Abstract
Allergic airway inflammation induced in mice is T-cell dependent and recruitment of eosinophils to airspaces requires both αβ and γδ T cells. From previous studies it is evident that αβ T cells are essential for the allergic T helper type 2 (Th2)-like response, while the mechanistic contribution of γδ T cells is still unclear. In this study, we have investigated the role of γδ T cells in allergic airway eosinophilia induced by ovalbumin hypersensitivity. By comparing the responsiveness to sensitizing allergen of wild-type mice with that of T-cell receptor γδ knockout mice (TCRγδ KO) we demonstrated that mice lacking γδ T cells are defective in the systemic ovalbumin-specific immunoglobulin E (IgE) response. Furthermore, after aerosol challenge with allergen, γδ T-cell deficient mice exhibited a significantly decreased migration of B cells and natural killer cells to airways and reduced levels of allergen-specific IgG and IgA in bronchoalveolar lavage fluid. The role for B cells in the airway inflammation was indicated by the impaired ability of mice lacking functional B cells to evoke an eosinophilic response. The diminished eosinophilia in TCRγδ KO mice could not be explained by a defective Th2 activation since these mice displayed a normal IgG response in serum and an unaffected IG2b/IgG1 ratio in airways. Analysis of immunoregulatory cytokines in isolated lung tissue, thoracic lymph nodes and spleen further supported the notion that these mice are able to evoke a sufficient activation of T helper cells and that γδ T cells are not required for maintaining the Th2 profile. These results indicate that γδ T cells contribute to allergic airway inflammation by pathways separate from classical Th2 immune activation.
Introduction
Lymphocytes expressing the γδ T-cell receptor (TCR) comprise less than 10% of the T-cell population in lymphoid organs and peripheral blood.1 A specific localization of γδ T cells in the epithelium has been postulated, based on the observations that these cells are increased among intraepithelial lymphocytes (IEL) in the gastrointestinal tract and by the skewed repertoire of TCR Vδ and Vγ genes expressed by human and mouse IEL.2–8 Several studies have demonstrated that γδ T cells expressing a similar Vδ and Vγ repertoire as in IEL are increased in the circulation of patients with inflammatory bowel disease and in the joints of patients with rheumatoid arthritis.2,9–18 Similarly, it is reported that this subset of γδ T cells is enriched in the airway epithelium of patients with allergic inflammation.19 Although it is strongly suggested from these studies that γδ T cells are involved in the progression or regulation of inflammatory disorders, it has not been possible to definitely define the role for γδ T cells in human diseases.
Experimentally induced inflammation in rodents may be helpful to unravel the role of γδ T cells in various inflammatory disorders. Adjuvant-induced arthritis in rats is a T-cell dependent disease affecting peripheral joints.20,21 Depletion of γδ T cells before injection of the adjuvants, mineral oil22 squalene23 or Freund's complete adjuvant24 does not affect the disease course measured as joint swelling, although microscopic aggravation of joint destruction has been observed in animals depleted of γδ T cells.24 Adjuvant-induced airway inflammation, provoked by inhalation of bacterial endotoxin (lipopolysaccharide; LPS), is similarly to adjuvant-induced arthritis controlled by CD4+ T cells.25 In this model of acute airway inflammation, we have recently demonstrated that mice lacking γδ T cells and the corresponding wild-type strain do not differ in the accumulation of neutrophils in airspaces.26 However, Penido et al. reported that γδ T cells are required for eosinophil migration into the lungs after inthrathoracic injection of LPS.27 Thus, under certain experimental conditions γδ T cells may contribute to the migration of granulocytes into the inflamed loci and it appears that eosinophils rather than neutrophils are controlled by this T-cell subset.
In mouse models of allergic hyperresponsiveness induced by sensitization to an allergen followed by inhalation challenge, it has been recently shown that γδ T cells participate in airway inflammation.28–30 This disease differs from adjuvant-induced inflammation by the dependency of a specific T helper 2 (Th2)-dominated T helper response against the immunized antigen and is characterized by accumulation of eosinophils rather than neutrophils in airspaces.31,32 Whether γδ T cells are involved in the type 2 response or promote the eosinophilia through some other mechanism is controversial. In a model of adoptive transfer of γδ T cells from ovalbumin (OVA)-tolerant mice, McMenamin et al. found that γδ T cells regulate IgE responsiveness to inhaled antigens by high production of interferon-γ (IFN-γ), suggesting that antigen-specific γδ T cells are able to suppress the pathogenic Th2 response in allergic asthma.33 In contrast, Zuany-Amorim et al. reported that γδ T cells contribute to type 2-mediated airway inflammation by inducing interleukin (IL)-4 dependent IgE and IgG1 responses,28 a finding which was recently supported in a study by Schramm et al.30 However, other studies reported that mice lacking γδ T cells are not defective in production of IL-4 and IL-5, suggesting that T helper cells with a Th2 profile can be sufficiently induced in the absence of γδ T cells.29,34
In the present study we used a murine model of allergen-induced sensitization to dissect and clarify the role of γδ T cells in eosinophilic airway inflammation. By comparing the response in mice lacking γδ T cells (TCRδ–/–) with that of wild-type controls on the same genetic background, we demonstrated that the decreased eosinophilia in γδ T cell deficient mice is associated with defective systemic antigen-specific IgE but a normal IgG response. In contrast, TCRβ–/– mice lacking αβ T cells were deficient in both IgE and IgG responses including the Th2-induced IgG1 isotype. Mice lacking γδ T cells were also defective in the recruitment of B cells and natural killer (NK) cells to airspaces and expressed decreased allergen-specific IgG and IgA in bronchoalveolar lavage fluid (BALF). γδ T-cell deficient mice were able to evoke a Th2 response, however, and displayed a normal balance of Th1- and Th2-promoting cytokines in thoracic lymph nodes. Collectively, our data indicate that γδ T cells promote allergic airway inflammation by enhancing the systemic IgE response and local antibody reactivity, but unlike αβ T cells, this occurs without a specific role in the shift of the immune response towards Th2. The contribution of B cells to the airway eosinophilia was demonstrated by the diminished inflammatory response in mice lacking functional B cells.
Materials and methods
Mice
For studies of T- and B-cell involvement in eosinophilic inflammation, knockout (KO) and corresponding wild-type strains, originally obtained from Jackson Laboratories (Bar Harbor, ME), were bred in our animal facility. KO mice with T-cell receptor deficiencies lacking either αβ T cells (TCRβ–/–), γδ T cells (TCRδ–/–), or both T cell subsets (TCRβ–/– δ–/–) were of C57BL/6 origin. Experiments with B-cell deficient mice (Igh-6tm1Cgn) were initially performed on mice with C57BL/10 origin and later with mice of C57BL/6 origin. Animals were fed with standard chow and water ad libitum. All mice were between 9 and 13 weeks old when experiments were initiated. The study was approved by the Regional Animal Research Ethics Committee according to national laws.
Immunization and aerosol challenge
Animals were immunized intraperitoneally with 200 µl OVA/aluminium hydroxide gel (alum) (1 : 3) on days 0 and 14. OVA (Chicken egg albumin grade V, Sigma, St. Louis, MO) was dissolved in saline and mixed with alum to a concentration of 50 µg/ml by rotation at 4° for 3 hr. On days 30, 33 and 35, mice were challenged in the lungs by inhalation of aerosolized OVA for 30 min using a nose-only Batelle exposure chamber. Aerosols were generated by a compressed-air nebulizer (Collision 6-jet) at an airflow of 7 l/min using a nebulizer concentration of 10 mg/ml OVA dissolved in phosphate-buffered saline (PBS). Control mice received no other treatment than OVA-aerosol challenge on days 30, 33 and 35.
Analysis of leucocytes in bronchoalveolar fluid
Mice were killed by cervical dislocation 18 hr after the last aerosol challenge and their tracheae were cannulated with polyethylene tubing. BAL was performed using 1-ml aliquots of Hank's balanced salt solution to a recovered volume of 4 ml. The BALF was centrifuged (400 g, 10 min, 4°) and the supernatants were collected for anti-OVA antibody analysis. Cells were resuspended in 0·4 ml PBS and total leucocytes counted using trypan blue exclusion in a Bürker chamber. For determination of BALF eosinophils, duplicate Cytospin (Cytospin 3, Shandon, Runcorn, UK) preparations (729, 5 min) were made of 30 000 cells from each sample and stained with May–Grünwald Giemsa. Percentage eosinophils were determined through differential counts of 300 cells per slide using standard morphological criteria. Lymphocytes in BALF were determined by flow cytometry analysis using the monoclonal antibodies, CD3-fluoroscein isothiocyanate (FITC; KT3), NK1.1-phycoerythrin (PE; PK136, Serotec, Kidlington, UK), TCRβ-CyChr (H57-597) and B220-CyChr (RA3–6B2; Pharmingen, San Diego, CA). Isotype-matched antibodies were used as negative controls. For staining, 250 000 cells were distributed into tubes and washed in PBS containing 0·1% bovine serum albumin (BSA). To avoid non-specific binding the cells were incubated with 5 µl Fc BlockTM (anti-CD16/CD32, Pharmingen) and 15 µl rat serum for 5 min at 4°. Optimal dilutions of antibodies were determined by pilot experiments. Properly diluted antibodies were added and the cells were further incubated for 30 min in the dark. After staining, cells were fixed and red blood cells were lysed with FACSTM Lysing solution (Becton Dickinson Immunocytometry Systems, San Jose, CA) and washed in PBS before analysis with a FACSort (Becton Dickinson). B cells were defined as B220+ and CD3–, αβ T cells as TCRβ+ and NK cells as NK1.1+ and CD3–. Total numbers of B, T and NK cells in BALF were determined as well as the percentage of these cells among cells in the lymphocyte gate.
Analysis of anti-OVA antibodies and total IgE
Serum samples were obtained by orbital puncture. Serial dilutions of serum and BALF samples were performed in order to keep OD values within the linear range of the dilution curve. Final analysis was performed with serum dilutions 1 : 10 000 (IgG), 1 : 100 000 (IgG1), 1 : 3 (IgE) or 1 : 100 (IgM). Analysis of OVA-specific immunoglobulin in BALF was performed with dilutions 1 : 3 or 1 : 9.
OVA (grade V) was purchased from Sigma. Microtitre 96-well enzyme-linked immunosorbent assay (ELISA) plates were Nunc-Immuno Plate with Max Sorb surface (Tamro MedLab AB, Mölndal, Sweden). A ready-to-use peroxidase substrate system with 3,3′,5,5′-tetramethylbenzidine (TMB) and hydrogen peroxide (Sigma) was used in ELISA. The soluble product was read after 40 min at 620 nm (OD620) in a Thermo Labsystems iEMS ELISA (Vantaa, Finland) reader. Washing solutions were saline/0·1% (v/v) Tween. Mouse antisera were analysed against OVA (3 µg/ml in coating buffer) with the Bio-Rad Mouse Typer Isotyping kit according to the manufacturer's description except that TMB (Biorad Richmond, CA) was used as peroxidase substrate.
Total IgE in mouse sera was analysed with a capture ELISA using an anti-mouse IgE capture monoclonal antibody (mAb; Pharmingen, clone R35-72, 2 µg/ml) and a biotinylated detecting anti-mouse IgE mAb (Pharmingen, clone R35-118, 2 µg/ml). Bound detecting antibody was quantified with a strepavidin-peroxidase conjugate (Strepavidin-POD, Roche Diagnostics Scand. AB, Bromma, Sweden, 0·05 U/ml) and the substrate TMB as described in Pharmingen's mouse IgE ELISA protocol. Amount total IgE in serum samples was calculated from a standard curve with a mouse IgE isotype standard (Pharmingen, cat.no. 03231D).
Anti-OVA antibodies of IgE isotype were analysed using biotin-labelled OVA. In the biotinylation procedure 10 mg OVA in 1 ml PBS was mixed with 2·5 mg biotinamidocaproic acid 3-sulpho-N-hydroxy-succinimide ester (Sigma) dissolved in 0·25 ml distilled water. The mixture was stirred for 2 hr at room temperature. To remove unreacted biotin the mixture was dialysed against PBS at 4° and then the biotinylated OVA was stored at 4° in 0·1% sodium azide. In a capture ELISA anti-IgE mAb (Pharmingen, clone R35-72, 8 µg/ml, 100 µl) was coated in the microtitre plates in coating buffer. Mouse immune sera, 100 µl, were diluted and were added and incubated for 2 hr at room temperature. Bound anti-OVA antibodies were quantified by first incubating with biotinylated OVA (2 µg/ml, 100 µl), then the strepavidin-peroxidase conjugate (0·05 U, 100 µl) and finally the TMB substrate (100 µl) were added.
Analysis of cytokine mRNA expression in lung tissue, thoracic lymph nodes and spleen
Animals were killed by cervical dislocation 18 hr after the last aerosol challenge, after which the lungs or thoracic lymph nodes (mediastinal) were removed. Spleens were removed either from non-immunized control mice or from immunized mice on day 28 after the first injection. The tissues were immediately frozen in liquid nitrogen and stored in −70°, or preserved in RNA stabilization reagent (RNAlater, QIAGEN GmbH, Hilden, Germany), until preparation of RNA. Total cytoplasmic RNA was purified from lung tissue using the TRIzol extraction protocol (Gibco BRL, Gaithersburg, MD)26 and from lymph nodes using the Qiagen extraction method (QIAGEN GmbH) according to the manufacturer's instructions. First strand cDNA was synthesized using oligo(dT) primers according to a standardized protocol.26 Two µl of cDNA was added to a 23-µl polymerase chain reaction (PCR) reaction mixture consisting of 12.5 µl SYBRgreen Mix including AmpliTaq gold and cybergreen (Applied Biosystems, Warrington, UK), 0·4 µm sense and antisense primer and 9,5 µl sterile distilled water. Target cDNA was amplified using primers specific for glyceraldehyde-3-phosphate dehydrogenase (GAPDH)26 IFN-γ,26 IL-4,26 IL-5,26 IL-1026 and IL-25 (5′-GTCGTGCATTCGTTCTCCCAG-3′ and 5′-CACATGCCCTGCATCTTCCCT-3′). Amplification of cDNA was performed by a two-step PCR protocol (95°, 10 min, followed by 40 cycles of 95° for 15 s and 60° for 60 s) using amplification equipment with a real-time monitor system (iCycler, Biorad). The reaction was followed in real-time by recording the increase in fluorescence when cybergreen was incorporated in the double-stranded PCR product. The number of cycles needed to increase the fluorescence intensity above the background threshold was calculated using the icycler software (cycle threshold: CT). This enables evaluation of the results in the early phase of the PCR reaction, i.e. when the amplification yields an exponential increase in PCR product. Since the CT value is inversely proportional to the number of target cDNA copies in the sample, we calculated a residual CT by subtracting the measured CT value from the total number of amplification cycles, giving an adjusted CT value which is directly proportional to the amount of target cDNA. The relative amount of cytokine cDNA in each sample was calculated by dividing the residual CT value by the corresponding value of the housekeeping gene GAPDH. For the determination of the relative balance of IL-4 and IFN-γ, the residual CT IL-4/IFN-γ ratio was calculated. Cytokine transcripts with no detectable fluorescence intensity after 40 cycles were assigned the value 0. The identity and purity of the PCR products was confirmed by agarose gel electrophoresis followed by staining in ethidium bromide.26 Only PCR products yielding a single band corresponding to the expected fragment size were further analysed.
Statistical analysis
Statistical comparisons were performed by analysis of means using unpaired Student's t-test (two-tailed). If more than two groups were compared, analysis of variances (anova) was performed. This was followed by Dunnet's multiple comparisons test when comparisons were performed versus a single control group. For comparison of cytokine/GAPDH and IL-4/IFN-γ ratios, we used the non-parametric Mann–Whitney U-test (two-tailed) when two groups were compared and Dunn's non-parametric multiple comparisons test when more than two groups were included in the analysis. Analysis of correlation between cytokine expression and eosinophil numbers in BALF was performed using the non-parametric Spearman rank test. P < 0·05 was regarded as significant.
Results
Both αβ and γδ T cells are essential for eosinophilic airway inflammation
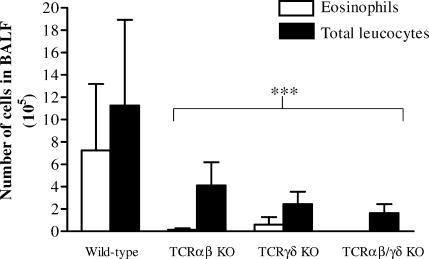
Mice deficient in defined T-cell populations and wild-type mice of the same genetic background (C57BL/6) were sensitized by i.p. injections of 10 µg OVA absorbed to alum adjuvant and thereafter challenged repeatedly with an aerosol of 10 mg/ml OVA. BAL was performed 18 hr after the last challenge followed by counting of cells in BALF and identification by morphological analysis. In untreated healthy animals, the total recovered leucocyte number in BALF was generally less than 400 000, with a predominance of alveolar macrophages (>95%) and only occasional appearance of eosinophils (data not included). Control mice receiving no other treatment than OVA-aerosol challenge did not differ in BALF leucocyte number from healthy untreated animals. Neither did a second control group, sensitized with OVA and challenged with house dust mite allergen (Dermatophagoides pteronyssinus antigen-2), evoke a detectable airway response, demonstrating that the airway eosinophilia requires both a sensitization reaction and a subsequent aerosol challenge with the immunized antigen. In sensitized and OVA challenged wild-type C57BL/6 mice, a dramatic increase in eosinophils was observed, rising to a proportion up to 80% of the total leukocytes in BALF. The eosinophilic responses in airways of C57BL/6 KO mice lacking αβ T cells (TCRβ–/–), γδ T cells (TCRδ–/–) and mice lacking all T cells (TCRβ–/–δ–/–) were significantly reduced when compared to the wild-type strain (Fig. 1). Most animals lacking αβ T cells (the TCRβ–/– and TCRβ–/–δ–/– strains) did not evoke a detectable airway eosinophilia.
Figure 1.
Airway eosinophilia is diminished in T-cell deficient mice. The number of eosinophils and total leukocytes in BALF was analysed 18 hr after the last of three repeated exposures of aerosolized OVA in presensitized T-cell deficient or wild-type C57BL/6 mice. Mean values and standard deviations are presented. Nine animals in the wild-type control group and five to eight animals in the T-cell deficient strains. ***P < 0·001 versus the wild-type control group.
The systemic antigen-specific IgE but not IgG response is defective in γδ T-cell deficient mice
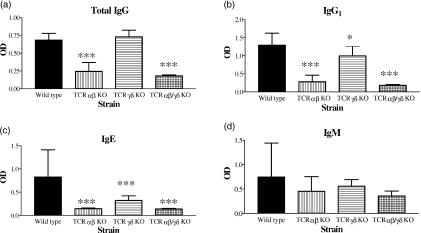
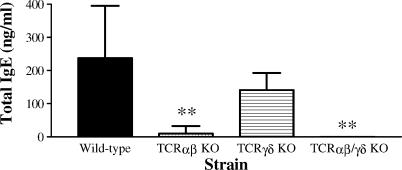
Immunization with OVA together with alum adjuvant was monitored in serum by analysis of OVA-specific antibodies. It is well established that i.p. injection of antigen mixed with aluminium hydroxide predominantly activates Th2 skewed T cells resulting in a strong humoral immune response. In concordance with a strong bias towards a Th2 response we detected an increase in OVA-specific IgG1 and IgE but undetectable levels of IgG2a. In sensitized and aerosol challenged mice the OVA-specific IgG response was diminished in the αβ T-cell deficient strain but not in animals lacking only the γδ T-cell subset (Fig. 2a), while the OVA-specific IgE response was significantly decreased in both TCRαβ and TCRγδ KO mice compared to wild-type control mice (Fig. 2c). The Th2-induced IgG1 response was severely affected in mice lacking αβ T cells but only to a limited degree in the γδ T-cell deficient strain (Fig. 2b). The antigen-specific IgM response, which is to a large extent T-cell independent, was not significantly affected in any of the T-cell deficient strains (Fig. 2d). Total IgE levels in serum were clearly attenuated in mice without αβ T cells, while in the TCRγδ KO strain a weak tendency of reduction was observed although this was not statistically significant (Fig. 3).
Figure 2.
Analysis of systemic anti-OVA immunoglobulin response in T-cell deficient and wild-type C57BL/6 mice. Serum samples were collected 18 hr after the last of three repeated exposures of aerosolized OVA in presensitized mice. The anti-OVA immunoglobulin response was analysed by isotype-specific immunoassays. OVA-specific total IgG (a), IgG1 (b), IgE (c) and IgM (d) responses were detected in serum from wild-type mice after immunization and aerosol challenge with OVA. Mean values and standard deviations are given. Nine animals in the wild-type control group and five to eight animals in the T-cell deficient strains. *P < 0·05; ***P < 0·001 versus the wild-type control group.
Figure 3.
Analysis of total IgE in serum of sensitized and aerosol challenged T cell deficient or wild-type control mice. Amount total IgE in serum samples was calculated from a standard curve with a mouse IgE isotype standard. Mean values and standard deviations are depicted. Seven animals in the wild-type control group and four to five animals in the T-cell deficient strains. **P < 0·01 versus the wild-type control group.
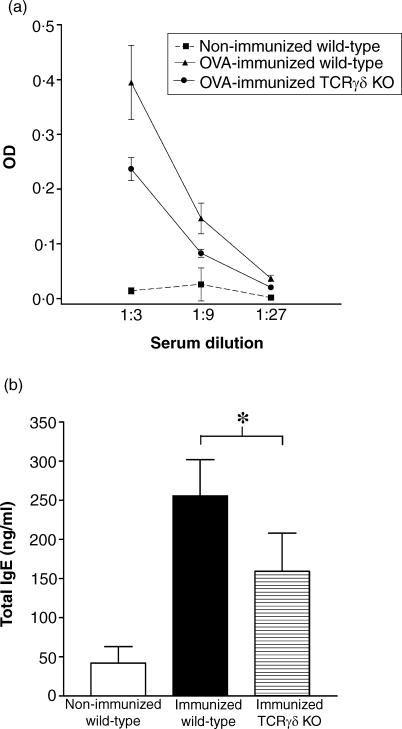
To investigate whether the contribution of γδ T cells to the systemic IgE response is exerted during the sensitization reaction or during aerosol challenge, we analysed OVA-specific and total IgE in serum from immunized but not aerosol-challenged mice (Fig. 4). Also in this experiment, TCRγδ KO mice dispalyed impaired IgE levels, demonstrating that the dependency of γδ T cells is exerted during the sensitization phase.
Figure 4.
Analysis of OVA-specific IgE (a) and total IgE (b) in serum from immunized but not aerosol challenged wild-type and knockout mice lacking γδ T cells (TCRγδ KO). OVA-specific IgE was measured at three different serum dilutions (1 : 3, 1 : 9 and 1 : 27). In this dilution range, the OD-values were significantly lower in OVA-immunized TCRγδ mice than in OVA-immunized wild-type mice (P < 0·01, anova). The amount of total IgE in serum samples was calculated from a standard curve with a mouse IgE isotype standard. *P < 0·05. Data are also shown for non-immunized wild-type control mice.
Local B cell reactivity in airways is severely affected in γδ T-cell deficient mice
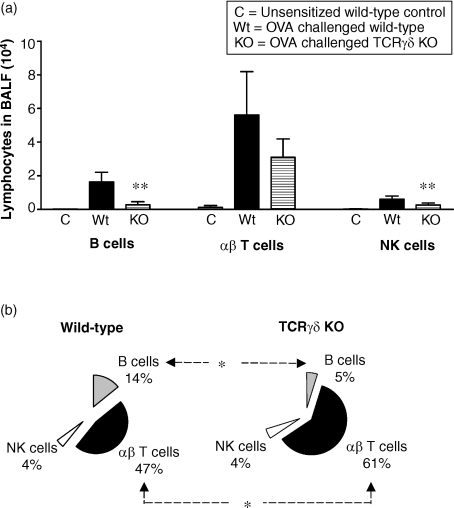
Along with the airway eosinophilia, a marked influx of lymphocytes to airspaces was observed in sensitized and aerosol challenged wild-type mice (Fig. 5a). Flow cytometry analysis of lymphocyte subsets in BALF revealed a predominance of T cells, but also a substantial increase of B cells and NK cells. The recruitment of lymphocytes into the airways was severely affected in mice lacking γδ T cells. Although all subsets of lymphocytes appeared to be decreased in BALF of TCRγδ KO mice, this effect was most pronounced for the B cells. The proportion of B cells among gated lymphocytes in the flow cytometry analysis was significantly decreased in γδ T-cell deficient mice compared to the wild-type controls, while the opposite was observed for the αβ T cells (Fig. 5b). The TCRγδ KO mice did not exhibit a general deficiency in B-cell numbers as indicated by the normal proportion of B cells in spleen and thoracic lymph nodes (data not included).
Figure 5.
Analysis of lymphocyte subsets in BALF of γδ T cell deficient and wild-type C57BL/6 mice. (a) The total number of B cells (B220+ CD3–), αβ T cells (TCRαβ+) and NK cells (NK1.1+ CD3–) in BALF was analysed by flow cytometry 18 hr after the last of three repeated exposures of aerosolized OVA in presensitized or untreated control mice. In unsensitized control mice receiving only OVA-aerosol challenge, the percentage of lymphocytes was generally less than 2% of the total leucocytes, yielding less than 8000 lymphocytes in 4 ml BALF. Among these lymphocytes, a small but substantial population of αβ T cells was observed while B cells and NK cells were barely detectable. In sensitized and aerosol challenged wild-type mice, a 30-fold increase in BALF lymphocytes was recorded. The increase in BALF total lymphocytes was significantly weaker in mice lacking γδ T cells than in the wild-type strain (P < 0·001 when comparing total number of cells in the lymphocyte population). Mean values and standard deviations are illustrated. (b) The distribution of lymphocyte subsets in BALF of sensitized and aerosol challenged mice was analysed by calculating the proportion of αβ Τ cells, B cells and NK cells among total lymphocytes. Electronic gates were set in the forward/side scatter plot according to standardized procedures for identification of lymphocytes by flow cytometry. Gated cells also included monocytes/macrophages with similar size and granulation as lymphocytes. The appearance of nonlymphocytes among the gated cells was similar in the two strains and did not influence the results. Mean values are shown. Six animals in the OVA treated wild-type and TCRγδ KO groups and two animals in the untreated group. The figure represents one of two experiments with similar results. *P < 0·05; **P < 0·01 versus the OVA challenged wild-type group.
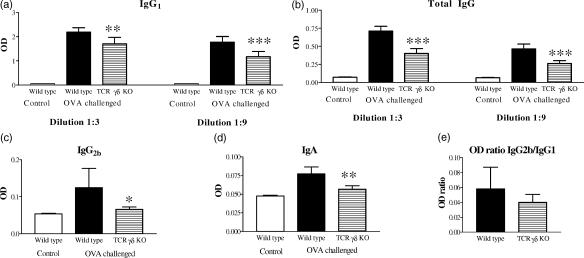
The local immunoglobulin response in airways was studied by analysis of OVA-specific antibodies in BALF. In Fig. 6, data from one of two experiments with similar results is shown. When comparing the immunoglobulin levels between sensitized and challenged wild-type mice with that of untreated healthy controls we detected elevated OVA-specific IgG, IgA as well as the IgG1 and IgG2b isotypes (Fig. 6a–d). The levels of IgE and IgG2a were undetectable in BALF, however. Because the appearance of OVA-specific IgG isotypes as well as IgA and IgE differed between serum and BALF, it is likely that the immunoglobulins detected in BALF were derived from local B cells rather than because of leakage from the blood. In mice lacking γδ T cells, the IgA and IgG responses (including both the IgG1 and IgG2b isotypes) were significantly reduced compared to the responses in wild-type mice. Notably, the IgG2b and IgA responses were decreased to levels similar to that in untreated healthy animals. The IgG2b/IgG1 ratio, which can be used as a marker for an IL-4 dependent shift to Th2, was not significantly different between the TCRγδ KO mice and the wild-type strain (Fig. 6e).
Figure 6.
Analysis of anti-OVA Ig in BALF of TCRγδ deficient and wild-type C57BL/6 mice. OVA-specific IgG1 (a), total IgG (b), IgG2b (c) and IgA (d) responses were detected in BALF from wild-type mice after immunization and aerosol challenge with OVA. Results from IgA and IgG2b analyses are shown for dilution 1 : 3 and from total IgG and IgG1 for dilutions 1 : 3 and 1 : 9. OVA-specific IgE, IgM or IgG2a were not detected in BALF. (e) The ratio of IgG2b : IgG1 did not differ between OVA treated γδ T cell deficient mice and wild-type controls. Mean values and standard deviations are shown. Six animals in the OVA treated wild-type and TCRγδ KO groups and two animals in the untreated group. The figure represents one of two experiments with similar results. *P < 0·05; **P < 0·01; ***P < 0·001 versus the OVA challenged wild-type group.
Analysis of cytokine gene expression in lung tissue, thoracic lymph nodes and spleen
We used a semiquantitative real-time PCR assay to investigate the mRNA expression of cytokines in lung tissue and lymph nodes draining the airways. The relative increase in cytokine mRNA was determined by calculation of the amplification cycle threshold (CT) versus the housekeeping gene GAPDH. This evaluation method is enabled by the real-time detection system and permits semiquantitative analysis. The expression of IL-4 mRNA but not IFN-γ was increased in lung tissue preparations 18 hr after the last of three aerosol exposures in presensitized mice compared to untreated control mice (Fig. 7a). When comparing cytokine gene expression between OVA challenged wild-type and TCRγδ KO mice, we detected lower levels of both IL-4 and IFN-γ in thoracic lymph nodes of mice lacking γδ T cells (Fig. 7b), while the expression in lung tissue preparations were similar in the two strains (Fig. 7a). The mRNA expression of other cytokines involved in the promotion of the type 2 immune response (IL-5, IL10 and IL-25) did not differ in lung tissue or lymph nodes between the γδ T-cell deficient and wild-type strains (data not included).
Figure 7.
Analysis of IL-4 and IFN-γ mRNA expression in lung tissue (a and c) and isolated thoracic lymph nodes (b and d). Expression of mRNA was semiquantitatively analysed 18 hr after the last of three repeated exposures of aerosolized OVA in presensitized γδ T-cell deficient (TCRγδ KO) or wild-type C57BL/6 mice. For comparison, the IL-4 and IFN-γ expression in lung tissue of untreated wild-type control mice is also shown. Thoracic lymph nodes could not be dissected from untreated healthy mice. The residual cycle threshold value (CT) was determined for each amplified transcript (see Materials and methods) and the relative levels of IL-4 and IFN-γ mRNA was calculated in relation to the expression of the housekeeping GAPDH gene (a and b). The residual CT ratio of IL-4/IFN-γ was used as a marker for a shift in the Th2/Th1 balance (c and d). *P < 0·05; **P < 0·01
To investigate the relative dominance of Th2 versus the Th1 immune response, we determined the mRNA expression of IL-4 in relation to IFN-γ. As indicated in Fig. 7(c), the IL-4/IFN-γ ratio was elevated in lung tissue of mice with established airway inflammation compared to that of untreated control mice. When comparing the ratio of IL-4 and IFN-γ between treated wild-type and TCRγδ KO mice, we observed no significant difference, either when analysing mRNA from lung tissue or from thoracic lymph nodes (Figs 7c,d).
By performing analysis of correlation between magnitude of airway eosinophilia and levels of cytokine mRNA in lymph nodes, we demonstrated that the number of eosinophils recovered from wild type mice was positively correlated with expression of the Th2-associated cytokine IL-5 and to some extent also IL-4, while the converse was observed when correlation was analysed between eosinophils in BALF and IFN-γ expression (Table 1). An increased IL-4/IFN-γ ratio, which we used to monitor a shift towards a Th2 immune response, was highly associated with an elevated number of eosinophils in airways. In TCRγδ KO mice, however, these correlations were less pronounced and did not reach the level of statistical significance.
Table 1.
Analysis of correlation between cytokine gene expression in thoracic lymph nodes and magnitude of airway eosinophilia
| Cytokine* | Correlation with Eosinophil number in BALF† | Statistical significance | |
|---|---|---|---|
| Wild-type | IFN-γ | −0·65 | P = 0·022 |
| IL-4 | 0·55 | P = 0·063 | |
| IL-5 | 0·71 | P = 0·01 | |
| IL-10 | 0·36 | P = 0·25 | |
| IL-25 | 0·34 | P = 0·29 | |
| IL-4/IFN-γ | 0·73 | P = 0·007 | |
| TCRγδ KO | IFN-γ | −0·29 | P = 0·37 |
| IL-4 | 0·49 | P = 0·11 | |
| IL-5 | 0·45 | P = 0·14 | |
| IL-10 | 0·028 | P = 0·93 | |
| IL-25 | 0·19 | P = 0·56 | |
| IL-4/IFN-γ | 0·49 | P = 0·11 |
Cytokine expression was calculated as a ratio versus GAPDH or as an IL-4/IFN-γ ratio.
Spearman rank correlation coefficient.
Statistical significant correlations are indicated by italics (n = 12).The number of eosinophils in BALF was determined 18 hr after the last of three repeated aerosol exposures of OVA in presensitized C57BL/6 wild-type or TCRγδ knockout mice. Immediately after performing BAL, thoracic lymph nodes were removed for semiquantitative analysis of cytokine mRNA.
We did not observe any significant change in mRNA levels of IL-4 and IFN-γ in spleen of immunized mice compared to non-immunized control mice (Table 2). Neither did immunized TCRγδ knockout mice differ significantly from the corresponding wild-type strain in spleen expression of IL-4 and IFN-γ.
Table 2.
Analysis of IFN-γ and IL-4 gene expression in the spleen of wild-type and TCRγδ knockout mice
| Treatment/strain | IFN-γ/GAPDH | IL-4/GAPDH | IL-4/IFN-γ | Statistical significance |
|---|---|---|---|---|
| Non immunized wild-type | 0·35 ± 0·08 | 0·29 ± 0·08 | 0·82 ± 0·13 | ns |
| Immunized wild-type | 0·36 ± 0·06 | 0·30 ± 0·06 | 0·83 ± 0·17 | |
| Immunized TCRγδ KO | 0·38 ± 0·07 | 0·36 ± 0·02 | 0·97 ± 0·16 | ns |
Statistical comparisons versus immunized wild-type mice (n = 5 in each group). Mean values ± standard deviations are indicated. ns = non-significant differences for all ratios.
Spleens were removed from OVA-immunized mice 28 days after the first injection. For comparison, data are also shown from non-immunized wild-type mice. The ratio versus the housekeeping gene GAPDH and the IL-4/IFN-γ ratio are shown.
B cells contribute to eosinophilic airway inflammation
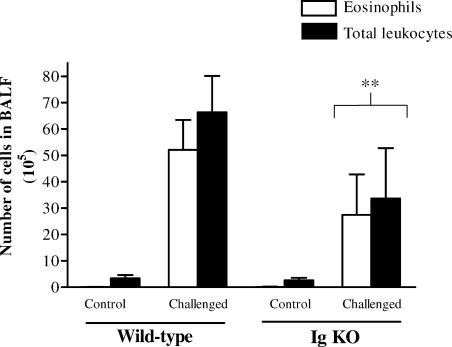
The role of B cells in airway eosinophilia was studied using genetically modified mice with deletions in the membrane exon of the immunoglobulin µ chain gene (Igh-6tm1Cgn). These mice are unable to produce immunoglobulins and exhibit a severe deficiency in B cells as indicated by the lack of B220+ cells in the spleen (data not included). Using accumulation of eosinophils in airspaces as the endpoint of the hypersensitivity reaction, we observed a diminished inflammatory response in the B-cell deficient C57BL/6 mice compared to wild-type mice on the same genetic background (Fig. 8 illustrates one out of three experiments). Similar results were obtained in one experiment comparing the response in Igh-6tm1Cgn C57BL/10 mice with that of C57BL/10 wild-type mice (data not included). Although these results demonstrate a role for B cells in our model of airway hypersensitivity, it should be noted that most mice lacking functional B cells were able to evoke a detectable inflammatory response.
Figure 8.
Airway eosinophilia is diminished in B cell deficient mice. The number of eosinophils and total leukocytes in BALF was analysed 18 hr after the last of three repeated exposures of aerosolised OVA in presensitized B-cell deficient (Igh-6tm1Cgn) or wild-type C57BL/6 mice. Mean values and standard deviations are depicted. Four animals in the untreated control groups. Nine animals in the OVA challenged wild-type group and seven animals in the OVA challenged B cell deficient group. The figure represents one of three experiments with similar results. Similar results were also obtained when B-cell deficient mice on the C57BL/10 background were compared with C57BL/10 wild-type mice (data not included). **P < 0·01 versus the OVA challenged wild-type group.
Discussion
Repeated challenge with aerosolized OVA in presensitized mice provokes an airway inflammation which is characterized by an extensive migration of eosinophils into the lungs and also a prominent influx of lymphocytes, mostly T cells. It is evident from our results that both αβ and γδ T cells contribute to the airway eosinophilia, but apparently through different mechanisms. Mice deficient in the αβ T-cell subset are severely immunocompromised as indicated by the lack of allergen-specific IgG and IgE responses, demonstrating that participation of the major T-cell subset is crucial for the sensitization reaction and subsequent airway inflammation. In contrast to the αβ T-cell deficient strain, mice lacking the minor γδ T-cell subset were able to evoke a normal systemic IgG response, which in similarity to wild-type C57BL/6 mice was strongly skewed against Th2 as shown by the dominance of the IgG1 isotype. However, TCRγδ KO mice exhibited a diminished allergen-specific IgE response, indicating that γδ T cells contribute to B-cell secretion of IgE, either by promoting Ig class switch to IgE or by providing activation signals to differentiated IgE-producing cells. Such a role in the systemic B cell reactivity against the allergen is compatible with a previous study by Zuany-Amorim et al.28 but contradicts recent results by Wang and HayGlass.34 The antigen-specific IgE levels are low in serum and difficult to detect using standard immunoassays. We improved the sensitivity of the sandwich ELISA and were thus able to detect both total IgE and allergen-specific IgE in serum. The divergent results by Wang and HayGlass may at least in part be explained by the methodology used for the analysis of OVA-specific IgE in serum, i.e. passive cutaneous anaphylaxis in rats as readout instead of the specific immunoassay employed by us and Zuany-Amorim et al.28 It is recently reported by Schramm et al. that also total IgE levels are suppressed in mice lacking γδ T cells.30 Although we observed reduced total IgE in the TCRγδ-deficient strain when compared to wild-type mice, the statistical significance of this finding was not evident. The apparent discrepancy between the data on OVA-specific IgE and total IgE in our study may reflect a more marked influence of γδ T cells on allergen-specific B cells than on the ‘non-specific’ bystander B-cell population.
In our model of allergic hyperresponsiveness, the challenge was performed using a compressed air nebulizer, yielding a size of the aerosolized particles between 0·1 and 2 µm. This particle size enables high uptake of the allergen in the lungs and favours deposition in the lower respiratory tract. The immune reactivity in bronchioles and alveoli give rise to an airway response, which can be divided in the immediate (within minutes) airway hyperresponsiveness (AHR) manifested as smooth muscle contraction, and the subsequent allergic inflammation beginning 3–6 hr after challenge.32 In the present study we used accumulation of eosinophils in BALF as readout to demonstrate that γδ T cells are required for the allergic airway response. In a previous study by Zuany-Amorim et al. it was shown that γδ T cells also influence pathology measured as infiltration of eosinophils in bronchial tissue.28 Similar results have been reported by Korsgren et al. when analysing eosinphil appearance in whole lung tissue although these investigators did not provide statistical evidence for a γδ T-cell dependent response.35 In addition to impaired eosinophilia in mice lacking γδ T cells we observed a deficient recruitment of lymphocytes to airspaces, most evident for B cells and NK cells. Concurrent with the defective influx of B cells into the lungs, the allergen-specific IgA and IgG responses were significantly reduced. These observations together with similar findings previously reported by Schramm et al.30 indicate that γδ T cells contribute strongly to the immunoglobulin-mediated response at the epithelial border of the respiratory tract. The mechanism for the γδ T cell dependency remains to be defined but it can be interpreted from our data that γδ T cells are particularly important for the recruitment of B cells to the lungs, possibly by producing factors involved in B-cell migration. It can not be excluded, however, that γδ T cells also play a role in the enhancement of the local NK cell and αβ T-cell activities. It is previously reported that high NK cell activity in patients with asthma is associated with exaggerated T cell responses to inhaled antigens.36–38 In a mouse model similar to ours, Korsgren et al. demonstrated that NK cells have a critical role in airway eosinophilia.35 Depletion of NK cells in vivo using a NK1.1-specific mAb inhibited pulmonary eosinophil and T-cell infiltration, activation of T cells in BALF and systemic levels of allergen-specific IgE and IgG2a. These effects were only observed when the mAb was administered before the primary phase of immunization with allergen, however, and not when given during the subsequent aerosol challenge, arguing for a major role of NK cells in the initiation of the systemic Th2-mediated immune response and against a direct involvement in the recruitment of inflammatory cells to the airways.
It has been previously suggested that γδ T cells contribute to airway eosinophilia by enhancing the local Th2 response. In favour of this hypothesis, Zuany-Amorim et al. reported impaired IL-5 release in BALF of TCRγδ KO mice following OVA sensitization and intranasal challenge, and also that the deficient inflammatory response in these mice could be restored by administration of exogenous IL-4.28 More recently, these data were challenged by Lahn et al. who reported that deficiency in γδ T cells was not associated with decreased levels of IL-4 and IL-5 in BALF.29 In this study we used the levels of OVA-specific IgG2b and IgG1 in BALF as markers for IL-4 dependency in the induction of an immunoglobulin-isotype switch, and the ratio of IL-4/IFN-γ mRNA in, lung tissue and regional lymph nodes to monitor the balance of Th2/Th1 immune responses in established airway inflammation. Using the semiquantitative reverse transcriptase (RT)–PCR technology, we did not detect induction of IL-4 and shaping of the immune response towards Th2 in the spleen. However, it is evident from our data of gene transcription in lung tissue that development of allergic airway eosinophilia is accompanied by increased local IL-4 expression and deviation towards Th2 dominance. By removing thoracic lymph nodes and performing BAL on the same animals, we were able to investigate the correlation between cytokine gene expression in draining lymph nodes and the magnitude of airway eosinophilia. In this experiment we observed that elevated number of BALF eosinophils in wild-type mice was highly associated with an increased IL-4/IFN-γ ratio, indicating that the Th2-dominated immune response evoked by sensitization with OVA is maintained in the lungs throughout the course of aerosol challenges, and is closely linked to the recruitment of eosinophils to airspaces.
Because IL-4 strongly promotes expression of IgG1 but markedly inhibits IgG2b,39 a specific role for γδ T cells in an IL-4-dependent isotype switch would be detected by an increased IgG2b/IgG1 ratio in γδ T-cell deficient mice. Such change in immunoglobulin isotype ratio did not occur, however, arguing for the notion that γδ T cells do not contribute to the IL-4-induced immune deviation. Our data on cytokine gene expression in lung tissue and thoracic lymph nodes supported that mice lacking γδ T cells are able to evoke a local IL-4 response and that these mice exhibit a Th2 dominance which is not different from the wild-type strain. The slightly decreased expression of both IL-4 and IFN-γ in lymph nodes from the TCRγδ KO strain might be of relevance for the diminished eosinophilia in these mice. However, this observation probably indicates a general contribution of γδ T cells to the local immune activation rather than a specific role in the skewing towards Th2. In a previous study by Wang and HayGlass. it was demonstrated that γδ T-cell deficient mice do not differ from the wild-type strain in their induction of systemic Th2-dominated response to OVA.34 Our results of an unaffected IL-4/IFN-γ ratio in the spleen of TCRγδ knockout mice are compatible with the data from Wang and HayGlass, providing additional support that mice lacking γδ T cells do not exhibit an aberrant balance of IL-4 and IFN-γ.
It is well established that B cells producing IgE play an important role in mast cell activation, thereby promoting the immediate AHR in human asthma.31,32 In patients, however, the severity of the asthma reaction does not correlate with eosinophilia40 and in experimentally induced allergic hyperresponsiveness it is proposed that the mechanisms inducing the late phase eosinophilia in airways are dissociated from the mechanisms underlying the early bronchospastic response.41–44 Because of several studies with conflicting results the exact role of B cells has not as yet been defined in the murine models, neither in the early nor in the late phase response. It is previously shown that anaphylaxis, bronchial hyperreactivity and eosinophilic inflammation can occur in mice selectively deficient in IgE,45,46 implicating that IgE-independent pathways for mast cell activation and hypersensitivity reactions exist in mice and can be activated in the absence of IgE. Nevertheless, in a model of passive sensitization by injection of allergen-specific antibodies followed by aerosol challenge with allergen, Oshiba et al. demonstrated that IgE and IgG1 contribute to both the immediate AHR and the airway inflammation.47 Our results of a diminished airway eosinophilia in B-cell deficient mice are consistent with such a role of immunoglobulin-producing cells in the sensitization phase of the hypersensitivity reaction. In other models for allergic hyperresponsiveness, which differ from our experimental system by performing repeated aerosol challenge without preceding systemic sensitization, it is reported that B-cell deficient mice48 or mice with selective mutations in IgE46 evoke airway eosinophilia similar to that of wild-type controls. The discordant results in these two studies can, thus, be explained by a contribution of B cells to the experimental allergic inflammation only when mice are sensitized by systemic immunization before aerosol challenge. In a recent study, Sung et al. compared the efficiency of OVA as the antigen with that of the major major histocompatibility complex class-II restricted OVA T-cell epitope OVA323−339 to induce AHR and lung eosinophilia.44 Priming with OVA and OVA-pulsed splenic dendritic cells, but not with OVA323−339 or OVA323−339-pulsed dendritic cells, induced a B-cell response measured as antigen-specific IgG and IgE in serum. With OVA as the antigen both the lung inflammatory response and AHR were higher than in animals immunized with the OVA323-339 peptide. Although the results by Sung et al., together with our own data, indicate that B cells are required for a full-blown allergic eosinophilia, it should be emphasized that in these experimental models the airway hyperresponsiveness can proceed to some extent in the absence of immunoglobulin-producing cells. Under certain experimental conditions it appears that other as yet undefined compensatory mechanisms may completely replace B cells in the presensitization reaction.49–51
In the present study we indicate a possible pathway for the contribution of γδ T cells to the allergic airway eosinophilia. In the systemic sensitization, γδ T cells promote the specific IgE response, and in the subsequent airway inflammation this T cell subset plays an important role in B-cell migration into the lungs and the occurrence of allergen-specific antibodies in airways. γδ T cells did not contribute to the systemic IgG response, however, and appeared not to be essential for the induction of αβ T cells with a Th2 profile. In our experimental model, we also demonstrated that B cells contribute to the airway eosinophilia, most likely by enhancing the efficiency of the allergic sensitization. We conclude that γδ T cells promote allergic airway inflammation by pathways separate from classical Th2-mediated immune activation, but nevertheless leading to systemic and local induction of B-cell responses. The molecular mechanism for such cross-talk between γδ T cells and B cells remains to be defined.
Acknowledgments
We thank Thorsten Johansson, Marlene Lundström, David Rocksén, Barbro Ekstrand-Hammarström for technical assistance and Assoc. Prof Robert A Harris for linguistic advice. The work was financially supported by the Swedish Heart Lung foundation and the Swedish Ministry of Defence.
Abbreviations
- BALF
bronchoalveolar lavage fluid
- KO
knockout
References
- 1.Groh V, Porcelli S, Fabbi M, et al. Human lymphocytes bearing T cell receptor γδ are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989;169:1277–94. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Söderström K, Bucht A, Halapi E, et al. High expression of Vγ8 is a shared feature of human γδ cells in the epithelium of the gut and in the inflamed synovial tissue. J Immunol. 1994;152:6017–27. [PubMed] [Google Scholar]
- 3.Spencer J, Isaacson PG, Diss TC, MacDonald TT. Expression of disulfide-linked and non-disulfide-linked forms of the T cell receptor γ/δ heterodimer in human intestinal intraepithelial lymphocytes. Eur J Immunol. 1989;19:1335–8. doi: 10.1002/eji.1830190728. [DOI] [PubMed] [Google Scholar]
- 4.Ullrich R, Scheiferdecker HL, Ziegler K, Riecken EO, Zeitz M. T cells in human intestine express surface markers of activation and are preferentially located in the epithelium. Cell Immunol. 1990;128:619–27. doi: 10.1016/0008-8749(90)90053-t. [DOI] [PubMed] [Google Scholar]
- 5.Jarry A, Cerf-Benussan N, Brouss N, Selz F, Guy-Grand D. Subsets of CD3+ (T cell receptor α/β or γ/δ) and CD3− lymphocytes isolated from normal human gut epithelium display phenotypic features different from their counterparts in peripheral blood. Eur J Immunol. 1990;20:1097–103. doi: 10.1002/eji.1830200523. [DOI] [PubMed] [Google Scholar]
- 6.Deusch K, Lüling F, Reich K, Classen M, Wagner H, Pfeffer K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the γ/δ T cell receptor, the CD8 accessory molecule and preferentially uses the Vδ1 gene segment. Eur J Immunol. 1991;21:1053–9. doi: 10.1002/eji.1830210429. [DOI] [PubMed] [Google Scholar]
- 7.Goodman T, Lefrancois L. Expression of the γδ T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988;333:855–8. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- 8.Bonneville M, Janeway CA, Ito K, Haser W, Ishida I, Nakanishi N, Tonegawa S. Intestinal intraepithelial lymphocytes are a distinct set of γδ T cells. Nature. 1988;336:479–81. doi: 10.1038/336479a0. [DOI] [PubMed] [Google Scholar]
- 9.Bucht A, Söderström K, Hultman T, Uhlén M, Nilsson E, Kiessling R, Grönberg A. T-cell receptor diversity and activation markers in the Vδ1 subset of rheumatoid synovial fluid and peripheral blood T lymphocytes. Eur J Immunol. 1992;22:567–74. doi: 10.1002/eji.1830220240. [DOI] [PubMed] [Google Scholar]
- 10.Bucht A, Söderström K, Esin S, et al. Analysis of γδ V region usage in normal and diseased human intestinal biopsies and peripheral blood by PCR and flow cytometry. Clin Exp Immunol. 1995;99:57–64. doi: 10.1111/j.1365-2249.1995.tb03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Söderström K, Bucht A, Halapi E, Grönberg A, Magnusson I, Kiessling R. Increased frequency of abnormal γδ T cells in blood of patients with inflammatory bowel diseases. J Immunol. 1996;156:2331–9. [PubMed] [Google Scholar]
- 12.Giacomelli R, Parzanese I, Frieri G, et al. Increase of circulating γ/δ T lymphocytes in the peripheral blood of patients affected by active inflammatory disease. Clin Exp Immunol. 1994;98:83–8. doi: 10.1111/j.1365-2249.1994.tb06611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sioud M, Kjeldsen-Kragh J, Quayle A, Kalvenes C, Waalen K, Förre Ö, Natvig JB. The Vδ gene usage by freshly isolated T lymphocytes from synovial fluids in rheumatoid synovitis: a preliminary report. Scand J Immunol. 1990;31:415–21. doi: 10.1111/j.1365-3083.1990.tb02787.x. [DOI] [PubMed] [Google Scholar]
- 14.Kjeldsen-Kragh J, Quayle A, Kalvenes C, Förre Ö, Sörskar D, Vinje O, Thoen J, Natvig JB. Tγδ cells in juvenile rheumatoid arthritis and rheumatoid arthritis. Scand J Immunol. 1990;32:651–60. doi: 10.1111/j.1365-3083.1990.tb03207.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith MD, Bröker B, Moretta L, et al. Tγδ cells and their subsets in blood and synovial tissue from rheumatoid arthritis patients. Scand J Immunol. 1990;32:585–93. doi: 10.1111/j.1365-3083.1990.tb03200.x. [DOI] [PubMed] [Google Scholar]
- 16.Keystone EC, Rittershaus C, Wood N, Snow KM, Flatow J, Purvis JC, Poplonski L, Kung PC. Elevation of a γδ T cell subset in peripheral blood and synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 1991;84:78–82. [PMC free article] [PubMed] [Google Scholar]
- 17.Lamour A, Jouen-Beades F, Lees O, Gilbert D, Le Loet X, Tron F. Analysis of T cell receptors in rheumatoid arthritis: the increased expression of HLA-DR antigen on circulating γδ+ T cells is correlated with disease activity. Clin Exp Immunol. 1992;89:217–22. doi: 10.1111/j.1365-2249.1992.tb06935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Gabalawy HS, Keillor J. Immunohistologic study of T-cell receptor δ-chain expression in rheumatoid synovial membranes. Semin Arthritis Rheum. 1992;21:239–45. doi: 10.1016/0049-0172(92)90054-h. [DOI] [PubMed] [Google Scholar]
- 19.Spinozzi F, Agea E, Bistoni O, Forenza N, Bertotto A. γδ T cells, allergen recognition and airway inflammation. Immunol Today. 1998;19:22–6. doi: 10.1016/s0167-5699(97)01182-1. [DOI] [PubMed] [Google Scholar]
- 20.Holmdahl R, Goldschmidt TJ, Kleinau S, Kvick C, Jonsson R. Arthritis induced in rats with adjuvant oil is a genetically restricted, alpha beta T-cell dependent autoimmune disease. Immunology. 1992;76:197–202. [PMC free article] [PubMed] [Google Scholar]
- 21.Svelander L, Mussener A, Erlandsson-Harris H, Kleinau S. Polyclonal Th1 cells transfer oil-induced arthritis. Immunology. 1997;91:260–5. doi: 10.1046/j.1365-2567.1997.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansson ÅM, Lorentzen JC, Bucht A. CD8+ cells suppress oil-induced arthritis. Clin Exp Immunol. 2000;120:532–6. doi: 10.1046/j.1365-2249.2000.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlson BC, Jansson ÅM, Larsson A, Bucht A, Lorentzen JC. The endogenous cholesterol precursor squalene can induce a chronic T cell mediated adjuvant arthritis in rats. Am J Pathol. 2000;156:2057–65. doi: 10.1016/S0002-9440(10)65077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelegri C, Kuhnlein P, Buchner E, et al. Depletion of gamma/delta T cells does not prevent or ameliorate, but rather aggravates, rat adjuvant arthritis. Arthritis Rheum. 1996;39:204–15. doi: 10.1002/art.1780390206. [DOI] [PubMed] [Google Scholar]
- 25.Dsouza NB, Mandujano FJ, Nelson S, Summer WR, Shellito JE. CD4 (+) T lymphocyte depletion attenuates lipopolysaccharide-induced tumor necrosis factor secretion by alveolar macrophages in the mouse. Lymphokine Cytokine Res. 1994;13:359–66. [PubMed] [Google Scholar]
- 26.Larsson R, Rocksén D, Lilliehöök B, Jonsson Å, Bucht A. Dose-dependent activation of lymphocytes in endotoxin-induced airway inflammation. Infect Immun. 2000;68:6962–9. doi: 10.1128/iai.68.12.6962-6969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penido C, Castro-Faria-Neto HC, Larangeira AP, Rosas EC, Ribeiro-dos-Santos R, Bozza PT, Henriques MG. The role of γδ T lymphocytes in lipopolysaccharide-induced eosinophil accumulation into the mouse pleural cavity. J Immunol. 1997;159:853–60. [PubMed] [Google Scholar]
- 28.Zuany-Amorim C, Ruffié C, Hailé S, Vargaftig BB, Prereira P, Pretolani M. Requirement for γδ T cells in allergic airway inflammation. Science. 1998;280:1265–7. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 29.Lahn M, Kanehio A, Takeda K, et al. Negative regulation of airway responsiveness that is dependent on γδ T cells and independent of αβ T cells. Nature Med. 1999;5:1150–6. doi: 10.1038/13476. [DOI] [PubMed] [Google Scholar]
- 30.Schramm CM, Puddington L, Yiamouyiannis CA, Lingenheld EG, Whiteley HE, Wolyniec WW, Noonan TC, Thrall RS. Proinflammatory roles of T-cell receptor (TCR) γδ and TCRαβ lymphocytes in a murine model of asthma. Am J Respir Cell Mol Biol. 2000;22:218–25. doi: 10.1165/ajrcmb.22.2.3620. [DOI] [PubMed] [Google Scholar]
- 31.Bochner BS, Undem BJ, Lichtenstein LM. Immunological aspects of allergic asthma. Annu Rev Immunol. 1994;12:295–335. doi: 10.1146/annurev.iy.12.040194.001455. [DOI] [PubMed] [Google Scholar]
- 32.Willis-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–81. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 33.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γδ T cells. Science. 1994;265:1869–71. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 34.Wang MD, HayGlass KT. γδ T-cell-deficient mice do not differ from normal controls in their induction or expression of type 2 dominant responses to exogenous antigen. Immunopharmacology. 2000;48:291–8. doi: 10.1016/s0162-3109(00)00226-5. [DOI] [PubMed] [Google Scholar]
- 35.Korsgren M, Persson CGA, Sundler F, et al. Natural killer cells determine development of allergen-induced eosinophilic airway inflammation in mice. J Exp Med. 1999;198:553–62. doi: 10.1084/jem.189.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timonen T, Stenius-Aarniala B. Natural killer cell activity in asthma. Clin Exp Immunol. 1985;59:85–0. [PMC free article] [PubMed] [Google Scholar]
- 37.Jira M, Antosova E, Vondra V, Strejcek J, Mazakova H, Prazakova J. Natural killer and interleukin-2 induced cytotoxicity in asthmatics. Allergy. 1988;43:294–8. doi: 10.1111/j.1398-9995.1988.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 38.Krejsek J, Král B, Vokurková D, Derner V, Tousková M, Paráková Z, Kopecký O. Decreased peripheral blood γδ T cells in patients with bronchial asthma. Allergy. 1998;53:73–7. doi: 10.1111/j.1398-9995.1998.tb03776.x. [DOI] [PubMed] [Google Scholar]
- 39.Snapper CM, Paul WE. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–7. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 40.McFadden ER., Jr Asthma: Morphologic–physiologic interactions. Am J Respir Crit Care Med. 1994;150:523–6. doi: 10.1164/ajrccm/150.5_Pt_2.S23. [DOI] [PubMed] [Google Scholar]
- 41.Coyle AJ, Köhler G, Tsuyuki S, Brombacher F, Kopf M. Eosinophils are not required to induce airway hyperresponsiveness after nematode infection. Eur J Immunol. 1998;28:2640–7. doi: 10.1002/(SICI)1521-4141(199809)28:09<2640::AID-IMMU2640>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 42.Wilder JA, Collie DS, Wilson BS, Bice DE, Lyons RC, Lipscomb MF. Dissociation of airway hyperresponsiveness from immunoglobulin E and airway eosinophilia in a murine model of allergic asthma. Am J Respir Cell Mol Biol. 1999;20:1326–34. doi: 10.1165/ajrcmb.20.6.3561. [DOI] [PubMed] [Google Scholar]
- 43.Tournoy KG, Kips JC, Schou C, Pauwels RA. Airway eosinophilia is not a requirement for allergen-induced airway hyperresponsiveness. Clin Exp Allergy. 2000;30:79–85. doi: 10.1046/j.1365-2222.2000.00772.x. [DOI] [PubMed] [Google Scholar]
- 44.Sung SJ, Rose EC, Jr, Fu SM. Intratracheal priming with ovalbumin- and ovalbumin 223–339 peptide-pulsed dendritic cells induces airway hyperresponsiveness, lung eosinophilia, Goblet cell hyperplasia and inflammation. J Immunol. 2001;166:1261–71. doi: 10.4049/jimmunol.166.2.1261. [DOI] [PubMed] [Google Scholar]
- 45.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–70. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 46.Melhop PD, Van de Rinj M, Goldberg AB, Brewer J, Kurup V, Martin TR, Oettgen HC. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Immunology. 1997;94:1344–9. doi: 10.1073/pnas.94.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oshiba A, Hamelmann E, Takeda K, Bradley KL, Loader JE, Larsen GL, Glefand EW. Passive transfer of immediate hypersensitivity and airway hyperresponsiveness by allergen-specific immunoglobulin (Ig) E and IgG1 in mice. J Clin Invest. 1996;6:1398–408. doi: 10.1172/JCI118560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamelmann E, Vella AT, Oshiba A, Kappler JW, Marrack P, Gelfand EW. Allergic airway sensitization induces T cell activation but not airway hyperresponsiveness in B cell-deficient mice. Proc Natl Acad Sci USA. 1997;94:1350–5. doi: 10.1073/pnas.94.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korsgren M, Erjefält JS, Korsgren O, Sundler F, Persson CGA. Allergic eosinophil-rich inflammation develops in lungs and airways of B cell-deficient mice. J Exp Med. 1997;5:885–92. doi: 10.1084/jem.185.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacLean JA, Sauty A, Luster AD, Drazen JM, De Sanctis GT. Antigen-induced airway hyperresponsiveness, pulmonary eosinophilia and chemokine expression in B cell-deficient mice. Am J Respir Cell Mol Biol. 1999;20:379–87. doi: 10.1165/ajrcmb.20.3.3291. [DOI] [PubMed] [Google Scholar]
- 51.Hamelmann E, Takeda K, Schwarze J, Vella AT, Irvin CG, Gelfland EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness requires interleukin-5 but not immunoglobulin E or B lymphocytes. Am J Respir Cell Mol Biol. 1999;21:480–9. doi: 10.1165/ajrcmb.21.4.3659. [DOI] [PubMed] [Google Scholar]