Abstract
Basophils are key effector cells of allergic reactions. Although proinflammatory cytokines, such as interleukin (IL)-3, granulocyte–macrophage colony-stimulating factor (GM–CSF) and IL-5, inhibit eosinophil apoptosis in vitro, little is known about basophil apoptosis, and the signalling mechanisms required for basophil survival remain undefined. To address this issue, we used a novel negative-selection system to isolate human basophils to a purity of >95%, and evaluated apoptosis by morphology using light and transmission electron microscopy, and by annexin-V binding and propidium iodide incorporation using flow cytometry. In this study, we demonstrated that the spontaneous rate of apoptotic basophils was higher than that of eosinophils as, at 24 hr, 57·6 ± 4·7% of basophils underwent apoptosis compared with 39·5 ± 3·8% of eosinophils. In addition, basophil cell death was significantly inhibited when cultured with IL-3 for 48 hr (84·6 ± 4·9% vehicle-treated cells versus 40·9 ± 3·9% IL-3-treated cells). IL-3 also up-regulated basophil CD69 surface expression. The effects of IL-3 on apoptosis and CD69 surface expression of human basophils were completely blocked by LY294002 (LY), a potent inhibitor of phosphatidylinositol 3-kinase (PI3-K), but only partially inhibited by lactacystin, a proteasome inhibitor that prevents degradation of IκB and NF-κB translocation. These observations reveal the novel finding that IL-3 prevents basophil apoptosis through the activation of PI3-K, which is only partially NF-κB dependent. As basophils are active participants in allergic reactions and IL-3 is one of the abundant proinflammatory cytokines in secretions from allergic tissue, we suggest that IL-3-mediated inhibition of basophil apoptosis may exacerbate the inflammation associated with allergic disorders.
Introduction
Since the first description by Ehrlich more than 100 years ago, basophils have been recognized to be unique white blood cells that stain and function differently from other blood cells.1,2 Recently, basophils have become of increasing interest as a result of the evidence provided by a number of studies to support the belief that human basophils play a unique role in allergic inflammation, particularly that associated with chronic disease.2–6 Basophils, like mast cells, contain high-affinity receptors for immunoglobulin E (IgE) (FcεRI) on their cell surface and secrete a host of inflammatory mediators of relevance to allergic diseases, such as asthma.1,3,4,7 These inflammatory mediators include preformed granular mediators, such as histamine, as well as rapidly synthesized lipid mediators, such as sulphidopepide leukotrienes and platelet-activating factor.1,3,4 Basophils contribute to allergic disease by infiltrating tissues and secreting granular products as well as proinflammatory cytokines, such as interleukin (IL)-4, IL-13 and macrophage inflammatory protein-1α (MIP-1α).8–10 Perhaps not surprisingly, owing to the difficulty in purifying adequate numbers of basophils for study, no published data exist on basophil apoptosis.
Apoptosis is a form of cell death in which the cell participates in its own demise. IL-3, which is derived from T cells and other sources, has been shown to inhibit apoptosis in many cell types, including eosinophils,11,12 mast cells13 and other haematopoietic cells.14,15 IL-3-dependent cell survival of haematopoietic cells is known to rely on the activation of multiple signalling pathways, including a pathway leading to activation of phosphatidylinositol 3-kinase (PI3-K).15–17 The protein kinase B (PKB)/Akt is a direct target of PI3-K.18 It has also been demonstrated that the PI3-kinase-PKB (Akt) pathway influences NF-κB DNA-binding activity,19 and IL-3 can initiate a signalling cascade that leads to the activation of the transcriptional factor NF-κB in haematopoietic cells.20 Other studies related to NF-κB have suggested that CD69 is produced by an NF-κB-dependent gene,21,22 and IL-3 can induce CD69 expression in T cells and eosinophils.23–25 Whether IL-3 exhibits the same anti-apoptotic effects on human basophils as it does in other cells is unexplored.
It has been reported that normal human basophils possess functional receptors for IL-3,26,27 and IL-3 exhibits diverse effects on human basophils, including development,28 differentiation,29 migration,30 histamine release31 and augmentation of cytokine generation,5 but there is a paucity of data regarding its effect on basophil apoptosis. In 1992, Yamaguchi and colleagues demonstrated that IL-3 and granulocyte–macrophage colony-stimulating factor (GM–CSF) maintained the numbers of viable basophils in vitro, as determined by Trypan blue exclusion.32 In 1998, MacGlashan and colleagues showed that IL-3 was not able to prevent basophils from entering apoptosis over a 3-week period.4 As the basophils they investigated were only 33% pure, there may have been confounding effects owing to the actions of IL-3 on other cell types. Having developed a method to purify human basophils and eosinophils to greater than 95%,33,34 we are in a unique position to answer many of the questions raised from earlier studies.
We hypothesize that IL-3 inhibits spontaneous apoptosis of human basophils via the PI3-K pathway, which is NF-κB dependent, and this is associated with IL-3-induced up-regulation of the early cell-activation marker CD69 on human basophils. To test this hypothesis, we examined the in vitro spontaneous apoptosis of human mature basophils versus eosinophils and the effects of IL-3 on basophil apoptosis and CD69 expression. Additional experiments used specific inhibitors of PI3-K activation and NF-κB translocation to evaluate their roles in IL-3 inhibition of apoptosis in human basophils.
Materials and methods
Reagents
All reagents were of the highest grade available and purchased from Sigma Chemical Co. (St. Louis, MO), unless specified otherwise. Recombinant human (rh)IL-3, rhIL-5 and rhGM–CSF were obtained from Pharmingen (San Diego, CA). Anti-CD16 magnetic beads were purchased from Miltenyi Biotech GmBH (Auburn, CA). Basophil isolation kits, including Magnetic Colloid, biotinylated anti-human CD15 and antibody cocktail, were from Stem Cell Technologies Inc. (Vancouver, Canada). The antibody cocktail includes antibiotin and bispecific tetrameteric antibody complexes directed against dextran (on the iron-dextran magnetic particles, and cell-surface markers including glycophorin A, CD3, CD24, CD14, CD15 biotin, CD34, CD45RA, CD2, CD56, CD19, CD16 and CD36. Specific mouse anti-human CD69 (MCA 736) and isotype-matched control antibody [mouse immunoglobulin G2b (IgG2b) negative control, MCA 691] were obtained from Serotec (Toronto, Ontario, Canada). Annexin-V–fluorescein isothiocyanate (FITC) was obtained from R & D Systems Inc. (Minneapolis, MN).
Human subjects
Basophils and eosinophils were isolated from the peripheral blood of volunteers after they gave informed consent. As sufficient basophil numbers could not be routinely obtained from non-atopics, 12 atopic volunteers were recruited for the study. Atopy was determined by positive skin-prick test responses to one or more of five common aeroallergens (cat, dog, moulds, trees, grasses, and dust mite), a history of seasonal or perennial allergic rhinitis or asthma, but no other significant medical illness.34
Basophil and eosinophil purification
Basophil purification
Basophils were isolated by negative immune-magnetic selection from the peripheral blood of human atopic subjects. Briefly, 50–75 ml of ACD anticoagulated peripheral blood was centrifuged for 20 min at 190 g to remove the platelet-rich plasma. After dilution with Hank's buffer (0·9% normal saline, 10 m HEPES), the blood was overlaid onto Ficoll–Hypaque 1077 and centrifuged at 500 g for 30 min at 4°. After centrifugation, the peripheral blood mononuclear cell (PBMC) fraction (containing basophils, lymphocytes and monocytes) was washed twice with lysis buffer (155 mm NH4Cl, 10 mm NaHCO3 and 0·1 mm EDTA) to lyse contaminating erythrocytes. This mononuclear cell fraction containing 1–3% basophils was incubated, in a tube rotator at 4 °C for 15 min, with 225 µl of phosphate-buffered saline (PBS) containing 0·5% (wt/vol) bovine serum albumin (BSA), 5 mm EDTA and 100 µl of StemSep™ antibody cocktail containing antibodies against the following: CD2, CD3, CD14, CD16, CD19, CD24, CD34, CD36, CD45RA, CD56 and glycophorin A, and 15 µl of anti-CD15. Following further incubation for 15 min at 4° with 60 µl of StemSep™ magnetic colloid in a total suspension volume of 1 ml, the unlabelled cells were passed through the magnetic column, while the labelled cells were retained on the magnetic column. The purity of isolated basophils, as determined by using Wright/Giemsa staining, was > 95% (see Fig. 1B, panel a).33
Figure 1.
Interleukin (IL)-3, but not granulocyte–macrophage colony-stimulating factor (GM–CSF) and IL-5, inhibits apoptosis in human mature basophils and eosinophils. (A). Differential sensitivity to apoptosis of basophils versus eosinophils in the presence of IL-3, or GM–CSF + IL-5. Freshly isolated basophils and eosinophils were cultured with or without IL-3 or GM–CSF + IL-5 for 48 hr or 72 hr. Apoptotic cell death was measured by annexin-V/propidium iodide (prop. iod.) and flow cytometry. Data are representative of five separate experiments and reported as the mean ± standard error (SE) of percentage of cells. (B) Morphological evidence of the effect of IL-3 on basophil apoptosis. The experimental protocol was the same as in (A), except that apoptotic cell death was identified by morphology, as described in the Materials and methods. (a) Freshly purified basophils; (b) purified basophils cultured in the presence of 300 pm of IL-3 for 48 hr; (c) purified basophils cultured in the absence of IL-3 for 48 hr. Arrow head: apoptotic basophils; arrow, necrotic basophils.
Eosinophil purification
Human peripheral blood eosinophils were isolated by negative selection using immunomagnetic beads, as previously described.34 Briefly, after the blood was overlaid onto Ficoll–Hypaque 1007 and centrifugation, the cell pellets containing neutrophils and eosinophils were subjected to erythrocyte lysis. After centrifugation, the resulting pellet of granulocytes was then incubated with anti-CD16 magnetic beads at 4° for 30 min. Neutrophils with the magnetic beads bound to their surface were separated from eosinophils on a magnetic column. This procedure produced eosinophil purity of > 97%, with the contaminating cells being lymphocytes.34
Cell culture
Purified human basophils and eosinophils were resuspended at a concentration of 1 × 105 cells/ml in RPMI-1640 supplemented with 10% fetal calf serum (FCS), 2 mm l-glutamine, 10 mm HEPES, 100 IU/ml penicillin and 100 µg/ml streptomycin. Aliquots (1 ml, 1 × 105 cells) were added to the wells of 24-well, flat-bottomed tissue-culture plates. Following 24, 48 or 72 hr of incubation with and without cytokines or inhibitors, or combinations of cytokine and inhibitors, at 37° in a humidified atmosphere of 5% CO2, cells were harvested by gentle pipetting for apoptosis analysis.
Apoptosis assay
Annexin-V/propidium iodide binding
Cells undergoing the early stage of apoptosis were quantified by staining with annexin-V–FITC, according to the manufacturer's protocol (R & D Systems, Minneapolis, MN) and measured by flow cytometry (EPIC XL-mcL; Beckman Coulter, Miami, FL). Late apoptotic cells were also distinguished by their ability to take up propidium iodide. Live cells were those staining negatively for annexin-V–FITC and propidium iodide (% of cell survival). Cells staining with annexin-V alone, and with both annexin-V and propidium iodide, were combined, giving the total number of cells at both early and late stages of apoptosis (% cell death) (Fig. 2).
Figure 2.
Time-course evaluation of spontaneous apoptosis of human basophils versus eosinophils. (A) Representative histograms are shown of basophils and eosinophils stained with annexin V and propidium iodide (prop. iod.). After 24 and 48 hr of culture, three populations of cells were observed: viable cells (negatively stained, lower left quadrants); early apoptotic cells (annexin-V positive and propidium iodide negative, lower right quadrant) and cells in the late stages of apoptosis (annexin-V and propidium iodide positive, upper right quadrants). At 48 hr, a greater number of basophils and eosinophils underwent the early and late stages of apoptosis than at 24 hr. (B) Kinetic evaluation of cells undergoing the early and late stage of apoptosis in basophils versus eosinophils. (a) Cells staining with annexin V only, showing the early stage of apoptosis (% early apoptosis); (b) cells staining with annexin V alone and with both annexin V and propidium iodide were combined, giving the total number of cells at both the early and late stages of apoptosis (% cell death). Data are reported as the mean ± standard error (SE) of percentage of cells. n= 5, *P < 0·05 when compared with eosinophils treated in an identical manner.
Morphology
As an ancillary method, apoptosis was also assessed morphologically, as described previously.35 Briefly, aliquots of basophils and eosinophils were cytospun and air-dried. Slides were fixed in methanol and stained with Wright's stain. Apoptosis was assessed based on the following features: loss of normal chromatin pattern with homogenous staining of nuclei; rounding of nuclear lobes or loss of nuclear lobation; and reduction of cytoplasm. Three-hundred cells on each slide were assessed in a blinded manner and classified as normal, apoptotic, or other. The other classification included cells with loss of cellular integrity and those with enlarged, smudged nuclei and poor preservation of cell membrane. For all studies, these cells were < 5% (Fig. 1B).
Electron microscopy
To further evaluate the effect of IL-3 on human basophil apoptosis, transmission electron microscopy was performed as reported previously.36,37 Briefly, 1·5 × 106 freshly purified human basophils were divided into three groups (0·5 × 106 cells in each group). Group 1 contained fresh basophils; the other two groups of cells were incubated with and without IL-3 for 48 hr at 37° in a humidified atmosphere of 5% CO2. Cells were then harvested by gentle pipetting for electron microscopy. Fresh cells and cultured cells were fixed for 2 hr in 2·5% gluteraldehyde (in 0·1 m cacodylate buffer, pH 7·4) and subsequently washed with PBS (by centrifugation at 600 g for 10 min). Cell pellets were mixed with 2% agarose and then cut into 1-mm cubes. Next, the cubes were fixed in 1% osmium tetroxide for 1 hr, the samples washed, dehydrated through a graded series of isopropyl alcohol and then embedded in Epon 812 epoxyl resin, as previously described.36 Sections were stained with uranyl acetate and lead citrate before observation using a Philips 400 transmission electron microscope (Philips, Mahwah, NJ).
Assessment of CD69 surface expression
Purified cells were resuspended at a concentration of 1 × 105 cells/ml in RPMI-1640 supplemented with 10% FCS, 2 mm l-glutamine, 10 mm HEPES, 100 IU/ml penicillin and 100 µg/ml streptomycin. Aliquots (1 ml, 1 × 105 cells) were added to the wells of 24-well, flat-bottomed tissue-culture plates. Following 18 hr of incubation with and without cytokines or inhibitors, or combinations of cytokine and inhibitors, at 37° in a humidified atmosphere of 5% CO2, cells were harvested by gentle pipetting. The cell suspensions were centrifuged and the cells resuspended in cold RPMI-1640 and placed on ice for immunofluorescence staining and flow cytometry. Expression of CD69 on eosinophils was measured using published methodology.34,38 Flow cytometry was performed on a fluorescence-activated cell sorter (FACS) analyser (EPICS XL-MCL; Beckman Coulter, Miami, FL) equipped with a 488-nm argon-ion laser. Fluorescence intensity was determined on at least 5000 cells from each sample.
All experiments were repeated two to five times, with similar results obtained on each occasion.
Statistical analysis
The results are expressed as the mean ± standard error (SE) of the number of independent experiments, using cells from different donors for each protocol. All the data were analysed using the paired or unpaired t-test, and P-values of <0·05 were considered significant.
Results
In vitro spontaneous apoptosis of human mature basophils versus eosinophils
When cultured in RPMI-1640 containing 10% FCS, basophils underwent apoptosis spontaneously, with 57·6 ± 4·7% of cell death occurring at 24 hr and 84·6 ± 4·9% of cell death occurring at 48 hr, as assessed by annexin-V/propidium iodide through flow cytometry (Fig. 2A, 2B, panel b). Similar results were observed for early basophil apoptosis, assessed by annexin-V binding only (Fig. 2A, 2B, panel a). Cells undergoing apoptosis quickly showed an increase in annexin-V binding, but excluded propidium iodide (early apoptosis). At later time-points, the percentage of propidium iodide-staining cells gradually increased (late apoptosis). Therefore, we report here both the percentage of early apoptosis (which indicates annexin-V-positive cells only) and the percentage cell death (which indicates the total number of annexin-V–FITC- plus propidium iodide-staining cells and is representative of populations containing cells at both early and late stages of apoptosis). The rates of spontaneous eosinophil cell death, as shown in Fig. 2(A) and 2(B) (panel b), with 39·5% ± 3·8 of cell death occurring at 24 hr and 66·9% ± 3·4 occurring at 48 hr, are similar to previous reports.11,12 The spontaneous rates of basophil apoptosis at 24 and 48 hr were higher than those of eosinophil apoptosis (n = 5; P < 0·05). Both early and late apoptosis gave the same results (Fig. 2B, panels a and b).
IL-3, but not GM–CSF + IL-5, inhibits apoptosis in human mature basophils
It has been known for many years that GM–CSF, IL-5 and IL-3 can inhibit apoptosis in many cell types, including eosinophils,11,12 mast cells13 and other haematopoietic cells.14,15 To determine whether IL-3 and GM–CSF + IL-5 exhibit the same anti-apoptotic effect in basophils as they do in eosinophils, freshly isolated human basophils and eosinophils were incubated with IL-3 (300 pm) or GM–CSF (10 pm) + IL-5 (100 pm) for 24, 48 and 72 hr. IL-3 (100 pm, 300 pm and 1000 pm) inhibited basophil apoptosis in a dose-dependent manner with the optimal concentration being 300 pm (data not shown). Apoptotic cells were detected by annexin-V/propidium iodide using flow cytometry (Fig. 1A), and morphology (Fig. 1B). At the 24-hr time-point, 300 pm IL-3 failed to alter the percentage of basophil cell death, but significantly inhibited eosinophil cell death (data not shown). After 48 and 72 hr of culture, however, 300 pm IL-3 significantly inhibited both basophil and eosinophil cell death (n = 5, P < 0·05). Furthermore, 10 pm GM–CSF and 100 pm IL-5 failed to alter the percentage of basophil cell death at 24, 48 and 72 hr time-points, when compared with appropriate time controls (n = 5, P > 0·05). However, at the same concentration and same time-points, GM–CSF and IL-5 significantly inhibited eosinophil cell death (n = 5, P < 0·05), Fig. 1(A). These results indicate that IL-3, but not GM–CSF + IL-5, inhibits human basophil apoptosis when cultured in vitro for 48 and 72 hr.
Ultrastructural observations indicated that culturing basophils with 300 pm IL-3 for 48 hr attenuated the apoptosis and necrosis observed in cells without IL-3 (Fig. 3a–3f). Freshly isolated basophils remained spherical with intact plasma membranes, nuclei and other organelles (Fig. 3a, 3b). Incubation of basophils for 48 hr led to the disruption of plasma membranes, nuclei and loss of heterochromatin and recognizable organelles (Fig. 3e). There was also evidence of cell fragmentation (Fig. 3f). In contrast, the addition of IL-3 to basophils for 48 hr preserved cellular integrity, although they did become irregularly shaped (Fig. 3c, 3d).
Figure 3.
Ultrastructural effects of interleukin-3 (IL-3) on basophil apoptosis. (a) and (b) Freshly isolated human basophils were essentially spherical with numerous small microvilli. Variable nuclear profiles showed normal amounts of peripheral heterochromatin and nucleoplasm. Variable amounts of granules and vesicles were visible. (c) and (d) Basophils incubated with IL-3 (300 pm) for 48 hr were irregularly shaped and microvilli were apparently absent. The cytoplasm and nuclei appeared to be similar to those of freshly isolated basophils. Basophils in culture for 48 hr were either necrotic with disrupted plasma membranes and nuclei without condensed heterchromatin (e), or fragmented (f). Scale = 1 µm.
IL-3 requires de novo gene expression for its cytoprotective effect
To determine whether the protective effect of IL-3 is post-translational or requires new gene expression, basophils were cultured in the presence of IL-3 and cycloheximide (an inhibitor of protein synthesis) or actinomycin D (an inhibitor of transcription). Figure 4 shows that basophil cell death was not inhibited by IL-3 in the presence of 2 µg/ml actinomycin D or 10 µg/ml cycloheximide. This observation indicated that the IL-3 anti-apoptotic effect on basophils requires de novo RNA and protein syntheses.
Figure 4.
Interleukin-3 (IL-3) does not inhibit basophil apoptosis in the presence of cycloheximide and actinomycin (Act-D). Basophils were cultured in medium alone (control), in medium containing 300 pm IL-3, or in medium containing 300 pm IL-3 and either 10 µg/ml cycloheximide (CHX) or 2 µg/ml Act-D, for 48 hr. Cell death was measured by annexin-V/propidium iodide (prop. iod.) incorporation using flow cytometry. Results represent the mean value ± standard error (SE). n= 3. *P < 0·05 when compared with the control.
IL-3 induces CD69 expression on human basophils and eosinophils
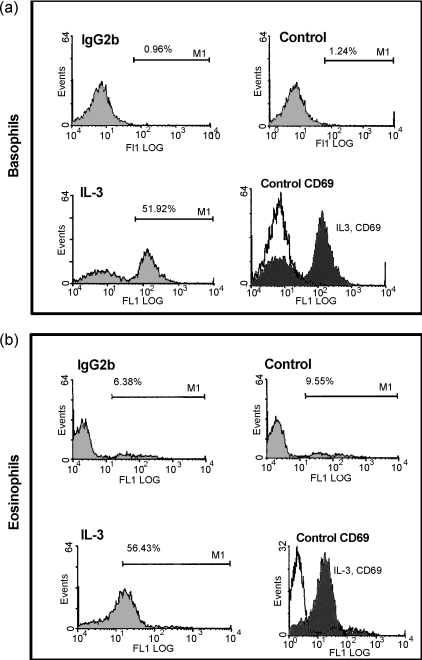
CD69 has been shown to be a marker of activation in eosinophils. It is not known whether basophils express CD69. To determine whether IL-3 can stimulate surface expression of CD69 in human basophils, freshly isolated human basophils or eosinophils were incubated with culture medium alone or with 300 pm IL-3 for 18 hr and then stained for CD69 expression. Figure 5 shows representative histograms of the flow cytometry analysis of basophils and eosinophils stained for CD69 after culture in medium alone (unstimulated group) or in the presence of IL-3 for 18 hr. This illustrates that after 18 hr of culture in medium alone, both basophils and eosinophils remained negative for CD69. In the presence of IL-3, however, most of the cell population expressed appreciable amounts of CD69.
Figure 5.
Interleukin-3 (IL-3) induces CD69 expression on human basophils and eosinophils. Flow cytometry analysis data are shown of CD69 expression in basophils and eosinophils after 18 hr of culture in medium alone or in medium containing 300 pm IL-3. The figure shows representative histograms of three independent experiments. The percentage of positive cells is indicated.
IL-3 activates basophils and promotes cell survival through PI3-K, but not MAPK
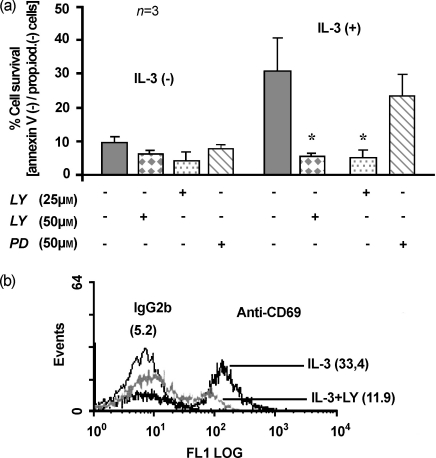
To determine if PI3-K or mitogen-activated protein kinase (MAPK)-signalling pathways are involved in IL-3-induced basophil survival and CD69 expression, cells were cultured with and without 300 pm IL-3 or inhibitors, LY294002 (LY; a potent inhibitor of PI3-K) and PD98059 [PD; a specific MAPK/ERK kinase (MEK) inhibitor], or combinations of IL-3 and inhibitors, for 18 or 48 hr. As shown in Fig. 6(a), 25 µm and 50 µm of LY completely abrogated the IL-3-induced basophil survival. However, PD, at a concentration of 50 µm, failed to block IL-3-induced inhibition of apoptosis in basophils. After 18 hr of culture, 50 µm LY inhibited IL-3-induced CD69 expression on basophils by 64·5% (Fig. 6b). This finding indicates that the PI3-K signalling pathway is involved in IL-3-induced basophil activation and survival.
Figure 6.
The phosphatidylinositol 3-kinase (PI3-K) inhibitor LY294002 (LY) blocks interleukin-3 (IL-3)-induced cell survival and CD69 expression in human basophils. (a) LY inhibits the IL-3-induced basophil survival. Freshly isolated human basophils were cultured in medium alone or with 300 pm IL-3, or in the presence or absence of LY294002 (25 µm or 50 µm), or 50 µm PD98059. At 48 hr, basophils were collected and stained with annexin-V and propidium iodide (prop. iod.), and analysed by flow cytometry. Annexin-V fluorescein isothiocyanate (FITC) and propidium iodide negativity indicates cell viability (% cell survival). *P < 0·05 when compared with IL-3 alone. (b) LY inhibits CD69 expression on basophils. The experimental condition was the same as in (a), except that the cells were cultured for 18 hr and then stained with anti-CD69 or isotype-control antibody (IgG2b). A representative histogram is shown of human basophils stained with anti-CD69 or control antibody after 18 hr of culture with IL-3 alone and IL-3 plus 50 µm LY. Values in parenthesis indicate the mean fluorescence intensity. The figure is representative of three independent experiments.
IL-3-induced basophil survival and activation is partially the result of NF-κB activation
PI3-K has been shown to signal a cytoprotective effect through transcription-dependent and -independent mechanisms.19 Recently, PI3-K has been shown to activate NF-κB,20 and induction of CD69 has been shown to be dependent, in part, on activation of NF-κB.21 To determine if NF-κB is involved in IL-3-induced basophil survival and CD69 expression, cells were cultured with and without 300 pm IL-3 or 10 µm lactacystin (a proteasome inhibitor that prevents degradation of IκB and NF-κB translocation) or combinations of IL-3 and lactacystin. CD69 was used as a reporter marker for NF-κB pathway activation. Figure 7(a) shows that the induction of basophil survival by IL-3 was partially inhibited by lactacystin. At 5 and 10 µm, lactacystin caused modest inhibition of IL-3-induced basophil survival. Lactacystin, at 20 µm, resulted in a significant degree of inhibition of IL-3-induced basophil survival (n = 3, P < 0·05). Overall, 20 µm lactacystin inhibited IL-3-induced basophil survival by 41·6%. However, complete inhibition was not observed, suggesting that besides NF-KB, there may be alternate pathways involved in IL-3-induced basophil survival. Lactacystin also inhibited the IL-3-induced basophil surface expression of CD69. Lactacystin, at 10 µm, inhibited IL-3-induced CD69 expression by 21·5% (Fig. 7b). These findings suggest that the IL-3 protective effect is mediated through PI3-K by NF-κB-dependent and -independent pathways.
Figure 7.
Lactacystin (LC) inhibits interleukin-3 (IL-3)-induced cell survival and CD69 surface expression in human basophils. (a) LC inhibits IL-3-induced cell survival. Freshly isolated human basophils were cultured in medium alone, or in medium containing IL-3 or IL-3 + LC, for 48 hr. Basophils were then collected and stained with annexin-V and propidium iodide, and analysed by using flow cytometry. Annexin-V fluorescein isothiocyanate (FITC) and propidium iodide (prop. iod.) negativity indicates cell viability (% cell survival). These data presented here are representative of three experiments. (b) LC inhibits IL-3-induced cell-surface expression of CD69. A representative histogram is shown of human basophils stained with anti-CD69 or control antibody after 18 hr of culture with IL-3 alone or IL-3 + 10 µm LC. The experimental conditions were the same as in (a), except that the cells were cultured for 18 hr and then stained with anti-CD69 or isotype-control antibody (IgG2b). Values in parenthesis indicate the mean fluorescence intensity. The figure is representative of three independent experiments.
Discussion
This study revealed several novel observations:
the spontaneous rate of apoptotic basophils is higher than that of eosinophils;
basophil cell death is significantly inhibited upon culture with IL-3 for 48 and 72 hr;
IL-3 induces basophil CD69 surface expression;
the effects of IL-3 on apoptosis and CD69 surface expression in human basophils are blocked by LY, a potent inhibitor of PI3-K, but only partially inhibited by lactacystin, a proteasome inhibitor which prevents degradation of Iκ-B and subsquent NF-κB translocation.
These findings, coupled with those of other previous reports,3,7,17,28,31 suggest a potentially important link between basophils and IL-3. Thus, control of basophil number through the inhibition of basophil apoptosis by IL-3 may contribute to allergic inflammation in diseases such as asthma.
Like eosinophils, basophils are terminally differentiated cells, which die rapidly in culture. It is well known that normal mature eosinophils separated from peripheral blood rapidly undergo spontaneous apoptosis, and do not survive for longer than 4 days in vitro without the addition of cytokines.11,12,39 Mature human neutrophils undergo spontaneous apoptosis very rapidly, resulting in the death of >70% of the population within 24 hr.35 Until now, no data have been published regarding spontaneous apoptosis in human basophils. Our data show that the spontaneous rate of basophil apoptosis is higher than that of eosinophils, but less than that of neutrophils. This perhaps is not surprising as these cells are known to be functionally different in the tissue. This may explain the histological findings of marked eosinophilia in asthmatic airways without significant basophils,40,41 as this could be a result of the rapid apoptosis of basophils.
Our finding, that IL-3 inhibits human basophil apoptosis, adds to the other known effects of IL-3 on human basophils.28,29,31 IL-3 can prevent apoptosis in many cell types.12,13,15 The reason why it takes 48 hr for IL-3 to prevent basophil apoptosis may be a result of the time it takes for IL-3 to induce anti-apoptotic gene expression and protein synthesis. In this study, new RNA and protein synthesis was required for the protection of basophil apoptosis by IL-3, because this process was completely blocked by cycloheximide, a protein synthesis inhibitor, and actinomycin D, an inhibitor of transcription. Meanwhile, our study also showed that 10 pm GM–CSF + 100 pm IL-5 significantly inhibited eosinophil cell death at 24, 48 and 72 hr, but, at the same concentration and same time-points, failed to alter the percentage of basophil cell death. It is known that IL-3, IL-5 and GM–CSF receptors share a common β-chain, but different target cells have different sensitivity to the three cytokines because they have different α-chain expression.42 Recent studies have demonstrated that basophils express a very prominent and much higher level of IL-3 receptor α-chain as compared to the α-chains of the receptors for IL-5 and GM–CSF,27,42 and basophils have a higher IL-3 surface receptor expression than eosinophils.26 Therefore, it is not surprising that there was a difference in the survival of basophils versus eosinophils in the presence of IL-3 and GM–CSF + IL-5. These observations indicate that normal human basophils possess a functional receptor for IL-3 and that IL-3 may modulate immediate- and delayed-type hypersensitivity by prolonging the life span of basophils.
The human activation inducer molecule (AIM/CD69) is a phosphorylated disulphide-linked 27 000/33 000 MW transmembrane homodimeric glycoprotein.21 It was first detected on the surface of activated T lymphocytes, but has subsequently been described on most haemopoietic cells23,43 and has been detected on eosinophils obtained by bronchoalveolar lavage from patients with mild asthma and pneumonia.38,43 Expression of the surface antigen can be induced following exposure to IL-5, GM–CSF, IL-3 and IL-13 in vitro, and these cytokines also prolong cell survival.23,43,44 These findings have led to the suggestion that CD69 may be a sensitive indicator of eosinophil activation and survival in response to cytokines.38,44 Although a physiological ligand for CD69 has not yet been identified, experiments with specific monoclonal antibodies have indicated that this antigen might act either as a signal-transmitting receptor in T cells23,44 or as an apoptosis-inducing receptor in monocytes45 and eosinophils.46 Inflammatory cytokines such as IL-3, IL-5, GM–CSF, IL-13, IL-4 and TNF-α not only activate eosinophils, as indicated by CD69 expression, but also prolong their life span by inhibiting their apoptotic cell death.39,44 Therefore, our results have shown that IL-3 can also inhibit basophil apoptosis as well as induce CD69 expression on human basophils. As for many other cell-surface receptors, CD69 appears to play a dual role in cellular physiology, on the one hand initiating cell activation and on the other hand mediating apoptosis.46 The nature of the intracellular pathway involved in the relationship between CD69 expression and cell survival should be investigated further.
In the present study, the CD69 surface antigen was chosen as a marker for basophil activation and as a sensitive indicator for NF-κB pathway activation, because recent reports have shown that some cytokines (including IL-3) can induce eosinophil CD69 expression and survival,23 and CD69 gene transcription occurs through the NF-κB-dependent pathway.21,22 Sequence analysis of the 5′ flanking region of the CD69 gene revealed the presence of a potential TATA element, 30 bp upstream of the major transcription initiation site, and several putative binding sequences for inducible transcription factors (NFκB, Egr-1, AP-1), which might mediate the inducible expression of CD69 gene.21 IL-3 has been reported to ameliorate apoptosis in haematopoietic cells through an NF-κB-dependent mechanism,20 and activation of NF-κB protects against TNF-α-induced apoptosis.47,48 In this study, the most selective proteasome inhibitor, lactacystin, which has been shown to inhibit IκB degradation and NF-κB translocation49 was found to partially inhibit IL-3-induced basophil survival and CD69 surface expression. Thus, NF-κB does not appear to be the only mechanism of gene induction, as lactacystin only partially inhibited the effect of IL-3 on human basophil apoptosis and CD69 expression. Other pro-survival mechanisms remain to be elucidated.
PI3-K regulates fundamental cellular responses such as proliferation, apoptosis, cell motility and adhesion.50,51 In this study, we have shown that LY completely blocked IL-3-induced basophil survival and partially blocked CD69 expression on human basophils. However, 50 µm PD failed to block IL-3-induced basophil survival. Several previous reports are consistent with this study. It is known that the PI3-K signalling pathway is required for cell survival in several cell types, including myeloid progenitor cell lines.16,17 Furthermore, the requirement for PI3-K signalling in the IL-3-induced survival of many cell types has been well documented in recent years.16,17,52 Akt activity is induced rapidly by the cytokine IL-3, and the activation of Akt by IL-3 is dependent upon the activity of PI3-K.17,52 When haematopoietic cells were stimulated with IL-3, blocking the activation of PI3-K inhibited cell survival and also partially reduced the phosphorylation of Bad.17,52 Meanwhile, Scheid et al. also indicated that although PI3-K may contribute to the phosphorylation of Bad in some instances, there is at least one other PI3-K-independent pathway involved, possibly via activation of MEK.52 Shimamura et al. reported that a specific MEK inhibitor, PD, could induce apoptosis and antagonized the IL-3 survival signal in EoL-1 cells.53 However, in our study, 50 µm PD failed to block IL-3-induced basophil survival. This is not surprising as, at this concentration and this time-point, IL-3 may support the survival of human basophils through activation of PI3-K, rather than MEK. The studies of intracellular signalling pathways for the induction of CD69 expression by cytokines are limited. A previous study showed that interferon-γ (IFN-γ) induced survival and CD69 expression through the activation of JAK2 in human eosinophils.54 As shown in Fig. 5(b), 50 µm of LY partially blocks CD69 expression on human basophils. This suggests that PI3-K is, at least partially, involved in IL-3-induced CD69 expression in human basophils. The effect of PI3-K on the association between IL-3-induced survival and CD69 expression in human basophils may explain one of the functions of PI3-K on IL-3-induced inhibition of basophil apoptosis.
Figure 8 is a schema which presents our hypothesis that IL-3 activates PI3-K, initiating signalling cascades that lead to the generation of survival genes through both an NF-κB pathway as well as through an alternate mechanism.
Figure 8.
A schema presenting our hypothesis that interleukin-3 (IL-3) inactivates phosphatidylinositol 3-kinase (PI3-K), initiating signalling cascades that lead to the generation of survival genes through both an NF-κB pathway, as well as through an alternate mechanism. In summary, we demonstrated that the in vitro spontaneous rate of apoptosis of human basophils is higher than that of eosinophils, and that IL-3 inhibits basophil apoptosis as well as up-regulating basophil CD69 surface expression via a PI3-K-dependent mechanism(s) that entails new RNA and protein synthesis, partially via NF-κB signalling. As basophils are active participants in allergic reactions, and IL-3 is one of the most abundant proinflammatory cytokines in the secretions from allergic tissue, IL-3-mediated inhibition of basophil apoptosis may exacerbate the inflammation associated with allergic disorders.
In summary, we have demonstrated that the in vitro spontaneous rate of apoptosis of human basophils is higher than that of eosinophils, and that IL-3 inhibits basophil apoptosis as well as up-regulating basophil CD69 surface expression via a PI3-K dependent mechanism(s) that entails new RNA and protein synthesis, partially via NFκB signalling. As basophils are active participants in allergic reactions, and IL-3 is one of the most abundant proinflammatory cytokines in the secretions from allergic tissue, IL-3-mediated inhibition of basophil apoptosis may exacerbate the inflammation associated with allergic disorders.
Acknowledgments
This work was supported by grants to R. Robert Schellenberg and Aly Karsan from the Canadian Institutes of Health Research (CIHR) and to R. Robert Schellenberg from the British Columbia Lung Association. Aly Karsan is a clinician–scientist of the CIHR. We would like to thank Dr Stephan Van Eeden, Beth Whalen, Samuel Tsang, Patrick J. Duriez and Christopher M. Hull for advice.
Abbreviations
- ERK
extracellular signal regulated kinase
- MAPK
mitogen-activated protein kinases
- MEK
MAPK/ERK kinase
- LY
LY294002
- PD
PD98059
- PI3-K
phosphatidylinositol 3-kinase
References
- 1.Schroeder JT, Kagey-Sobotka A, Lichtenstein LM. The role of the basophil in allergic inflammation. Allergy. 1995;50:463. doi: 10.1111/j.1398-9995.1995.tb01180.x. [DOI] [PubMed] [Google Scholar]
- 2.Abraham SN, Arock M. Mast cells and basophils in innate immunity. Semin Immunol. 1998;10:373. doi: 10.1006/smim.1998.0140. [DOI] [PubMed] [Google Scholar]
- 3.Chang TW. The pharmacological basis of anti-IgE therapy. Nat Biotechnol. 2000;18:157. doi: 10.1038/72601. [DOI] [PubMed] [Google Scholar]
- 4.MacGlashan D, Jr, McKenzie-White J, Chichester K, Bochner BS, Davis FM, Schroeder JT, Lichtenstein LM. In vitro regulation of FcepsilonRIalpha expression on human basophils by IgE antibody. Blood. 1998;91:1633. [PubMed] [Google Scholar]
- 5.Schroeder JT, MacGlashan DW., Jr New concepts: the basophil. J Allergy Clin Immunol. 1997;99:429. doi: 10.1016/s0091-6749(97)70065-4. [DOI] [PubMed] [Google Scholar]
- 6.Ochensberger B, Rihs S, Brunner T, Dahinden CA. IgE-independent interleukin-4 expression and induction of a late phase of leukotriene C4 formation in human blood basophils. Blood. 1995;86:4039. [PubMed] [Google Scholar]
- 7.Iikura M, Yamaguchi M, Fujisawa T, et al. Secretory IgA induces degranulation of IL-3-primed basophils. J Immunol. 1998;161:1510. [PubMed] [Google Scholar]
- 8.Schroeder JT, MacGlashan DW, Jr, Kagey-Sobotka A, White JM, Lichtenstein LM. Cytokine generation by human basophils. J Allergy Clin Immunol. 1994;94:1189. doi: 10.1016/0091-6749(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 9.MacGlashan D, Jr, White JM, Huang SK, Ono SJ, Schroeder JT, Lichtenstein LM. Secretion of IL-4 from human basophils. The relationship between IL-4 mRNA and protein in resting and stimulated basophils. J Immunol. 1994;152:3006. [PubMed] [Google Scholar]
- 10.Li H, Sim TC, Grant JA, Alam R. The production of macrophage inflammatory protein-1 alpha by human basophils. J Immunol. 1996;157:1207. [PubMed] [Google Scholar]
- 11.Rothenberg ME, Owen WF, Jr, Silberstein DS, Woods J, Soberman RJ, Austen KF, Stevens RL. Human eosinophils have prolonged survival, enhanced functional properties, and become hypodense when exposed to human interleukin 3. J Clin Invest. 1988;81:1986. doi: 10.1172/JCI113547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh GM. Mechanisms of human eosinophil survival and apoptosis. Clin Exp Allergy. 1997;27:482. [PubMed] [Google Scholar]
- 13.Park BS, Kim GC, Back SJ, et al. Murine bone marrow-derived mast cells exhibit evidence of both apoptosis and oncosis after IL-3 deprivation. Immunol Invest. 2000;29:51. doi: 10.3109/08820130009105144. [DOI] [PubMed] [Google Scholar]
- 14.Berardi AC, Wang A, Levine JD, Lopez P, Scadden DT. Functional isolation and characterization of human hematopoietic stem cells. Science. 1995;267:104. doi: 10.1126/science.7528940. [DOI] [PubMed] [Google Scholar]
- 15.Blalock WL, Weinstein-Oppenheimer C, Chang F, et al. Signal transduction, cell cycle regulatory, and anti-apoptotic pathways regulated by IL-3 in hematopoietic cells: possible sites for intervention with anti-neoplastic drugs. Leukemia. 1999;13:1109. doi: 10.1038/sj.leu.2401493. [DOI] [PubMed] [Google Scholar]
- 16.Scheid MP, Lauener RW, Duronio V. Role of phosphatidylinositol 3-OH-kinase activity in the inhibition of apoptosis in haemopoietic cells: phosphatidylinositol 3-OH-kinase inhibitors reveal a difference in signalling between interleukin-3 and granulocyte–macrophage colony-stimulating factor. Biochem J. 1995;312:159. doi: 10.1042/bj3120159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Songyang Z, Baltimore D, Cantley LC, Kaplan DR, Franke TF. Interleukin 3-dependent survival by the Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:11345. doi: 10.1073/pnas.94.21.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 19.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 20.Besancon F, Atfi A, Gespach C, Cayre YE, Bourgeade MF. Evidence for a role of NF-kappaB in the survival of hematopoietic cells mediated by interleukin 3 and the oncogenic TEL/platelet-derived growth factor receptor beta fusion protein. Proc Natl Acad Sci USA. 1998;95:8081. doi: 10.1073/pnas.95.14.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Cabrera M, Munoz E, Blazquez MV, Ursa MA, Santis AG, Sanchez-Madrid F. Transcriptional regulation of the gene encoding the human C-type lectin leukocyte receptor AIM/CD69 and functional characterization of its tumor necrosis factor-alpha-responsive elements. J Biol Chem. 1995;270:21545. doi: 10.1074/jbc.270.37.21545. [DOI] [PubMed] [Google Scholar]
- 22.Blazquez MV, Macho A, Ortiz C, Lucena C, Lopez-Cabrera M, Sanchez-Madrid F, Munoz E. Extracellular HIV type 1 Tat protein induces CD69 expression through NF-kappaB activation: possible correlation with cell surface Tat-binding proteins. AIDS Res Hum Retroviruses. 1999;15:1209. doi: 10.1089/088922299310304. [DOI] [PubMed] [Google Scholar]
- 23.Marzio R, Mauel J, Betz-Corradin S. CD69 and regulation of the immune function. Immunopharmacol Immunotoxicol. 1999;21:565. doi: 10.3109/08923979909007126. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto K, Appiah-Pippim J, Schleimer RP, Bickel CA, Beck LA, Bochner BS. CD44 and CD69 represent different types of cell-surface activation markers for human eosinophils. Am J Respir Cell Mol Biol. 1998;18:860. doi: 10.1165/ajrcmb.18.6.3159. [DOI] [PubMed] [Google Scholar]
- 25.Julius P, Luttmann W, Knoechel B, Kroegel C, Matthys H, Virchow JC., Jr CD69 surface expression on human lung eosinophils after segmental allergen provocation. Eur Respir J. 1999;13:1253. doi: 10.1183/09031936.99.13612609. [DOI] [PubMed] [Google Scholar]
- 26.Toba K, Koike T, Shibata A, et al. Novel technique for the direct flow cytofluorometric analysis of human basophils in unseparated blood and bone marrow, and the characterization of phenotype and peroxidase of human basophils. Cytometry. 1999;35:249. [PubMed] [Google Scholar]
- 27.Ochensberger B, Tassera L, Bifrare D, Rihs S, Dahinden CA. Regulation of cytokine expression and leukotriene formation in human basophils by growth factors, chemokines and chemotactic agonists. Eur Respir J. 1999;29:11. doi: 10.1002/(SICI)1521-4141(199901)29:01<11::AID-IMMU11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 28.Lantz CS, Boesiger J, Song CH, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 29.Valent P, Schmidt G, Besemer J, et al. Interleukin-3 is a differentiation factor for human basophils. Blood. 1989;73:1763. [PubMed] [Google Scholar]
- 30.Yamaguchi M, Hirai K, Shoji S, Takaishi T, Ohta K, Morita Y, Suzuki S, Ito K. Haemopoietic growth factors induce human basophil migrationin vitro. Clin Exp Allergy. 1992;22:379. doi: 10.1111/j.1365-2222.1992.tb03099.x. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi M, Hirai K, Ohta K, et al. Nonreleasing basophils convert to releasing basophils by culturing with IL-3. J Allergy Clin Immunol. 1996;97:1279. doi: 10.1016/s0091-6749(96)70196-3. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi M, Hirai K, Morita Y, et al. Hemopoietic growth factors regulate the survival of human basophilsin vitro. Int Arch Allergy Immunol. 1992;97:322. doi: 10.1159/000236140. [DOI] [PubMed] [Google Scholar]
- 33.Tsang S, Hayashi M, Zheng X, Campbell A, Schellenberg RR. Simplified purification of human basophils. J Immunol Methods. 2000;233:13. doi: 10.1016/s0022-1759(99)00182-9. [DOI] [PubMed] [Google Scholar]
- 34.Zheng X, Knight DA, Zohou D, Weir T, Peacock C, Schellenberg RR, Bai TR. Leukemia inhibitory factor is synthesized and released by human eosinophils and modulates activation state and chemotaxis. J Allergy Clin Immunol. 1999;104:136. doi: 10.1016/s0091-6749(99)70125-9. [DOI] [PubMed] [Google Scholar]
- 35.Walker BA, Rocchini C, Boone RH, Ip S, Jacobson MA. Adenosine A2a receptor activation delays apoptosis in human neutrophils. J Immunol. 1997;158:2926. [PubMed] [Google Scholar]
- 36.Walker DC, Behzad AR, Chu F. Neutrophil migration through preexisting holes in the basal laminae of alveolar capillaries and epithelium during streptococcal pneumonia. Microvasc Res. 1995;50:397. doi: 10.1006/mvre.1995.1067. [DOI] [PubMed] [Google Scholar]
- 37.Schedle A, Samorapoompichit P, Fureder W, et al. Metal ion-induced toxic histamine release from human basophils and mast cells. J Biomed Mater Res. 1998;39:560. doi: 10.1002/(sici)1097-4636(19980315)39:4<560::aid-jbm9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 38.Hartnell A, Robinson DS, Kay AB, Wardlaw AJ. CD69 is expressed by human eosinophils activatedin vivo in asthma andin vitro by cytokines. Immunology. 1993;80:281. [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaguchi Y, Suda T, Ohta S, Tominaga K, Miura Y, Kasahara T. Analysis of the survival of mature human eosinophils: interleukin-5 prevents apoptosis in mature human eosinophils. Blood. 1991;78:2542. [PubMed] [Google Scholar]
- 40.Denburg JA, Woolley M, Leber B, Linden M, O'Byrne P. Basophil and eosinophil differentiation in allergic reactions. J Allergy Clin Immunol. 1994;94:1135. doi: 10.1016/0091-6749(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 41.Koshino T, Arai Y, Miyamoto Y, et al. Airway basophil and mast cell density in patients with bronchial asthma: relationship to bronchial hyperresponsiveness. J Asthma. 1996;33:89. doi: 10.3109/02770909609054536. [DOI] [PubMed] [Google Scholar]
- 42.Miyajima A, Mui AL, Ogorochi T, Sakamaki K. Receptors for granulocyte–macrophage colony-stimulating factor, interleukin-3, and interleukin-5. Blood. 1993;82:1960. [PubMed] [Google Scholar]
- 43.Nishikawa K, Morii T, Ako H, Hamada K, Saito S, Narita N. In vivo expression of CD69 on lung eosinophils in eosinophilic pneumonia: CD69 as a possible activation marker for eosinophils. J Allergy Clin Immunol. 1992;90:169. doi: 10.1016/0091-6749(92)90068-d. [DOI] [PubMed] [Google Scholar]
- 44.Luttmann W, Knoechel B, Foerster M, Matthys H, Virchow JC, Jr, Kroegel C. Activation of human eosinophils by IL-13. Induction of CD69 surface antigen, its relationship to messenger RNA expression, and promotion of cellular viability. J Immunol. 1996;157:1678. [PubMed] [Google Scholar]
- 45.Ramirez R, Carracedo J, Castedo M, Zamzami N, Kroemer G. CD69-induced monocyte apoptosis involves multiple nonredundant signaling pathways. Cell Immunol. 1996;172:192. doi: 10.1006/cimm.1996.0232. [DOI] [PubMed] [Google Scholar]
- 46.Walsh GM, Williamson ML, Symon FA, Willars GB, Wardlaw AJ. Ligation of CD69 induces apoptosis and cell death in human eosinophils cultured with granulocyte–macrophage colony-stimulating factor. Blood. 1996;87:2815. [PubMed] [Google Scholar]
- 47.Karin M. The NF-kappa B activation pathway: its regulation and role in inflammation and cell survival. Cancer J Sci Am. 1998;4:S92. [PubMed] [Google Scholar]
- 48.Duriez PJ, Wong F, Dorovini-Zis K, Shahidi R, Karsan A. A1 functions at the mitochondria to delay endothelial apoptosis in response to tumor necrosis factor. J Biol Chem. 2000;275:18099. doi: 10.1074/jbc.M908925199. [DOI] [PubMed] [Google Scholar]
- 49.Cui H, Matsui K, Omura S, Schauer SL, Matulka RA, Sonenshein GE, Ju ST. Proteasome regulation of activation-induced T cell death. Proc Natl Acad Sci USA. 1997;94:7515. doi: 10.1073/pnas.94.14.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasaki T, Irie-Sasaki J, Jones RG, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040. doi: 10.1126/science.287.5455.1040. [See comments] [DOI] [PubMed] [Google Scholar]
- 51.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 52.Scheid MP, Duronio V. Dissociation of cytokine-induced phosphorylation of Bad and activation of PKB/akt: involvement of MEK upstream of Bad phosphorylation. Proc Natl Acad Sci USA. 1998;95:7439. doi: 10.1073/pnas.95.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimamura A, Ballif BA, Richards SA, Blenis J. Rsk1 mediates a MEK-MAP kinase cell survival signal. Curr Biol. 2000;10:127. doi: 10.1016/s0960-9822(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 54.Ochiai K, Tanabe E, Ishihara C, Kagami M, Sugiyama T, Sueishi M, Koya N, Tomioka H. Role of JAK2 signal transductional pathway in activation and survival of human peripheral eosinophils by interferon-gamma (IFN-gamma) Clin Exp Immunol. 1999;118:340. doi: 10.1046/j.1365-2249.1999.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]