Lopez MD, Martinez MT, Lopez G, de los Rios MG. Genetic predisposition and environmental factors leading to the development of insulin-dependent diabetes mellitus in Chilean children. J Mol Med. 1996;74:105. doi: 10.1007/BF00196786. [DOI] [PubMed] [Google Scholar]
- 28.Homann D, Holz A, Bot A, et al. Autoreactive CD4+ T cells protect from autoimmune diabetes via bystander suppression using the IL-4/Stat6 pathway. Immunity. 1999;11:463–72. doi: 10.1016/s1074-7613(00)80121-1. [DOI] [PubMed] [Google Scholar]
- 29.von Herrath MG. Bystander suppression induced by oral tolerance. Res Immunol. 1997;148:541. doi: 10.1016/s0923-2494(98)80148-x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang ZJ, Davidson L, Eisenbarth G, Weiner HL. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci USA. 1991;88:10252–526. doi: 10.1073/pnas.88.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergerot I, Fabien N, Maguer V, Thivolet C. Oral administration of human insulin to NOD mice generates CD4+ T cells that suppress adoptive transfer of diabetes. J Autoimmun. 1994;7:655–63. doi: 10.1006/jaut.1994.1050. [DOI] [PubMed] [Google Scholar]
- 32.Strobel S, Mowat AM. Immune responses to dietary antigens: oral tolerance. Immunol Today. 1998;19:173–81. doi: 10.1016/s0167-5699(97)01239-5. [DOI] [PubMed] [Google Scholar]
- 33.Boirivant M, Fuss IJ, Ferroni L, De Pascale M, Strober W. Oral administration of recombinant cholera toxin subunit B inhibits IL-12-mediated murine experimental (trinitrobenzene sulfonic acid) colitis. J Immunol. 2001;166:3522–32. doi: 10.4049/jimmunol.166.5.3522. [DOI] [PubMed] [Google Scholar]
- 34.Garside P, Mowat AM, Khoruts A. Oral tolerance in disease. Gut. 1999;44:137–42. doi: 10.1136/gut.44.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramiya VK, Lan MS, Wasserfall CH, Notkins AL, Maclaren NK. Immunization therapies in the prevention of diabetes. J Autoimmun. 1997;10:287–92. doi: 10.1006/jaut.1997.0127. [DOI] [PubMed] [Google Scholar]
- 36.Chaillous L, Lefevre H, Thivolet C, et al. Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabete Insuline Orale group. Lancet. 2000;356:545–9. doi: 10.1016/s0140-6736(00)02579-4. [DOI] [PubMed] [Google Scholar]
- 37.Pozzilli P, Pitocco D, Visalli N, et al. No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII). IMDIAB Group. Diabetologia. 2000;43:1000–4. doi: 10.1007/s001250051482. [DOI] [PubMed] [Google Scholar]
- 38.Pozzilli P, Gisella Cavallo M. Oral insulin and the induction of tolerance in man: reality or fantasy? Diabetes Metab Res Rev. 2000;16:306–7. doi: 10.1002/1520-7560(200009/10)16:5<306::aid-dmrr150>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 39.Chatenoud L. Restoration of self-tolerance is a feasible approach to control ongoing beta-cell specific autoreactivity: its relevance for treatment in established diabetes and islet transplantation. Diabetologia. 2001;44:521–36. doi: 10.1007/s001250051658. [DOI] [PubMed] [Google Scholar]