Abstract
The ability of the cell cycle inhibitor n-butyrate to induce T helper 1 (Th1) cell anergy is dependent upon its ability to block the cell cycle progression of activated Th1 cells in G1. Results reported here show that although both interleukin (IL)-2 and antigen (Ag) push Th1 cells into G1 where they are blocked by n-butyrate, only the Ag-activated Th1 cells demonstrate functional anergy once the n-butyrate has been removed from the culture. Because n-butyrate-induced Th1 cell anergy has been linked to increased expression of the cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1, mechanistic experiments focused on the role of these inhibitors. It was found that when Th1 cells were reincubated in Ag-stimulated secondary cultures, the Th1 cells previously exposed to Ag and n-butyrate (anergic Th1 cells) demonstrated a cumulative increase in p21Cip1 and p27Kip1 when compared with Th1 cells previously exposed to recombinant (r)IL-2 and n-butyrate (non-anergic Th1 cells). p27Kip1 in the anergic Th1 cells from the secondary cultures was associated with cyclin-dependent kinases (cdks). In contrast, p21Cip1 in the anergic Th1 cells, although present at high levels, did not associate significantly with cdks, suggesting that p21Cip1 may target some other protein in the anergic Th1 cells. Taken together, these findings suggest that Th1 cell exposure to Ag and n-butyrate, rather than IL-2 and n-butyrate, is needed to induce the cumulative increase in p21Cip1 and p27Kip1 that is associated with the proliferative unresponsiveness in anergic Th1 cells. In addition, p21Cip1 may inhibit proliferation in the anergic Th1 cells by some mechanism other than suppression of cdks that is unique to the induction of Th1 cell anergy.
Introduction
T helper 1 (Th1) cell anergy is induced when Th1 cells are exposed to antigen (Ag) presented by antigen-presenting cells (APC) deficient in costimulatory molecules.1,2 Alternatively, Th1 cell anergy can be induced by exposing Th1 cells to Ag presented by intact APC in the presence of the G1 blocker n-butyrate.3,4 Mechanistic studies revealed that anergy induction by n-butyrate was an active process requiring protein synthesis, and was associated with increases in the cyclin-dependent kinase inhibitors (CDKIs) p21Cip1 and p27Kip1 (see ref. 4). Cells may respond to various stimulatory or inhibitory events by altering the levels and/or association patterns of these CDKIs. One function of these CDKIs is to inhibit the activity of cyclin-dependent kinases (cdks), thereby inhibiting cdk-dependent retinoblastoma protein (pRb) phosphorylation and S-phase entry.5 cdk activity, pRb phosphorylation and S-phase entry were all suppressed in anergic Th1 cells in association with increased levels of p21Cip1 and p27Kip1 (see ref. 4).
n-Butyrate-induced anergy requires Th1 cell activation, and is not observed in resting Th1 cells exposed to n-butyrate.3,4 It appears that the Th1 cells must progress to the G1 phase of the cell cycle, where they are blocked by n-butyrate, in order for anergy to be induced. Other methods of initiating Th1 cell anergy depend upon exposing the Th1 cells to Ag in a way that is only minimally stimulatory. In contrast, n-butyrate induces anergy in Th1 cells exposed to Ag in a fully stimulatory manner. Therefore, it seemed possible that it is the event of the G1 blockade itself, regardless of how the Th1 cells get pushed into G1, that is crucial for n-butyrate-induced Th1 cell anergy. Experiments to address this possibility examined whether Th1 cells exposed to rIL-2 and n-butyrate, and similar Th1 cells exposed to Ag and n-butyrate, became anergic. In addition, because n-butyrate-induced Th1 cell anergy induction has been shown to be associated with increased levels of p21Cip1 and p27Kip1, we performed mechanistic studies that compared Th1 cells exposed to n-butyrate and rIL-2, or Ag, or both, with regard to the expression, association pattern and activity of these CDKIs. Determining whether the T-cell activation and cell cycle entry required for n-butyrate-induced anergy occurs through the general stimulation provided by IL-2, or requires Ag-specific T-cell stimulation, has important implications for the potential immunotherapeutic use of n-butyrate.
Materials and methods
Animals and reagents
Male C57BL/10 mice (6–8 weeks of age) were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN). Keyhole limpet haemocyanin (KLH) (Imject) was purchased from Pierce (Rockford, IL). The anti-p21Cip1 monoclonal antibody (mAb) [clone SMX30; mouse immunoglobulin G1 (IgG1)] and anti-pRb mAb (clone G3-245, mouse IgG1) were purchased from PharMingen (San Diego, CA). The anti-p27Kip1 mAb (clone G173-524, mouse IgG1), and the horseradish peroxidase (HRP)-labelled goat anti-mouse IgG antibody (Ab), were purchased from Transduction Laboratories (Lexington, KY). The anti-cdk2 Ab (rabbit IgG), anti-cdk4 Ab (rabbit IgG), anti-cdk6 Ab (rabbit IgG), anti-actin Ab (goat IgG), anti-cyclin D2 mAb (clone34B1-3; rat IgG2a), anti-cyclin D3 mAb (clone 18B6-10; rat IgG2a), anti-cyclin E Ab (rabbit IgG), HRP-labelled goat anti-rat IgG Ab, and the HRP-labelled donkey anti-goat IgG Ab were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The HRP-labelled goat anti-rabbit IgG was purchased from Sigma (St. Louis, MO). The Western Blot Recycling Kit was purchased from Alpha Diagnostic (San Antonio, TX).
Th1 clones
The KLH-specific Th1 cells (clone D9) were developed as described previously.4 The Th1 clones were derived from C57BL/10 mice and passaged every 7–14 days using Ag (KLH, 25 µg/ml), irradiated syngeneic splenic APC and IL-2-containing concanavalin A (Con A)-stimulated conditioned medium.
Inducing Th1 cell anergy
The Th1 cells were incubated in primary cultures at 5×105 cells/ml together with 5×106/ml irradiated syngeneic spleen cells as APC, KLH (20 µg/ml) and/or rIL-2 (1·25 ng/ml; R & D Systems, Minneapolis, MN), and 1·1 mm n-butyrate (Sigma). Control Th1 cells received APC and either rIL-2 or n-butyrate alone in primary cultures. After incubation for 48 hr at 37°, the cells in the primary cultures were washed and reincubated [at a concentration of 5×105/ml along with 5×106/ml irradiated syngeneic spleen cells as APC and KLH (20 µg/ml unless otherwise stated)] in medium without n-butyrate. After various time intervals in the secondary cultures, the Th1 cells were assessed for DNA synthesis (pulsed with [3H]TdR for 12 hr), or passed over Ficoll–Hypaque to remove the irradiated APC and disrupted with lysis buffer containing 10 mm KCl, 10 mm HEPES, 1·5 mm MgCl2, 0·5% Nonidet P-40, 1 mm NaVO4, aprotinin (10 mg/ml), leupeptin (10 mg/ml) and 0·5 mm phenylmethylsulphonyl fluoride (PMSF).
Western blotting analysis and immunoprecipitation
For Western blot analysis, equivalent amounts of protein (30–50 µg) from Th1 cell lysates were resolved on 12% sodium dodecyl sulphate (SDS)–polyacrylamide gels, electrotransferred onto nitrocellulose (Amersham Life Sciences, Bucks., UK), and immunoblotted with different primary Abs (2 µg/ml) and appropriate HRP-conjugated secondary Abs (diluted 1 : 2000). Immunodetection was performed by enhanced chemiluminescence (ECL) (Pierce) using Hyperfilm ECL (Amersham Life Sciences). To test for appropriate protein loading, some blots were stripped using the Western Blot Recycling Kit and reprobed with the anti-actin Ab. Densitometric analysis was performed using an IS-1000 Digital Imaging System and AlphaImager Software (Alpha Innotech Corporation, San Leandro, CA).
The pRb phosphorylation status in Th1 cell lysates was analysed using cell equivalents (2·5×105 cells/sample) disrupted in pRb lysis buffer containing 5% 2-mercaptoethanol, 10% glycerol, 0·625 mm Tris–HCl, pH 6·8, and 2% SDS. The proteins were resolved on 7·5% SDS–polyacrylamide gels, and subsequently immunoblotted with mouse anti-pRb mAb and goat anti-mouse IgG–HRP.
To examine association patterns of CDKIs and other G1 regulatory components, co-immunoprecipitation experiments were conducted. Lysates (200 µg/sample), obtained from different Th1 cell preparations, were incubated overnight at 4° with 30 µl of streptavidin-coated magnetic beads (Dynal, Great Neck, NY) that had been previously incubated (30 min at 4°) with the appropriate biotinylated secondary Ab [either goat anti-rabbit IgG Fc Ab (Jackson ImmunoResearch, West Grove, PA), or rat anti-mouse IgG1 mAb (clone A85-1; PharMingen)], followed by incubation (30 min at 4°) with the appropriate primary antibody directed against the target protein. The magnetic beads were washed three times in lysis buffer, boiled with loading buffer for 5 min, resolved on 12% SDS–polyacrylamide gels and immunoblotted with Abs specific for p21Cip1, p27Kip1, cdk2, cdk4, cdk6, cyclin D2, or cyclin D3.
Kinase assay
CDKI activity was measured as previously described.4 Briefly, cdk2 immunoprecipitated from a lysate (100 µg/sample) of asynchronous EL4 cells was mixed with 100 µg of preboiled lysates prepared from different Th1 cell preparations and incubated for 30 min at 37°. In some cases boiled lysates used in the mixing experiments had been depleted of p21Cip1 and p27Kip1 by immunoprecipitation. The immunoprecipitated cdk2 was next washed twice with lysis buffer and once with kinase buffer and tested for activity in a kinase assay kit (Upstate Biotechnology, Lake Placid, NY) using H1 as a substrate and 10 µCi of [γ-32P]ATP. The level of [γ-32P] incorporation was quantified using a scintillation counter (Beckman LS6000TA; Beckman, Fullerton, CA). The results are presented as total counts per minute (c.p.m.) minus the background c.p.m. measured in samples containing immunoprecipitated cdk2 but no substrate.
DNA analysis
At various time-points following stimulation Th1 cells were isolated from primary cultures and fixed in prechilled 70% ethanol at 4° overnight. The fixed Th1 cells were then washed in phosphate-buffered saline (PBS), resuspended in 1 ml of PBS containing RNAse (1 mg/ml; Sigma) and propidium iodide (50 µg/ml; Sigma), incubated for 20 min in the dark at room temperature and analysed by flow cytometry using a FACSCalibur (Becton-Dickinson, Mountain View, CA). The data was analysed using modfit (Verity Software House, Topsham, ME).
Results
IL-2 stimulation does not induce or interfere with n-butyrate-induced anergy
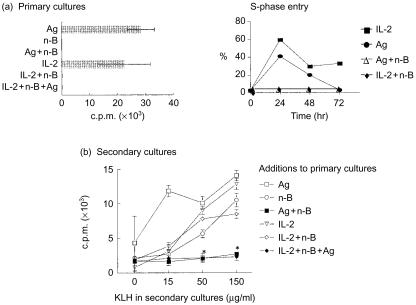
Experiments were conducted to determine whether n-butyrate could induce anergy in Th1 cells exposed to an Ag non-specific stimulus such as exogenous IL-2. As expected, based on the ability of n-butyrate to block cells in the G1 phase of the cell cycle, Th1 cells exposed to n-butyrate alone, n-butyrate and rIL-2, or n-butyrate and Ag, with or without rIL-2, did not proliferate in primary cultures (Fig. 1a). Only Th1 cells exposed to Ag or IL-2 alone proliferated in the primary cultures. DNA analysis revealed that peak S-phase entry in both the Ag- and IL-2-stimulated Th1 cells occurred at 24 hr, while Th1 cells exposed to IL-2 and n-butyrate or Ag and n-butyrate remained in G1 (Fig. 1a). In addition, as described previously, Th1 cells isolated from the primary cultures containing Ag and n-butyrate were unable to proliferate when restimulated with Ag in secondary cultures (Fig. 1b). In contrast, Th1 cells isolated from primary cultures containing IL-2 and n-butyrate did not lose their ability to proliferate following subsequent Ag stimulation. Control Th1 cells exposed to n-butyrate, Ag, or IL-2 alone in the primary cultures did not become anergic. These results suggest that n-butyrate-induced anergy requires Ag-specific Th1 cell activation, not general Th1 cell activation provided by IL-2. Furthermore, n-butyrate-induced Th1 cell anergy cannot be overcome by exposure to IL-2 stimulation. Based on these data, subsequent experiments utilized Th1 cells exposed to IL-2 and n-butyrate in primary cultures as a non-anergic control group with which to compare expression and association patterns of anergic (treated with Ag and n-butyrate or treated with Ag, n-butyrate and IL-2 in primary cultures) Th1 cells.
Figure 1.
n-Butyrate does not induce anergy in interleukin-2 (IL-2)-stimulated T helper 1 (Th1) cells. (a) Th1 cells (clone D9) were incubated for 48 hr in primary culture with antigen (Ag) alone, n-butyrate alone, Ag+n-butyrate, recombinant (r)IL-2 alone, rIL-2+n-butyrate, or rIL-2+n-butyrate+Ag. In addition, Th1 cells were stimulated with IL-2 or Ag + n-butyrate for 24, 48, or 72 hr. Following isolation from the primary cultures, the Th1 cells were fixed in 70% ethanol at 4° overnight. Th1 cells were then washed in phosphate-buffered saline (PBS), resuspended in RNAse (1 mg/ml) and propidium iodide (50 µg/ml), incubated for 20 min at room temperature in the dark, and analysed for their DNA content by flow cytometry. (b) Th1 cells were then isolated from the primary cultures and stimulated in secondary cultures with Ag for 48 hr. [3H]Thymidine ([3H]TdR) uptake by the Th1 cells was assessed and is presented as counts per minute (c.p.m.)±SD from a representative experiment. *The responses generated by the Th1 cells pretreated with Ag+n-butyrate or with rIL-2+Ag+n-butyrate and then restimulated with 50 or 150 µg/ml of keyhole limpet haemocyanin (KLH) in secondary cultures were determined by the Student's t-test to be statistically different at a P-value of 0·05 from their non-Ag control values. This experiment has been repeated twice with similar results.
Expression of G1 cell cycle regulatory proteins in IL-2-activated Th1 cells
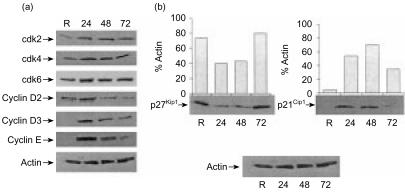
It seemed possible that differences in G1 cell cycle regulatory protein expression and/or function could account for the inability of Th1 cells treated with IL-2 and n-butyrate in primary cultures to become anergic. Before this possibility could be investigated it was necessary to determine the baseline levels of the G1 cell cycle regulatory proteins in IL-2-stimulated Th1 cells. The expression patterns of the G1 cell cycle regulatory proteins in IL-2-stimulated Th1 cells were similar to those observed in Ag-stimulated Th1 cells. cdk4 and cdk6 were detected in resting Th1 cells and their expression levels did not fluctuate noticeably during the IL-2-stimulated cell cycle (Fig. 2a). cdk2 was detected at low levels in resting Th1 cells, but was up-regulated at 24 hr following IL-2 stimulation and remained relatively constant during the remainder of the cell cycle. Expression of cyclin D2, detected at low levels in resting Th1 cells, peaked 24 hr following IL-2 stimulation. Cyclins D3 and E were not detected in resting Th1 cells; however, they were present 24 hr following stimulation with IL-2, 24 hr earlier than their detection in Ag-stimulated Th1 cells, demonstrated in an earlier study conducted in this laboratory.4
Figure 2.
Expression of G1 cell cycle regulatory proteins in interleukin-2 (IL-2)-stimulated Th1 cells. (a) Lysates were prepared from resting (R) T helper 1 (Th1) cells, or from Th1 cells stimulated for 24, 48, or 72 hr with IL-2. Expression of the various G1 cell-cycle regulatory proteins was assessed by immunoblotting. Equal loading of cell extracts was confirmed using an antibody (Ab) that recognized actin. (b). Lysates were prepared from resting Th1 cells, or from Th1 cells stimulated for 24, 48, or 72 hr with IL-2. Expression of p21Cip1 and p27Kip1 was assessed by immunoblotting. Densitometric analysis was performed, and the results are presented as percentage actin. This experiment was been repeated twice with similar results obtained on each occasion.
Expression patterns of the CDKIs, p21Cip1 and p27Kip1, in IL-2-stimulated Th1 cells were also similar to those previously seen in Ag-stimulated Th1 cells. p21Cip1 was not detectable in resting Th1 cell lysates, appeared by 24 hr poststimulation with IL-2 and began to decrease by 72 hr poststimulation (Fig. 2b). In contrast, protein levels of p27Kip1 were high in resting Th1 cell lysates, down-regulated by 24 hr poststimulation and began to rise again by 72 hr poststimulation. Taken together, these results suggest that Th1 cells stimulated with IL-2 express a similar pattern of cell cycle regulatory proteins and DNA synthesis as compared with Th1 cells stimulated with Ag.
The effects of n-butyrate on the expression of p21Cip1 and p27Kip1 in IL-2-treated Th1 cells were next compared to the expression pattern in Ag-stimulated Th1 cells. In addition, association of p21Cip1 and p27Kip1 with cdk4 was examined in order to help evaluate the functional consequences of any n-butyrate-induced alteration in CDKI expression. Similarly to the previously described IL-2-induced down-regulation of p27Kip1, Th1 cells exposed in primary cultures to IL-2 and n-butyrate, or to Ag and n-butyrate (with or without IL-2), showed a low level of p27Kip1 at the 24-hr time-point (Fig. 3). In contrast to p27Kip1, the level of p21Cip1 in Th1 cells exposed in primary cultures to IL-2 and n-butyrate, or to Ag and n-butyrate (with or without IL-2), was dramatically increased. Furthermore, p21Cip1 and p27Kip1 (albeit to a lesser degree) were associated with cdk4 in all three groups of Th1 cells. Therefore, no significant differences between Th1 cells exposed to n-butyrate and Ag (even in the presence of IL-2), or to n-butyrate and IL-2, with regard to the expression of p21Cip1 and p27Kip1 or the association of these CDKIs with cdk4, were revealed in the primary cultures.
Figure 3.
Effects of n-butyrate on cyclin-dependent kinase inhibitors (CDKIs) in interleukin-2 (IL-2)-stimulated T helper 1 (Th1) cells. Th1 cells were incubated in primary cultures containing IL-2+n-butyrate, or antigen (Ag)+n-butyrate, with or without IL-2. After 24 hr, the Th1 cells were isolated and lysates were prepared. p21Cip1 and p27Kip1 were immunoprecipitated, and the expression of p21Cip1 and p27Kip1, as well as their association with cyclin-dependent kinase 4 (cdk4), was assessed by immunoblotting. This experiment was been repeated, with similar results obtained on each occasion.
CDKI expression in Ag-restimulated Th1 cells pretreated with Ag and n-butyrate or with IL-2 and n-butyrate
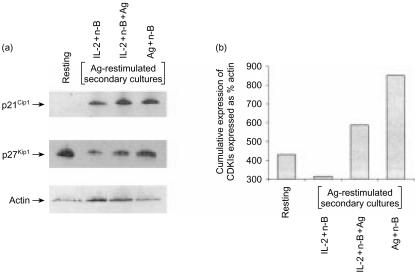
As examination of CDKIs in primary culture could not account for the functional differences between non-anergic Th1 cells (treated with IL-2 and n-butyrate) and anergic Th1 cells (treated with Ag and n-butyrate, with or without IL-2), it seemed probable that if differential expression of CDKIs existed it might only be detected once the Th1 cells had been restimulated. To examine this possibility, lysates were prepared from non-anergic Th1 cells (treated with IL-2 and n-butyrate in primary cultures) and anergic Th1 cells (treated with Ag and n-butyrate, with or without IL-2 in primary cultures) following Ag stimulation in secondary cultures (Fig. 4a). At 24 hr postrestimulation, which represents the main stage of S-phase entry, anergic Th1 cells contained notably elevated levels of p21Cip1 and p27Kip1 in comparison with non-anergic Th1 cells. The cumulative increase in expression of p21Cip1 and p27Kip1 in anergic Th1 cells, as compared to non-anergic Th1 cells, following Ag restimulation (Fig. 4b), may account for the observed differences in their proliferative capacities in the secondary cultures. In addition, these results demonstrate that the addition of IL-2 to the primary cultures treated with Ag and n-butyrate is not capable of overcoming the increase in p21Cip1 and p27Kip1 found in Ag-restimulated Th1 cells treated in primary cultures with Ag and n-butyrate alone.
Figure 4.
Expression of cyclin-dependent kinase inhibitors in anergic and non-anergic T helper 1 (Th1) cells following restimulation with antigen (Ag). (a) Th1 cells were incubated for 48 hr in primary culture with interleukin-2 (IL-2)+n-butyrate, or with Ag+n-butyrate (with or without IL-2). These Th1 cells were then restimulated with Ag for 24 hr, at which time they were lysed. Some Th1 cells (resting) were left untreated before being lysed. Expression of p21Cip1 and p27Kip1 was assessed by immunoblotting. (b) Densitometric analysis was performed, and the cumulative expression of p21Cip1 and p27Kip1 is presented as percentage actin. This experiment was repeated three times, with similar results obtained on each occasion.
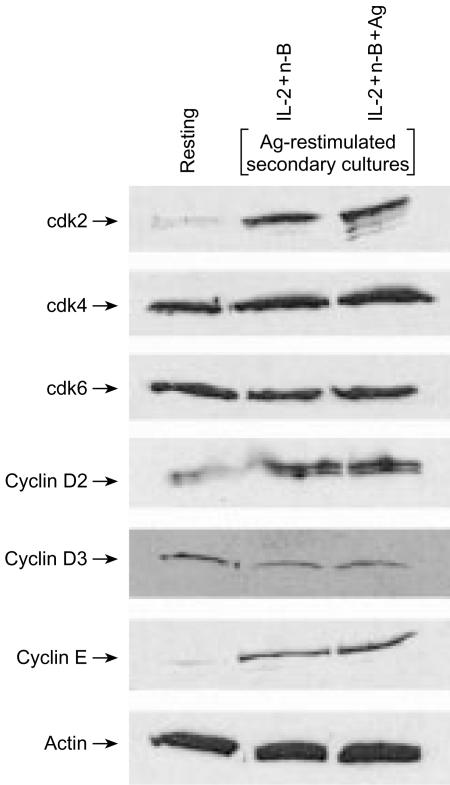
Besides the decreased expression of CDKIs in non-anergic Th1 cells, different functional outcomes of Th1 cells treated with IL-2 and n-butyrate, and those treated with Ag and n-butyrate, might reflect differential expression of some other G1 cell-cycle regulatory components. However, Western blot analysis revealed essentially no differences in the expression of the G1 cdks or cyclins between Ag-restimulated Th1 cells treated in primary cultures with Ag and n-butyrate, even in the presence of IL-2, and those treated in primary cultures with IL-2 and n-butyrate (Fig. 5).
Figure 5.
Expression of G1 cell-cycle regulatory proteins in anergic and non-anergic T helper 1 (Th1) cells following restimulation with antigen (Ag). Th1 cells were incubated for 48 hr in primary culture with interleukin-2 (IL-2)+n-butyrate, or with Ag+IL-2+n-butyrate. The Th1 cells were then restimulated with Ag for 24 hr, at which time they were lysed. Expression of cyclin-dependent kinase (cdk)2, cdk4, cdk6, cyclin D2, cyclin D3, and cyclin E was assessed by immunoblotting. Equal loading of protein was confirmed by staining with an anti-actin antibody.
CDKI activity in anergic and non-anergic Th1 cells
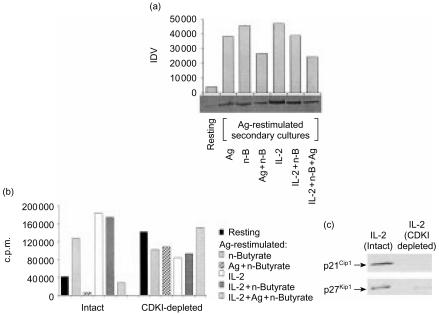
In addition to comparing the protein levels of p21Cip1 and p27Kip1, functional CDKI activity was compared in Th1 cells treated with Ag and n-butyrate (with or without IL-2) and in Th1 cells treated with IL-2 and n-butyrate. CDKI activity in the Th1 cell lysates was evaluated as a function of endogenous pRb phosphorylation and as the ability to inhibit exogenous cdk activity. In correlation with their increased level of p21Cip1 and p27Kip1, the Ag-restimulated anergic Th1 cells exhibited decreased endogenous pRb phosphorylation levels when compared with the different groups of non-anergic or control Th1 cells (Fig. 6a). In addition, lysates from Ag-restimulated anergic Th1 cells (exposed in primary cultures to Ag and n-butyrate, with or without IL-2) successfully inhibited exogenous cdk2 activity in a kinase assay (Fig. 6b). Furthermore, removal of the CDKIs p21Cip1 and p27Kip1 by immunoprecipitation from the anergic Th1 cell lysates eliminated their ability to suppress exogenous cdk2 kinase activity. In contrast to lysates from anergic Th1 cells, lysates from Ag-restimulated Th1 cells exposed in primary cultures to either n-butyrate alone or to IL-2 and n-butyrate, were unable to suppress the activity of preformed exogenous cdk2 complexes. Lysates from resting Th1 cells, shown to contain high levels of p27Kip1, also suppressed exogenous cdk2 activity. Taken together, these results demonstrated that anergic Th1 cells, unlike Th1 cells treated with IL-2 and n-butyrate in primary cultures, exhibited functional CDKI activity, capable of inhibiting both endogenous and exogenous cdk activity.
Figure 6.
(a) Endogenous retinoblastoma protein (pRb) levels in anergic and non-anergic T helper 1 (Th1) cells following restimulation with antigen (Ag). Lysates were prepared from resting Th1 cells, or from Th1 cells exposed in primary cultures to Ag alone, n-butyrate alone, Ag+n-butyrate, interleukin-2 (IL-2) alone, IL-2+n-butyrate, Ag+n-butyrate, or IL-2+Ag+n-butyrate, and which had been restimulated with Ag for 24 hr. These lysates were immunoblotted with anti-pRb Ab. Densitometric analysis was performed, and the results are presented as integrated density value (IDV). (b) Activity of cyclin-dependent kinase inhibitors (CDKIs) in anergic and non-anergic Th1 cells following restimulation with Ag. Lysates were prepared from resting Th1 cells, or from Th1 cells exposed in primary cultures to n-butyrate alone, Ag+n-butyrate, IL-2+n-butyrate, or IL-2+Ag+n-butyrate, and then recultured with Ag for 24 hr. The Th1 cell lysates, either intact or depleted of both p21Cip1 and p27Kip1 by immunoprecipitation, were mixed in equal protein amounts with cdk2 immunoprecipitated from lysates of asynchronous EL4 cells for 30 min, and then tested for activity in an H1 kinase assay. The results are presented as total counts per minute (c.p.m.) minus the background c.p.m. measured in samples containing immunoprecipitated cdk2 but no substrate. This experiment was repeated with similar results obtained. (c) Representative experiment demonstrating the immunodepletion of p27Kip1 and p21Cip1 from the Th1 cell lysates (treated with IL-2) confirmed by Western blotting.
Examining the cause of the decreased cdk activity in the Ag-restimulated anergic Th1 cells
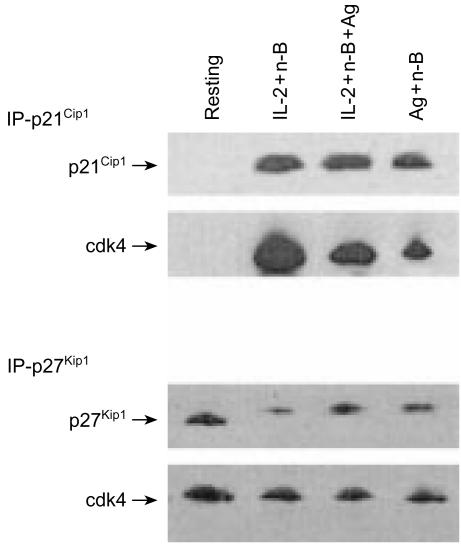
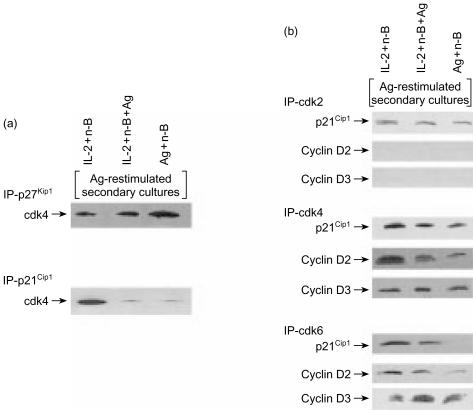
It seemed probable that the decreased pRb phosphorylation observed in the Ag-resimulated anergic Th1 cells was a result of suppression of G1 cdk activity by p21Cip1 and p27Kip1 in these Th1 cells. Co-immunoprecipitation experiments conducted to examine the association of p21Cip1 and p27Kip1 with cdks revealed that cdk4 was associated with p27Kip1 in all groups of Ag-restimulated Th1 cells (Fig. 7a). However, unlike p27Kip1, significantly less p21Cip1 was found in association with cdk4 in the Ag-restimulated anergic Th1 cells. Thus, the inability of the anergic Th1 cells (treated with Ag and n-butyrate, with or without IL-2 in primary cultures) to proliferate in the Ag-restimulated secondary cultures cannot be explained by the association of p21Cip1 with cdk4.
Figure 7.
(a) Association of cyclin-dependent kinase (cdk)4 with p21Cip1 and p27Kip1 in anergic and non-anergic T helper 1 (Th1) cells following restimulation with antigen (Ag). Lysates were prepared from Th1 cells exposed in primary cultures to interleukin-2 (IL-2)+n-butyrate, or from Th1 cells exposed in primary cultures to Ag+n-butyrate (with or without IL-2) that had been restimulated with Ag for 24 hr. p27Kip1 or p21cip1 (200 µg/sample) was immunoprecipitated from these lysate preparations and analysed via Western blotting for expression of cdk4. (b) Association of p21Cip1 cyclin D2, and cyclin D3 with cdk2, cdk4, and cdk6 in anergic and non-anergic Th1 cells following restimulation with Ag. Lysates were prepared from Th1 cells exposed in primary cultures to IL-2+n-butyrate, or from anergic Th1 cells exposed in primary cultures to Ag+n-butyrate (with or without IL-2) that had been restimulated with Ag for 24 hr. cdk2, cdk4, or cdk6 (200 µg/sample) were immunoprecipitated from these lysates and analysed via Western blotting for expression of p21Cip1 cyclin D2 and cyclin D3.
As p21Cip1 did not associate with cdk4 to any great degree in the Ag-restimulated anergic Th1 cells, it was expected that p21Cip1 was binding to and inhibiting some other G1 cdk/cyclin complex in the Ag-restimulated anergic Th1 cells. However, experiments carried out to test this possibility found that p21Cip1 did not associate with cdk2 and cdk6 in the Ag-restimulated anergic Th1 cells at levels greater than those observed in non-anergic Th1 cells (Fig. 7b). Thus, p21Cip1 in the anergic Th1 cells did not appear to exert its suppressive effects in the Ag-stimulated secondary cultures by binding to G1 cyclin/cdk complexes.
The association of G1 cdks with cyclin D2 and cyclin D3 was also examined as these interactions correlate with cdk activity, and the lack of such an interaction could help to explain the decrease in cdk-induced pRb phosphorylation observed in the anergic Th1 cells. Less cyclin D2 was found to be associated with cdk4 and cdk6 in the Ag-restimulated anergic Th1 cells, including those treated with IL-2 in the primary cultures, in comparison with non-anergic Th1 cells (Fig. 7b). No cyclin D2 was found to be associated with cdk2 in either group. Essentially no differences were observed between anergic Th1 cells and non-anergic Th1 cells following Ag rechallenge, with regard to the levels of cyclin D3 associated with cdk2, cdk4, or cdk6 (Fig. 7b). Taken together, these results suggest that the decreased pRb phosphorylation observed in Ag-restimulated anergic Th1 cells, including those treated with rIL-2 during the primary culture, may reflect decreased association of cyclin D2 with cdk4 and cdk6 rather than p21Cip1 suppression of G1 cyclin/cdk complexes.
Discussion
The data presented here demonstrate that the relatively novel method of Th1 cell anergy induction using the G1a blocker, n-butyrate, requires T-cell receptor (TCR) ligation. Although Th1 cells exposed to the general stimulant, IL-2, and n-butyrate fail to proliferate during primary cultures, these Th1 cells retain their ability to proliferate following removal of n-butyrate from the cultures and subsequent Ag challenge. In contrast, Th1 cells exposed to Ag and n-butyrate in the primary cultures fail to proliferate in response to a subsequent Ag challenge, demonstrating that n-butyrate-induced Th1 cell anergy requires Ag-specific T-cell activation. This finding is consistent with previously described data showing that early activation events associated with TCR ligation, such as protein kinase C (PKC) activation and [Ca2+]i mobilization, are necessary for Th1 cell anergy.6 Although IL-2 stimulation was not sufficient to induce anergy in n-butyrate-treated Th1 cells, it seemed possible that the addition of IL-2 to primary cultures of Th1 cells treated with Ag and n-butyrate would prevent the induction of anergy. This possibility was based on the fact that Th1 cell anergy has been associated with the lack of IL-2 receptor (IL-2R) occupancy.7 However, the inclusion of IL-2 did not interfere with anergy induction of the Th1 cells. These results demonstrated that addition of exogenous IL-2 to primary cultures containing n-butyrate alone, or Ag and n-butyrate, has very different functional outcomes.
The direct effects of n-butyrate in conjunction with either IL-2 or Ag on Th1 cell expression of p27Kip1 and p21Cip1 were compared in order to determine whether differential sensitivity to anergy induction was caused by differences in the expression and/or association patterns of these CDKIs. In primary cultures, Th1 cells treated with IL-2 and n-butyrate, or with Ag and n-butyrate (with or without IL-2), down-regulated p27Kip1 by 24 hr in the primary cultures. In contrast, p21Cip1 was dramatically up-regulated in Th1 cells treated in primary cultures with either IL-2 and n-butyrate or Ag and n-butyrate. Increased p21Cip1 expression has been shown to be induced in response to a variety of stimuli, including IL-28 and mitogen-activated protein kinase (MAPK) signalling.9,10 p21Cip1 has also been shown to be up-regulated by n-butyrate in cycling cancer cells and fibroblasts.11,12 We have shown previously that p21Cip1 is not increased when resting Th1 cells are treated with n-butyrate alone, but is up-regulated when Th1 cells are treated with both n-butyrate and Ag.4 The p21Cip1 response observed in Th1 cells exposed to both n-butyrate and Ag is up-regulated sooner and more vigorously than that induced by Ag alone. Consequently, it is not surprising that Th1 cells exposed here in primary cultures to both IL-2 and n-butyrate, or to both Ag and n-butyrate (with or without IL-2), up-regulated their expression of p21Cip1. The p21Cip1 observed in the Th1 cells treated with IL-2 and n-butyrate, and the Th1 cells treated with Ag and n-butyrate (with or without IL-2), in primary cultures was found to associate with cdk4. Taken together, these data imply that the increased levels of p21Cip1 in n-butyrate-treated stimulated Th1 cells prevents cell cycle progression in the primary cultures by suppressing the G1 cdk activity required for S-phase entry. This data correlates with the lack of proliferation observed in all of the groups treated with n-butyrate in the primary cultures.
Although the results obtained adequately explained the lack of proliferation by the n-butyrate-treated Th1 cells in the primary cultures, they did not explain why Th1 cells treated with IL-2 and n-butyrate, as compared to Ag and n-butyrate, with or without IL-2, differed in their Ag-induced proliferation in secondary cultures. Examining the levels of p21Cip1 and p27Kip1 in Th1 cells in Ag-stimulated secondary cultures revealed that the anergic Th1 cells (treated with Ag and n-butyrate in primary cultures, even in the presence of IL-2) contained higher cumulative levels of p21Cip1 and p27Kip1 than the non-anergic Th1 cells (exposed in primary cultures to IL-2 and n-butyrate). This observed cumulative increase in the levels of p21Cip1 and p27Kip1 following exposure of Th1 cells in primary cultures to n- butyrate and Ag, but not IL-2, occurs at a time (24 hr after Ag restimulation) when p21Cip1 and p27Kip1 are normally present at low or undetectable levels. The unusually elevated levels of these CDKIs in the anergic Th1 cells at this critical time-point would severely inhibit the ability of the anergic Th1 cells to enter the S phase. The increased levels of p27Kip1 observed in the Ag-stimulated anergic Th1 cells compared with non-anergic Th1 cells may represent a failure to down-regulate p27Kip1. Such a failure may be explained by the fact that p27Kip1 ubiquitination and degradation requires activation of cyclin E/cdk2 complexes by IL-2,13 a cytokine that is suppressed in the Ag-restimulated anergic Th1 cells.3 One potential mechanism for the increased levels of p21Cip1 seen in the Ag-restimulated anergic Th1 cells, compared to the non-anergic Th1 cells, may be activation of PKC. PKC activation is primarily associated with TCR cross-linking rather than IL-2 signalling, and appears to increase the levels of p21Cip1 by actually stabilizing its mRNA.14 Thus, activation of PKC by TCR ligation in the anergic Th1 cells may contribute to the maintenance of the increased levels of p21Cip1 in the anergic Th1 cells. Furthermore, activation of PKC has been shown to augment n-butyrate-induced effects in other cell types.15 Experiments are currently underway to investigate this possibility. Taken together, these findings suggest that the differential susceptibility to tolerance induction between anergic Th1 cells (treated with Ag and n-butyrate in the primary cultures, with or without IL-2) and non-anergic Th1 cells (treated with IL-2 and n-butyrate in primary cultures) may be a result of the fact that Ag, unlike IL-2, leads to sustained high levels of the CDKIs p27Kip1 and p21Cip1 at a time-point (i.e. 24 hr following Ag restimulation) that would otherwise represent the main stage of S-phase entry in these Th1 cells. These results further show that the addition of exogenous IL-2 to primary cultures containing Th1 cells treated with Ag and n-butyrate, although possibly capable of down-regulating p27Kip1 to some degree by the aforementioned mechanism, is not sufficient to overcome the cumulative increase in the CDKIs induced by Ag and n-butyrate.
Investigating the mechanisms by which the increased levels of p27Kip1 and p21Cip1 in the anergic Th1 cells inhibited proliferation in the Ag-stimulated secondary cultures revealed a decreased association of cdk4 and cdk6 with the positive regulator, cyclin D2, in the anergic Th1 cells. An anergy-induced increase in the levels of p27Kip1 associated with cdk4, as well as a decrease in the association of cyclin D2 with cdks, could account for the low level of pRb phosphorylation observed in the anergic Th1 cells. The decrease in pRb phosphorylation would, in turn, inhibit S-phase entry in the secondary cultures. Surprisingly, the increased levels of p21Cip1 in the anergic Th1 cells following Ag restimulation may not play a role in suppressing pRb phosphorylation, as significantly less p21Cip1 was found in association with the G1 cdks in the secondary cultures. These data suggest that if p21Cip1 plays a role in suppressing proliferation in the Ag-stimulated anergic Th1 cells, it does so by a mechanism that does not involve inhibition of G1 cyclin/cdk complexes. Although more p21Cip1 appears to be associated with cdk4 immunoprecipitated from the Ag-restimulated anergic Th1 cell lysates (Fig. 7b) in comparison with the reciprocal blot shown in Fig. 7(a), this difference may be explained by the fact that the levels of p21Cip1, but not cdk4, are increased in these cells. The cdk4 present in these anergic Th1 cells may bind to some of the p21Cip1, such that immunoprecipitation of cdk4 reveals an association with p21Cip1. However, the elevated levels of p21Cip1 in the anergic Th1 cells means that the relative amount of p21Cip1 associated with cdk4 is decreased, suggesting that p21Cip1 in these cells may be associating with other target proteins. Moreover, the reduction in p21Cip1 association with cdk6 immunoprecipitated from the Ag-restimulated anergic Th1 cells, especially those treated with Ag and n-butyrate in the primary cultures (shown in Fig. 7), compared with the already limited association of p21Cip1 with the other G1 cdks, further supports the idea that the increased levels of p21Cip1 found in the Ag-restimulated anergic Th1 cells may be associating with other target proteins. In addition to its inhibitory effects on cdks, p21Cip1 can inhibit the activity of other proteins important for cell proliferation, including mitogen-activated protein kinases and proliferating cell nuclear antigen.16–18 The possibility that p21Cip1 helps to block proliferation in anergic Th1 cells by suppressing one of these other regulatory proteins, is presently being investigated.
Taken together, the results of this study suggest that differences in the expression and alterations in the association patterns of CDKIs, p21Cip1 and p27Kip1 may be responsible for the different functional outcomes associated with exposure of Th1 cells to IL-2 and n-butyrate, or to Ag and n-butyrate, with or without IL-2 (activation or anergy, respectively). The correlation between anergy and increased levels of both p21Cip1 and p27Kip1 has been previously demonstrated by ourselves and others.4,19 Increased levels of p27Kip1 present in the anergic Th1 cells shown here appear to associate with G1 cdks, thereby inhibiting pRb phosphorylation and cell cycle progression, whereas the mechanism by which increased levels of p21Cip1 suppress proliferation in the Ag-restimulated anergic Th1 cells has yet to be elucidated.
Acknowledgments
We thank Dr Tim Chambers, UAMS, for helpful discussions and technical advice. This work was supported by grants from the National Science Foundation (MCB-9817191), and the Arthritis Foundation.
References
- 1.Quill H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987;138:3704. [PubMed] [Google Scholar]
- 2.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–19. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert KM, Weigle WO. Th1 cell anergy and blockade in G1a phase of the cell cycle. J Immunol. 1993;151:1245–54. [PubMed] [Google Scholar]
- 4.Jackson SK, DeLoose A, Gilbert KM. Induction of anergy in Th1 cells associated with increased levels of cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1. J Immunol. 2001;166:952–8. doi: 10.4049/jimmunol.166.2.952. [DOI] [PubMed] [Google Scholar]
- 5.Aprelikova O, Xiong Y, Liu ET. Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J Biol Chem. 1995;270:18195–7. doi: 10.1074/jbc.270.31.18195. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins MK, Pardoll DM, Mizuguchi J, Chused TM, Schwartz RH. Molecular events in the induction of a nonresponsive state in interleukin 2-producing helper T-lymphocyte clones. Proc Natl Acad Sci USA. 1987;84:5409–13. doi: 10.1073/pnas.84.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madrenas J, Schwartz RH, Germain RN. Interleukin 2 production, not the pattern of early T-cell antigen receptor-dependent tyrosine phosphorylation, controls anergy induction by both agonists and partial agonists. Proc Natl Acad Sci USA. 1996;93:9763–41. doi: 10.1073/pnas.93.18.9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570–3. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 9.Kivinen L, Tsubari M, Haapajarvi T, Datto MB, Wang XF, Laiho M. Ras induces p21Cip1/Waf1 cyclin kinase inhibitor transcriptionally through Sp1-binding sites. Oncogene. 1999;18:6251–61. doi: 10.1038/sj.onc.1203000. [DOI] [PubMed] [Google Scholar]
- 10.Chen D, Heath V, O'Garra A, Johnston J, McMahon M. Sustained activation of the raf-MEK-ERK pathway elicits cytokine unresponsiveness in T cells. J Immunol. 1999;163:5796–805. [PubMed] [Google Scholar]
- 11.Xiao H, Hasegawa T, Miyaishi O, Ohkusu K, Isobe K. Sodium butyrate induces NIH3T3 cells to senescence-like state and enhances promoter activity of p21WAF/CIP1 in p53-independent manner. Bichem Biophys Res Commun. 1997;237:457–60. doi: 10.1006/bbrc.1997.7158. [DOI] [PubMed] [Google Scholar]
- 12.Archer SY, Meng S, Shei A, Hodin RA. p21 (Waf1) is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc Natl Acad Sci USA. 1998;95:6791–6. doi: 10.1073/pnas.95.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–78. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 14.Akashi M, Osawa Y, Koeffler HP, Hachiya M. p21WAF1 expression by an activator of protein kinase C is regulated mainly at the post-transcriptional level in cells lacking p53: important role of RNA stabilization. Biochem J. 1999;337:607–16. [PMC free article] [PubMed] [Google Scholar]
- 15.Rickard KL, Gibson PR, Young GP, Phillips WA. Activation of protein kinase C augments butyrate-induced differentiation and turnover in human colonic epithelial cells in vitro. Carcinogenesis. 1999;20:977–84. doi: 10.1093/carcin/20.6.977. 10.1093/carcin/20.6.977. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Jackson PK, Kirschner MW, Dutta A. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature. 1995;374:386–8. doi: 10.1038/374386a0. [DOI] [PubMed] [Google Scholar]
- 17.Shim J, Lee H, Park J, Kim H, Choi EJ. A non-enzymatic p21 protein inhibitor of stress-activated protein kinases. Nature. 1996;381:804–6. doi: 10.1038/381804a0. [DOI] [PubMed] [Google Scholar]
- 18.Patel R, Bartosch B, Blank JL. p21WAF1 is dynamically associated with JNK in human T-lymphocytes during cell cycle progression. J Cell Sci. 1998;111:2247–55. doi: 10.1242/jcs.111.15.2247. [DOI] [PubMed] [Google Scholar]
- 19.Boussiotis VA, Freeman GJ, Taylor PA, Berezovskaya A, Grass I, Blazar BR, Nadler LM. p27Kip1 functions as an anergy factor inhibiting interleukin 2 transcription and clonal expansion of alloreactive human and mouse helper T lymphocytes. Nat Med. 2000;6:290–6. doi: 10.1038/73144. 10.1038/73144. [DOI] [PubMed] [Google Scholar]