Abstract
Exposing bovine dendritic cells (DC) and macrophages (MΦ) to Salmonella typhimurium at a ratio of 1 cell to 10 bacteria had a cytotoxic effect that was not evident with a ratio of 1000 cells to 1 bacterium. This lower dose was considered to mimic more closely the in vivo situation and a comparison was made with this model of the consequences of infection for MΦ and DC. DC infected with S. typhimurium up-regulated cell surface expression of major histocompatibility class I (MHC-I), MHC-II, CD40, CD80 and CD86. In contrast, infected MΦ did not exhibit detectable changes in expression of cell surface molecules, except for a marginal increase in CD40. mRNA transcription for tumour necrosis factor-α, interleukin (IL)-1β, IL-6 and inducible nitric oxide synthase was up-regulated in both infected DC and infected MΦ, although mRNA transcription for granulocyte–macrophage colony-stimulating factor and IL-12p40 was up-regulated only in infected DC and for IL-10 was only in infected MΦ. Infected DC had an increased ability to stimulate both allogeneic and antigen-specific T-cell responses compared to non-infected controls. In contrast, infected MΦ showed an increased ability to induce allogeneic responses but this was less than seen for DC and no enhancement of ability to induce antigen-specific T cell responses was seen. Thus, in a low-dose infection model that does not result in the cytotoxicity of a substantial percentage of antigen presenting cells, bovine MΦ and DC respond differently to infection with S. typhimurium and this could have important implications for the development of the immune response.
Introduction
Salmonella enterica serovar Typhimurium (Salmonella typhimurium) is a Gram-negative facultative intracellular bacterium that can infect a variety of hosts. It causes gastroenteritis in calves similar to the disease induced by this Salmonella serovar in humans, and that is a serious problem in both the livestock industry and for public health. In mice, it causes a typhoid-like systemic infection that provides a surrogate model of typhoid fever in humans, but is of limited value in elucidating the mechanisms of gastroenteritis caused by S. typhimurium in humans and cattle. Thus, the study of S. typhimurium infection in cattle, as well as being important in its own right, is a useful model for understanding S. typhimurium infection in humans.1,2
Macrophages (MΦ) and dendritic cells (DC) are not only important as antigen-presenting cells (APC), but they can also provide a site where Salmonella can potentially escape from attack by immune effector cells.3 MΦ have been used to study the host–pathogen interaction and the pathogenicity of Salmonella4,5 because MΦ are actively phagocytic and crucial in host defence. Following infection with Salmonella in vitro, murine MΦ produce proinflammatory cytokines, and up-regulate major histocompatibility complex class I (MHC-I), MHC-II and costimulatory molecules in a manner similar to that noted following exposure to lipopolysaccharide (LPS).6 Recent evidence suggests that DC also phagocytose Salmonella and can process and present bacterial antigen. Cytokines critical to the immune response such as interleukin (IL)-6 and IL-12 are produced by both DC and MΦ infected with Salmonella.3,7 Thus, it would appear that infection of DC and MΦ by Salmonella have a number of similar consequences for the ensuing immune response to the pathogen.
The majority of studies on the interaction between Salmonella and MΦ and DC have used a ratio of 0·5 bacterium to 1 host cell or higher.3,7–9 These doses have been useful in elucidating many aspects of bacterial pathogenicity. However, use of a high ratio of bacteria to host cells can result in a toxic effect on host cells. DC are considered to be the only APC population capable of inducing primary immune responses10–12 and appropriate in vitro models would be expected to depict differences between the interactions of Salmonella and DC and MΦ that are related to pathogenicity in vivo and development of the immune response.
In this study, we compared the interaction of S. typhimurium with either MΦ or DC using a low dose infection model that does not result in cytotoxicity of a substantial percentage of APC. MΦ and DC reacted differently to infection with S. typhimurium and these differences are in line with the expected roles of each cell type during an infection in vivo.
Materials and methods
Bacteria and preparation of bacterial inocula
The virulent S. typhimurium strain 4/7413 was used throughout. Bacteria for inocula were grown overnight at 37° in static Luria Bertani (LB) broth cultures without shaking. Appropriate dilutions were made in RPMI-1640 media to obtain the number of bacteria required and the inocula numbers were confirmed by colony counts on LB agar.
Preparation of monocyte-derived MΦ and DC
All animal experiments were conducted according to the requirements of the Animal Scientific Procedures Act (1986). Friesian calves were reared conventionally.14 Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by density-gradient centrifugation (1·083 g/ml of Histopaque; Sigma Chemical Co., Poole, UK) and then MΦ and DC were generated as described by Werling et al.15 with some modifications. CD14+ cells were isolated using super-paramagnetic particles labelled with antibodies to human CD14 (Miltenyi-Biotech, Bergisch Gladbach, Germany). The purity of the cells was evaluated by flow cytometry and shown in each case to be > 90%. Cells were adjusted to 106/ml in RPMI-1640 medium containing Glutamax-1 (Life Technologies, Paisley, UK), 10% heat-inactivated fetal calf serum (FCS), 5 × 10−5 m 2-mercaptoethanol and 50 µg/ml of gentamicin (tissue culture medium; TCM). To generate MΦ and DC, 0·2 U/ml of recombinant (r) bovine (b) granulocyte–macrophage colony-stimulating factor (GM-CSF) alone or 0·2 U/ml of rbGM-CSF and 200 U/ml of rbIL-4 were added to TCM, respectively. After 3–4 days, fresh TCM and cytokines was added to cells. Cells were harvested with Cell Dissociation Solution (Sigma) after 6–8 days of culture, washed three times, adjusted to 2·2 × 105/ml in TCM without gentamicin. Unless otherwise stated, 0·9 ml of this suspension was added to each well of 24-well plate. Cells were incubated overnight and used for bacterial infection.
Infection of MΦ and DC with S. typhimurium
MΦ and DC cultures prepared as above were added bacteria in 0·1 ml of RPMI-1640. RPMI-1640 alone was added for non-infected negative control. The ratios of bacteria to APC were 1 bacterium to 1000 APC (200 bacteria/well) for low-dose infection and 10 bacteria to 1 APC (2 × 106 bacteria/well) for high dose infection. In the low dose infection, APC were incubated with bacteria for 4 hr, this allowed the interaction of bacteria and APC to occur without the bacteria overgrowing the culture. After 4 hr gentamicin was added at 50 µg/ml and incubated for an additional 20 hr. In the high-dose infection, APC were infected with bacteria for 1 hr to allow the interaction between bacteria and APC to occur without the bacteria overgrowing the culture. After 1 hr gentamicin at 50 µg/ml and incubated for a further 23 hr. Cells were harvested using cell dissociation solution for further analysis.
Determination of intracellular and extracellular viable bacteria
To quantify extracellular viable bacteria, supernatants of cultures were collected 1 hr after infection (high dose infection) or 4 hr after infection (low-dose infection and negative control) and plated onto LB agar after serial dilution. To quantify intracellular viable bacteria after infection, gentamicin at 50 µg/ml was added and the cultures were incubated for 2 h more. Cells were washed 3 times with sterile phosphate-buffered saline (PBS) to remove the gentamicin and lysed with 1% Triton-X-100 (Sigma). Lysates were titrated on LB agar. The number of colony forming units was determined after overnight incubation of the agar plates at 37°.
Determination of cytotoxicity in infected MΦ and DC using the lactate dehydrogenase (LDH) assay
Cytotoxicity in MΦ and DC cultures was evaluated by determining the release of LDH into the culture supernatant using a Cytotoxicity Detection Kit (Boehringer Mannheim, Mannheim, Germany). Supernatants were collected by centrifugation at 1 and 24 hr following high dose infection or 4 and 24 hr after low dose infection. Maximum LDH release was determined by measuring the amount of LDH in non-infected control cell lysates, prepared by lysing the cells with 1% Triton-X-100 after the supernatants were taken. Percentage cytotoxicity was determined according to the following formula: [(OD sample − OD medium)/(OD maximum LDH release − OD medium)] × 100.
Confocal microscopy
After 6–8 days of culture, APC were washed and adjusted to 2·2 × 105/ml in TCM without gentamicin and 0·45 ml added to wells in a 24-well plate containing a sterile 13-mm diameter coverslip. After overnight incubation, 100 S. typhimurim 4/74 in 50 µl of RPMI-1640 was added to wells. After 4 hr incubation, gentamicin was added and the cells were incubated for a further 20 hr. Cells were fixed with 1% paraformaldehyde in PBS for 10 min at room temperature. To stain the cytoskeleton, Phalloidin-Alexa Fluor 488 conjugate (Molecular Probes, Eugene, OR) was used according to the manufacturer's instructions. To determine the presence of S. typhimurium, cells were stained with anti-S. typhimurium LPS rabbit serum followed by incubation with Alexa Fluor 568-conjugated goat IgG anti-rabbit immunoglobulin (Molecular Probes). To stain the nuclei, To-PRO-3 (1/10 000 PBS, Molecular Probes) was used. Confocal microscopy was performed using a Leica TCSNT Confocal Microscope (Leica Microsystem, Milton Keynes, UK).
Flow cytometry
The sources of mouse mAb and their isotypes, secondary reagents and methods for flow cytometry have been described in detail.16 The defined surface antigens assessed and monoclonal antibodies (mAb) used to detect the molecules were as follows: MHC class I (IL-A88; immunoglobulin G2a (IgG2a)), MHC class II (CC158; IgG2a), CD40 (IL-A156; IgG1), CD80 (N32/52-3; IgG1) and CD86 (IL-A190; IgG1). Isotype-matched controls were murine mAb against chicken surface proteins AV20 (Bu-1, IgG1) and AV37 (chicken spleen cell subset, IgG2a). Bound mAb were detected with rabbit anti-mouse immunoglobulin conjugated to fluorescein isothiocyanate (FITC). APC were also stained with propidium iodide (PI) to determine viability. Immunofluorescent staining was analysed using the FACSCalibur (Becton-Dickinson, San Jose, CA) and FCS Express software (De Novo Software, Ontario, Canada). PI negative cells with high forward and side scatter angle were used for analysis.
T-cell proliferation assay
CD4+ T cells were isolated from PBMC following staining with a mouse mAb to bovine CD4 (CC8; IgG2a). PBMC were incubated with super-paramagnetic beads coated with rat immunoglobulin to mouse IgG2a (Miltenyi-Biotech) according to the manufacturer's instructions. Either allogeneic or S. typhimurium-specific CD4+ T cells (105/well) were incubated in triplicate with 104/well MΦ or DC in a total volume of 200 µl of TCM. Cultures were incubated for 5 days at 37°. Thirty-seven mBq of [3H]thymidine (3H-TdR; DuPont, Stevenage, UK) was added to each well for the final 18 hr of culture.
Reverse transcriptase–polymerase chain reaction (RT–PCR)
mRNA was extracted from APC using the DynabeadsTM mRNA DIRECT Kit (Dynal UK Ltd, Wirral, UK). The mRNA was processed for reverse transcription with Reverse Transcription System (Promega, Southampton, UK) using oligo-(dT)15 primer. cDNA was then analysed for the presence of sequences encoding for bovine iNOS, IL-1β, IL-6, IL-10, IL-12 p40, GM-CSF and tumour necrosis factor-α (TNF-α) by PCR, as described previously17 with some modifications. The primers used to detect bovine cytokines and their annealing temperatures are shown in Table 1. The performance of RT–PCR was monitored using plasmids containing the expected product as positive control or diethyl pyrocarbonate-treated water as negative control. Products were analysed by electrophoresis on 1% agarose gel with ethidium bromide staining and compared by normalizing the target signal to the β-actin signal from each sample.
Table 1.
Sequences and annealing temperature for primers used in RT–PCR analysis
| Primer | ||||
|---|---|---|---|---|
| Target | Sense (5′−3′) | Antisense (5′−3′) | Annealing temp (°) | Product size (bp) |
| IL-1β | AGTGAAGTTCAGGCTGCAGCT | TGACGCACCCGTTCAGTCAAT | 59 | 564 |
| IL-6 | AGGCAGACTACTTCTGACCAC | CAGCTACTTCATCCGAATAGC | 59 | 505 |
| IL-10 | GCAGCTCAGCACTGCTCTGTT | CGTTGTCATGTAGGTTTCTAT | 59 | 518 |
| IL-12 p40 | GCAGTACACCTGTCACAAAG | GACCACGACCTCAATAAGC | 55 | 325 |
| GM–CSF | AGTCCTCAAGAGGATGTGGC | TGGCCTGCTTCACTTCTGG | 59 | 457 |
| iNOS | TAGAGGAACATCTGGCCAGG | TGGCAGGGTCCCCTCTGATG | 59 | 372 |
| TNF-α | ACTCAGGTCCTCTTCTCAAGCC | ATGATCCCAAAGTAGACCTGCC | 60 | 464 |
| β-actin | CCAGACAGCACTGTGTTGGC | GAGAAGCTGTGCTACGTCGC | 55 | 270 |
Statistical analysis
Statistics was performed by analysis of variance using the StatView 4·0 (Abacus Concepts, Inc., Berkeley, CA).
Results
MΦ and DC survive better in a low-dose infection model than in a high-dose infection model
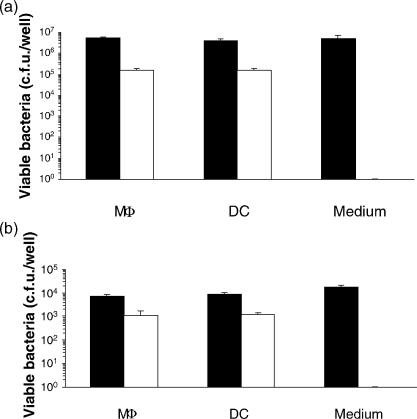
MΦ and DC were infected with S. typhimurium at a multiplicity of infection (MOI) of 0·001 (low dose infection) or 10 (high dose infection). Similar proportions of S. typhimurium were recovered from both MΦ and DC depending on the inoculation dose (Fig. 1a,b), of which approximately 10% were intracellular. As can be seen from Fig. 1, in the low-dose infection the number of S. typhimurium recovered intracellularly is approximately 103 colony forming units (c.f.u.). Assuming 1 c.f.u. would be recovered from one cell, then at most 1% of the DC or MΦ were infected with S. typhimurium.
Figure 1.
Bacterial recovery from MΦ and DC after exposure to S. typhimurium. MΦ and DC were exposed to S. typhimurium 4/74 at a ratio of 10 bacteria to 1 APC and incubated for 1 hr in a high-dose infection (a) or at a ratio of 1 bacterium to 1000 APC and incubated for 4 hr in a low-dose infection (b). As positive controls, each dose of inoculum was added to medium only without APC and processed following the same procedure. Closed bars: extracellular bacteria, Open bars: intracellular bacteria. Results presented are representative data from three experiments. Values are expressed as mean + SD of triplicates. Notice the different scales between a and b.
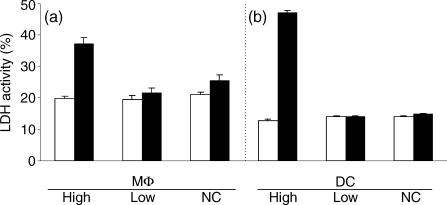
Host cell viability was determined by measuring the level of LDH in the culture supernatants. No difference in host cell viability was detected 1 or 4 hr after high- or low-dose infection compared to non-infected controls (Fig. 2a,b). However, 24 hr after infection, more cytotoxic effects were seen in cells infected with the high dose of bacteria compared to the non-infected control. In contrast, no significant cytotoxic effect was seen in cells exposed to the low dose of infection compared to the negative controls. Thus, the high-dose infection generated more cytotoxic effect than the low-dose infection. Therefore, the low-dose infection model was used for subsequent analysis of the response of MΦ and DC to S. typhimurium.
Figure 2.
LDH released to supernatant after exposure to S. typhimurium. LDH in supernatants from the high dose and the low dose cultures was measured 1 (High) or 4 (Low and NC) hr after infection (open bars) and 24 hr after infection (closed bars). Gentamicin was added to all the cells 1 (High) or 4 (Low and NC) hr after infection. Multiplicity of infection was 10 (High), 10−3 (Low) and 0 (NC), respectively. Data presented are representative data from three experiments. Values are expressed as percentage relative to cell lysate of negative control (mean + SD of triplicates).
DC but not MΦ undergo morphological changes in response to infection with S. typhimurium
Twenty-four hr after infection with S. typhimurium, DC, but not MΦ, had developed distinctive processes (Fig. 3a) which were not present in the non-infected controls (Fig. 3b). To determine the time point at which cells underwent change, cultures were monitored using time-lapse photography. Five hr after infection approximately 10% of the cell population were mobile and changing shape. Twelve to 18 hr after infection approximately 80% of cells had undergone morphological changes. Although most DC in the culture were affected, it is clear that, given the number of bacteria, not all the cells had been infected with S. typhimurium. The association between cells undergoing morphological changes and infection with S. typhimurium was determined by staining with phalloidin, to stain F-actin, and with antibodies to S. typhimurium, to determine the presence of S. typhimurium (Fig. 3c). We confirmed that not all DC were infected with Salmonella and that not all Salmonella were intracellular. No difference was observed in the distribution of F-actin compared with negative controls.
Figure 3.
Morphological change of DC 24 hr after exposure to S. typhimurium. Morphological change of DC 24 hr after exposure to low dose of S. typhimurium(a) and negative control (b). Bar 25 µm. Confocal microscopy showed morphological change occurred to some cells without intracellular bacteria (c). Equally, there were some extracellular Salmonella. Green: phalloidin, Red: anti-S. typhimurium LPS, Blue: To-PRO-3 (nuclei). Bar 50 µm.
Cell surface expression of costimulatory molecules increased in DC but not MΦ in response to S. typhimurium
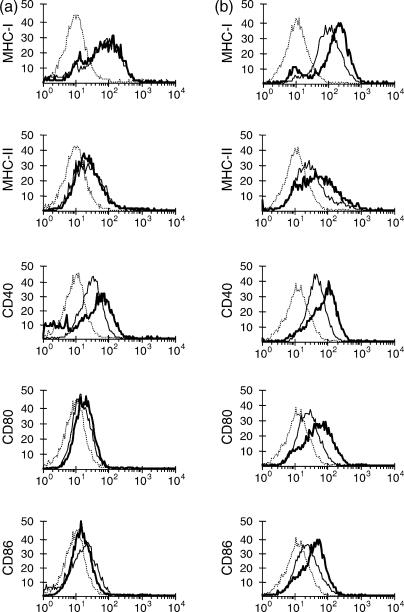
The changes in expression of a number of molecules involved in antigen presentation and stimulation of T cells in MΦ and DC 24 hr after S. typhimurium infection were evaluated by flow cytometry. Compared to non-infected cultures, infected DC showed increased expression of MHC-I, MHC-II, CD40, CD80 and CD86 (Fig. 4b). On the other hand, infected MΦ showed a marginal increase of CD40, but no detectable increase in expression of the other surface markers compared to non-infected control cultures (Fig. 4a).
Figure 4.
Expression of surface molecules on MΦ and DC 24 h after exposure to S. typhimurium. MΦ (a) and DC (b) were stained with monoclonal antibodies to bovine MHC-I, MHC-II, CD40, CD80 and CD86 24 hr after exposure to a low dose of Salmonella (thick lines) or non-infected negative control (thin lines). These cells were also stained with isotype-matched control antibody, of which data only for non-infected cells were shown (dotted lines) since infected cells showed the same staining pattern with the isotype-matched control antibody. Data are representative of at least four independent experiments.
MΦ and DC increase transcription of pro-inflammatory cytokine mRNA after infection with S. typhimurium
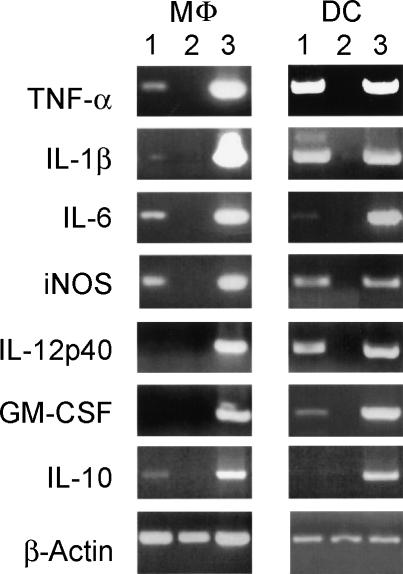
Expression of cytokine mRNA was analysed by RT-PCR 24 h after infection (Fig. 5). Infected MΦ and DC up-regulated transcription of mRNA for TNF-α, IL-1β, IL-6 and iNOS compared to non-infected control cultures, suggesting a proinflammatory response was induced in both cell types. IL-12 p40 mRNA and GM-CSF mRNA were up-regulated in infected DC, but not in infected MΦ. However, MΦ but not DC up-regulated transcription of IL-10 mRNA, suggesting MΦ have the potential to down-regulate the initial pro-inflammatory responses.
Figure 5.
Synthesis of mRNA transcripts for cytokines 24 h after exposure to S. typhimurium by RT–PCR. MΦ: macrophages, DC: dendritic cells, Lanes 1: cells infected with S. typhimurium, 2: non-infected cells, 3: positive plasmid control. Data are representative of at least three independent experiments.
MΦ and DC show increased ability to stimulate T-cell responses after infection with S. typhimurium
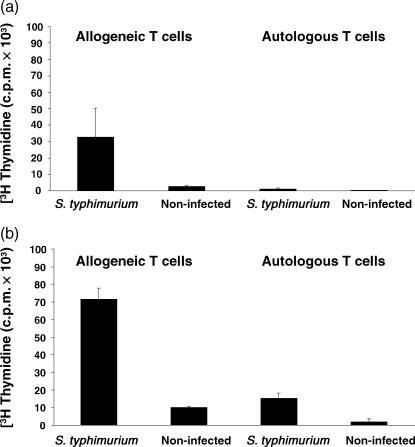
The response of MΦ and DC to infection with S. typhimurium in terms of morphology, expression of costimulatory molecules and cytokine transcription, suggests that these cells had been activated and might be more effective APC. To test this, the ability of DC and MΦ exposed to Salmonella to stimulate proliferation of CD4+ T cells was tested and compared to that of non-infected MΦ and DC. Both MΦ (Fig. 6a) and DC (Fig. 6b) exposed to S. typhimurium for 24 hr stimulated greater proliferative responses of allogeneic CD4+ T cells than their non-infected counterpart. DC induced more proliferation than MΦ. Furthermore, DC but not MΦ exposed to S. typhimurium stimulated Salmonella-specific autologous T-cell proliferative responses, suggesting that DC are more efficient APC than MΦ in immunized cattle.
Figure 6.
Stimulatory capacity of MΦ and DC for CD4+ T cells. MΦ (a) and DC (b) were harvested 24 hr after exposure to a low dose of S. typhimurium. Cells were washed and processed for a T-cell proliferation assay. Either allogeneic or autologous CD4+ T cells (105 cells/well) were incubated in triplicate with 104 cells/well of MΦ or DC. Autologous CD4+ T cells were isolated from a calf previously vaccinated with an isogenic aroA aroD mutant strain. Cultures were incubated for 6 days as indicated in Materials and Methods and pulsed with [3H]TdR for the final 18 hr of culture. Results are expressed as average of counts per minute (c.p.m.) + SD. Data are representative of at least three independent experiments.
Discussion
Salmonella species have been reported to infect both DC and MΦ derived from mice and humans.3,7,8,18 However, the interaction of Salmonella with MΦ or DC may differ in many ways, being influenced by the properties of the APC. Broadly, the role of DC is to uptake and present the processed antigens to T cells while MΦ are highly developed for the killing and degrading of bacteria.19,20 The effect on cells is also affected by bacterial virulence.8,21,22 Given the different role of MΦ and DC as APC19 it would be expected that infection of these APC with salmonellae would cause different effects in the cells and these would influence the development of the immune response.
In models in which ratios of 10 Salmonella to 1 APC have been used with murine DC, infection with salmonellae can reduce the viability of APC cells by up to 50%.3 Others have reported that no cytotoxicity is observed in infected DC even when using ratios of 15 bacteria to 1 eukaryotic cell.18 High ratios of bacteria: APC are unlikely to equate to in vivo ratios in early stages but maybe more relevant to later stages when marked pathological changes are evident. A low-dose infection model might be a more useful tool for the study of host pathogen interactions more similar to the in vivo situation.
Despite only a small percentage of cells being infected with Salmonella, morphological changes were evident in DC. None were seen in MΦ indicating different responses of the two types of APC to the bacteria. The time frame over which these changes took place and the observation that only some DC were infected indicates that, following infection/contact with salmonellae, cells were able to secrete factors that induced the remainder of the cells to change their morphology. It is known that some cytokines such as TNF-α and some bacterial components such as LPS and bacterial DNA can induce maturation of DC accompanied by morphological change.11 Further analysis is necessary to elucidate the exact mechanism of induction of the changes induced in the cytoskeleton of DC triggered in response to S. typhimurium infection.
Changes in the expression of MHC-I, MHC-II, CD80 and CD86 after infection with S. typhimurium were evident with DC. This is in accordance with reported data in mice with a high ratio of bacteria: APC that showed maturation of DC, induced by bacterial components, is accompanied by increased expression of costimulatory molecules.11 Our data suggest that DC respond in a similar fashion to a low dose Salmonella infection. On the other hand, in contrast to published observations in the murine model7 MΦ did not show an increase in the expression of cell surface MHC-I, MHC-II, CD80 or CD86 after low-dose S. typhimurium infection.
S. typhimurium infection of both DC and MΦ resulted in an increase in the levels of mRNA transcripts for TNF-α, IL-1β, IL-6 and iNOS. This was in contrast to murine DC which have been reported not to up-regulate iNOS or indeed not to produce nitric oxide (NO) in response to Salmonella.3,7 This may be caused by a species difference because it is known that iNOS in bovine MΦ is induced by cytokines under much more restricted conditions than in rodents.23
Of potentially great importance for the induction of an immune response were the differences noted for DC and MΦ in the low-dose model in the synthesis of IL-12, IL-10 and GM-CSF. DC exposed to Salmonella up-regulated synthesis of IL-12 and GM-CSF, but MΦ did not. In contrast, MΦ up-regulated synthesis of IL-10 but DC did not. This has significant implications for the developing immune responses and contrasts with observation in mice with high dose infection in which IL-12 was also produced by MΦ.3,7 GM-CSF would promote T-cell responses and IL-12 bias a T helper 1 (Th1) response. IL-10 is considered to be anti-inflammatory in cattle, rather than biasing a Th2 response, and would down-regulate responses.24
The changes detected in morphology, cell surface molecule expression and cytokine transcription would be expected to have consequences for the ability of MΦ and DC to present antigens to T cells. Indeed, DC and MΦ exposed to low-dose Salmonella infection showed an increased ability to induce allogeneic T-cell responses. However, only DC were able to induce Salmonella-specific autologous proliferative responses. These data are in agreement with published observations that DC are more potent antigen presenting cells than MΦ.25 Given that MΦ showed little changes in the expression of cell surface markers, it is of interest that these cells were able to induce allogeneic proliferative responses. This might be the combined result of small changes in cell surface markers (CD40), along with the expression of some pro-inflammatory and anti-inflammatory cytokines. Thus, we report for the first time that clear differences can be detected in the manner in which bovine DC and MΦ respond to infection with Salmonella. This is of importance for understanding how S. typhimurium induces disease. From the results presented, it could be predicted that infection of DC with Salmonella would result in the development of a rapid immune response with a Th1 bias, given the production of IL-12, which might result in the control of the infection. On the other hand, infection of MΦ might result in a delay in the establishment of the immune response, and this response might not necessarily be a strong Th1 type response, given the lack of IL-12. This in turn might result in dissemination of the infection and disease. Thus, with a low-dose infection as would be expected in vivo, the nature of the APC and its response to infection with Salmonella could affect the outcome of the infection.
Acknowledgments
This work was supported by a grant from the Biotechnology and Biological Sciences Research Council. The authors thank Thomas W. Jungi, University of Berne, Switzerland for his kind gift of the 372-bp iNOS plasmid and Neil D. MacHugh, ILRI, Kenya for his kind gift of the mAbs to bovine CD40, CD80 and CD86.
Abbreviations
- DC
dendritic cells
- MΦ
macrophages
- APC
antigen-presenting cells
References
- 1.Tsolis RM, Kingsley RA, Townsend SM, Ficht TA, Adams LG, Baumler AJ. of mice, calves, and men. Comparison of the mouse typhoid model with other Salmonella infections. Adv Exp Med Biol. 1999;473:261–74. [PubMed] [Google Scholar]
- 2.Wallis TS, Galyov EE. Molecular basis of Salmonella-induced enteritis. Mol Microbiol. 2000;36:997–1005. doi: 10.1046/j.1365-2958.2000.01892.x. [DOI] [PubMed] [Google Scholar]
- 3.Marriott I, Hammond TG, Thomas EK, Bost KL. Salmonella efficiently enter and survive within cultured CD11c+ dendritic cells initiating cytokine expression. Eur J Immunol. 1999;29:1107–15. doi: 10.1002/(SICI)1521-4141(199904)29:04<1107::AID-IMMU1107>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Pieters J. Evasion of host cell defense mechanisms by pathogenic bacteria. Curr Opin Immunol. 2001;13:37–44. doi: 10.1016/s0952-7915(00)00179-5. [DOI] [PubMed] [Google Scholar]
- 5.Lalmanach AC, Lantier F. Host cytokine response and resistance to Salmonella infection. Microbes Infect. 1999;1:719–26. doi: 10.1016/s1286-4579(99)80073-2. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberger CM, Scott MG, Gold MR, Hancock RE, Finlay BB. Salmonella typhimurium infection and lipopolysaccharide stimulation induce similar changes in macrophage gene expression. J Immunol. 2000;164:5894–904. doi: 10.4049/jimmunol.164.11.5894. [DOI] [PubMed] [Google Scholar]
- 7.Yrlid U, Svensson M, Johansson C, Wick MJ. Salmonella infection of bone marrow-derived macrophages and dendritic cells. influence on antigen presentation and initiating an immune response. FEMS Immunol Med Microbiol. 2000;27:313–20. doi: 10.1111/j.1574-695X.2000.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 8.Dreher D, Kok M, Cochand L, Kiama SG, Gehr P, Pechere JC, Nicod LP. Genetic background of attenuated Salmonella typhimurium has profound influence on infection and cytokine patterns in human dendritic cells. J Leukoc Biol. 2001;69:583–9. [PubMed] [Google Scholar]
- 9.Finlay BB, Brumell JH. Salmonella interactions with host cells: in vitro to in vivo. Phil Trans R Soc Lond B Biol Sci. 2000;355:623–31. doi: 10.1098/rstb.2000.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rescigno M, Granucci F, Citterio S, Foti M, Ricciardi-Castagnoli P. Coordinated events during bacteria-induced DC maturation. Immunol Today. 1999;20:200–3. doi: 10.1016/s0167-5699(98)01427-3. [DOI] [PubMed] [Google Scholar]
- 11.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 12.Rescigno M, Granucci F, Ricciardi-Castagnoli P. Molecular events of bacterial-induced maturation of dendritic cells. J Clin Immunol. 2000;20:161–6. doi: 10.1023/a:1006629328178. [DOI] [PubMed] [Google Scholar]
- 13.Villarreal-Ramos B, Manser JM, Collins RA, Chance V, Eckersall D, Jones PW, Dougan G. Susceptibility of calves to challenge with Salmonella typhimurium 4/74 and derivatives harbouring mutations in htrA or purE. Microbiology. 2000;146:2775–83. doi: 10.1099/00221287-146-11-2775. [DOI] [PubMed] [Google Scholar]
- 14.Villarreal-Ramos B, Manser J, Collins RA, Dougan G, Chatfield SN, Howard CJ. Immune responses in calves immunised orally or subcutaneously with a live Salmonella typhimurium aro vaccine. Vaccine. 1998;16:45–54. doi: 10.1016/s0264-410x(97)00156-4. [DOI] [PubMed] [Google Scholar]
- 15.Werling D, Hope JC, Chaplin P, Collins RA, Taylor G, Howard CJ. Involvement of caveolae in the uptake of respiratory syncytial virus antigen by dendritic cells. J Leukoc Biol. 1999;66:50–8. doi: 10.1002/jlb.66.1.50. [DOI] [PubMed] [Google Scholar]
- 16.Howard CJ, Sopp P, Brownlie J, Kwong LS, Parsons KR, Taylor G. Identification of two distinct populations of dendritic cells in afferent lymph that vary in their ability to stimulate T cells. J Immunol. 1997;159:5372–82. [PubMed] [Google Scholar]
- 17.McInnes E, Collins RA, Taylor G. Cytokine expression in pulmonary and peripheral blood mononuclear cells from calves infected with bovine respiratory syncytial virus. Res Vet Sci. 1998;64:163–6. doi: 10.1016/s0034-5288(98)90013-3. [DOI] [PubMed] [Google Scholar]
- 18.Svensson M, Johansson C, Wick MJ. Salmonella enterica serovar typhimurium-induced maturation of bone marrow-derived dendritic cells. Infect Immun. 2000;68:6311–20. doi: 10.1128/iai.68.11.6311-6320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellman I, Turley SJ, Steinman RM. Antigen processing for amateurs and professionals. Trends Cell Biol. 1998;8:231–7. doi: 10.1016/s0962-8924(98)01276-8. [DOI] [PubMed] [Google Scholar]
- 20.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 21.Hopkins SA, Niedergang F, Corthesy-Theulaz IE, Kraehenbuhl JP. A recombinant Salmonella typhimurium vaccine strain is taken up and survives within murine Peyer's patch dendritic cells. Cell Microbiol. 2000;2:59–68. doi: 10.1046/j.1462-5822.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- 22.Niedergang F, Sirard JC, Blanc CT, Kraehenbuhl JP. Entry and survival of Salmonella typhimurium in dendritic cells and presentation of recombinant antigens do not require macrophage-specific virulence factors. Proc Natl Acad Sci USA. 2000;97:14650–5. doi: 10.1073/pnas.97.26.14650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adler H, Frech B, Thony M, Pfister H, Peterhans E, Jungi TW. Inducible nitric oxide synthase in cattle. Differential cytokine regulation of nitric oxide synthase in bovine and murine macrophages. J Immunol. 1995;154:4710–8. [PubMed] [Google Scholar]
- 24.Brown WC, Rice-Ficht AC, Estes DM. Bovine type 1 and type 2 responses. Vet Immunol Immunopathol. 1998;63:45–55. doi: 10.1016/s0165-2427(98)00081-6. [DOI] [PubMed] [Google Scholar]
- 25.Giacomini E, Iona E, Ferroni L, Miettinen M, Fattorini L, Orefici G, Julkunen I, Coccia EM. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J Immunol. 2001;166:7033–41. doi: 10.4049/jimmunol.166.12.7033. [DOI] [PubMed] [Google Scholar]