Introduction
The immune system of any organism must maintain a fine balance between activation and inhibition. It must possess adequate reactivity to raise an effective immune response to target non-self molecules while not harming the organism itself. Essential to this process is the ability to control the timing and place of activation and to limit the extent of, and eventually terminate, activation. Failure to maintain this balance will result in either immunodeficiency or autoimmunity. Inhibitory receptors are involved in this regulation, a number having been shown to be critical in controlling the B cell immune response.
There are two broad classes of inhibitory receptor − most are of the immunoglobulin (Ig) superfamily while the remainder are lectin-like molecules. They share a number of structural and functional similarities. Each inhibitory receptor contains one or more immunoreceptor tyrosine-based inhibitory motifs (ITIMs) within its cytoplasmic domain essential for generation and transduction of inhibitory signals. The ITIM consists of a six amino acid consensus sequence (Ile/Val/Leu/Ser)-X-Tyr-X-X-(Leu/Val).1 Ligation of the inhibitory receptor to an immunoreceptor tyrosine-based activatory motif (ITAM)-containing activatory molecule results in tyrosine kinase phosphorylation of the tyrosine residue within the ITIM2 by lyn.3 Tyrosine phosphorylation of the ITIM allows it to bind and activate phosphatases containing an src homology 2 (SH2) domain. Two classes of SH2-containing inhibitory phosphatases have been identified: the protein tyrosine phosphatases SHP-1 and SHP-2, and the phosphoinositol phosphatases SHIP and SHIP2. These classes have separate downstream signalling pathways through which they modulate cellular inhibition. In general, each class of phosphatase interacts with the ITIMs of different inhibitory receptors but each inhibitory receptor appears to act predominantly through only one class of phosphatase.4
A number of inhibitory receptors have been described on B cells, the details of which are summarized in Fig. 1 and Table 1. We will concentrate on three of these, FcγRII, CD22 and PD−1, and in addition will discuss lyn, SHP-1 and SHIP, which are crucial elements in the signalling pathways of the inhibitory receptors. We will describe their probable physiological roles in immune regulation, and then review the evidence from knockout mice, spontaneous mouse models of autoimmunity and human disease that defective regulation by B cell inhibitory receptors can lead to autoimmunity.
Figure 1.
B cell inhibitory receptors.
Table 1.
B cell inhibitory receptors
| Phenotype of knockout mice | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Inhibitory receptor | Ligand | Expressed in humans (H) or mice (M) | Cellular distribution | Molecular weight in humans (kDa) | Putative number of ITIMs | Tyrosine phosphatase | Hyperactive B cells | Autoimmunity | Autoimmune disease |
| FcγRIIb | IgG immune complex | H + M | B cells, myeloid & mast cells | 40 | 1 | SHIP | ✓ | ✓ | ✓ |
| CD22 | Sialic acid | H + M | B cells | 140 | ✓ | SHP-1 | ✓ | ✓ | × |
| CD72 | CD100, CD5 | H + M | B cells (+ subpop T cells) | 45 | 2 | SHP-1 | ✓ | × | × |
| PD-1 | Ig superfamily protein (PD-L) | H + M | B cells, T cells and myeloid cells | 55 | 1 | SHP-2 | ✓ | ✓ | ✓ |
| CD5 | CD72 (+ ?others) | H + M | B1 cells | 67 | 1 | SHP-1 | ✓ | ✓ | × |
| PIR-B | ?MHC | M | Myeloid cells and B cells | 120 | 4 | SHP-1 (+ ?SHP-2) | ✓ | × | × |
| ILT-2 | HLA-A,HLA-B and HLA-G | H | B cells, T cells, NK and myeloid cells | 110 | 4 | SHP-1 | NA | ||
| LAIR-1 | Ep-CAM | H + M | Leukocytes | 32 | 2 | SHP-1 | NA | ||
| CD66a | CD66a | H + M | Myeloid cells, B cells, T cells NK cells,platelets, endothelia and epithelia | 160 | 2 | SHP-1 and SHP-2 | NA | ||
Inhibitory receptors and autoimmunity
FcγRIIb
FcγRIIb is a single-chain, low-affinity receptor for the Fc portion of IgG5 and a member of the Ig superfamily. It is a 40-kDa protein that consists of two extracellular Ig-like domains, a transmembrane domain and an intracytoplasmic domain that contains a single ITIM (Fig. 1). It binds IgG either complexed to multivalent soluble antigen as immune complexes (ICs) or bound to cell membranes.6 There are several isoforms that are found on different cell types, but the isoform on B cells is unique in containing an intracytoplasmic motif that prevents its internalization.7,8
In B cells, which do not express any other Fc receptors, it acts to inhibit signalling through the B cell receptor (BCR), whereas in myeloid cells FcγRIIb inhibits activation through activatory Fc receptors. It is cross-linked to the BCR by ICs containing IgG and antigen recognized by the BCR. Coligation of FcγRIIb to the BCR leads to tyrosine phosphorylation of the ITIM by the tyrosine kinase lyn, recruitment of SHIP and inhibition of Ca2+ flux and proliferation. The blockade of Ca2+ flux and calcium-dependent signalling is produced by dephosphorylation of PIP3 by SHIP and subsequent disassociation of Btk and PLCγ. The precise mechanism by which SHIP prevents B cell proliferation is uncertain. By dephosphorylation of PIP3, SHIP can prevent recruitment of the survival factor Akt. It also functions to recruit p62dok to the membrane where it is activated by lyn to down-regulate MAP kinase activity (Fig. 2). Under some circumstances in vitro FcγRII can bind both SHP-1 and SHP-2 instead of SHIP. The physiological significance of this observation is uncertain, but it may be that the conditions of co-aggregation may determine phosphatase recruitment, allowing further refinement of inhibitory responses. FcγRIIb also induces apoptosis on aggregation of the receptor in the absence of BCR signalling. In this circumstance an apoptotic signal is generated through Btk and Jnk independent of the ITIM, which is abrogated when FcγRIIb is cross-linked with the BCR. Coligation of FcγRIIb is thought to provide feedback control of the B cell immune response, shutting off or preventing a response if sufficient antigen-specific IgG is present (Fig. 2, reviewed in ref. 9).
Figure 2.
The FcγRII inhibitory pathway. Cross-linking of FcγRII to the BCR by immune complexes containing IgG inhibits B cell activation. This occurs through a number of mechanisms that are mediated by SHIP and lyn at key stages.
Evidence of a role for defective FcγRIIb inhibition in the pathogenesis of autoimmunity is found in studies of FcγRII-deficient mice, in mouse models of autoimmune disease and in human systemic lupus erythematosus (SLE) and rheumatoid arthritis. FcγRIIb-deficient mice derived on a 129Sv/C57BL/6 background have augmented humoral responses to immunization with both T-dependent and -independent antigens, but they do not develop autoantibodies.10 FcγRIIb deficiency renders normally resistant strains of mice susceptible to two antibody-dependent models of inducible autoimmunity: collagen-induced arthritis11 and Goodpasture's syndrome.12 The collagen-induced arthritis model involves the development of antibody-dependent arthritis after immunization with bovine type II collagen. H2q haplotype mice (e.g. DBA/1) are susceptible to this disease, but H2b (e.g. C57BL/6, 129) mice are resistant. Deficiency in FcγRII renders H2b mice as susceptible to disease as H2q mice.11 In a model of Goodpasture's syndrome FcγRIIb-deficient mice develop pulmonary haemorrhage and crescentic glomerulonephritis in response to immunization with bovine type IV collagen, whereas no control animals developed disease.12
While FcγRIIb-/- mice derived on a 129Sv/C57BL/6 background do not develop overt autoimmunity, when back-crossed onto a C57BL/6 background the mice produce autoantibodies and develop immune complex-mediated autoimmune disease resembling SLE.13 The peripheral B cell repertoire of the C57BL/6 animals appears normal, but with age an increased percentage of IgM low/IgD low ‘activated’ B cells are seen, as is hypergammaglobulinaemia and isotype switched autoantibodies (to antigens such as chromatin and dsDNA). These animals develop an immune complex-mediated glomerulonephritis and renal failure, with 50% dying before 9 months of age. The differences seen between the knockout mice on different backgrounds are postulated to be produced by differences in strain-specific epistatic modifiers of autoimmunity.13 Transfer studies show that the disease is fully transferable and dependent on B cells-while FcγRIIb-/- myeloid cells are not necessary for disease development, a role for them in determining severity has not been excluded.13
Genetic studies of polygenic murine models of human autoimmune diseases implicate FcγRIIb in pathogenesis. A number of independent linkage studies in murine models of SLE and rheumatoid arthritis have identified disease susceptibility loci that contain fcgr2 (Table 2). It should be emphasized that the region at the distal end of chromosome 1 containing fcgr2 also contains a large number of other candidate genes, for example complement receptor 2 (Cr2).14 It is now clear that contributions to disease pathogenesis are made by at least four independent subdivisions of this locus that have been identified by congenic studies15 and that a number of genetic polymorphisms are involved.
Table 2.
Susceptibility loci containing inhibitory receptors in mouse models of autoimmune disease
| Inhibitory receptors and related molecules | Chromosome position | Mouse strain | Disease | Locus name (position) | Reference |
|---|---|---|---|---|---|
| FcγRIIb | 1 92·3 | NZB | SLE | Nba2 (1 95) | 88 |
| Lbw7 (1 90) | 37 | ||||
| NZW | Sle1 (1 88) | 38 | |||
| BXSB | Bxs3 (1100) | 89 | |||
| NOD | BCG-induced SLE | Babs2, Bana3 (1 95) | 90 | ||
| NODx | Collagen-induced arthritis* | Cia9 (1 92·3) | 91 | ||
| C57BL/10 | |||||
| CD22 | 7 9 | NZW | SLE | Sle3 (7 15) | 18 |
| CD66a | 7 5·5 | NOD | IDDM | Idd7 (7 4) | 40 |
| PIR-B | 7 1 | SJL/J | EAE† | Eae12 (7 16) | 41 |
| CD72 | 4 22·5 | NZBW/F1 | SLE | Lbw3 (4 23) | 37 |
| SHP-1 | 6 60·2 | NZBW/F1 | SLE | Lbw4 (6 71) | 37 |
| DBA | Collagen-induced arthritis* | Cia3 (6 48·7) | 75 | ||
| NOD | IDDM | Idd19 (6 60·5) | 76 |
Mouse model of rheumatoid arthritis.
Mouse model of multiple sclerosis.
In several murine models of lupus16,17 and the non-obese diabetic (NOD) mouse18 a polymorphism of the fcgr2 gene promoter has been shown to reduce expression of the receptor on macrophages,16in vitro activated16 and germinal centre17 B cells. The reduction in expression seen on macrophages was associated with macrophage hyperactivity.16 C57BL/6 mice congenic for a region containing this promoter polymorphism (among others) have been shown recently to have reduced FcγRIIB expression on germinal centre B cells and increased IgG antibody responses.19 While almost all autoimmune-prone strains carry this polymorphism, the New Zealand White (NZW) mouse does not. This is consistent with FcγRII having been excluded as a susceptibility gene in the NZW mouse by fine congenic mapping,20 indirectly supporting a role for polymorphic FcγRIIb in the other autoimmune-prone strains.
Genetic studies of the human autoimmune diseases SLE and IDDM have shown significant linkage to the region of chromosome 1 (1q23) containing the low-affinity Fc receptors (both activatory and inhibitory)21–24 and are outlined in Table 3
Table 3.
Susceptibility loci potential containing inhibitory receptors in human autoimmune disease
| Inhibitory receptors and related molecules | Chromosome position | Disease* | Position of peak linkage | Reference |
|---|---|---|---|---|
| FcγRIIb | 1q23–24 | IDDM | 1q25.2 | 26 |
| SLE | 1q22–24 | 23, 56, 92 | ||
| CD22 | 19q13.1 | IDDM | 19q11 | 43 |
| ILT-2 | 19q13.4 | MS | 19q12 | 44 |
| LAIR-1 | 19q13.4 | 19q13.31 | 45 | |
| CD66a | 19q13.2 | |||
| CD72 | 9p | MS | 9p22.2 | 45 |
| PD-1 | 2q37.3 | SLE | 2q32 | 56 |
| SHIP | 2q36 | IDDM | 2q32 | 85 |
| CD5 | 11q13 | AS | 11q12.1 | 93 |
| IDDM | 11q13.1 | 22, 43, 94 | ||
| MS | 11q14.3 | 95 |
IDDM = insulin-dependent diabetes mellitus, SLE = systemic lupus erythematosus, MS = multiple sclerosis, CD = Crohn's disease, AS = asthma.
A number of studies have found a correlation between specific polymorphisms in FcγRIIA, FcγRIIIA and FcγRIIIB and the development of a number of different autoimmune diseases (reviewed in refs 25 and 26), although this has not been a consistent finding.27 While none of the studies above have implicated FcγRIIB directly, all three of the Fc receptor genes are clustered very tightly at 1q23 and thus in linkage dysequilibrium with each other.26
Genetic studies have linked polymorphisms in FcγRIIB to disease pathogenesis in humans. A recent study has identified a single nucleotide polymorphism in the Fcgr2b gene that results in an Ile232Thr substitution.28 The 232T/T genotype was found at a significantly higher frequency in Japanese SLE patients compared with controls. The precise effect of this mutation is unknown, but it lies within the trans-membrane region of the molecule and it is known that an intact TM region is required for induction of apoptotic signals through FcγRIIB in the mouse. Thus Fcgr2b is clearly a candidate gene for human autoimmune disease.
Three strands of evidence point to a role for FcγRIIb in the development of spontaneous autoimmune disease. The Fcgr2 knockout mouse has hyperactive B cells and can develop SLE, which demonstrates that defects in FcγRIIb have the potential to cause autoimmunity. An FcγRIIb defect occurs in all mouse models of SLE (except NZW) and this, taken together with human genetic studies implicating FcγRIIb in disease pathogenesis, provide strong circumstantial evidence that defects in FcγRIIb function may contribute to autoimmune disease.
CD22
CD22 is a B cell surface glycoprotein of the Ig superfamily in the sialoadhesin subclass.29 It is made up of seven extracellular Ig-like domains, a transmembrane region and an intracytoplasmic tail that contains six highly conserved tyrosine residues, three of which are part of ITIMs30 (Fig. 1). The ligand for CD22 is Siaα26Galβ1–4GlcNAc, a glycosylated sialic acid residue expressed at high levels on lymphocytes and inflamed endothelial cells.31
CD22 is associated constitutively with the BCR and is phosphorylated on stimulation through it by the tyrosine kinase lyn.3 Lyn also controls the basal level of CD22 phosphorylation and SHP-1 association. Phosphorylation of the ITIMs within the intracytoplasmic tail of CD22 allows association with and phosphorylation of SHP-1. Ligation of CD22 to the BCR, and subsequent SHP-1 activation inhibits B cell activation by inhibiting the MAP kinases ERK2, JNK and p38 and dephosphorylating molecules involved in the early events of BCR mediated activation. These include the BCR itself, tyrosine kinases activated by phosphorylation of Ig α/β (such as syk) and the targets of these kinases (including the adaptor protein BLNK and PLCγ). This suppresses Ca2+ mobilization initiated by BCR stimulation (Fig. 3, reviewed in ref. 30). Coligation of CD22 to the BCR reduces B cell activation while sequestering CD22 away from the BCR, as would occur if CD22 bound its ligand onto adjacent cells, results in B cell hyperactivity. Thus the interaction of CD22 with its ligand may promote B cell activation in appropriate lymphoid environments.30 Alternatively, increased levels of ligand on inflamed endothelium would recruit CD22 and make B cell activation by inflamed self less likely.32
Figure 3.
The CD22 inhibitory pathway. Ligation of CD22 to the BCR and subsequent SHP-1 activation by lyn inhibits B cell activation through a number of mechanisms.
CD22-deficient mice have an expanded B1 cell population and increased serum IgM, and their B cells are hyper-responsive to stimulation through the BCR.33 With age they develop high-affinity isotype-switched autoantibodies to dsDNA, myeloperoxidase and cardiolipin, although not overt autoimmune disease.34 Heterozygous CD22 knockout mice have a reduced but significant autoimmune phenotype35 and mice heterozygous for CD22, lyn and SHP-1 show reduced B cell tolerance in the HEL–anti-HEL transgenic system.36 These data imply that even a partial defect in CD22 function may contribute to autoimmune disease.
Cd22 has been shown to lie within a susceptibility locus for development of lupus in the NZBW/F1 and related NZM2410 models of lupus,37,38 the relevant locus in the NZM2410 genome being of NZW origin.39 This region has also been linked to the development of insulin-dependent diabetes mellitus (IDDM) in the NOD mouse and to experimental autoimmune encephalomyelitis (EAE) (Table 2).40–42 A number of autoimmune prone strains of mice, including the NZW mouse, express the Cd22a or Cd22c alleles which are associated with abnormal processing of CD22 mRNA leading to heterogeneous 5′-UTRs and truncated exon-4 encoded sequence.35 This defect is associated with a reduced surface expression of CD22 on resting B cells and reduced ability of LPS-activated B cells to up-regulate CD22.35 Heterozygous expression of cd22a with the Y chromosome-linked autoimmune acceleration gene Yaa promoted autoantibody production,35 supporting the link between this cd22 allele and autoimmune disease.
Genetic studies of linkage to human diseases have mapped susceptibility loci for the development of IDDM and multiple sclerosis close to the region of chromosome 19 that contains cd22 although, of course, this area contains other candidates (Table 3).43–45
Therefore, data from the CD22 knockout mouse link defects in CD22 expression to the development of autoimmunity, genetic studies have identified certain CD22 alleles that are associated with autoimmunity in mice, and the region containing CD22 has been linked genetically to disease in both mice and humans. All these point to a possible role for defects in CD22 contributing to the development of autoimmunity.
PD-1
The PD-1 receptor is a 55-kDa inhibitory receptor of the Ig superfamily that is highly conserved between humans and mice.46,47 It is expressed on resting B cells, T cells and macrophages and is induced strongly on activation.47 It is composed of a single extracellular Ig-like domain, a transmembrane region and has two tyrosine residues in the cytoplasmic tail, one of which forms part of an ITIM (Fig. 1). Two PD-1 ligands (PD-Ls) have been identified. These are transmembrane proteins of the Ig superfamily expressed constitutively on dendritic cells and on heart, lung, thymus and kidney and also on monocytes after IFN-γ stimulation.48,49In vitro studies on a B cell lymphoma line using a chimæric molecule with the FcγRII extracellular domain and the PD-1 cytoplasmic domain have shown that ligation of the PD-1 cytoplasmic domain to the BCR can inhibit signalling through it. This inhibition prevented BCR-mediated proliferation, Ca2+ mobilization and tyrosine phosphorylation of molecules, including CD79β, syk, PLCγ2 and ERK1/2. It is mediated by recruitment of SHP-2 to a non-ITIM cytoplasmic tyrosine residue.50 The physiological role of PD-1 in B cells is unclear, but it may play a role in maintaining peripheral tolerance by limiting activation of autoreactive B cells by cross-linking PD-1 during interactions with PD-L-expressing cells.51
The phenotype of PD-1 deficient mice supports a role for PD-1 in the prevention of autoimmunity.52–54 The mice have defects in T cell selection and maintenance of peripheral T cell tolerance.48,55 Splenic B cells from the deficient mice showed enhanced responses to anti-IgM stimulation in vitro, hypergammaglobulinaemia and an enhanced IgG3 antibody response to T-independent antigen.64 PD-1 knockout mice on a C57BL/6 background develop autoantibodies, an immune complex-mediated glomerulonephritis similar to that seen in human SLE, and a deforming arthritis resembling rheumatoid arthritis.53 When the C57BL/6 PD-1 knockout was crossed onto the lpr/lpr mouse they developed high titres of antidsDNA autoantibodies and accelerated glomerulonephritis and arthritis.53 BALB/c PD-1-/- mice develop dilated cardiomyopathy, with IgG deposition on the myocardium associated with the development of isotype switched autoantibodies to a cardiac myocyte-specific protein.54 PD-1-/- mice were protected from disease and the development of autoantibodies when on the RAG2-/- background, and disease could be transferred successfully to these mice with spleen or bone marrow cells from diseased mice.54 Therefore, lymphoid cells appear to be crucial for the development of autoimmune disease in PD-1-deficient mice. While defective PD-1 on myeloid cells may not be critical to the transfer of disease, this does not rule out a less critical role for them in the disease process.
Linkage studies in human autoimmune diseases have identified susceptibility loci for both SLE56 and IDDM43 that lie close PD-1 (Table 3), but no direct evidence for abnormal PD-1 function in human disease exists. Nonetheless that PD-1 knockout mice develop autoantibodies and autoimmune disease. This illustrates the potential for PD-1 deficiency to contribute to the pathogenesis of autoimmune disease.
Inhibitory receptor pathways and autoimmunity
Inhibitory receptors are subserved by remarkably similar signalling pathways. To date, lyn is the only tyrosine kinase that has been identified as phosphorylating ITIMs on the B cell inhibitory receptors, and most of these ITIMs then associate with SHP-1 or SHIP. The possible contribution that these three signalling molecules might make to the development of autoimmune disease will now be discussed.
Lyn
Lyn is an src family kinase that phosphorylates ITIMs in both the SHIP and SHP-1-mediated inhibitory receptor pathways. Lyn is expressed widely in haemopoietic cells57 and in B cells a significant proportion is associated constitutively with the BCR and becomes activated rapidly on BCR cross-linking.58 Lyn is involved in both the activation and inhibition of the B cell. Cross-linking of the BCR in lyn-deficient mice leads to delayed and reduced phosphorylation of syk and several other substrates within the activatory pathway. However, there is sufficient phosphorylation by other src family kinase members to generate a B cell response.59 The non-redundant role of lyn appears to be inhibitory, however, because the B cells of lyn-deficient mice are hyper-responsive to BCR cross-linking.3,60
B cells in lyn-/- mice show exaggerated proliferative responses following BCR cross-linking,61 have increased numbers of peripheral mature B cells and elevated serum IgM and IgA. B cells from lyn-deficient anti-HEL transgenic mice show a delay in the initial antigen-induced Ca2+ flux, but overall Ca2+ flux was increased.36 This suggests that lyn may be involved in the initiation of intracellular Ca2+ release but overall has an inhibitory effect upon it. The lyn knockout mice develop isotype switched autoantibodies, lymphadenopathy, splenomegaly and immune complex-mediated glomerulonephritis similar to that seen in SLE.62 Lyn-deficient mice develop worse disease than do mice deficient in single inhibitory receptors, presumably because lyn deficiency interrupts the function of multiple inhibitory receptors.
There are no clear data showing genetic linkage between lyn and the development of autoimmune disease in either murine models of disease or in human autoimmune conditions. Two studies have shown abnormal expression of lyn in SLE patients. The first demonstrated reduced levels of lyn in the lymphocytes of SLE patients with inactive disease.63 The second showed reduced expression in B cell-enriched cell lysates obtained from 66% of patients with SLE. This deficiency appeared to be disease-specific and unrelated to disease activity.64
SHP-1
SHP-1 is a protein tyrosine phosphatase and is similar in structure to SHP-2. SHP-1 is the phosphatase that is utilized most widely in the inhibitory receptor signalling pathways (see Table 1). These phosphatases contain two amino terminal SH2 domains, a phosphatase domain and two conserved carboxy-terminal tyrosine residues.4 SHP-2 has an additional carboxy-terminal domain that may allow interaction with SH3-containing proteins.65 While both molecules are activated after binding through their amino-terminal SH2 domains to phosphorylated ITIMs,66 they appear to bind with different affinities.67 These differences in structure and binding affinities appear to confer significant differences in the signalling functions of the two molecules. SHP-1 is a broadly inhibitory molecule and plays the predominant role of the two in regulation through ITIMs, while increasing evidence suggests that SHP-2 may well have an additional activatory role.68 Clearly, these molecules have an important role in regulation of a normal immune system which is due, at least in part, to their recruitment by inhibitory receptors.
Consistent with its role in mediating inhibitory receptor function, SHP-1 deficiency results in the development of spontaneous autoimmune disease. However, the situation is complicated, as this is not the only group of receptors it subserves. SHP-1 also associates with BCR, FcR, growth factor, complement and cytokine receptors.69 Despite these complicating factors, much of the knockout phenotype is consistent with SHP-1 having a predominant role in the inhibitory receptor pathways. The ‘moth-eaten’ (me) and ‘moth-eaten viable’ (mev) mice are naturally occurring SHP-1 mutants.70,71 The me mutation stops production of SHP-1 completely, whereas the mev mutation is a single base-pair deletion that disrupts an mRNA splice site leading to production of aberrant SHP-1 protein with 10–20% of normal activity.72 The me and mev mice have a broadly similar phenotype, although it is milder in the mev. These mice have reduced numbers of B cells but a higher proportion of B1 cells. The mice have B cells that are hyper-responsive to BCR stimulation,73 raised levels of serum immunoglobulin73 and develop autoantibodies.70 Both strains develop severe autoimmune disease with immune complex deposition in skin, lung and kidney,71 patchy alopecia,70 splenomegaly and inflamed paws. The life span of a homozygous me mouse is 3 weeks, while that of a homozygous mev mouse is 9 weeks.71 The double mutant mev and RAG-1-/- mice develop the full phenotype but without the development of autoantibodies. Thus SHP-1 deficiency produces such severe immune dysregulation that B cells do not appear to be necessary for the development of disease, although their contribution is demonstrated by the fact its phenotype is altered in their absence.74 The severity of the disease that is seen in SHP-1-deficient mice is clearly worse than that seen in mice with deficiencies of individual inhibitory receptors. This is due most probably to the effects of disrupting multiple inhibitory receptor pathways, although it should be kept in mind that SHP-1 has functions in addition to mediating inhibitory receptor suppression.
There are a number of genetic studies of mouse models of disease that identify susceptibility loci that contain SHP-1 (Table 2),37,75,76 although a role for it in disease pathogenesis has not been proved. There are no clear data that show linkage between SHP-1 and the development of autoimmune disease in humans. However, defects in SHP-1 expression have been associated with SLE in humans; reduced levels of SHP-1 (and lyn) are seen in the lymphocytes of patients with SLE during inactive phases of the disease,63 suggesting a possible role in pathogenesis.
SHIP
SHIP is an SH2-containing inositol phosphatase related to SHIP-277 and they share a conserved N-terminal SH2 catalytic domain. In the B cell inhibitory receptors SHIP acts predominantly on the FcγRIIb signalling pathway. In humans it occurs in a number of isoforms, the most common of which is 145 kDa in size.78 The molecule is highly conserved between humans and mice (96% homology) and is expressed widely in myeloid and lymphoid lineages, including B cells.79
SHIP acts to dephosphorylate PIP3 and inositol-1, 3, 4, 5-tetrakisphosphate (IP4),80 and because PIP3 is produced by the action of PI3K on PIP2, in so doing serves to counteract PI3K activity. Through this mechanism, activation of SHIP leads to reduced BCR-mediated phosphoinositide hydrolysis and Ca2+ mobilization.81
The pattern of B cell abnormalities seen in the SHIP-deficient mouse are consistent with this inhibitory role in B cell signalling. Splenic B cells have an activated phenotype with lower surface levels of IgM and higher levels of IgD and are hyper-responsive to BCR-mediated stimulation measured by the activation markers CD69 and CD86.82 SHIP-deficient B cells also demonstrate prolonged Ca2+ influx and enhanced proliferation in vitro in response to BCR stimulation that was associated with increased phosphorylation of MAP kinase and Akt and also with increased cell cycling and survival.83,84 SHIP-deficient mice also have elevated serum immunoglobulin levels with enhanced IgG responses to TI antigen.84 However, the mice do not develop autoantibodies or B cell-mediated autoimmune disease. They die prematurely (50% mortality by 10–12 weeks), with consolidation of the lungs brought about by myeloid cell infiltration.82 The B cell phenotype of these mice is comparable with mice susceptible to SLE, but their premature death with a myeloid-mediated autoimmune disease means that the potential for SHIP deficiency in B cells to contribute to disease has not been confirmed.
Genetic studies in humans have identified susceptibility loci for both diabetes85 and SLE56 mapping to the region of the genome containing SHIP (Table 3), but no direct evidence exists for abnormal SHIP function in human disease.
Thus, while mice deficient in these three signalling molecules develop autoimmune disease consistent with the interruption of inhibitory receptor function, little direct genetic evidence implicates them in autoimmune pathogenesis in humans. However, their expression is altered in patients with SLE, suggesting indirect dysregulation of them may play a role in disease development.
Concluding discussion
Inhibitory receptors control the activation threshold of many immune cells, including B cells. There are many similarities in the signalling pathways subserving these inhibitory receptors. Consistent with this is the fact that B cells from inhibitory receptor-deficient mice have similarities in phenotype, in particular lowering thresholds for activation. Inhibitory receptors also have specific effects, as they bind different ligands, and signal through different phosphatases. The exact physiological roles of the individual inhibitory receptors are incompletely understood.
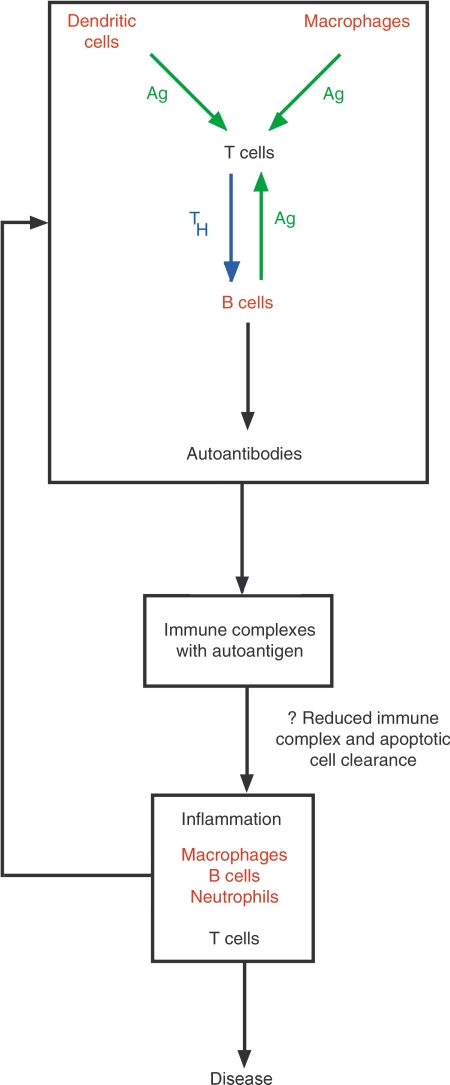
Almost all the inhibitory receptor knockout mice have a tendency to autoimmune disease. This broad similarity in phenotype is consistent with the similarity seen in the signalling pathways used by these molecules, and their common effect on activation threshold. The autoimmune disease developed by these mice has a striking similarity to that seen in human SLE. SLE is an autoimmune connective tissue disease which presents commonly with features including a skin rash, arthritis and glomerulonephritis (Fig. 4). Patients with SLE have hyperactive B cells, hypergammaglobulinaemia and develop autoantibodies, often directed against nuclear antigens. These are deposited in the form of immune complexes, initiating an inflammatory reaction which gives rise to end organ damage and the clinical features of the disease (Fig. 5).
Figure 4.
SLE in mice and humans. (a) Antinuclear antibodies in the serum of Lyn deficient mice, shown binding to HEp2 cells (courtesy of Dr D. M. Tarlinton). (b) Immunofluorescence microscopy for IgG demonstrating the deposition of immune complexes in a glomerulus from an SLE patient with membranous (Type V) lupus nephritis. (c) A normal glomerulus. (d) A glomerulus from a patient with SLE showing the inflammatory response to immune complex deposition (diffuse proliferative glomerulonephritis). (e) Crescentic glomerulonephritis with scarring following lupus glomerulonephritis. (f) Discoid lupus; a form of skin rash seen in SLE.
Figure 5.
The pathogenesis of SLE. The breakdown in tolerance responsible for the development of autoantibodies (upper panel) is necessary but insufficient to cause disease in the absence of an abnormal inflammatory response to immune complexes (lower panel). Cell types in which FcγRIIb is likely to control antibody production or immune complex handling are shown in red.
Differences in phenotypes are due no doubt to the different physiological functions of each inhibitory receptor and to the fact that they are not all expressed solely on B cells. FcγRIIb, for example, also controls macrophages and probably dendritic cells, which could explain why FcγRIIb knockout mice have a more severe autoimmune phenotype than CD22 knockout mice, as CD22 is expressed only on B cells (Fig. 5). Defects in molecules which mediate inhibitory receptor signalling also cause autoimmune disease, but as these molecules are responsible for the function of more than one inhibitory receptor, deficiencies in them generally result in a more severe phenotype than that seen in individual inhibitory receptor knockout mice.
Knockout mouse studies have shown that these partial defects in more than one inhibitory receptor can combine to predispose to autoimmunity.35,36 This raises the possibility that defects in the regulation of inhibitory receptor function might also predispose to disease. We have shown recently that interleukin-4 (IL-4) reduces the expression of FcγRII, CD22, PIR-B and CD72 on B cells, and abolishes the inhibitory function of CD22 and FcγRII.86 It would be expected, therefore, that excessive production of IL-4 might lead to autoimmunity, by co-ordinated interference with these four inhibitory receptors. This is consistent with the observation that transgenic overexpression of IL-4 leads to SLE.87
Mounting evidence implicates inhibitory receptor defects in spontaneous autoimmune disease in mice. The phenotype of most mouse models of SLE suggests impaired inhibitory receptor function, with hyperactive B cells and a similar pattern of autoantibody production and glomerular disease to that seen in the inhibitory receptor knockout mice. Nonetheless, at almost every genetic susceptibility locus containing an inhibitory receptor implicated in SLE there are large numbers of other immunologically relevant molecules which may also play a role in disease pathogenesis. The role of inhibitory receptors in spontaneous disease is therefore yet to be established firmly, but nonetheless the evidence favours contributions by defective inhibitory receptor function to the pathogenesis of such diseases. Much work remains to be undertaken to understand the physiological function of each inhibitory receptor, and to confirm the role that these receptors might play in the pathogenesis of autoimmunity, before strategies based on this knowledge can be devised to prevent or treat disease.
References
- 1.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JV. A 13-amino-acid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling. Nature. 1994;368:70–3. doi: 10.1038/368070a0. [DOI] [PubMed] [Google Scholar]
- 2.Doody GM, Justement LB, Delibrias CC, Matthews RJ, Lin J, Thomas ML, Fearon DT. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995;269:242–4. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- 3.Smith KGC, Tarlinton DM, Doody GM, Hibbs ML, Fearon DT. Inhibition of the B cell by CD22: a requirement for Lyn. J Exp Med. 1998;187:807–11. doi: 10.1084/jem.187.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamir I, Dal Porto JM, Cambier JC. Cytoplasmic protein tyrosine phosphatases SHP-1 and SHP-2: regulators of B cell signal transduction. Curr Opin Immunol. 2000;12:307–15. doi: 10.1016/s0952-7915(00)00092-3. [DOI] [PubMed] [Google Scholar]
- 5.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–34. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 6.Malbec O, Fridman WH, Daeron M. Negative regulation of hematopoietic cell activation and proliferation by Fc gamma RIIB. Curr Top Microbiol Immunol. 1999;244:13–27. [PubMed] [Google Scholar]
- 7.Brooks DG, Qiu WQ, Luster AD, Ravetch JV. Structure and expression of human IgG FcRII (CD32). Functional heterogeneity is encoded by the alternatively spliced products of multiple genes. J Exp Med. 1989;170:1369–85. doi: 10.1084/jem.170.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latour S, Fridman WH, Daeron M. Identification, molecular cloning, biologic properties, and tissue distribution of a novel isoform of murine low-affinity IgG receptor homologous to human Fc gamma RIIB1. J Immunol. 1996;157:189–97. [PubMed] [Google Scholar]
- 9.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–90. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 10.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–9. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 11.Yuasa T, Kubo S, Yoshino T, Ujike A, Matsumura K, Ono M, Ravetch JV, Takai T. Deletion of fcgamma receptor IIB renders H-2(b) mice susceptible to collagen-induced arthritis. J Exp Med. 1999;189:187–94. doi: 10.1084/jem.189.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura A, Yuasa T, Ujike A, Ono M, Nukiwa T, Ravetch JV, Takai T. Fcgamma receptor IIB-deficient mice develop Goodpasture's syndrome upon immunization with type IV collagen: a novel murine model for autoimmune glomerular basement membrane disease. J Exp Med. 2000;191:899–906. doi: 10.1084/jem.191.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc (gamma) RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–85. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 14.Boackle SA, Holers VM, Chen X, Szakonyi G, Karp DR, Wakeland EK, Morel L. Cr2, a candidate gene in the murine Sle1c lupus susceptibility locus, encodes a dysfunctional protein. Immunity. 2001;15:775–85. doi: 10.1016/s1074-7613(01)00228-x. [DOI] [PubMed] [Google Scholar]
- 15.Morel L, Blenman KR, Croker BP, Wakeland EK. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proc Natl Acad Sci USA. 2001;98:1787–92. doi: 10.1073/pnas.031336098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pritchard NR, Cutler AJ, Uribe S, Chadban SJ, Morley BJ, Smith KGC. Autoimmune-prone mice share a promoter haplotype associated with reduced expression and function of the Fc receptor FcgammaRII. Curr Biol. 2000;10:227–30. doi: 10.1016/s0960-9822(00)00344-4. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Y, Hirose S, Sanokawa-Akakura R, et al. Genetically determined aberrant down-regulation of FcgammaRIIB1 in germinal center B cells associated with hyper-IgG and IgG autoantibodies in murine systemic lupus erythematosus. Int Immunol. 1999;11:1685–91. doi: 10.1093/intimm/11.10.1685. [DOI] [PubMed] [Google Scholar]
- 18.Luan JJ, Monteiro RC, Sautes C, Fluteau G, Eloy L, Fridman WH, Bach JF, Garchon HJ. Defective Fc gamma RII gene expression in macrophages of NOD mice: genetic linkage with up-regulation of IgG1 and IgG2b in serum. J Immunol. 1996;157:4707–16. [PubMed] [Google Scholar]
- 19.Xiu Y, Nakamura K, Abe M, et al. Transcriptional regulation of Fcgr2b gene by polymorphic promoter region and its contribution to humoral immune responses. J Immunol. 2002;169:4340–6. doi: 10.4049/jimmunol.169.8.4340. [DOI] [PubMed] [Google Scholar]
- 20.Bolland S, Yim Y, Tus K, Wakeland E, Ravetch J. Genetic modifiers of systemic lupus erythematosus in FcgammaRIIB (-/-) mice. J Exp Med. 2002;195:1167–74. doi: 10.1084/jem.20020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto L, Habita C, Beressi JP, et al. Genetic mapping of a susceptibility locus for insulin-dependent diabetes mellitus on chromosome 11q. Nature. 1994;371:161–4. doi: 10.1038/371161a0. [DOI] [PubMed] [Google Scholar]
- 22.Shai R, Quismorio FP, Jr, Li L, et al. Genome-wide screen for systemic lupus erythematosus susceptibility genes in multiplex families. Hum Mol Genet. 1999;8:639–44. doi: 10.1093/hmg/8.4.639. [DOI] [PubMed] [Google Scholar]
- 23.Gaffney PM, Kearns GM, Shark KB, et al. A genome-wide search for susceptibility genes in human systemic lupus erythematosus sib-pair families. Proc Natl Acad Sci USA. 1998;95:14875–9. doi: 10.1073/pnas.95.25.14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaffney PM, Ortmann WA, Selby SA, et al. Genome screening in human systemic lupus erythematosus results from a second Minnesota cohort and combined analyses of 187 sib-pair families. Am J Hum Genet. 2000;66:547–56. doi: 10.1086/302767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harley JB, Moser KL, Gaffney PM, Behrens TW. The genetics of human systemic lupus erythematosus. Curr Opin Immunol. 1998;10:690–6. doi: 10.1016/s0952-7915(98)80090-3. [DOI] [PubMed] [Google Scholar]
- 26.Wakeland EK, Liu K, Graham RR, Behrens TW. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 2001;15:397–408. doi: 10.1016/s1074-7613(01)00201-1. [DOI] [PubMed] [Google Scholar]
- 27.Botto M, Theodoridis E, Thompson EM, Beynon HL, Briggs D, Isenberg DA, Walport MJ, Davies KA. Fc gamma RIIa polymorphism in systemic lupus erythematosus (SLE): no association with disease. Clin Exp Immunol. 1996;104:264–8. doi: 10.1046/j.1365-2249.1996.33740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyogoku C, Dijstelbloem H, Tsuchiya N, et al. Fcγ receptor gene polymorphisms in Japanese patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:1242–54. doi: 10.1002/art.10257. [DOI] [PubMed] [Google Scholar]
- 29.Dorken B, Moldenhauer G, Pezzutto A, Schwartz R, Feller A, Kiesel S, Nadler LM. HD39 (B3), a B lineage-restricted antigen whose cell surface expression is limited to resting and activated human B lymphocytes. J Immunol. 1986;136:4470–9. [PubMed] [Google Scholar]
- 30.Smith KGC, Fearon DT. Receptor modulators of BCR signalling − CD19/22. Curr Top Microbiol Immunol. 1999;245:195–212. doi: 10.1007/978-3-642-57066-7_6. [DOI] [PubMed] [Google Scholar]
- 31.Hanasaki K, Varki A, Stamenkovic I, Bevilacqua MP. Cytokine-induced beta-galactoside alpha-2,6-sialyltransferase in human endothelial cells mediates alpha 2,6-sialylation of adhesion molecules and CD22 ligands. J Biol Chem. 1994;269:10637–43. [PubMed] [Google Scholar]
- 32.Neuberger MS, Lanoue A, Ehrenstein MR, Batista FD, Sale JE, Williams GT. Antibody diversification and selection in the mature B-cell compartment. Cold Spring Harb Symp Quant Biol. 1999;64:211–16. doi: 10.1101/sqb.1999.64.211. [DOI] [PubMed] [Google Scholar]
- 33.O'Keefe TL, Williams GT, Davies SL, Neuberger MS. Hyperresponsive B cells in CD22-deficient mice. Science. 1996;274:798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- 34.O'Keefe TL, Williams GT, Batista FD, Neuberger MS. Deficiency in CD22, a B cell-specific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. J Exp Med. 1999;189:1307–13. doi: 10.1084/jem.189.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mary C, Laporte C, Parzy D, et al. Dysregulated expression of the Cd22 gene as a result of a short interspersed nucleotide element insertion in Cd22a lupus-prone mice. J Immunol. 2000;165:2987–96. doi: 10.4049/jimmunol.165.6.2987. [DOI] [PubMed] [Google Scholar]
- 36.Cornall RJ, Cyster JG, Hibbs ML, Dunn AR, Otipoby KL, Clark EA, Goodnow CC. Polygenic autoimmune traits. Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signalling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- 37.Kono DH, Burlingame RW, Owens DG, Kuramochi A, Balderas RS, Balomenos D, Theofilopoulos AN. Lupus susceptibility loci in New Zealand mice. Proc Natl Acad Sci USA. 1994;91:10168–72. doi: 10.1073/pnas.91.21.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morel L, Rudofsky UH, Longmate JA, Schiffenbauer J, Wakeland EK. Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity. 1994;1:219–29. doi: 10.1016/1074-7613(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 39.Morel L, Mohan C, Yu Y, Croker BP, Tian N, Deng A, Wakeland EK. Functional dissection of systemic lupus erythematosus using congenic mouse strains. J Immunol. 1997;158:6019–28. [PubMed] [Google Scholar]
- 40.Ghosh S, Palmer SM, Rodrigues NR, et al. Polygenic control of autoimmune diabetes in nonobese diabetic mice. Nat Genet. 1993;4:404–9. doi: 10.1038/ng0893-404. [DOI] [PubMed] [Google Scholar]
- 41.Butterfield RJ, Blankenhorn EP, Roper RJ, et al. Genetic analysis of disease subtypes and sexual dimorphisms in mouse experimental allergic encephalomyelitis (EAE): relapsing/remitting and monophasic remitting/nonrelapsing EAE are immunogenetically distinct. J Immunol. 1999;162:3096–102. [PubMed] [Google Scholar]
- 42.Baker D, Rosenwasser OA, O'Neill JK, Turk JL. Genetic analysis of experimental allergic encephalomyelitis in mice. J Immunol. 1995;155:4046–51. [PubMed] [Google Scholar]
- 43.Davies JL, Kawaguchi Y, Bennett ST, et al. A genome-wide search for human type 1 diabetes susceptibility genes. Nature. 1994;371:130–6. doi: 10.1038/371130a0. [DOI] [PubMed] [Google Scholar]
- 44.Sawcer S, Jones HB, Feakes R, et al. A genome screen in multiple sclerosis reveals susceptibility loci on chromosome 6p21 and 17q22. Nat Genet. 1996;13:464–8. doi: 10.1038/ng0896-464. [DOI] [PubMed] [Google Scholar]
- 45.Haines JL, Ter-Minassian M, Bazyk A, et al. A complete genomic screen for multiple sclerosis underscores a role for the major histocompatability complex. The Multiple Sclerosis Genetics Group. Nat Genet. 1996;13:469–71. doi: 10.1038/ng0896-469. [DOI] [PubMed] [Google Scholar]
- 46.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. Embo J. 1992;11:3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–72. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 48.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-I and inhibits T cell activation. Nat Immunol. 2001;2:261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 50.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signalling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci USA. 2001;98:13866–7. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001;22:265–8. doi: 10.1016/s1471-4906(01)01888-9. [DOI] [PubMed] [Google Scholar]
- 52.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563–72. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 53.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 54.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–22. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 55.Nishimura H, Honjo T, Minato N. Facilitation of beta selection and modification of positive selection in the thymus of PD-1-deficient mice. J Exp Med. 2000;191:891–8. doi: 10.1084/jem.191.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moser KL, Neas BR, Salmon JE, et al. Genome scan of human systemic lupus erythematosus: evidence for linkage on chromosome 1q in African-American pedigrees. Proc Natl Acad Sci USA. 1998;95:14869–74. doi: 10.1073/pnas.95.25.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bolen JB, Rowley RB, Spana C, Tsygankov AY. The Src family of tyrosine protein kinases in hemopoietic signal transduction. Faseb J. 1992;6:3403–9. doi: 10.1096/fasebj.6.15.1281458. [DOI] [PubMed] [Google Scholar]
- 58.Burkhardt AL, Brunswick M, Bolen JB, Mond JJ. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc Natl Acad Sci USA. 1991;88:7410–14. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takata M, Sabe H, Hata A, et al. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 1994;13:1341–9. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malbec O, Fong DC, Turner M, Tybulewicz VL, Cambier JC, Fridman WH, Daeron M. Fc epsilon receptor I-associated lyn-dependent phosphorylation of Fc gamma receptor IIB during negative regulation of mast cell activation. J Immunol. 1998;160:1647–58. [PubMed] [Google Scholar]
- 61.Wang J, Koizumi T, Watanabe T. Altered antigen receptor signalling and impaired Fas-mediated apoptosis of B cells in Lyn-deficient mice. J Exp Med. 1996;184:831–8. doi: 10.1084/jem.184.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–11. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 63.Huck S, Le Corre R, Youinou P, Zouali M. Expression of B cell receptor-associated signalling molecules in human lupus. Autoimmunity. 2001;33:213–24. doi: 10.3109/08916930109008048. [DOI] [PubMed] [Google Scholar]
- 64.Liossis SN, Solomou EE, Dimopoulos MA, Panayiotidis P, Mavrikakis MM, Sfikakis PP. B-cell kinase lyn deficiency in patients with systemic lupus erythematosus. J Invest Med. 2001;49:157–65. doi: 10.2310/6650.2001.34042. [DOI] [PubMed] [Google Scholar]
- 65.Hof P, Pluskey S, Dhe-Paganon S, Eck MJ, Shoelson SE. Crystal structure of the tyrosine phosphatase SHP-2. Cell. 1998;92:441–50. doi: 10.1016/s0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 66.Barford D, Neel BG. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure. 1998;6:249–54. doi: 10.1016/s0969-2126(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 67.Vely F, Olivero S, Olcese L, et al. Differential association of phosphatases with hematopoietic co-receptors bearing immunoreceptor tyrosine-based inhibition motifs. Eur J Immunol. 1997;27:1994–2000. doi: 10.1002/eji.1830270825. [DOI] [PubMed] [Google Scholar]
- 68.Huyer G, Alexander DR. Immune signalling: SHP-2 docks at multiple ports. Curr Biol. 1999;9:R129–32. doi: 10.1016/s0960-9822(99)80080-3. [DOI] [PubMed] [Google Scholar]
- 69.Bolland S, Ravetch JV. Inhibitory pathways triggered by ITIM-containing receptors. Adv Immunol. 1999;72:149–77. doi: 10.1016/s0065-2776(08)60019-x. [DOI] [PubMed] [Google Scholar]
- 70.Green MC, Shultz LD. Motheaten, an immunodeficient mutant of the mouse. I. Genetics and pathology. J Hered. 1975;66:250–8. doi: 10.1093/oxfordjournals.jhered.a108625. [DOI] [PubMed] [Google Scholar]
- 71.Shultz LD, Coman DR, Bailey CL, Beamer WG, Sidman CL. ‘Viable motheaten’; a new allele at the motheaten locus. I. Pathology. Am J Pathol. 1984;116:179–92. [PMC free article] [PubMed] [Google Scholar]
- 72.Tsui HW, Siminovitch KA, de Souza L, Tsui FW. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet. 1993;4:124–9. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 73.Sidman CL, Shultz LD, Hardy RR, Hayakawa K, Herzenberg LA. Production of immunoglobulin isotypes by Ly-1+ B cells in viable motheaten and normal mice. Science. 1986;232:1423–5. doi: 10.1126/science.3487115. [DOI] [PubMed] [Google Scholar]
- 74.YuCC, Tsui HW, Ngan BY, Shulman MJ, Wu GE, Tsui FW. B and T cells are not required for the viable motheaten phenotype. J Exp Med. 1996;183:371–80. doi: 10.1084/jem.183.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McIndoe RA, Bohlman B, Chi E, Schuster E, Lindhardt M, Hood L. Localization of non-MHC collagen-induced arthritis susceptibility loci in DBA/1j mice. Proc Natl Acad Sci USA. 1999;96:2210–14. doi: 10.1073/pnas.96.5.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Melanitou E, Joly F, Lathrop M, Boitard C, Avner P. Evidence for the presence of insulin-dependent diabetes-associated alleles on the distal part of mouse chromosome 6. Genome Res. 1998;8:608–20. doi: 10.1101/gr.8.6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lioubin MN, Algate PA, Tsai S, Carlberg K, Aebersold A, Rohrschneider LR. p150Ship, a signal transduction molecule with inositol polyphosphate-5-phosphatase activity. Genes Dev. 1996;10:1084–95. doi: 10.1101/gad.10.9.1084. [DOI] [PubMed] [Google Scholar]
- 78.Kavanaugh W, Pot D, Chin S, et al. Multiple forms of an inositol polyphosphate 5-phosphatase form signalling complexes with Shc and Grb2. Curr Biol. 1996;6:438–45. doi: 10.1016/s0960-9822(02)00511-0. [DOI] [PubMed] [Google Scholar]
- 79.Huber M, Helgason C, Damen J, et al. The role of SHIP in growth factor induced signalling. Prog Biophys Mol Biol. 1999;71:423–4. doi: 10.1016/s0079-6107(98)00049-2. [DOI] [PubMed] [Google Scholar]
- 80.Damen J, Liu L, Rosten P, Humphries R, Jefferson A, Majerus P, Krystal G. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci USA. 1996;93:1689–93. doi: 10.1073/pnas.93.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brauweiler AM, Tamir I, Cambier JC. Bilevel control of B-cell activation by the inositol 5-phosphatase SHIP. Immunol Rev. 2000;176:69–74. doi: 10.1034/j.1600-065x.2000.00612.x. [DOI] [PubMed] [Google Scholar]
- 82.Helgason CD, Damen JE, Rosten P, et al. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 1998;12:1610–20. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Q, Oliveira-Dos-Santos AJ, Mariathasan S, et al. The inositol polyphosphate 5-phosphatase ship is a crucial negative regulator of B cell antigen receptor signalling. J Exp Med. 1998;188:1333–42. doi: 10.1084/jem.188.7.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Helgason CD, Kalberer CP, Damen JE, Chappel SM, Pineault N, Krystal G, Humphries RK. A dual role for Src homology 2 domain-containing inositol-5-phosphatase (SHIP) in immunity aberrant development and enhanced function of B lymphocytes in ship -/- mice. J Exp Med. 2000;191:781–94. doi: 10.1084/jem.191.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo D, Maclaren N, Huang H, Muir A, She J. Intrafamilial and case–control association analysis of D2S152 in insulin-dependent diabetes. Autoimmunity. 1995;2:143–7. doi: 10.3109/08916939508993363. [DOI] [PubMed] [Google Scholar]
- 86.Rudge EU, Cutler AJ, Pritchard NR, Smith KGC. Interleukin 4 reduces expression of inhibitory receptors on B cells and abolishes CD22 and Fc gamma RII-mediated B cell suppression. J Exp Med. 2002;195:1079–85. doi: 10.1084/jem.20011435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Erb KJ, Ruger B, von Brevern M, Ryffel B, Schimpl A, Rivett K. Constitutive expression of interleukin (IL)-4 in vivo causes autoimmune-type disorders in mice. J Exp Med. 1997;185:329–39. doi: 10.1084/jem.185.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Drake CG, Rozzo SJ, Hirschfeld HF, Smarnworawong NP, Palmer E, Kotzin BL. Analysis of the New Zealand Black contribution to lupus-like renal disease. Multiple genes that operate in a threshold manner. J Immunol. 1995;154:2441–7. [PubMed] [Google Scholar]
- 89.Hogarth MB, Slingsby JH, Allen PJ, et al. Multiple lupus susceptibility loci map to chromosome 1 in BXSB mice. J Immunol. 1998;161:2753–61. [PubMed] [Google Scholar]
- 90.Jordan MA, Silveira PA, Shepherd DP, et al. Linkage analysis of systemic lupus erythematosus induced in diabetes-prone nonobese diabetic mice by Mycobacterium bovis. J Immunol. 2000;165:1673–84. doi: 10.4049/jimmunol.165.3.1673. [DOI] [PubMed] [Google Scholar]
- 91.Johansson AC, Sundler M, Kjellen P, et al. Genetic control of collagen-induced arthritis in a cross with NOD and C57BL/10 mice is dependent on gene regions encoding complement factor 5 and FcgammaRIIb and is not associated with loci controlling diabetes. Eur J Immunol. 2001;31:1847–56. doi: 10.1002/1521-4141(200106)31:6<1847::aid-immu1847>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 92.Gray-McGuire C, Moser KL, Gaffney PM, et al. Genome scan of human systemic lupus erythematosus by regression modeling: evidence of linkage and epistasis at 4p16–15.2. Am J Hum Genet. 2000;67:1460–9. doi: 10.1086/316891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Daniels SE, Bhattacharrya S, James A, et al. A genome-wide search for quantitative trait loci underlying asthma. Nature. 1996;383:247–50. doi: 10.1038/383247a0. [DOI] [PubMed] [Google Scholar]
- 94.Field LL, Tobias R, Magnus T. A locus on chromosome 15q26 (IDDM3) produces susceptibility to insulin-dependent diabetes mellitus. Nat Genet. 1994;8:189–94. doi: 10.1038/ng1094-189. [DOI] [PubMed] [Google Scholar]
- 95.Ebers GC, Kukay K, Bulman DE, et al. A full genome search in multiple sclerosis. Nat Genet. 1996;13:472–6. doi: 10.1038/ng0896-472. [DOI] [PubMed] [Google Scholar]