Abstract
Activation of human Vγ9/Vδ2 T cells by many pathogens depends on the presence of small phosphorylated non-peptide compounds derived from the 2-C-methyl-d-erythritol 4-phosphate (MEP) pathway of isoprenoid biosynthesis. We here demonstrate that in Escherichia coli mutants deficient in lytB, an essential gene of the MEP pathway, a potent Vγ9/Vδ2 T-cell activator accumulates by a factor of approximately 150 compared to wild-type E. coli. The compound responsible for the strong immunogenicity of this E. coli mutant was subsequently characterized and identified as a small pyrophosphorylated metabolite, with a molecular mass of 262 Da, derived from the MEP pathway. Stimulation of human peripheral blood mononuclear cells (PBMC) with extracts prepared from the lytB-deficient E. coli mutant led to upregulation of T-cell activation markers on the surface of Vγ9/Vδ2 T cells as well as proliferation and expansion of Vγ9/Vδ2 T cells. This response was dependent on costimulatory growth factors, such as interleukin (IL)-2, IL-15 and IL-21. Significant levels of interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) were secreted in the presence of IL-2 and IL-15, but not in the presence of IL-21, demonstrating that proliferating phosphoantigen-reactive Vγ9/Vδ2 T cells do not necessarily produce proinflammatory cytokines.
Introduction
γδ T cells play a crucial role in the immune response to microbial pathogens, many of which are capable of establishing chronic and debilitating diseases, such as tuberculosis and malaria.1,2 In humans, activation of Vγ9/Vδ2 T cells by most pathogenic micro-organisms depends on compounds derived from the 2-C-methyl-d-erythritol 4-phosphate (MEP)1 pathway of isoprenoid biosynthesis.3,4 Beside the chloroplasts of algae and higher plants, this pathway is utilized by many bacteria,5,6 as well as apicomplexan protozoa such as Plasmodium falciparum,7 but is apparently absent in all vertebrates.8–11 Thus, the unconventional γδ T-cell reactivity to common metabolites ensures a quick and efficient immune response to a broad range of evolutionarily distant pathogens that may otherwise escape classical major histocompatibility complex-restricted mechanisms.12 While isopentenyl pyrophosphate (IPP), the precursor of all isoprenoids, was the first ligand described for the Vγ9/Vδ2 T-cell receptor (TCR),13–15 the natural amounts of IPP in bacteria do not reach the minimum required for inducing T-cell activation.3 Fournié and co-workers showed that a mycobacterial molecule likely to be 3-formyl-1-butyl pyrophosphate (FBPP) is approximately 1000-fold more active than IPP;16,17 because of its structural similarity to IPP, FBPP was suggested to be an intermediate of the MEP pathway.17 However, the terminal steps of the MEP pathway still remain to be elucidated, with 2-C-methyl-d-erythritol 2,4-cyclopyrophosphate (MEcPP) being the last metabolite in the reaction sequence known so far, and evidence that FBPP is a natural intermediate of IPP biosynthesis is missing.18
Most recently, a role for the genes dxr, gcpE and lytB in the MEP pathway has been demonstrated by us and others.19–23 We constructed Escherichia coli mutant strains utilizing exogenously provided mevalonate for producing IPP, owing to complementation with a plasmid that provides the yeast enzymes of the classical mevalonate pathway of isoprenoid synthesis. Consequently, mutants deficient in the genes dxr, gcpE and lytB, respectively, are viable when the culture medium is supplemented with mevalonate.20,21 Low-molecular-weight (LMW) extracts prepared from Δdxr and ΔgcpE mutants have a significantly reduced capacity to stimulate Vγ9/Vδ2 T cells, compared to the parent E. coli strain,4 thus proving the importance of the MEP pathway for biosynthesis of the γδ T-cell activator. In striking contrast, we show here that in ΔlytB bacteria, the Vγ9/Vδ2 T cell-stimulating molecule is greatly accumulated.
Materials and Methods
Bacteria and plasmids
Bacterial strains E. coli ΔgcpE and E. coli ΔlytB with precise in-frame deletions of gcpE and lytB, respectively, were derived from the wild-type E. coli K-12 strain, DSM no. 498, ATCC 23716.20,21 All mutant and wild-type (WT) strains harboured plasmid pSC-MVA with a synthetic operon to express Saccharomyces cerevisiae ERG12 (mevalonate kinase), ERG8 (phosphomevalonate kinase) and ERG19 (mevalonate pyrophosphate decarboxylase).20 Bacteria were grown in Standard 1 medium (Merck, Darmstadt, Germany) in the presence of 150 µg/ml ampicillin (Sigma, Taufkirchen, Germany) and 200 µm mevalonate; a stock solution of 1 m mevalonate was prepared by hydrolysing mevalonolactone (Fluka, Taufkirchen, Germany) with 1 m KOH at 37° for 30 min.
Synthesis of 14C-labelled MEP
For enzymatic synthesis of [2-14C]1-deoxy-d-xylulose 5-phosphate (DOXP), 0·8 mg of pyruvate (Sigma), 1·4 mg of [2-14C]pyruvate (585 MBq/mmol; NEN Life Science Products, Zaventem, Belgium) and 5·5 mg of d-fructose-1,6-bisphosphate (Sigma) were incubated with 0·44 U rabbit aldolase (d-fructose-1,6-bisphosphate-d-glyceraldehyde-3-phosphate-lyase; Sigma), 22·4 U rabbit triose phosphate isomerase (d-glyceraldehyde-3-phosphate-ketol-isomerase; Sigma) and 40 µg of recombinant E. coli DOXP synthase.24 [2-14C]DOXP was precipitated with 100 mm BaCl2 and 80% ethanol, and subsequently transformed into [2-14C]MEP in the presence of 25 mm NADPH (Sigma) using 300 µg of recombinant E. coli DOXP reductoisomerase (Dxr).7 Samples were then passed over a porous graphitized carbon column (Hypercarb; Thermo Hypersil, Runcorn, UK). Finally, [2-14C]MEP was purified on a MonoQ column (Amersham Pharmacia, Freiburg, Germany), using 20 mm Tris–HCl, pH 8·0, and rising concentrations of NaCl, on a Gynkotech HPLC device (Gynkotech, Germering, Germany). This procedure yielded ≈1·6 mg of [2-14C]MEP with an activity of ≈ 344 MBq/mmol (data not shown).
Metabolic 14C labelling of metabolites derived from the MEP pathway
A fresh overnight culture of ΔgcpE and ΔlytB mutants, respectively, was diluted 1 : 100 in 2 ml of medium supplemented with 100 µm mevalonate and 100 µg/ml ampicillin. As MEP cannot be taken up by E. coli, [2-14C]MEP was treated with 2 U shrimp alkaline phosphatase (Roche Diagnostics, Mannheim, Germany) at 37° for 30 min, and the resulting product, [2-14C]-methyl-d-erythritol ([2-14C]ME), was added to the cultures at a concentration of 50 µm (corresponding to c. 17·1 kBq/ml). Fosmidomycin, a potent inhibitor of the Dxr enzyme,7 was used at 100 µm to block the endogenous biosynthesis of MEP and consequently to ensure an optimum incorporation of [2-14C]ME into metabolites produced via the MEP pathway. After incubation at 37° for 6 hr, bacterial cells were pelleted, resuspended in 200 µl of 5 mm Tris–HCl, pH 7·5, and lysed by repeated freeze–thaw cycles in the presence of 2 mg of lysozyme (Merck). Cell debris was pelleted and the supernatants were loaded onto Microcon YM-3 filters (Millipore, Eschborn, Germany). The flow-through was lyophilized, resuspended in 3 µl of H2O and analysed on high performance thin layer chromatography silica gel plates (Kieselgel 60; Merck) developed with propanol : ethylacetate : H2O (6 : 1 : 3). Radioactive spots were visualized by autoradiography after exposure for 4 weeks.
γδ T-cell stimulation assays
Bacteria were harvested from fresh liquid cultures at an OD600 of ≈0·8 and sonicated in a 1 : 10 volume of phosphate-buffered saline (PBS). LMW fractions were obtained using Centriprep 3-kDa filters (Millipore). Blood samples from healthy donors were kindly provided by the Institut für Klinische Immunologie und Transfusionsmedizin (Klinikum der Justus-Liebig-Universität Giessen, Giessen, Germany). Peripheral blood mononuclear cells (PBMC) were isolated from heparinized peripheral blood by density centrifugation over LSM medium (ICN Biomedicals, Eschwege, Germany). PBMC (2×105/well) were seeded in RPMI-1640 (200 µl) supplemented with 25 mm HEPES, 2 mm l-glutamine, 25 µg/ml gentamycin (all from Life Technologies, Karlsruhe, Germany) and 10% human AB serum (Bayerisches Rotes Kreuz, Augsburg, Germany). For routine assays, recombinant human interleukin-2 (IL-2) (Life Technologies) was used at 10 U/ml for short-term cultures (18–72 hr), and at 100 U/ml for longer culture periods. For comparison between different growth factors, IL-2, IL-15 (both Promocell, Heidelberg, Germany), or IL-2125 was added at a concentration of 0·1–10 ng/ml. LMW preparations were added at dilutions of 1 in 200 (corresponding to ≈1·0×107 bacteria/well) to 1 in 5×106 (≈400 bacteria/well). Cells incubated in medium alone, and cells stimulated with 10 µm IPP (Sigma), served as negative and positive controls, repectively. Cells were harvested at different time-points and analysed for γδ T-cell activation and γδ T-cell outgrowth on an Epics XL flow cytometer supported with Expo32 software (Beckman Coulter, Krefeld, Germany), using the following Beckman Coulter monoclonal antibodies (mAbs): CD3-PC5 (UCHT1), Vγ9-fluorescein isothiocyanate (FITC) (Immu360), Vδ2-FITC (Immu389), CD25-phycoerythrin (PE) (B1·49·9), CD69-PE (TP1·55·3), CD94-PE (HP-3B1), and HLA-DR-PE (Immu357), together with the appropriate isotype controls. Gates were set on total lymphocytes and on CD3+ lymphocytes, and results obtained from 10 to 30 000 gated events were expressed as percentage of Vγ9+ cells among CD3+ lymphocytes on day 6, and of CD69+ (day 1), CD25+ (day 3), CD94+ (day 6), or HLA-DR+ (day 6) cells among Vγ9+ CD3+ lymphocytes. For cytokine analysis, culture supernatants were taken after 18 and 72 hr, and tested for tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), respectively, using the human OptEIA sets (Becton-Dickinson, Hamburg, Germany).
Chromatography and mass spectrometry
Cultures of ΔgcpE and ΔlytB mutants, respectively, were grown in 100 ml of Standard 1 medium to an OD600 of ≈0·8. LMW extracts were prepared as described above (except that 20 mm ammonium formiate, pH 8·0, was used instead of PBS) and passed over an Isolute C18 reverse-phase (RP) column (International Sorbent Technology, Mid Glamorgan, UK). The flow-through was then loaded onto a Source 15Q anion-exchange column (Amersham Pharmacia) and fractionated using a Waters HPLC device coupled to a PDA 996 photodiode array detector (Waters, Eschborn, Germany), scanning 1 spectrum/second between λ210 and 500 nm with a 1·2-nm resolution. Buffers used were: A, 20 mm ammonium formiate, pH 8·0; B, 500 mm ammonium formiate, pH 8·0; C, 1 m ammonium formiate; and chromatography was performed with a flow rate of 10 ml/min as follows: wash, 30 min 100% A; elution, linear gradient from 100% A to 100% B in 30 min; 10 min 100% C; equilibration, 10 min 100% A. Fractions collected were first tested in standard γδ T-cell activation assays at a dilution of 1 in 400 000, using pools consisting of four fractions each. The individual fractions from the only pool inducing upregulation of CD69 expression within 18 hr were then tested at a dilution of 1 in 400 000. The active sample, ΔlytB #20, and the corresponding negative control, ΔgcpE #20, were analysed by electrospray ionization orthogonal-time-of-flight mass spectrometry (ESI-o-ToF MS) (Mariner; PE Biosystems, Framingham, MA) equipped with a nano-ESI-source (Protana, Odense, Denmark). Sample solutions (3 µl) were filled in gold-coated nanospray capillaries and sprayed at flow rates of 10–30 nl/min. The voltage of the nanospray needle was set to 900 V; the temperature of the heated transfer capillary was 200°C. Spectra were taken in the negative ion mode and averaged over 10 scans of 3 seconds each. Structural analysis by MS-MS of selected ions was performed using a quadruple ion trap MS (LCQ; Finnigan MAT, San Jose, CA) with a relative collision energy of 20–30%.
Statistical analysis
Data were expressed as mean value±standard error of the mean (SEM). Statistical analysis was performed using a two-tailed Student's t-test for unequal variance, with differences considered statistically significant at a P-value of <0·05. R2 values were calculated using Microsoft Excel 2000 software.
Results
E. coli ΔlytB mutants accumulate a metabolite that is derived from methyl-d-erythritol
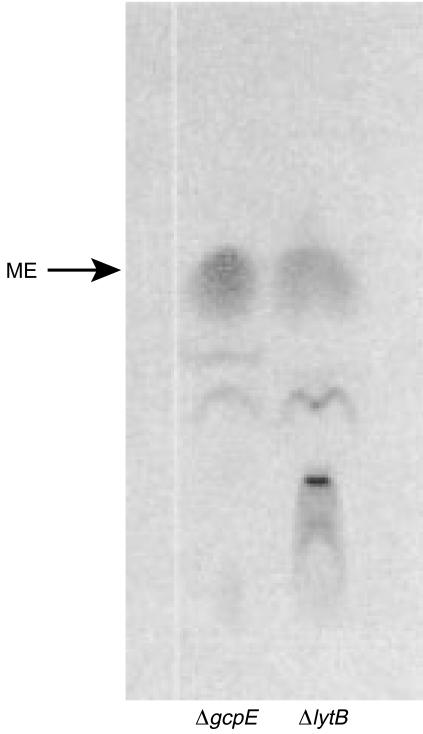
In recent studies, we demonstrated that the enzymes encoded by the E. coli genes gcpE and lytB are essential for isoprenoid synthesis via the MEP pathway.20,21 As the final biochemical steps in this pathway still remain to be unveiled, labelling experiments were performed in order to gain a more detailed insight into the function of the LytB and GcpE proteins. Thus, ΔlytB and ΔgcpE mutants were grown in medium supplemented with 14C-labelled ME. Separation of the corresponding LMW extracts by thin-layer chromatography revealed that in ΔgcpE mutants, most of the [2-14C]ME was not metabolized (Fig. 1). In contrast, in the ΔlytB mutant there was an additional spot indicating the accumulation of an unknown metabolite with reduced chromatographic mobility. Unfortunately, the amounts of labelled material were too limited to allow quantification of radioactivity by liquid scintillation or further characterization by mass spectrometry (data not shown).
Figure 1.
(a) Thin-layer chromatography analysis of Escherichia coli ΔgcpE and ΔlytB grown in the presence of [2-14C]2-C-methyl-d-erythritol (ME). Low molecular weight preparations were examined on an HPTLC silica gel plate, and radioactive spots were visualized by autoradiography.
Disruption of the lytB gene leads to a >100-fold accumulation of a γδ T-cell stimulating molecule
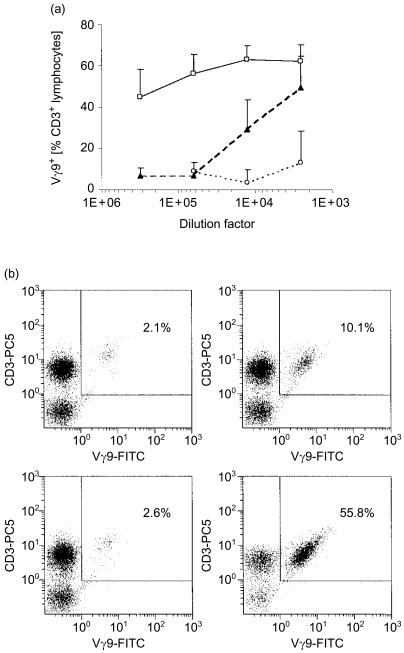
From previous work we know that a compound derived from the MEP pathway represents the natural activator for phosphoantigen-reactive human γδ T cells.3,4 Thus, we speculated that the metabolite accumulating in ΔlytB bacteria (Fig. 1) might in fact be this highly immunogenic substance. Here, in γδ T-cell stimulation assays, LMW extracts prepared from WT bacteria induced expansion of Vγ9/Vδ2 T cells at dilutions down to 1 : 12 500, corresponding to 1·6×105 bacteria/well (Fig. 2); in accordance with our previous observations,4 LMW extracts from ΔgcpE mutants were less active than those from WT E. coli. However, in striking contrast, ΔlytB mutants exhibited a γδ T-cell activity that was considerably above that of the WT bacteria; significant outgrowth of Vγ9/Vδ2 T cells could be detected at dilutions down to 1 : 312 500, corresponding to 6·4×103 bacteria/well. The relative increase in γδ T cells detected did reflect a true expansion of this population, as no substantial death of αβ T cells occurred (data not shown), which is in accordance with similar observations from other groups.2,17,26–29
Figure 2.
Disruption of the lytB gene increases the γδ T-cell stimulatory potential of Escherichia coli. (a) Vγ9+ T-cell outgrowth in the presence of bacterial extracts. Peripheral blood mononuclear cells (PBMC) were incubated over a period of 6 days with medium alone, or with low molecular weight extracts prepared from wild-type E. coli (black triangles), ΔgcpE (open circles), and ΔlytB (open squares), at different dilutions. Data shown represent mean values+standard error of the mean from three donors analysed. (b) Flow cytometry analysis. PBMC were incubated with medium alone (top left panel), or with LMW extracts prepared from WT E. coli (top right panel), ΔgcpE (bottom left panel), or ΔlytB (bottom right panel), at a dilution of 1 : 12 500. Panels shown are representative data obtained from one blood donor.
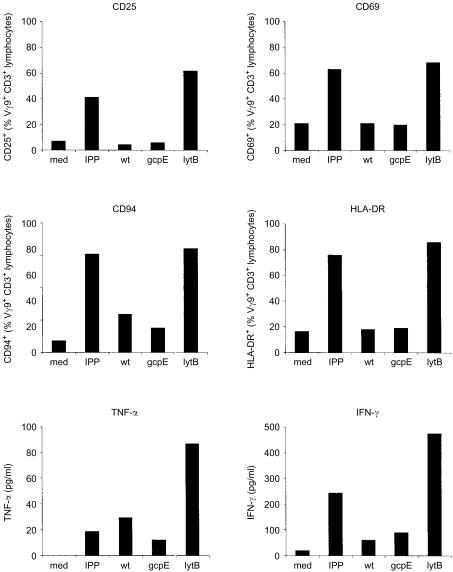
We then further investigated the LMW extracts prepared from ΔlytB bacteria, by measuring immunological parameters correlated with γδ T-cell activation by phosphoantigens. Thus, at a dilution of 1 : 62 500, the ΔlytB extract did not only stimulate proliferation of γδ T cells, but also induced upregulation of the activation markers CD25, CD69 and HLA-DR, and the C-type lectin CD94 on the surface of Vγ9/Vδ2 T cells, as well as secretion of TNF-α and IFN-γ by PMBC (Fig. 3). This activity was not present in extracts from WT or ΔgcpE bacteria tested at the same dilution.
Figure 3.
Effect of ΔlytB low molecular weight extract on a range of immunological parameters. Peripheral blood mononuclear cells (PBMC) were incubated with medium alone, with isopentenyl pyrophosphate (IPP) at 2 µm, or with LMW extract prepared from wild-type (WT) Escherichia coli, ΔgcpE, or ΔlytB, at a dilution of 1 : 62 500. Data represent results from one donor, for expression of CD25 (day 3), CD69 (day 1), CD94 (day 6) and human leucocyte antigen (HLA)-DR (day 6) on Vγ9+ T cells, and secretion of tumour necrosis factor (TNF-α) (day 1) and interferon-γ (IFN-γ) (day 3) by PBMC. Note: the pattern shown is typical for a number of blood donors analysed (n = 3–6); however, with absolute levels of CD94 and HLA-DR expression, as well as absolute amounts of cytokines secreted, greater biological variation was found between individuals than in the data for γδ T-cell expansion and expression of CD25 or CD69. Also, for some donors changes in CD94 and HLA-DR expression in the presence of serial dilutions of phosphoantigens was inconsistent despite good dose-dependent responses, as judged by CD25 and CD69 expression, thus making CD25 and CD69 the more reproducible activation markers tested.
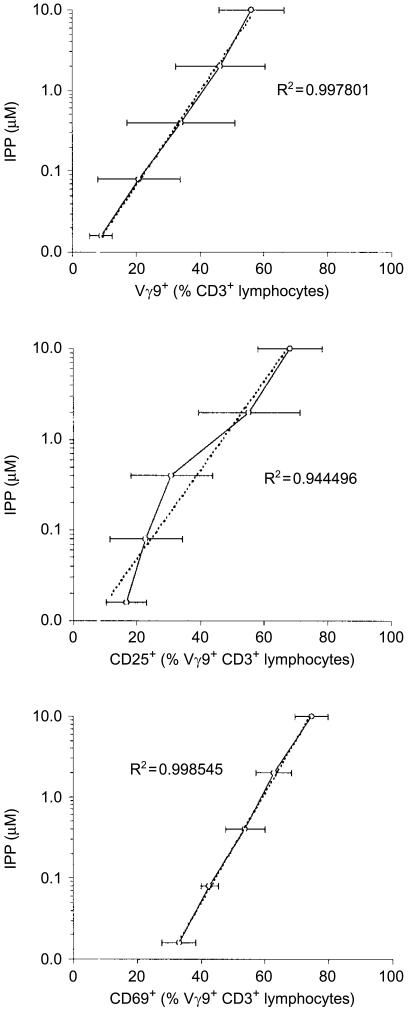
Using serial dilutions of an IPP standard, we obtained positive correlations between antigen concentration and γδ T-cell activation, measured as γδ T-cell expansion and expression of CD25 and CD69 (Fig. 4). These calibration curves enabled us to estimate the activity of the bacterial extracts tested and express it as IPP equivalents; importantly, all three analyses performed gave very similar levels (Table 1). Thus, LMW extracts from WT E. coli had a γδ T-cell stimulatory potential comparable to an IPP concentration of ≈3 mm, whereas the activity of the ΔgcpE mutant was significantly lower, equivalent to ≈0·8 mm. Strikingly, LMW extracts from bacteria deficient in lytB were greater than 100-fold more active than extracts from the WT bacteria, containing an antigenic compound that was as potent as ≈450 mm IPP.
Figure 4.
Dose–response to isopentenyl pyrophosphate (IPP). Peripheral blood mononuclear cells were incubated with medium alone, or with IPP at different concentrations. The data represent the mean value±SEM from three donors analysed, for Vγ9+ T-cell outgrowth, and expression of CD25 and CD69 on Vγ9+ T cells. Trend lines and R2 values were calculated using Microsoft Excel software.
Table 1. Immunological activity of extracts from different strains of Escherichia coli¶.
| Equivalent to IPP (mm) | WT | ΔgcpE | ΔlytB |
|---|---|---|---|
| Expt. 1† | 4·04 | 0·69 | 462·58 |
| Expt. 2‡ | 2·54 | 0·85 | 475·11 |
| Expt. 3§ | 2·66 | 0·79 | 394·64 |
| Mean±SEM | 3·08±0·48 | 0·78±0·05* | 444·11±25·00* |
Calibration curves were obtained using serial dilutions of an isopentenyl pyrophosphate (IPP) standard (Fig. 4). Bioactivity of the bacterial extracts analysed is expressed as IPP equivalents from three experiments performed.
Outgrowth of Vγ9 + CD3+ T cells, day 6 (n=3).
Upregulation of CD25 on Vγ9 + CD3+ T cells, day 3 (n=3).
Upregulation of CD69 on Vγ9 + CD3+ T cells, day 1 (n=3).
P<0·05, using a two-tailed Student's t-test for unequal variance.
SEM, standard error of the mean; WT, wild type.
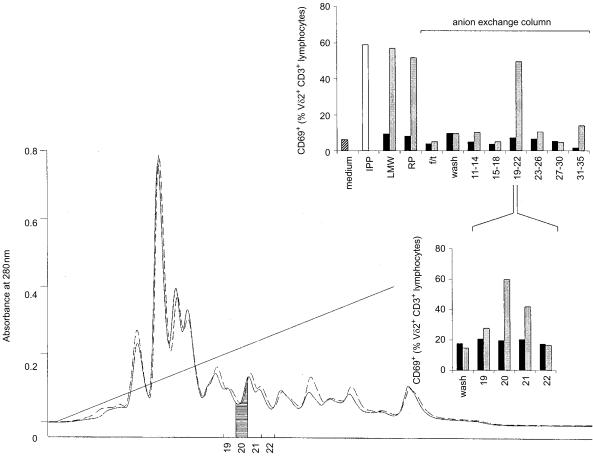
The γδ T-cell stimulating molecule can be purified by anion-exchange chromatography
In order to further characterize the immunogenic molecule accumulating in ΔlytB mutants, the 3-kDa LMW filtrate was passed over a C18 reversed-phase column and fractionated subsequently by anion-exchange chromatography, using ammonium formiate as eluent. An LMW preparation from ΔgcpE bacteria was subjected to the same protocol and served as a control. Unexpectedly, there was no significant difference in the absorbance profiles between the ΔgcpE and ΔlytB mutants, measured between 210 and 500 nm (Fig. 5, and data not shown). Consequently, all fractions collected were then tested in pools for their γδ T-cell reactivity. No sample obtained from ΔgcpE bacteria stimulated γδ T cells above background levels. In contrast, a peak of bioactivity was recovered in the pool consisting of fractions 19–22, whereas all other samples collected, as well as the flow-through of the column and the wash fractions, remained negative (Fig. 5). Testing the individual fractions of the positive pool showed that the γδ T-cell-stimulating molecule was eluted in fraction no. 20, at an ammonium formiate concentration of ≈250 mm. Titration of fraction no. 20 against an IPP standard gave a bioactivity corresponding to ≈2500 mm IPP (Table 2); the side fraction no. 21 still had ≈500 mm IPP bioactivity (data not shown).
Figure 5.
Fractionation of bacterial extracts by anion-exchange chromatography. ΔgcpE and ΔlytB low molecular weight (LMW) extracts, respectively, were passed over a reverse phase (RP) column and fractionated on a Source 15Q column, using an ammonium formiate gradient (20–500 mm). Absorbance at 280 nm is shown as a solid line for ΔlytB and a dashed line for ΔgcpE. Fractions collected were first tested in pools consisting of four each; fractions 19 to 22 were subsequently tested individually for their ability to upregulate CD69 expression on Vδ2+ T cells. Samples tested were: medium control; isopentenyl pyrophosphate (IPP) as positive control, 10 µm; 3-kDa LMW filtrate, 1 in 50 000 (LMW) extract; LMW extract after RP column chromatography (=sample loaded onto a Source 15Q column), 1 in 50 000 (RP); Source 15Q flow-through, 1 in 50 000 (f/t); Source 15Q wash fraction, 1 in 50 000; fractions 11–35, in pools or individually, 1 in 400 000. Solid bars, ΔgcpE; hatched bars, ΔlytB.
Table 2. Immunological activity of fraction ΔlytB #20*.
| Dilution factor | CD69 expression (% Vγ9+ CD3+)† | Bioactivity (mm IPP)‡ |
|---|---|---|
| Medium control | 29·8±8·4 | – |
| 6·25×107 | 29·9±12·4 | – |
| 1·25×107 | 35·1±7·0 | 3759·5 |
| 2·5×106 | 40·4±1·4 | 1823·8 |
| 5·0×105 | 52·2±0·2 | 2592·5 |
| 1·0×105 | 63·0±1·3 | 3104·2 |
| 2·0×104 | 66·2±0·6 | 1065·9 |
| Mean±SEM§ | – | 2469·2±472·7 |
A calibration curve was obtained using serial dilutions of an isopentenyl pyrophosphate (IPP) standard, ranging from 80 nm to 10 µm and yielding the formula (at R2=0·99): Bioactivity (µm IPP)=0·000869×e0·167×(CD69 expression level).
Upregulation of CD69 expression was monitored after incubation of peripheral blood mononuclear cells for 18 hr in the presence of serial dilutions of fraction ΔlytB #20 (n=3).
Bioactivity of fraction ΔlytB #20 was calculated for each dilution using the formula: Bioactivity ΔlytB #20 (mm IPP)=(1/1000×[bioactivity (µm IPP)×dilution factor]).
Mean bioactivity from the five dilutions tested that resulted in upregulation of CD69 expression above the background value. An analogous experiment measuring γδ T-cell expansion after 6 days resulted in a bioactivity value of 2487·6±911·4 mm IPP.
SEM, standard error of the mean.
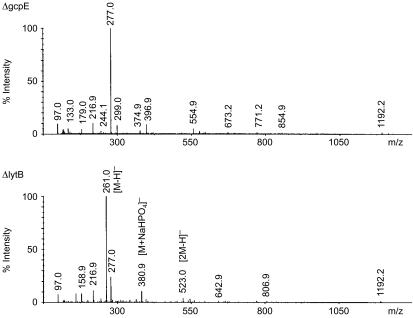
Mass spectrometry analysis identifies a molecule of Mr 262
To obtain structural information of the bioactive metabolite, ESI-MS analysis was performed on the active fraction derived from ΔlytB bacteria, ΔlytB #20, and the corresponding fraction from ΔgcpE mutants, ΔgcpE #20. In the negative ion spectrum of ΔlytB #20, the most abundant peak was at m/z 261 [M-H]−; two other peaks at m/z 381 [M+NaHPO4]− and m/z 523 [2M-H]− could be correlated to this molecule (Fig. 6). These signals were not detectable in fraction ΔgcpE #20, where the most prominent peak was at m/z 277, with common impurity peaks (e.g. m/z 216·9 and m/z 1192·2) at comparable intensities as in fraction ΔlytB #20; most of the residual UV absorbance of fractions ΔlytB #20 and ΔgcpE #20 could be ascribed to the ion at m/z 1192·2 (data not shown). Negative mode ion trap MS/MS from m/z 261 yielded ions at m/z 243 (corresponding to H2O loss) and m/z 159 (corresponding to a pyrophosphate anion, ) (data not shown). Subsequent large-scale purification of the γδ T-cell activator from a 1-l culture of E. coli ΔlytB mutants by anion-exchange chromatography, and its structural characterization by 1H, 13C and 31P nuclear magnetic resonance spectroscopy and nuclear Overhauser effect spectroscopy analysis, as (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) has been described elsewhere.30
Figure 6.
Negative mode electrospray ionization-mass spectrometry (ESI-MS) analysis of fractions ΔgcpE #20 and ΔlytB #20.
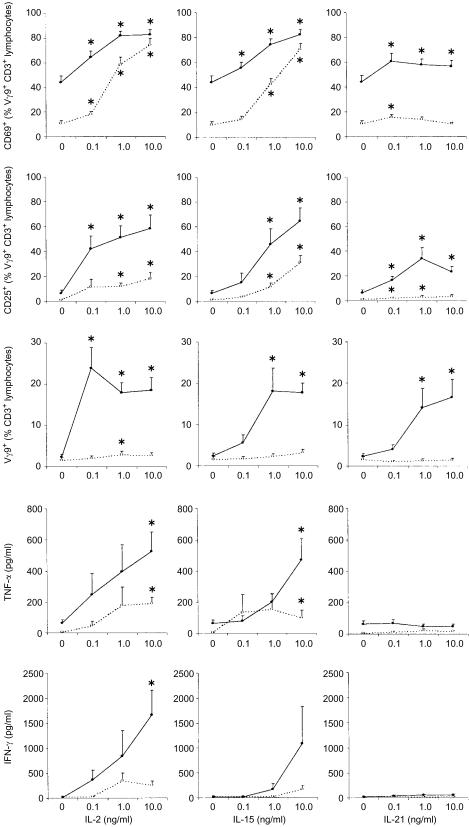
ΔlytB #20-driven γδ T-cell activation does not result in secretion of proinflammatory cytokines in the presence of IL-21
Apart from their reactivity towards small pyrophosphorylated molecules, Vγ9/Vδ2 T cells also depend on cytokines in the local microenvironment, a fact that may bias the read-outs of bioactivity assays. Consequently, we investigated the influence of different interleukins on the γδ T-cell response towards the novel activator present in fraction ΔlytB #20. IL-2 and IL-15 are clearly the most important growth factors known for γδ T cells, both of which synergize with IL-12.26 IL-21 is a novel cytokine that is closely related to IL-2 and IL-15, and produced by activated CD4+ T cells but not by CD19+ B cells or CD14+ monocytes. 25 Here, all three cytokines, IL-2, IL-15 and IL-21, costimulated the expansion of Vγ9/Vδ2 T cells among CD3+ T cells in the presence of fraction ΔlytB #20, while neither antigen nor cytokines alone had a significant proliferative effect (Fig. 7). Moreover, IL-21 increased the ΔlytB #20-driven upregulation of CD25 and CD69 on Vγ9/Vδ2 T cells, similarly to IL-2 and IL-15, although an effect on CD25 and CD69 expression in the absence of antigenic stimulation was barely detectable, in contrast to the other cytokines tested. Addition of IL-21 to PBMC cultures enhanced the IL-2- or IL-15-induced upregulation of CD69 expression, both in the presence or absence of antigenic stimulation; the additive effect on CD25 expression and γδ T-cell proliferation was less pronounced (data not shown). While IL-2 and IL-15 costimulated the ΔlytB #20-induced synthesis of both TNF-α and IFN-γ by PBMC, IL-21 did not enhance the ΔlytB #20-driven TNF-α production, and maximum IL-21 induced IFN-γ levels were much lower than those achieved in the presence of IL-2 or IL-15.
Figure 7.
Dependence of γδ T-cell activity on costimulation with interleukin (IL)-2, IL-15 and IL-21. Peripheral blood mononuclear cells were incubated with medium alone (open circles), or with ΔlytB #20 at a dilution of 1 in 100 000, corresponding to a bioactivity equivalent to 25 µm isopentenyl pyrophosphate (IPP) (solid circles), in the presence of different concentrations of the recombinant cytokines. Data shown represent the mean value+SEM for expression of CD69 and CD25 on Vγ9+ CD3+ T cells, for outgrowth of Vγ9+ T cells among CD3+ T cells, and for secretion of interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) (all n = 6 except n = 3 for IFN-γ). *P<0·05 compared to the corresponding medium controls, using a two-tailed Student's t-test for paired data.
Discussion
Over the past few years, Vγ9/Vδ2 T cells have been shown to react towards many pathogenic bacteria, such as Brucella, Coxiella, Ehrlichia, E. coli, Francisella, Legionella, Listeria, Mycobacterium, Pseudomonas, Salmonella and Yersinia, as well as to the protozoan parasites Plasmodium and Toxoplasma.12,31,32 Although some bacteria lacking the alternative MEP pathway of isoprenoid synthesis, such as Staphylococcus or Streptococcus, may be capable of stimulating Vγ9/Vδ2 T cells via superantigen recognition sites,33,34 recent studies have established a general link between Vγ9/Vδ2 T-cell reactivity35 and the presence of the MEP pathway.3,4 Accordingly, from our current data it is now clear that the LytB enzyme21 plays a crucial role in the metabolism of the natural Vγ9/Vδ2 TCR ligand. Stimulation of human PBMC with minute quantities of ΔlytB LMW preparations led to a drastic upregulation of the known T-cell activation markers CD25,26 CD6926 and HLA-DR,36 as well as the killer inhibitory receptor, CD94,37 on the surface of Vγ9/Vδ2 T cells, to proliferation and expansion of Vγ9/Vδ2 T cells,4 and to secretion of the proinflammatory cytokines IFN-γ and TNF-α.27,38,39
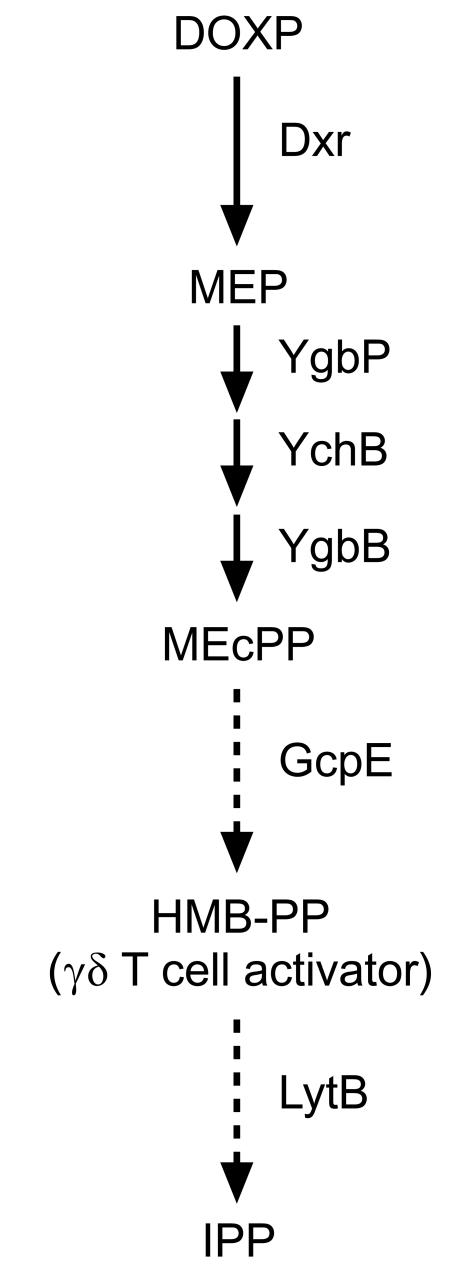
Recently, a molecule of Mr 262 (TUBag1) was identified in Mycobacterium fortuitum. Data from ion-trap ESI-MS and comparative photodiode array absorption analysis of TUBag1, together with NMR spectroscopy signals that were obtained using the corresponding γ-UTP and γ-TTP phosphodiesters, TUBag3 and TUBag4, suggested that this mycobacterial γδ T-cell activator is FBPP.13,16,17 As the most potent natural Vγ9/Vδ2 T-cell stimulator identified to date, this compound is active in the low nm range, whereas most other natural and synthetic substances (such as IPP and other prenyl pyrophosphates, alkylphosphates, alkylamines, and aminobisphosphonates) have bioactivities between 1 and 100 µm.13,15,28,29,40–42 Only now, some synthetic FBPP derivatives such as halohydrin pyrophosphates with bioactivities similar to, or even above, the naturally occurring γδ T-cell activator, have been described.43–46 However, the connection between FBPP and the MEP pathway of isoprenoid synthesis has only been deduced indirectly from the close structural similarity between FBPP and IPP.17 A mass of 262 atomic units as well as fragments at m/z 243 and m/z 159, as found in our present study, are in accordance with the chemical structure of FBPP;17 however, related molecules such as 3,4-epoxy-3-methyl-1-butyl pyrophosphate (EpoxPP), may give identical fragmentation patterns,44 thereby making the MS-based approach insufficient to support any proposed structure.45 Indeed, recent 1H, 13C and 31P NMR spectroscopy studies on the purified substance have identified the γδ TCR ligand accumulating in E. coli ΔlytB mutants as HMB-PP, a novel compound derived from the MEP pathway that is ≈104 times more potent in activating Vγ9/Vδ2 T cells than IPP.30 In contrast to FBPP, the chemical structure of HMB-PP is in accordance with incorporation studies using deuterium-labelled ME isotopomers,18 and insinuates a very elegant mechanism for the still unclear biosynthetic reaction sequence leading from MEcPP to IPP (Fig. 8). However, at present we do not know whether the Vγ9/Vδ2 TCR ligand is the actual substrate for LytB or merely represents a side product of the MEP pathway, utilizing an intermediate produced by GcpE (or another yet-unidentified enzyme downstream of GcpE). Yet, recently Hecht et al. have detected HMB-PP in bacteria overexpressing the gcpE gene,47 thus supporting our own conclusions.30
Figure 8.
Proposed scheme for the final biosynthetic reactions of the 2-C-methyl-d-erythritol 4-phosphate (MEP) pathway in Escherichia coli. Initially, 1-deoxy-d-xylulose 5-phosphate (DOXP) is converted into MEP by DOXP reductoisomerase (Dxr). Subsequently, the enzymes YgbP, YchB and YgbB catalyse the transformation of MEP into 2-C-methyl-d-erythritol 2,4-cyclopyrophosphate (MEcPP), with a molecular mass (Mr) of 278. GcpE is involved in the next step(s) using MEcPP as substrate to produce the γδ T-cell activator, (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) with Mr 262, whereas LytB mediates a/the subsequent reaction leading to IPP.
Finally, it is important to note that E. coli ΔgcpE mutants seem to accumulate another metabolite derived from the MEP pathway, detectable as a mass at m/z 277 eluted from the anion-exchange column under the same conditions as for the m/z 261 compound; this correspondence in the chromatographical behaviour may be a result of the close chemical similarity between the metabolites of the MEP pathway. The presence of a pyrophosphate group in the m/z 277 molecule was confirmed by ion trap MS/MS (U. Bahr and M. Hintz, unpublished). These findings suggest that in E. coli ΔgcpE there is an overproduction of MEcPP that may serve as the putative substrate for the GcpE enzyme catalysing the synthesis of the natural γδ TCR ligand, or its precursor.47 This conclusion is corroborated by recent data indicating that MEcPP itself does not display any γδ T-cell activity.48
In addition to the molecular characterization of the γδ T-cell activator, we have identified IL-21 as a potent costimulator of human phosphoantigen-reactive Vγ9/Vδ2 T cells. This confirms that expansion of activated γδ T cells depends on additional growth factors which may derive from bystander CD4+ T cells during an antigen-specific immune response to the pathogen, such as IL-2, IL-4 or IL-7, or from cells of the innate immune system at early stages of an infection when CD4+ T cells have not yet been recruited, like IL-12 and IL-15.26 The synergism between IL-21 and IL-2 or IL-15 is in accordance with previous findings showing that combinations of IL-21 and IL-2 or IL-15 have an additive effect on the lytic function of human natural killer cells; also, IL-21 potentiates the costimulation of murine T-cell proliferation by IL-2 or IL-15.25,49 The biological implication for this seemingly redundant utilization of IL-21 as another Vγ9/Vδ2 T-cell costimulator remains to be elucidated, as both IL-2 and IL-21 are produced by activated CD4+ T cells, and signalling of all three interleukins, IL-2, IL-15 and IL-21, via their respective receptors involves activation of STAT1, STAT3 and STAT5, as well as JAK1 and JAK3.50,51 In this respect, it is intriguing that IL-21 has similar effects as IL-2 and IL-15 on the ΔlytB #20-driven γδ T-cell proliferation and CD25 and CD69 surface expression, but is far less active in stimulating the ΔlytB #20-induced secretion of IFN-γ and TNF-α by PBMC. These findings demonstrate that the production of proinflammatory cytokines, which has been considered a key function of Vγ9/Vδ2 T cells and is a widely used read-out in bioactivity assays,27,38,39 is not necessarily associated with proliferating Vγ9/Vδ2 T cells. The immunology of IL-21 costimulated phosphoantigen-reactive Vγ9/Vδ2 T cells is well worth further attention.
Taken together, we have demonstrated that a pyrophosphorylated molecule of Mr 262 accounts for the strong immunogenicity of a mutant E. coli strain deficient in lytB. As there is increasing evidence that Vγ9/Vδ2 T cells contribute to the immune response against some of the most harmful pathogens infecting humans, the unexpected accessibility of the natural Vγ9/Vδ2 TCR ligand in large quantities, and the identification of IL-21 as a potent growth factor, will further aid our understanding of the unconventional function of Vγ9/Vδ2 T cells in diseases such as tuberculosis, tularemia, malaria, or Crohn's disease.52–55 The fact that in the presence of IL-21, ΔlytB #20-reactive Vγ9/Vδ2 T-cells proliferate without secreting significant levels of proinflammatory cytokines may be of considerable relevance for immunotherapeutic approaches in these infections.
Acknowledgments
This study was supported by the Bundesministerium für Bildung und Forschung (BioChance 0312588), and by a studentship from the Deutsche Forschungsgemeinschaft (to A.K.K). We gratefully acknowledge Rosel Engel, Christian Haug, Dajana Henschker, Ursula Jost, Irina Steinbrecher, and Stefanie Wagner for excellent technical assistance; and Erhard Dreiseidler, Claus Weidemeyer, and Oliver Wolf for helpful discussions.
Abbreviations
- DOXP
1-deoxy-d-xylulose 5-phosphate
- Dxr
1-deoxy-d-xylulose 5-phosphate reductoisomerase
- ESI
electrospray ionization
- FBPP
3-formyl-1-butyl pyrophosphate
- HMB-PP
(E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate
- IFN-γ
interferon-γ
- IL
interleukin
- IPP
isopentenyl pyrophosphate
- LMW
low molecular weight
- ME
2-C-methyl-d-erythritol
- MEcPP
2-C-methyl-d-erythritol 2,4-cyclopyrophosphate
- MEP
2-C-methyl-d-erythritol 4-phosphate
- MS
mass spectrometry
- TNF-α
tumour necrosis factor-α
References
- 1.Goerlich R, Hacker G, Pfeffer K, Heeg K, Wagner H. Plasmodium falciparum merozoites primarily stimulate the Vγ9 subset of human γ/δ T cells. Eur J Immunol. 1991;21:2613–6. doi: 10.1002/eji.1830211045. [DOI] [PubMed] [Google Scholar]
- 2.Kabelitz D, Bender A, Schondelmaier S, Schoel B, Kaufmann SH. A large fraction of human peripheral blood γ/δ+ T cells is activated by Mycobacterium tuberculosis but not by its 65-kDa heat shock protein. J Exp Med. 1990;171:667–75. doi: 10.1084/jem.171.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jomaa H, Feurle J, Luhs K, Kunzmann V, Tony HP, Herderich M, Wilhelm M. Vγ9/Vδ2 T cell activation induced by bacterial low molecular mass compounds depends on the 1-deoxy-d-xylulose 5-phosphate pathway of isoprenoid biosynthesis. FEMS Immunol Med Microbiol. 1999;25:371–8. doi: 10.1111/j.1574-695X.1999.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 4.Altincicek B, Moll J, Campos N, et al. Human γδ T cells are activated by intermediates of the 2-C-methyl-d-erythritol 4-phosphate pathway of isoprenoid biosynthesis. J Immunol. 2001;166:3655–8. doi: 10.4049/jimmunol.166.6.3655. [DOI] [PubMed] [Google Scholar]
- 5.Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J. 1993;295:517–24. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosa Putra S, Disch A, Bravo JM, Rohmer M. Distribution of mevalonate and glyceraldehyde 3-phosphate/pyruvate routes for isoprenoid biosynthesis in some Gram-negative bacteria and mycobacteria. FEMS Microbiol Lett. 1998;164:169–75. doi: 10.1111/j.1574-6968.1998.tb13082.x. [DOI] [PubMed] [Google Scholar]
- 7.Jomaa H, Wiesner J, Sanderbrand S, et al. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science. 1999;285:1573–6. doi: 10.1126/science.285.5433.1573. 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- 8.Rohmer M. The discovery of a mevalonate-independent pathway for isoprenoid synthesis in bacteria, algae and higher plants. Nat Prod Rep. 1999;16:565–74. doi: 10.1039/a709175c. [DOI] [PubMed] [Google Scholar]
- 9.Lange BM, Rujan T, Martin W, Croteau R. Isoprenoid biosynthesis: the evolution of two ancient and distinct pathways across genomes. Proc Natl Acad Sci USA. 2000;97:13172–7. doi: 10.1073/pnas.240454797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boucher Y, Doolittle WF. The role of lateral gene transfer in the evolution of isoprenoid biosynthesis pathways. Mol Microbiol. 2000;37:703–16. doi: 10.1046/j.1365-2958.2000.02004.x. [DOI] [PubMed] [Google Scholar]
- 11.Eisenreich W, Rohdich F, Bacher A. Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci. 2001;6:78–84. doi: 10.1016/s1360-1385(00)01812-4. [DOI] [PubMed] [Google Scholar]
- 12.Sicard H, Fournié JJ. Metabolic routes as targets for immunological discrimination of host and parasite. Infect Immun. 2000;68:4375–7. doi: 10.1128/iai.68.8.4375-4377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournié JJ. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–70. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka Y, Sano S, Nieves E, et al. Nonpeptide ligands for human γδ T cells. Proc Natl Acad Sci USA. 1994;91:8175–9. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 16.Poquet Y, Constant P, Halary F, et al. A novel nucleotide-containing antigen for human blood γδ T lymphocytes. Eur J Immunol. 1996;26:2344–9. doi: 10.1002/eji.1830261011. [DOI] [PubMed] [Google Scholar]
- 17.Belmant C, Espinosa E, Poupot R, Peyrat MA, Guiraud M, Poquet Y, Bonneville M, Fournié JJ. 3-Formyl-1-butyl pyrophosphate: a novel mycobacterial metabolite-activating human γδ T cells. J Biol Chem. 1999;274:32079–84. doi: 10.1074/jbc.274.45.32079. [DOI] [PubMed] [Google Scholar]
- 18.Charon L, Hoeffler JF, Pale-Grosdemange C, Lois LM, Campos N, Boronat A, Rohmer M. Deuterium-labelled isotopomers of 2-C-methyl-d-erythritol as tools for the elucidation of the 2-C-methyl-d-erythritol 4-phosphate pathway for isoprenoid biosynthesis. Biochem J. 2000;346:737–42. [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi S, Kuzuyama T, Watanabe H, Seto H. An 1-deoxy-d-xylulose 5-phosphate reductoisomerase catalyzing the formation of 2-C-methyl-d-erythritol 4-phosphate in an alternative nonmevalonate pathway for terpenoid biosynthesis. Proc Natl Acad Sci USA. 1998;95:9879–84. doi: 10.1073/pnas.95.17.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altincicek B, Kollas AK, Sanderbrand S, Wiesner J, Hintz M, Beck E, Jomaa H. GcpE is involved in the 2-C-methyl-d-erythritol 4-phosphate pathway of isoprenoid biosynthesis in Escherichia coli. J Bacteriol. 2001;183:2411–6. doi: 10.1128/JB.183.8.2411-2416.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altincicek B, Kollas AK, Eberl M, Wiesner J, Sanderbrand S, Hintz M, Beck E, Jomaa H. LytB, a novel gene of the 2-C-methyl-d-erythritol 4-phosphate pathway of isoprenoid biosynthesis in Escherichia coli. FEBS Lett. 2001;499:37–40. doi: 10.1016/s0014-5793(01)02516-9. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham FX, Jr, Lafond TP, Gantt E. Evidence of a role for LytB in the nonmevalonate pathway of isoprenoid biosynthesis. J Bacteriol. 2000;182:5841–8. doi: 10.1128/jb.182.20.5841-5848.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campos N, Rodriíguez-Concepción M, Seemann M, Rohmer M, Boronat A. Identification of gcpE as a novel gene of the 2-C-methyl-d-erythritol 4-phosphate pathway for isoprenoid biosynthesis in Escherichia coli. FEBS Lett. 2001;488:170–3. doi: 10.1016/s0014-5793(00)02420-0. [DOI] [PubMed] [Google Scholar]
- 24.Altincicek B, Hintz M, Sanderbrand S, Wiesner J, Beck E, Jomaa H. Tools for discovery of inhibitors of the 1-deoxy-d-xylulose 5-phosphate (DXP) synthase and DXP reductoisomerase: an approach with enzymes from the pathogenic bacterium Pseudomonas aeruginosa. FEMS Microbiol Lett. 2000;190:329–33. doi: 10.1111/j.1574-6968.2000.tb09307.x. [DOI] [PubMed] [Google Scholar]
- 25.Parrish-Novak J, Dillon SR, Nelson A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2001;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 26.Wesch D, Marx S, Kabelitz D. Comparative analysis of αβ and γδ T cell activation by Mycobacterium tuberculosis and isopentenyl pyrophosphate. Eur J Immunol. 1997;27:952–6. doi: 10.1002/eji.1830270422. [DOI] [PubMed] [Google Scholar]
- 27.García VE, Sieling PA, Gong J, et al. Single-cell cytokine analysis of γδ T cell responses to nonpeptide mycobacterial antigens. J Immunol. 1997;159:1328–35. [PubMed] [Google Scholar]
- 28.Bukowski JF, Morita CT, Brenner MB. Human γδ T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 29.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–92. [PubMed] [Google Scholar]
- 30.Hintz M, Reichenberg A, Altincicek B, et al. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 2001;509:317–22. doi: 10.1016/s0014-5793(01)03191-x. [DOI] [PubMed] [Google Scholar]
- 31.Kaufmann SH. γ/δ and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci USA. 1996;93:2272–9. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kabelitz D, Glatzel A, Wesch D. Antigen recognition by human γδ T lymphocytes. Int Arch Allergy Immunol. 2000;122:1–7. doi: 10.1159/000024353. [DOI] [PubMed] [Google Scholar]
- 33.Munk ME, Gatrill AJ, Kaufmann SH. Target cell lysis and IL-2 secretion by γ/δ T lymphocytes after activation with bacteria. J Immunol. 1990;145:2434–9. [PubMed] [Google Scholar]
- 34.Morita CT, Li H, Lamphear JG, Rich RR, Fraser JD, Mariuzza RA, Lee HK. Superantigen recognition by γδ T cells: SEA recognition site for human Vγ2 T cell receptors. Immunity. 2001;14:331–44. doi: 10.1016/s1074-7613(01)00113-3. [DOI] [PubMed] [Google Scholar]
- 35.Allison TJ, Winter CC, Fournié JJ, Bonneville M, Garboczi DN. Structure of a human γδ T-cell antigen receptor. Nature. 2001;411:820–4. doi: 10.1038/35081115. [DOI] [PubMed] [Google Scholar]
- 36.Borsellino G, Poccia F, Placido R, et al. Phenotypic and functional properties of γδ T cells from patients with Guillain Barré syndrome. J Neuroimmunol. 2000;102:199–207. doi: 10.1016/s0165-5728(99)00165-4. [DOI] [PubMed] [Google Scholar]
- 37.Boullier S, Poquet Y, Halary F, Bonneville M, Fournie JJ, Gougeon ML. Phosphoantigen activation induces surface translocation of intracellular CD94/NKG2A class I receptor on CD94− peripheral Vγ9Vδ2 T cells but not on CD94− thymic or mature γδ T cell clones. Eur J Immunol. 1998;28:3399–410. doi: 10.1002/(SICI)1521-4141(199811)28:11<3399::AID-IMMU3399>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 38.Sireci G, Champagne E, Fournié JJ, Dieli F, Salerno A. Patterns of phosphoantigen stimulation of human Vγ9/Vδ2 T cell clones include Th0 cytokines. Hum Immunol. 1997;58:70–82. doi: 10.1016/s0198-8859(97)00211-5. [DOI] [PubMed] [Google Scholar]
- 39.Lafont V, Liautard J, Liautard JP, Favero J. Production of TNF-α by human Vγ9Vδ2 T cells via engagement of FcγRIIIA, the low affinity type 3 receptor for the Fc portion of IgG, expressed upon TCR activation by nonpeptidic antigen. J Immunol. 2001;166:7190–9. doi: 10.4049/jimmunol.166.12.7190. [DOI] [PubMed] [Google Scholar]
- 40.Bukowski JF, Morita CT, Tanaka Y, Bloom BR, Brenner MB, Band H. Vγ2Vδ2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J Immunol. 1995;154:998–1006. [PubMed] [Google Scholar]
- 41.Morita CT, Lee HK, Wang H, Li H, Mariuzza RA, Tanaka Y. Structural features of nonpeptide prenyl pyrophosphates that determine their antigenicity for human γδ T cells. J Immunol. 2001;167:36–41. doi: 10.4049/jimmunol.167.1.36. [DOI] [PubMed] [Google Scholar]
- 42.Espinosa E, Belmant C, Sicard H, Poupot R, Bonneville M, Fournié JJ. Y2K+1 state-of-the-art on non-peptide phosphoantigens, a novel category of immunostimulatory molecules. Microbes Infect. 2001;3:645–54. doi: 10.1016/s1286-4579(01)01420-4. [DOI] [PubMed] [Google Scholar]
- 43.Belmant C, Espinosa E, Halary F, et al. A chemical basis for selective recognition of nonpeptide antigens by human γδ T cells. FASEB J. 2000;14:1669–70. doi: 10.1096/fj.99-0909fje. [DOI] [PubMed] [Google Scholar]
- 44.Pont F, Luciani B, Belmant C, Fournié JJ. Characterization of phosphoantigens by high-performance anion-exchange chromatography-electrospray ionization ion trap mass spectrometry and nanoelectrospray ionization ion trap mass spectrometry. Anal Chem. 2001;73:3562–9. doi: 10.1021/ac010184f. [DOI] [PubMed] [Google Scholar]
- 45.Feurle J, Espinosa E, Eckstein S, et al. E. coli produces phosphoantigens activating human γδ T cells. J Biol Chem. 2002;277:148–54. doi: 10.1074/jbc.M106443200. [DOI] [PubMed] [Google Scholar]
- 46.Espinosa E, Belmant C, Pont F, et al. Chemical synthesis and biological activity of bromohydrin pyrophosphate, a potent stimulator of human γδ T cells. J Biol Chem. 2001;276:18337–44. doi: 10.1074/jbc.M100495200. [DOI] [PubMed] [Google Scholar]
- 47.Hecht S, Eisenreich W, Adam P, et al. Studies on the nonmevalonate pathway to terpenes: the role of the GcpE (IspG) protein. Proc Natl Acad Sci USA. 2001;98:14837–42. doi: 10.1073/pnas.201399298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potapov VD, Biketov SF, Demina GR, Lysak EI, Titareva GM, Bakhteeva IV, Ostrovsky DN. Immunomodulatory properties of 2-C-methyl-d-erythritol 2,4-cyclopyrophosphate and the search for its new derivatives. Appl Biochem Microbiol. 2001;37:238–41. [PubMed] [Google Scholar]
- 49.Voßhenrich CA, di Santo JP. Cytokines: IL-21 joins the γc-dependent network? Curr Biol. 2001;11:R175–7. doi: 10.1016/s0960-9822(01)00087-2. [DOI] [PubMed] [Google Scholar]
- 50.Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci USA. 2000;97:11439–44. doi: 10.1073/pnas.200360997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, Sugamura K. The common γ-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. 2001;167:1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- 52.Boom WH. γδ T cells and Mycobacterium tuberculosis. Microbes Infect. 1999;1:187–95. doi: 10.1016/s1286-4579(99)80033-1. [DOI] [PubMed] [Google Scholar]
- 53.Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 54.Kabelitz D. Effector functions and control of human γδ T-cell activation. Microbes Infect. 1999;1:255–61. doi: 10.1016/s1286-4579(99)80042-2. [DOI] [PubMed] [Google Scholar]
- 55.Kanazawa H, Ishiguro Y, Munakata A, Morita CT. Multiple accumulation of Vδ2+ γδ T-cell clonotypes in intestinal mucosa from patients with Crohn's disease. Dig Dis Sci. 2001;46:410–6. doi: 10.1023/a:1005669319556. [DOI] [PubMed] [Google Scholar]