Abstract
Leishmania major infected BALB/c mice were treated with N-acetyl-l-cysteine (NAC), a glutathione precursor, to evaluate the role of in vivo glutathione on lesion pathology and cytokine profiles following infection. Mice were maintained on NAC-containing water 2 days before infection for a total of 14 weeks. The BALB/c response to L. major infection was improved by oral administration of NAC, at the level of histopathological outcome, lesion progression and cytokine profile. A significantly improved histopathological outcome of the footpad lesion, characterized by a mixed inflammatory infiltrate organized in a focal pattern with little tissue destruction and a reduced parasite load, was observed in NAC-treated BALB/c mice. Histopathological modulation was accompanied by a modified cytokine pattern from popliteal lymph node cells, demonstrated by a sustained higher frequency of interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α)-producing cells. This work points to an important role for glutathione in the modulation of effector responses in BALB/c mice.
Introduction
Leishmaniasis is a parasitic disease caused by several species of the genus Leishmania. It has recently been declared as one of the world's most serious parasitic diseases by the World Health Organization, with an annual incidence of new cases of about 2 million, and a worldwide prevalence of 12 million individuals.1 Infection of mice with L. major is a well-established model for the investigation of factors that control disease development. Most inbred strains of mice, termed resistant or healer strains, develop a local inflammation at the site of L. major inoculation that resolves over a period of 4–8 weeks, as a consequence of interleukin-12 (IL-12)-dependent expansion of interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α)-producing cells (Th1 cells).2,3 These cytokines are essential for nitric oxide-dependent killing of the intracellular amastigote forms of Leishmania.4–6 In contrast, infection of susceptible strains such as BALB/c leads to the establishment of severe uncontrolled cutaneous lesions. These mice mount a strong Th2 response after L. major infection, characterized by the production of high levels of IL-4, IL-10 and IL-13·7,8 In these mice, IL-4 and IL-10 interfere with IFN-γ-dependent activation of macrophages.9
Many factors are involved in the differentiation of Th1 and Th2 responses. Recently Peterson et al.10 demonstrated that intracellular glutathione levels in antigen-presenting cells influence the Th1/Th2 cytokine response pattern. It was demonstrated that glutathione depletion inhibits Th1-associated cytokine production and favours Th2-associated responses. Replenishing glutathione levels, using N-acetyl-l-cysteine (NAC), restored the Th1 response pattern in their system.
To examine whether increased in vivo glutathione would influence the outcome of disease and progression of pathology during infection of BALB/c mice with L. major, control and NAC treated BALB/c mice were infected with L. major. NAC is a thiol antioxidant, converted in the body into metabolites capable of stimulating glutathione synthesis.11 It has been used historically as a mucolytic agent in a variety of human respiratory illnesses and is used today in a number of diseases.12–14 Our results show that oral administration of NAC in BALB/c mice infected with L. major improves the histopathology of the lesion, and correlated with changes in the balance between IL-4 and IFN-γ- or TNF-α-producing cells. These findings suggest that glutathione levels can modulate the effector response in Leishmania infections. Our data also suggest a potential immunotherapeutic application for NAC.
Materials and methods
Leishmania parasite and infection of mice
Leishmania major (MHOM/SU/73/5-ASKH) was the strain used. Specific pathogen free 7–8-week-old female BALB/c mice were infected with 2 × 106 viable stationary-phase promastigotes s.c. in the right hind footpad. The progression of the infection was monitored weekly by the measurement of the thickness of the resulting cutaneous lesion.
Chemicals and antibodies
N-acetyl-l-cysteine (Zambom Group, Vicenza, Italy) was dissolved in filtered water at 1 mg/ml. Brefeldin A (Sigma) was dissolved in ethanol at 1 mg/ml and stored at −80°. Saponin was purchased from Sigma. All flow cytometry antibodies were purchased from Caltag (CA). Eosin and haematoxilin were purchased from Merk (Germany).
NAC consumption model
Control groups were fed a solid diet and water ad libitum. NAC-treated group had the water substituted by a solution of N-acetyl-l-cysteine (1 mg/ml). Mice were switched to the NAC containing water two days before L. major infection, and subsequently for 13 weeks.
Glutathione measurement
Reduced glutathione levels of popliteal lymph node cells were determined spectrophotometrically using a kit for GSH concentration determination from Oxis International Inc (Portland), according to the manufacturer's instructions.
Histopathological analysis
Morphometric analysis were employed to quantify tissue vacuolization and parasitism. Fragments of footpad lesion were fixed in Bouin's solution 4% (picric acid 75%, glacial acetic acid 5% and 10% formaldehyde) and embedded in paraffin. Sections of 5–6 µm were stained with haematoxilin and eosin (H&E) for histopathological analysis. Images from slides were captured in a Zeiss Axiolab microscope connected to a video camera and a computer-digitized plate. Quantitative morphometric analysis were performed using the digitalized images and analysis by the Gel Expert software (Nucleotech Corp.). The parasite load for each lesion was measured in 10 non-contiguous fields (magnification 630 ×), measuring the total parasite number per field. Tissue vacuolization area for each lesion was also measured in 10 non-contiguous fields per section (magnification 200 ×). The means of at least three lesions per group were compared using Student's t-test, contained within the statistical software package JMP from SAS. Significant differences where those that returned P-values < 0·05.
Flow cytometry analysis
Popliteal lymph node cells were evaluated for intracellular cytokine expression patterns as described by Openshaw et al.15 Briefly, lymph node cells were cultured in 24-well plates in 1-ml culture for 20 h in the presence of 1/50 diluted freeze/thawed soluble leishmania antigen (SLA) at an initial concentration of 4 × 108/ml as determined previously by us and personal communication with R.L. Coffman, Dynavax, Berkely, CA. During the last 4 hr of incubation, Brefeldin A (1 µg/ml) was added to the cultures. Cells were harvested, stained for surface markers, fixed with 2% formaldehyde and then permeabilized with a solution of saponin and stained for 30 min at 4°, using anticytokine monoclonal antibodies directly conjugated with phycoerythrin (PE). Preparations were then analysed using a FACSVantage (Becton Dickinson), gating on a total lymphocyte/monocyte population. In all cases, 30 000 gated events were acquired for later analysis. The antibodies used for the staining were rat immunoglobulin control(s), anti-CD11b-FITC, anti-IFN-γ-PE, anti-IL4-PE, anti-IL10-PE and anti-TNF-α-PE. Lymph node cells were analysed for their intracellular cytokine expression patterns and frequencies using the program Cell Quest (Becton Dickinson, San Jose, CA). The frequency of positive cells was analysed using a gate that included lymphocytes, large blast lymphocytes and monocytes/macrophages. Limits for the quadrant markers were always set based on negative populations and isotype controls.
Results
NAC-treatment leads to improved histopathology
Infection of BALB/c mice with L. major leads to the development of progressive, uncontrolled cutaneous lesions, which are correlated with the establishment of Th2 responses, and the absence of effective cellular immunity. In the present work a relatively mild strain of L. major was used to increase the window for studying the ability of increased glutathione levels to modulate lesion pathology and cellular responses during the course of infection. BALB/c mice were started on NAC containing water 2 days before L. major infection and maintained on the NAC water up to 13 weeks of infection. Consumption of NAC containing water by the experimental group was similar to water consumption by the control group (4·11 ± 0·37 ml/day/animal and 4·39 ± 0·59 ml/day/animal, respectively). At the time of infection, in vivo glutathione levels as measured using popliteal lymph node cells were increased by approximately 35% in NAC-treated BALB/c mice (2·3 × 10−2µmol/l/106 cells) when compared to the control group (1·7 × 10−2µmol/l/106 cells). These increased glutathione levels in the NAC-treated mice were maintained at later time-points as measured at 6 weeks (1·9 × 10−2µmol/l/106 cells versus 1·1 × 10−2µmol/l/106 cells in NAC-treated and control mice, respectively) and 8 weeks (2·5 × 10−2µmol/l/106 cells versus 1·9 × 10−2µmol/l/106 cells in NAC-treated and control mice, respectively) following infection.
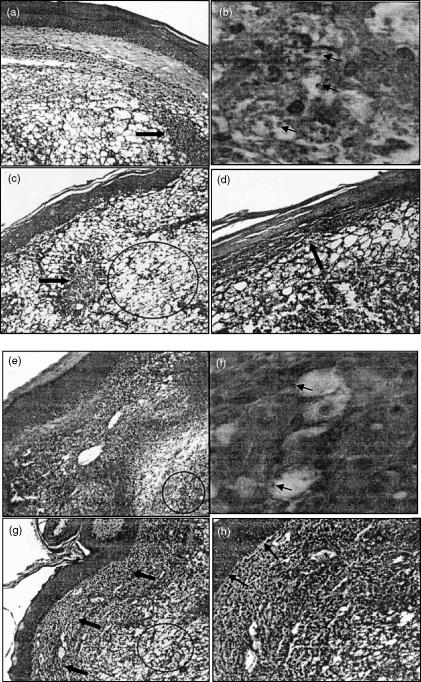
The effects of the glutathione modulation on the histopathology of L. major-infected BALB/c mice was determined through direct analysis of the footpad lesions at several time-points following infection. Control infected BALB/c mice, following 7 weeks of infection, displayed the presence of numerous macrophages in the dermis, presenting with large, clear, vacuolated cytoplasm (Fig. 1a,b). Moreover, the macrophages present in the lesion were strongly parasitized, as indicated by the presence of the large number of Leishmania amastigotes in macrophage vacuoles (Fig. 1b, arrows). Many coagulated necrotic areas could also be observed (Fig. 1a, arrow). In contrast, the NAC-treated BALB/c infected mice demonstrated a clear improvement in the histopathology of footpad lesion following the 7th week of infection, compared to infected control animals (Fig. 1e,f versus Fig. 1a,b, respectively). The histopathological analysis revealed the presence of a population of inflammatory cells lacking parasites and with much less vacuolization (Fig. 1f) than that seen in the control mice (Fig. 1b). Moreover, inflammatory cells formed a focal pattern, isolating restricted infected areas (Fig. 1e, circle), where vacuolated macrophages harboring Leishmania could be observed (Fig. 1f, arrows).
Figure 1.
NAC-treated BALB/c mice display improved histopathology following L. major infection. Histopathological analysis of footpad lesion fragments from control (a–d) and NAC-treated (e–h) BALB/c mice, after the 7th (a, b, e and f) and 10th (c, d, g and h) weeks of infection are represented. Histopathological outcome of footpad lesion of control BALB/c mice on the 7th week (a and b) is marked by numerous parasitized macrophages in the dermis (b, arrows) and presence of many coagulative necrotic areas (a, arrow). After 10 weeks (c and d) increased vacuolization and intense tissue destruction of the dermis (c, circle) and epidermis (d, arrow) is observed. In NAC-treated BALB/c mice the presence of a less-infected (f, arrows), less-vacuolated inflammatory cell population, restricting the infected area (e, circle), can be observed by the 7th week. By the 10th week of infection (g and h) the non-infected inflammatory population seen on the 7th week is still present (g, arrows), although increased vacuolization could be also observed (g, circle). No epidermis destruction was observed (h, arrows). Figures are representative of at least three animals analysed in each group at each time-point. Similar profiles have also been observed in another independent experiment. (H&E × 200, 1 cm = 200 µm, for Fig. a,c,e,g × 400 for Fig. d,h and × 630 for Fig. b,f). Arrows indicate necrotic areas (a and c), macrophages harboring parasites (b and f), epidermis degeneration (d), nonvacuolated inflammatory cells (g) and intact epidermis (h).
Following the 10th week of infection, clear improvements in the NAC-treated mice could still be seen compared to the control infected animals. The control infected BALB/c mice now presented with severe vacuolization, leading to intense tissue destruction of the dermis (Fig. 1c, circle), which had now progressed to the epidermis, as can be seen by destruction of the basal layer of the epidermis (Fig. 1d, arrow). Moreover, at this time-point, necrotic areas could also be observed (Fig. 1c, arrow). In contrast, by the 10th week of infection, the histopathology in the NAC-treated mice was greatly improved compared to the control infected mice, with less tissue vacuolization (Fig. 1g, circle), and the maintenance of a well-preserved inflammatory cell population (Fig. 1g, arrows). Moreover, the epidermis in the NAC-treated mice was completely intact (Fig. 1h, arrows) in contrast to the control (Fig. 1d, arrow).
To quantify further the differences observed in the histopathology of the lesions from NAC-treated and control mice, we performed a series of morphometric analysis, evaluating the area of vacuolated tissue and the number of parasites in a defined tissue area (Table 1). As seen in the histopathological analysis, the extent of tissue vacuolization in the footpad lesions of NAC-treated BALB/c mice was reduced significantly (P = 0·007) compared to the control group, both at the 7th and 10th weeks of infection (Table 1). Finally, the parasite load in NAC-treated BALB/c lesions, as measured by the total parasite number per field, was approximately 3·5-fold lower (P = 0·02) than that seen in control BALB/c lesions, on the 7th week of infection (Table 1).
Table 1.
Tissue vacuolization and parasite load at the site of L. major infection is reduced in NAC-treated BALB/c mice
| Vacuolated area (µm2)‡ | |||
|---|---|---|---|
| 7th week | 10th week | Parasite load (no. parasites/field)§ 7th week | |
| BALB/c H2O† | 316 016 ± 44 643 | 361 635 ± 31 040 | 1469 ± 197 |
| BALB/c NAC† | 142 343 ± 37 242* | 198 279 ± 34 832* | 424 ± 104* |
Mice were euthanized on the 7th and 10th weeks following the primary infection and their lesions were harvested for morphometric analysis. The data presented represent the mean ± SD for at least three animals in each group, at each time-point.
The values represent the means of vacuolated areas obtained through the analysis of 10 fields/lesion ± the standard deviation, using a magnification of 200 ×, covering a total area of 759 310 µm2, which accounts for more than 60% of the tissue fragment area.
The values represent the means parasite number obtained through the analysis of 10 fields/lesion ± the standard deviation, using a magnification of 630 ×
Statistically significant when compared to infected control mice using Student's t-test (P < 0·05) as provided by the statistical analysis software by SAS, JMP.
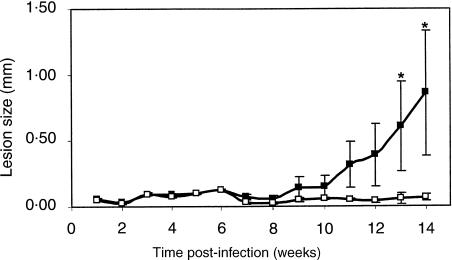
These histopathological alterations were accompanied by a modulation of lesion size. Figure 2 shows the kinetics of footpad swelling in control (shaded) and NAC-treated (white symbol) BALB/c mice. Footpad swelling in control mice was first detected at the 9th week, at which time the cutaneous lesions became progressively larger. In contrast, the onset of footpad swelling in the NAC-treated BALB/c mice was delayed up to the 14th week, at which time the average lesion size was 10 times smaller than in control mice (P = 0·001).
Figure 2.
NAC-treatment modifies the kinetics of lesion development in L. major-infected BALB/c mice. Control (black dots) and NAC-treated (white dots) BALB/c mice were infected with 2 × 106 promastigotes s.c. in the right hind footpad 2 days after the beginning of NAC administration. Lesion progression was monitored weekly and expressed as the difference between the thickness of the infected (right) and the contra lateral (left) non-infected footpad. Both groups began with 26 animals in each group and the experiment ended at 14 weeks with a total of seven animals in each group. The data are represented as mean ± SE. *Statistically significant differences (P < 0·05) between the control and NAC treated groups using the Tukey–Kramer, comparison of all pairs test from SAS, JMP software.
Draining lymph node lymphocytes from NAC-treated, L. major-infected BALB/c mice express an altered cytokine profile
To evaluate whether the observed NAC-induced alterations in the footpad lesion histopathology were correlated to the modulation of the specific lymphocyte cytokine response to L. major infection, mice from both groups were killed after the 6th, 8th, and 11th week of infection, and the cytokine pattern of the draining popliteal lymph node cells were evaluated using flow cytometry.
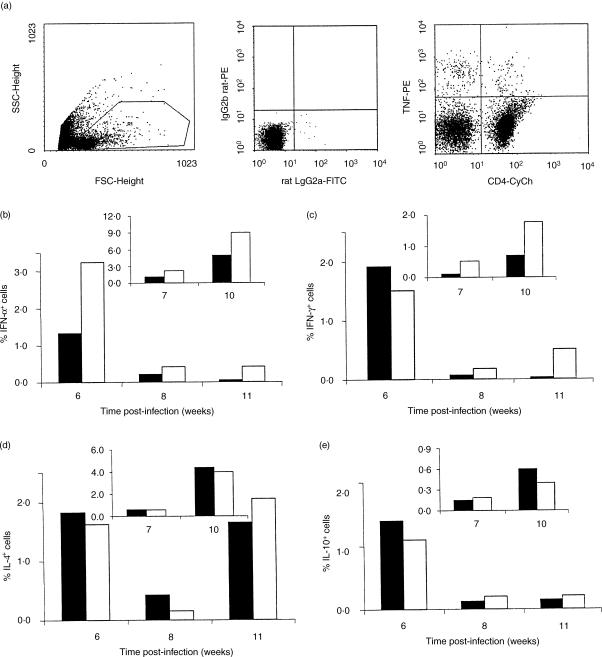
NAC treatment increased the frequency of TNF-α+ cells early in infection, with at least a 2·5-fold increase in the frequency of TNF-α-producing cells compared to the control infected group (Fig. 3b). This increased frequency in the NAC treated group was maintained throughout the infection, as shown in two independent experiments (Fig. 3b). Moreover, these cells could still be detected in NAC-treated BALB/c mice from the 11th week onwards, while TNF-α-producing cells could not be detected reliably in control BALB/c mice.
Figure 3.
The frequency of cytokine expressing lymph node cells from L. major-infected BALB/c mice is modified by NAC-treatment. Popliteal lymph node cells from control (shaded bars) and NAC-treated (white bars) BALB/c mice were submitted to intracytoplasmic cytokine staining and analysed by flow cytometry. Lymph node cells from five, four, and three animals were pooled on the 6th, 8th and 11th weeks of infection, respectively, and stimulated with SLA as described in Materials and methods. The frequency of positive cells was analysed using a gate (R1) that included lymphocytes, large blast lymphocytes and monocytes/macrophages (a, left dotplot). Limits for the quadrant markers were always set based on negative populations and isotype controls (a, middle dotplot). The frequency of cytokine positive cells in the total cell population was calculated by the number of cells positioned in the upper right and upper left quadrants of dot plots for cytokine (PE-stained) versus cell markers (CY-stained) (a, right dotplot). The frequencies of total cells producing TNF-α (b), IFN-γ (c), IL-4(d) and IL-10(c) were determined. The inserts represent the data obtained from another set of independent experiments.
Similar frequencies of IFN-γ-producing cells were detected at the 6th week of infection, both in control and NAC-treated BALB/c mice, in one experiment followed by an increase in the NAC-treated mice at the other time-points (Fig. 3c). However, as observed for TNF-α, the frequency of IFN-γ-producing cells was down-modulated during the course of infection in both groups, and on the 11th week no IFN-γ-producing cells could be detected in popliteal lymph node cells of control mice (Fig. 3c). Moreover, in an independent experiment, there was a higher frequency of IFN-γ-producing cells at all time-points tested in the NAC-treated mice than in control animals.
The frequencies of IL-4-producing cells in the control and NAC-treated mice were similar (Fig. 3d). An early high frequency of cells expressing IL-4 was observed in both groups on the 8th week, which was maintained to the 11th week. The frequency of IL-10+ cells was similar in both groups (Fig. 3e).
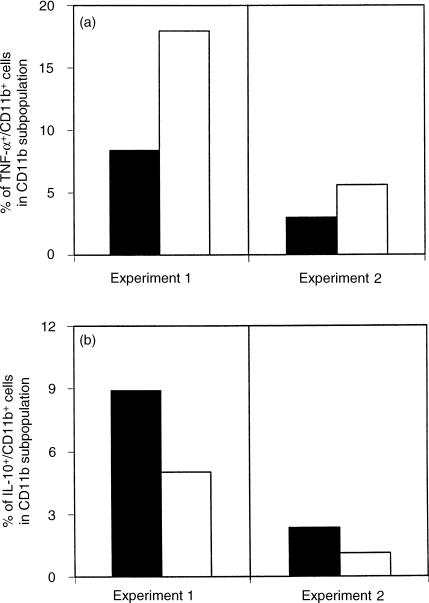
Given that macrophages are the main effector cells responsible for the control of Leishmania replication, the production of TNF-α and IL-10 by CD11b+ cells was also determined (Fig. 4a,b, respectively). A twofold increase in the frequency of TNF-α-producing CD11b+ cells was observed in NAC-treated mice compared to the control group (Fig. 4a), accompanied by a twofold decrease in the expression of IL-10 by NAC-treated macrophages (Fig. 4b). Moreover, a similar frequency of TNF-α and IL-10 expressing cells was observed for macrophages from control mice (Fig. 4a,b, shaded bars), while in NAC-treated BALB/c mice a marked increase (3–4 times) in the frequency of TNF-α expressing macrophages was seen over the frequency of IL-10-producing cells (Fig. 4a,b, white bars).
Figure 4.
The cytokine expression pattern of macrophages is modulated by NAC treatment. Popliteal lymph node cells from control (shaded bars) and NAC-treated (white bars) BALB/c mice were submitted to intracytoplasmic cytokine staining and analysed by flow cytometry. The frequency of TNF-α (a) and IL-10 (b) expression by macrophages within the CD11b+ subpopulation was determined. Data from two independent sets of experiments (experiment 1 and experiment 2) are presented. The data from experiment 1 and experiment 2 were obtained after 6 and 10 weeks of infection, respectively. Lymph node cells from at least three animals were pooled in each experiment.
Discussion
Leishmaniasis is a disease affecting millions of people in developing countries and represents a serious public health problem. In humans, distinct profiles of disease development are related to the immune response of the host, among other factors, with resistance associated commonly with Th1-like responses and susceptibility to Th2-like responses.16–18 Recently, it has been shown that Th1/Th2 development can be influenced through modulation of glutathione levels.10 We now demonstrate, using the murine model of L. major infection, that the increase in glutathione levels through NAC treatment improves the BALB/c mice response to infection as analysed using several critical parameters: histopathological outcome of the footpad lesion and cytokine profile of popliteal lymph node cells.
Histopathological and morphometric analysis of the footpad lesions revealed the improvement in the NAC-treated L. major-infected BALB/c mice. NAC-treated BALB/c mice presented with greatly improved histopathology, characterized by a less intense vacuolization and the maintenance of non--infected inflammatory cell populations in an organized focal pattern (Fig. 1). This was similar to the mixed inflammatory infiltrate with granulomatous patterns that are associated commonly with healer strains or in vaccinated susceptible strains made resistant to reinfection.19–21 On the other hand, a monomorphous inflammatory infiltrate, observed in susceptible strains, is a direct consequence of poor/inefficient macrophage activation, leading to uncontrolled parasite replication/spreading at the site of infection as seen in our control animals (Fig. 1a,b).
The histopathological outcome of murine leishmaniasis is a direct consequence of the cytokine response established. The development of severe and uncontrolled cutaneous lesions in response to L. major infection of BALB/c mice occurs as a consequence of the establishment of a Th2 response, allowing for poorly controlled parasite replication at the site of infection and consequently the visceralization of the disease.22 The histopathological findings, and the delay in the appearance of footpad swelling, in the NAC-treated BALB/c mice (Fig. 2) suggested that the immune response to L. major infection was modulated by NAC treatment.
In agreement with this hypothesis, NAC-treatment induced quantitative and qualitative modifications in the cytokine response to L. major infection. Early in infection an increased frequency of TNF-α+-producing cells was detected in NAC-treated mice (Fig. 3b), and by 11 weeks a higher frequency of IFN-γ- and TNF-α-producing cells was maintained as compared to the control mice. The control of L. major infection is associated strongly with IFN-γ production,3 a potent activator of the leishmanicidal macrophage function, that is mediated ultimately by nitric oxide.4,5 TNF-α strongly synergises with IFN-γ to induce maximal macrophage activation and control of Leishmania replication at the site of infection.23,24 In contrast, IL-4 and IL-10 are potent inhibitors of macrophage functions and abrogate the activating capacity of IFN-γ.9
We suggest that the NAC-induced alterations established early in infection created a cytokine microenvironment that, different from control mice, led to macrophage activation and control of parasite replication at the site of infection. This, in turn, probably led to the observed delay in the onset of lesion formation, as indicated by the delay in lesion size, as well as the clear histopathological findings, including the lower parasite number at the site of infection by the 7th week of infection and the lower degree of tissue destruction (Table 1). Moreover, changes in the cytokine expression pattern of macrophages were also observed (Fig. 4a,b), indicating that in NAC-treated mice macrophages will be exposed to an environment rich in TNF-α and IFN-γ, with less IL-10 than the control animals. Thus, acounting for the increased leishmanicidal activity in the NAC-treated mice.
Recently, Murata et al.25 have demonstrated that macrophage function, like nitric oxide and IL-12 production, can be improved by increasing glutathione levels. It is possible that macrophages in Leishmania-infected NAC-treated BALB/c mice were activated to induce IL-12 production, which could account for the higher IFN-γ and TNF-α expression observed.
Historically, L. major-infected BALB/c mice are resistant to immune deviation, reflecting many genetic factors related to the background of this strain. In particular, it probably reflects a down-regulation of IL-12 receptor function and a continual self-renewal of a Th2 type response due to the biasing effects of IL-4 on the expansion of precursor CD4+ T cells towards Th2 cells.26 Immunomodulatory manipulations that lead to resolution of L. major infection in BALB/c mice have in common the ability to reduce or abrogate IL-4 production,27,28 and consistent with this is the finding that healer strains rapidly down-regulate IL-4 expression following infection.29 Here, the frequency of IL-4-producing cells was not down-modulated following infection of NAC-treated BALB/c mice. The failure to modulate the frequency of IL-4-producing cells could be reflecting an intrinsic defect of BALB/c mice, related to an overexpression of, or oversensitization to, IL-4.30,31 The frequency of both TNF-α- and IFN-γ-producing cells was increased in the NAC-treated mice (Fig. 3). This increase of two key cytokines for the induction of optimal leishmanicidal activity is consistent with the induction of a better effector response, leading to more efficient macrophage activation and control of leishmania infection. However, the increase of these two cytokines, in the absence of a decrease in the frequency of the Th2 cytokine, IL-4, do not demonstrate a absolute shift in the classical Th2 toward Th1 profile.
In our model of glutathione modulation NAC treatment was started before infection. It is well documented that the modulation of immune responses, particularly the Th1/Th2 decision, can be achieved only during the first days after antigen encounter. Thus, NAC treatment was initiated before L. major infection to guarantee that GSH levels would be increased at the time of infection. Moreover, NAC treatment was maintained throughout the infection to maintain the effects during the immune response against Leishmania. The NAC treatment led clearly to an improvement in the histopathology and cytokine profiles; however, it did not lead to a complete protection from infection, as seen in resistant C57Bl/6 mice.
In conclusion, the data presented here show that NAC-treatment can induce changes in the cytokine microenvironment that allow for a more efficient control of parasite replication at the site of infection (more efficient effector response) and a delay in the onset of the disease. The molecular mechanisms acting in the observed modulation could involve modulation of signalling pathways, and/or changes in gene expression, that would in turn alter the cytokine profile, cell migration patterns and effector activities, among other possibilities. A potential therapeutic application for NAC as an adjuvant in parasitic diseases could be considered, and it is hoped that the experience gained here will help in future efforts to design immunotherapeutic approaches that use endogenous biochemical processes for cell- or tissue-specific therapy.
Acknowledgments
We thank our colleagues from UFMG, Dr Maria de Fátima M. Horta for kindly providing the L. major strain, Dr Elizabeth R. da Silva Camargos and Dr Ricardo S. Gomez for morphometric/histopathological facilities, Dr Leda Quercia Vieira and Dr Jaqueline Alvarez-Leite for mice facilities, and Casio M. F. Costa for technical assistance with the FACS. We also thank Dr Leonore Herzenberg, Stanford University, for critical review of the manuscript. This study was supportwed by PRONEX/CNPq-FINEP (Brazilian Research Financing Agency), UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR), and CNPq (Brazilian Research Financing Agency supplying student fellowships), TMRC-National Institutes of Health, USA.
Abbreviations
- GSH
glutathione
- IFN-γ
interferon-γ
- IL-12
interleukin-12
- NAC
N-acetyl-l-cysteine
- SLA
soluble leishmania antigen
- TNF-α
tumour necrosis factor-α
References
- 1.World Health Organization. 2001. WHO online. http://www.who.org.
- 2.Wang ZE, Reiner SL, Zheng S, Dalton DK, Locksley RM. CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J Exp Med. 1994;179:1367–71. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belosevic M, Finbloom DS, Van Der Meide PH, Slayter MV, Nacy CA. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989;143:266–74. [PubMed] [Google Scholar]
- 4.Assreuy J, Cunha FQ, Epperlein M, Noronha-Dutra A, O'Donnell CA, Liew FY, Moncada S. Production of nitric oxide and superoxide by activated macrophages and killing of Leishmania major. Eur J Immunol. 1994;24:672–6. doi: 10.1002/eji.1830240328. [DOI] [PubMed] [Google Scholar]
- 5.Green SJ, Meltzer MS, Hibbs JB, Jr, Nacy CA. Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J Immunol. 1990;144:278–83. [PubMed] [Google Scholar]
- 6.Liew FY, Millott S, Parkinson C, Palmer RM, Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from l-arginine. J Immunol. 1990;144:4794–7. [PubMed] [Google Scholar]
- 7.Wang ZE, Zheng S, Corry DB, Dalton DK, Seder RA, Reiner SL, Locksley RM. Interferon gamma-independent effects of interleukin 12 administered during acute or established infection due to Leishmania major. Proc Natl Acad Sci USA. 1994;91:12932–6. doi: 10.1073/pnas.91.26.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinzel FP, Sadick MD, Mutha SS, Locksley RM. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci USA. 1991;88:7011–5. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liew FY, Millott S, Li Y, Lelchuk R, Chan WL, Ziltener H. Macrophage activation by interferon-gamma from host-protective T cells is inhibited by interleukin IL3 and IL4 produced by disease-promoting T cells in leishmaniasis. Eur J Immunol. 1989;19:1227–32. doi: 10.1002/eji.1830190712. [DOI] [PubMed] [Google Scholar]
- 10.Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci USA. 1998;95:3071–6. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotgreave IA. N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharmacol. 1997;38:205–27. [PubMed] [Google Scholar]
- 12.Droge W, Eck WP, Mihm S. HIV-induced cysteine deficiency and T-cell dysfunction − a rationale for treatment with N-acetylcysteine. Immunol Today. 1992;13:211–4. doi: 10.1016/0167-5699(92)90156-2. [DOI] [PubMed] [Google Scholar]
- 13.Staal FJ, Ela SW, Roederer M, Anderson MT, Herzenberg LA. Glutathione deficiency and human immunodeficiency virus infection. Lancet. 1992;339:909–12. doi: 10.1016/0140-6736(92)90939-z. [DOI] [PubMed] [Google Scholar]
- 14.Martinez E, Domingo P. N-acetylcysteine as chemoprotectant in cancer chemoterapy. Lancet. 1991;338:249–56. doi: 10.1016/0140-6736(91)90383-z. [DOI] [PubMed] [Google Scholar]
- 15.Openshaw P, Murphy EE, Hosken NA, Maino V, Davis K, Murphy K, O'Garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–67. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bottrel RLA, Dutra WO, Martins FA, et al. Flow cytometric determination of cellular sources and frequencies of key cytokine producing lymphocytes against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis. Infect Immun. 2001;69:3232–9. doi: 10.1128/IAI.69.5.3232-3239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kemp K. Cytokine-producing T cell subsets in human leishmaniasis. Arch Immunol Ther Exp (Warsz) 2000;48:173–6. [PubMed] [Google Scholar]
- 18.Ribeiro-de-Jesus A, Almeida RP, Lessa H, Bacellar O, Carvalho EM. Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res. 1998;31:143–8. doi: 10.1590/s0100-879x1998000100020. [DOI] [PubMed] [Google Scholar]
- 19.Lemos de Souza V, Ascençao Souza J, Correia Silva TM, Sampaio Tavares Veras P, Rodrigues de-Freitas LA. Different Leishmania species determine distinct profiles of immune and histopathological responses in CBA mice. Microbes Infect. 2000;2:1807–15. doi: 10.1016/s1286-4579(00)01340-x. [DOI] [PubMed] [Google Scholar]
- 20.Barral-Netto M, de Freitas LA, Andrade ZA. Histopathologic changes induced by vaccination in experimental cutaneous leishmaniasis of BALB/c mice. Am J Pathol. 1987;127:271–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Andrade ZA, Reed SG, Roters SB, Sadigursky M. Immunopathology of experimental cutaneous leishmaniasis. Am J Pathol. 1984;114:137–48. [PMC free article] [PubMed] [Google Scholar]
- 22.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annu Rev Immunol. 1995;13:151–77. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 23.Deng W, Thiel B, Tannenbaum CS, Hamilton TA, Stuehr DJ. Synergistic cooperation between T cell lymphokines for induction of the nitric oxide synthase gene in murine peritoneal macrophages. J Immunol. 1993;151:322–9. [PubMed] [Google Scholar]
- 24.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–12. [PubMed] [Google Scholar]
- 25.Murata Y, Toshiro S, Hamuro J. The polarization of Th1/Th2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. Int Immunol. 2001;14:201–12. doi: 10.1093/intimm/14.2.201. [DOI] [PubMed] [Google Scholar]
- 26.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–24. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinzel FP, Rerko RM. Cure of progressive murine leishmaniasis: interleukin 4 dominance is abolished by transient CD4 (+) T cell depletion and T helper cell type 1-selective cytokine therapy. J Exp Med. 1999;189:1895–906. doi: 10.1084/jem.189.12.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakil AE, Wang ZE, Locksley RM. Leishmania major. targeting IL-4 in successful immunomodulation of murine infection. Exp Parasitol. 1996;84:214–22. doi: 10.1006/expr.1996.0107. [DOI] [PubMed] [Google Scholar]
- 29.Morris L, Troutt AB, Handman E, Kelso A. Changes in the precursor frequencies of IL-4 and IFN-gamma secreting CD4+ cells correlate with resolution of lesions in murine cutaneous leishmaniasis. J Immunol. 1992;149:2715–21. [PubMed] [Google Scholar]
- 30.Reiner SL, Zheng S, Wang ZE, Stowring L, Locksley RM. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med. 1994;179:447–56. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seder RA, Paul WE, Davis MM, Fazekas de St G. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091–8. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]