Abstract
Previously we have shown that vaccination with the poorly immunogenic B16BL6-D5 melanoma (D5) elicits a dominant type 2 (T2) cytokine response that fails to protect the host from a subsequent tumour challenge. Here we investigated whether the inherent immunogenicity of a tumour can be correlated with its ability to bias the anti-tumour cytokine response towards either a type 1 (T1) or a T2 profile. The immune response to six tumours of different inherent immunogenicity was assayed. By isolating l-selectinlow T cells from tumour vaccine draining lymph nodes (TVDLN), it was possible to detect tumour-specific cytokine responses from both immunogenic, poorly immunogenic and non-immunogenic tumours. Immunogenic tumours (MCA-304, MCA-309, MPR-4) induced a predominant tumour-specific T1 cytokine response. In contrast, weakly (MCA-310, MPR-3) and poorly/non-immunogenic tumours (MPR-5, D5) sensitized T cells with a predominant tumour-specific T2 cytokine response. A significant correlation (P < 0·025) between immunogenicity and the ratio of tumour-specific interferon-γ: interleukin-4 (IL-4) secretion by TVDLN T cells was identified. We then documented that non-therapeutic T cells primed by the poorly immunogenic D5, recognized ‘tumour-rejection’ antigens and that reprogramming their cytokine response, by in vitro culture with IL-12 and anti-IL-4, to a T1 profile uncovered therapeutic efficacy. In contrast, TVDLN T cells primed by a therapeutic vaccine lose therapeutic efficacy when cultured with IL-4. These results provide insights into the development of a protective anti-tumour immune response and strengthen the hypothesis that a T1 cytokine response is critical for T-cell-mediated tumour regression.
Introduction
A tumour is considered strongly immunogenic when vaccination with irradiated/unmodified tumour efficiently protects the host from a challenge with that same tumour. Tumour vaccines that protect a minority of animals are considered weakly immunogenic and those that uniformly fail to protect the host are generally characterized as poorly immunogenic or non-immunogenic. This last designation suggests that the host has not recognized, or is tolerant of, the tumour antigens. Recently, we identified that the poorly immunogenic B16BL6-D5 (D5) melanoma elicits a T2-biased immune response that appears to be non-destructive and incapable of protecting the host.1 The development of a non-destructive immune response is a form of tolerance and has been referred to as immune deviation.2
It is well established that the immune response generated by T helper (Th) cells and T cytotoxic (Tc) cells can be segregated into two general categories based on their cytokine release patterns.3–6 A type 1 (T1) cell selectively secretes interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α) and leukotriene-α (LT-α), whereas type 2 (T2) cells secrete interleukin-4 (IL-4), IL-5, IL-6, IL-9, IL-10 and IL-13. They appear to share a common precursor that can differentiate along either pathway. The final pathway is determined by the cytokine milieu in which the T cells are activated and undergo differentiation.7 The presence of T1 or T2 cytokines can drive uncommitted T cells to develop a cytokine profile similar to the one to which they are exposed, while at the same time inhibiting the development of cells with the reciprocal phenotype. Thus, IFN-γ selectively expands T1 cells and inhibits proliferation of T2 cells, while IL-4 and IL-10 can selectively inhibit cytokine secretion by T1 cells.3,8,9 This ability to inhibit the maturation of cells producing cytokines of the alternate type may account for the tendency to see a predominant cytokine profile during most immune responses. Therefore, the presence of T2 cytokines during the initial interaction between a T cell and a tumour antigen presented by antigen-presenting cells (APC) in the draining lymph node would facilitate the development of a T2 anti-tumour response and vice versa for T1 cytokines.
Our previous experiments exploited reduced l-selectin expression as an approach to enrich for the tumour vaccine draining lymph node (TVDLN) T cells that were responding to the tumour. Effector T cells generated from the l-selectinlow TVDLN from mice vaccinated with D5 tumour cells were found to exhibit a tumour-specific T2 cytokine profile and were non-therapeutic in adoptive transfer studies, while lymph nodes draining a gene-modified D5 vaccine expressed a T1 cytokine profile and mediated regression of pulmonary metastases.1 These results led us to develop the hypothesis that a tumour-specific T1 response is critical for T-cell-mediated tumour regression. Here we wanted to investigate whether the observations made in the D5 tumour model might represent a paradigm that could be applied to other tumour models. To do this we have examined the immune response to vaccination with six additional tumour cell lines. Additionally, we wanted to test the hypothesis that tumour-specific T1 cells were responsible for mediating regression of established pulmonary metastases. Thus, we examined whether the T cells primed by the non-therapeutic D5 vaccine could be induced to mediate therapeutic activity by shifting them towards a T1 cytokine profile by incubation with IL-12 and anti-IL-4. The reverse was also investigated, by culturing tumour-primed T cells with therapeutic potential, under conditions that promoted a T2 cytokine response.
Materials and methods
Mice
Female C57BL/6J (wild-type; wt) and IFN-γ knock-out (GKO) mice (C57BL/6-IFN-γtmiTs) were purchased from the Jackson Laboratory (Bar Harbor, ME) and maintained in a specific pathogen-free environment. Mice were generally 8–12 weeks old at the time of experimentation. Recognized principles of laboratory animal care were followed (Guide for the Care and Use of Laboratory Animals, National Research Council, 1996), and all animal protocols were approved by the Earle A. Chiles Research Institute Animal Care and Use Committee.
Tumour cell lines
D5 is a poorly immunogenic subclone of the spontaneously arising B16BL6 melanoma1,10 (provided by Dr S. Shu, Cleveland Clinic Foundation, Cleveland, OH). D5-G6 is a stable clone of D5 that was originally transduced with a murine granulocyte–macrophage colony-stimulating factor (GM-CSF) retroviral MFG vector (provided by Dr M. Arca, University of Michigan, Ann Arbor, MI).11 D5-G6 cells secrete approximately 200 ng/ml/106 cells/24 hr GM-CSF. MPR-3 MPR-4 and MPR-5 are transformed prostate tumour cell lines (generously provided by Dr Thompson, Baylor College of Medicine, Houston, TX).12 MCA-304, MCA 309 and MCA-310 are 3-methylcholanthrene-induced tumours of C57BL/6J mice.
Reagents
The 145-2c11 hybridoma (anti-CD3) was a gift of Dr J. A. Bluestone (University of Chicago, Chicago, IL). Recombinant human IL-2 was generously provided by the Chiron Corporation (Dr S. Wilson, Emeryville, CA) and recombinant murine IL-4 was purchased from Peprotech (Rocky Hill, NJ, USA). The anti-CD4 (GK1.5, TIB-207), anti-CD8 (2.43, TIB-210), anti-IL-4 (11B11) and anti-IFN-γ (R4-6A2) hybridomas were obtained from the American Type Culture Collection (Rockville, MD). Ascites were prepared in DBA/2 mice primed with pristane and immunosuppressed by injection with 200 mg/kg cyclophosphamide. Fluorescein isothiocyanate (FITC) and phycoerythrin (PE) -labelled isotype control rat immunoglobulin G (IgG), hamster IgG and a monoclonal antibody (mAb) against CD3, CD4, CD8, IFN-γ and TNF-α were purchased from Pharmingen (San Diego, CA). Freshly isolated TVDLN cells were blocked with anti-mouse Fc receptor hybridoma 2.4G2 (HB-197, ATCC) culture supernatant before incubation with directly labelled specific antibodies. Magnetic beads conjugated with anti-CD62L(l-selectin) were generously provided by Miltenyi Biotec (Auburn, CA).
Culture conditions
Lymphocytes and tumour cells were cultured in complete medium (CM), which consisted of RPMI-1640 containing 0·1 mm non-essential amino acids, 1 mm sodium pyruvate, 2 mm l-glutamine, and 50 μg/ml of gentamycin sulphate (all from BioWhittaker, Walkersville, MD.). This was further supplemented with 50 mm 2-mercaptoethanol (Aldrich, Milwaukee, WI), and 10% fetal bovine serum (Gibco BRL, Grand Island, NY). Tumour cells were harvested two or three times per week by brief trypsinization (Trypsin, Bio Whittaker, Walkersville, MD.) and were maintained in T-75 or T-150 culture flasks. For some experiments MCA tumours were serially passaged by subcutaneous injection in C57BL/6 mice. In vivo passaged tumours were harvested, minced and incubated with collagenase, hyaluronidase, and DNAase (Sigma, St Louis, MO) for several hours. The resulting cell suspension was filtered through a sterile Nytex filter, washed three times and used for vaccination experiments.
Tumour vaccination and challenge experiments
Tumour cells were harvested as specified above, washed twice with Hanks' balanced salt solution (HBSS), irradiated with 10 000 rads and resuspended at 108 cells/ml. Ten million tumour cells were injected subcutaneously into the hind flanks of naïve C57BL/6 mice. The mice were rechallenged 14 days after vaccination with a dose of tumour cells that was five to 10 times the TD100 dose for the specified tumour. (The TD100 is the dose at which 100% of the subjected animals will develop tumour.) Tumour size was determined by measurement of two perpendicular diameters using a digital calliper.
Separation and activation of TVDLN
Eight days following subcutaneous tumour inoculation with 106 viable cells, the superficial inguinal lymph nodes were harvested and l-Selectinlow lymphocytes were isolated using CD62L-MACS microbeads (Miltenyi, Biotec, Auburn, CA). Lymphocytes were resuspended at 108 cells/ml in CM, mixed with 40 μl/ml of CD62L-MicroBeads (497-01, Miltenyi Biotec), and incubated for 20 min at 4°. Cells were washed once with 50 ml of CM and resuspended at 108/ml and passed over a VarioMACS magnetic depletion column (Type BS 413-04 or CS 413-05, Miltenyi Biotec) held by a high-energy magnet (VarioMACS separator, Miltenyi Biotec). The column was washed extensively with CM and non-adherent cells (l-selectinlow/− cells) were collected. Samples of unseparated and separated cells were stained with an FITC-labelled anti-CD62L and analysed on a Coulter flow cytometer for efficiency of separation. Unfractionated and l-selectinlow/− TVDLN cells were resuspended at 2 × 106 cells per ml in CM and cultured in 24-well plates with 50 μl of a 1 : 40 dilution of 2C11 ascites (anti-CD3). This dilution was determined previously to be optimal for T-cell activation. After 2 days of activation the T cells were harvested and then expanded in CM containing 60 IU recombinant human interlsukin-2 (rhIL-2)/ml for three additional days. Culture supernatant was saved for determination of the cytokines released. T cells were then harvested, washed twice in HBSS, counted and used in cytokine release assays. For reprogramming experiments TVDLN cells were incubated with IL-12 (100 pg/ml) and anti-IL-4 (10 µg/ml) or IL-4 (20 ng/ml) and anti-IFN-γ (20 µg/ml) during activation with 50 μl of 1 : 40 dilution of 2C11 ascites (anti-CD3).
Adoptive immunotherapy
Experimental pulmonary metastases were established by intravenous inoculation of 2 × 105 D5 tumour cells. Three days later T cells were adoptively transferred intravenously. Starting on the day of T-cell infusion, mice received 90 000 IU IL-2 i.p. once per day for four days. Animals were killed 11–13 days following tumour inoculation by CO2 narcosis and their lungs were harvested and fixed in Fekete's solution. The number of pulmonary metastases was counted in a blinded fashion. Metastases that were too numerous to count accurately were known to be greater than 250 metastases and were assigned a value of 250.
Measurement of cytokines
After activation and expansion TVDLN were washed, resuspended in CM containing 60 IU/ml IL-2 and seeded at 4 × 106/2 ml/well in a 24-well plate. The cells were either cultured without further stimulation or stimulated with 2 × 105 of the immunizing tumour or a syngeneic but unrelated tumour. Immobilized anti-CD3 was included as a positive control in each experiment. Supernatants were harvested after 18–24 hr and assayed for the release of IFN-γ, IL-10 and IL-4 by enzyme-linked immunosorbent assay (ELISA) using commercially available reagents (IFN-γ, Pharmingen or Genzyme; IL-4, Genzyme; IL-10, Pharmingen). The concentration of cytokines in the supernatant was determined by regression analysis. For determination of intracellular cytokine expression, effector T cells, generated as described above, were stimulated for 12 hr in the presence of 5 μm Brefeldin A (Sigma), harvested and surface stained with anti-CD8-CyChrome and anti-CD3-FITC mAb, then fixed and permabilized in Cytofix/Cytoperm™ and stained with a control anti-IgG1-PE, anti-IFN-γ-PE or anti-TNF-α-PE (Pharmingen, CA). Fixed cells were washed twice in FACS buffer and analysis performed using a FACS™Calibur and Cellquest software (Becton & Dickinson, San Diego, CA).
Statistical analysis
The statistical significance of differences in the number of metastases between experimental groups was determined by the Wilcoxon rank sum test. Two-sided P-values of <0·05 were considered significant. Each treatment group consisted of at least five mice, and no animal was excluded from the statistical evaluations. Statistical analysis of the tumour growth in naïve and vaccinated wt and GKO mice was determined by non-parametric (distribution-free) tumour growth analysis performed on medians and rank order statistics. The statistical significance of the correlation between the IFN-γ/IL-4 ratio and immunogenicity was determined using a Fisher exact test.
Results
Determination of tumour immunogenicity
To test our hypothesis that a tumour-specific T1 cytokine response was critical for T-cell-mediated tumour regression, we first characterized the inherent immunogenicity of six tumour cell lines of C57BL/6 mice. Three 3-methylcholanthrene-induced tumours (MCA-304, MCA-309, MCA-310) and three prostate tumours (MPR-3, MPR-4, MPR-5) were characterized using a standard immunization/challenge protocol described previously.1 Naïve C57BL/6 mice were injected subcutaneously with 107 irradiated (10 000 rads) tumour cells and challenged 14 days after vaccination with a number of viable tumour cells that was 5–10 times the TD100 predetermined for each tumour cell line. The tumour size was determined every second day. MCA-309, MPR-4 and MCA-304 were found to be strongly immunogenic, with vaccination protecting 69, 80 and 92% of the vaccinated animals, respectively. MPR-3 and MCA-310 exhibited a low level of inherent immunogenicity, protecting 20 and 25% of vaccinated animals, respectively. Therefore these tumours were designated as being weakly immunogenic. In contrast, vaccination with MPR-5 failed to protect any animal from a subsequent tumour challenge and, like the D5 tumour, was defined as poorly immunogenic.1
Polarization of T cells in TVDLN correlates with their immunogenicity
Previous studies have demonstrated that the cytokine environment in which T cells are first activated determines the subsequent cytokine profile of those cells.3,5,6 As freshly isolated TVDLN require additional activation in vitro with tumour cells, microbial superantigens, or antibodies to CD3 or the T-cell receptor to develop therapeutic activity, they are not fully differentiated effector T cells.13–15 Therefore, to investigate whether the cytokines released by these fresh lymph node cells during the in vitro activation might account for effector T cells exhibiting a particular cytokine profile, we examined the concentration of IFN-γ and IL-4 released into the culture medium during the in vitro polyclonal activation with anti-CD3. Tumour-specific T cells were first enriched by isolating l-selectinlow cells from TVDLN and then were stimulated with anti-CD3 for 2 days. After 2 days culture, supernatants were collected and the concentration of IFN-γ and IL-4 in the supernatant was determined by ELISA. Analysis of the cytokine profile for the three MCA tumours studied identified a pattern of IFN-γ and IL-4 secretion by TVDLN that directly correlated with the immunogenicity of these tumours. TVDLN from mice immunized with MCA-304, the most immunogenic tumour studied, released 297 pg/ml IFN-γ and 85 pg/ml IL-4. TVDLN from MCA-309-vaccinated mice released about half as much IFN-γ (151 pg/ml) and almost twice as much IL-4 (142 pg/ml), while anti-CD3-stimulated supernatants of MCA-310 TVDLN contained only 28 pg/ml of IFN-γ and 228 pg/ml IL-4. Since the relative concentrations of IFN-γ and IL-4 are likely to be important in regulating or predicting the polarization of T cells generated in these cultures, we examined the ratio of IFN-γ to IL-4. As shown in Table 1, the ratio of IFN-γ to IL-4 for the most immunogenic tumour, MCA-304, was 3·5, while for the least immunogenic MCA tumour, MCA-310, the IFN-γ to IL-4 ratio was 0·1. The ratio for MCA-309 was 1·1. A similar pattern was also seen for MPR tumours. The TVDLN for the most immunogenic MPR tumour, MPR-4, released 246 pg/ml IFN-γ and only 45 pg/ml IL-4, generating a ratio of 5·5. In contrast, the TVDLN primed by vaccination with the weakly and poorly immunogenic tumours, MPR-3 and MPR-5, each exhibited an IFN-γ: IL-4 ratio of 1·0. These results support our earlier observation that the poorly immunogenic D5 tumour primed a non-therapeutic type 2 cytokine response in TVDLN.1 Combined with these results obtained from two serial of tumour cell lines with different histology and immunogenicity, we conclude that tumour immunogenicity is determined not only by tumour antigenicity but also the type of cytokine responses induced by tumour vaccines.
Table 1.
Cytokine profile of anti-CD3 stimulated TVDLN correlates with immunogenicity
| Tumour vaccine | Immuno- genicity* | IFN-γ† | IL-4† | Ratio‡ |
|---|---|---|---|---|
| MCA 304 | 92% | 297(111) | 85(45) | 3·5 |
| MCA 309 | 69% | 151(28) | 142(8) | 1·1 |
| MCA 310 | 25% | 28(14) | 228(93) | 0·1 |
| MPR-4 | 80% | 246(19) | 45(21) | 5·5 |
| MPR-3 | 20% | 203(82) | 119(114) | 1·0 |
| MPR-5 | 0% | 169(30) | 175(55) | 0·97 |
Tumour immunogenicity was determined by vaccination of B6 mice with irradiated tumour cells and challenge with live tumour cells 2 weeks later. For MCA experiments, groups of 11 mice were used. Data represent one out of three experiments with variation less 20%. For MPR experiments, five mice per group were used for each experiments. Percentage of protection is derived from combined tumour-free mice divided by total of mice used in three experiments performed consecutively. The variation of percentage of protection between each experiment is less 20%.
TVDLN T cells were stimulated with anti-CD3 for 48 hr, and the supernatant was harvested. The concentration of IFN-γ and IL-4 was determined by ELISA. The results represent mean and standard error of three independent experiments.
Ratios are calculated by dividing mean of IFN-γ concentration by IL-4 concentration.
Tumour-specific cytokine release correlates with tumour immunogenicity
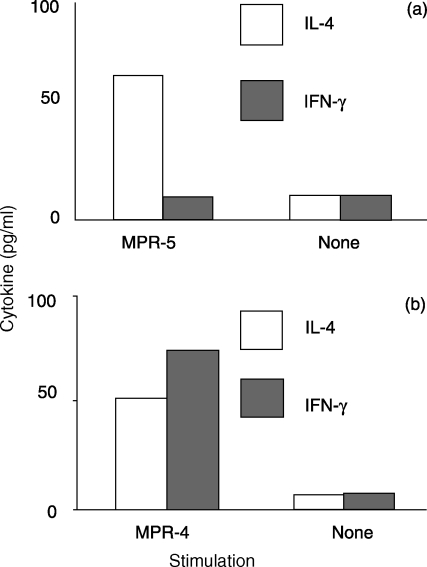
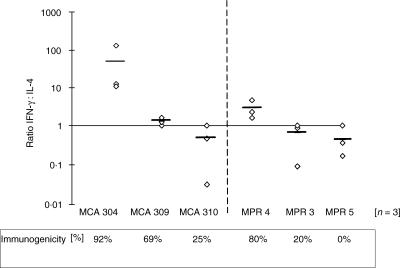
When the total population of T cells from lymph nodes draining the poorly immunognenic D5 tumour are activated and expanded with IL-2 there is usually no evidence of tumour-specific cytokine release. However, we have previously reported that by enriching for the small percentage of tumour-reactive T cells present in the TVDLN, based on reduced expression of l-selectin, it is possible to study the cytokines produced by T cells responding to tumour, an opportunity that might otherwise be missed because of the low frequency of tumour-reactive T cells or high background cytokine secretion in an unselected population of T cells.1 Thus, to evaluate whether mice vaccinated with the six different tumours primed tumour-specific T cells, effector T cells generated from l-selectinlow TVDLN were assayed for reactivity against the immunizing tumour cells. Effector T cells from the immunogenic tumours, MCA-304, MPR-4 and MCA-309, exhibited a tumour-specific cytokine profile that was polarized towards a T1 phenotype while effector T cells from TVDLN of the weakly and poorly immunogenic tumours exhibited a tumour-specific T2 profile. An example of representative experiments with MPR-4 and MPR-5 are presented in Fig. 1. To evaluate further the nature of this tumour-specific cytokine response three independent experiments were performed for each of the six tumours studied. The results of these studies are graphed as the IFN-γ: IL-4 ratios for each experiment (Fig. 2). While there are some striking differences in the magnitude of the responses to the three strongly immunogenic tumours, the IFN-γ: IL-4 ratio for all these tumours was greater than 1, indicating that the induction of IFN-γ-secreting T1 T cells was more pronounced than the induction of IL-4-secreting T2 T cells. In contrast, weakly (MCA 310 and MPR 3) and poorly (MPR 5) immunogenic tumours exhibited an IFN-γ: IL-4 ratio that was skewed towards a T2 phenotype. This last observation of MPR-5 inducing a tumour-specific T2 cytokine response is important as it further discounts the concept that poorly/non-immunogenic tumours are ignored by the immune system. Furthermore, the analysis of the IFN-γ: IL-4 ratios and inherent immunogenicity identified a significant correlation (P < 0·025) between the type of immune response induced by the tumour in the TVDLN and the inherent level of immunogenicity defined by induction of protective immunity (mean of 18 experiments) Immunogenic tumours show an IFN-γ: IL-4 ratio >1, whereas weakly/non-immunogenic tumours show a ratio <1.
Figure 1.
Tumour-specific cytokine profile of effector T cells generated from MPR-5 (a) or MPR-4 (b). l-Selectinlow/− TVDLN were activated with anti-CD3 for 2 days and expanded in 60 IU IL-2/ml for 3 days. Resulting effector T cells were then cultured alone or with specific tumour and 18–20 hr supernatants were assayed for IFN-γ and IL-4 release. Results for a single experiment are shown.
Figure 2.
The IFN-γ: IL-4 ratio correlates with tumour immunogenicity. The ratio of tumour-specific release of IFN-γ to IL-4 by effector T cells generated from l-selectinlow TVDLN is plotted for each of the six tumours studied. Each diamond represents the ratio for an independent experiment. The bar in each group is the mean ratio for that group. The immunogenicity of the tumour cells as determined by the percentage of animals protected in vaccination/challenge experiments is listed at the bottom of the graph.
T2-polarized D5 TVDLN can be shifted by in vitro culture to a T1 cytokine profile
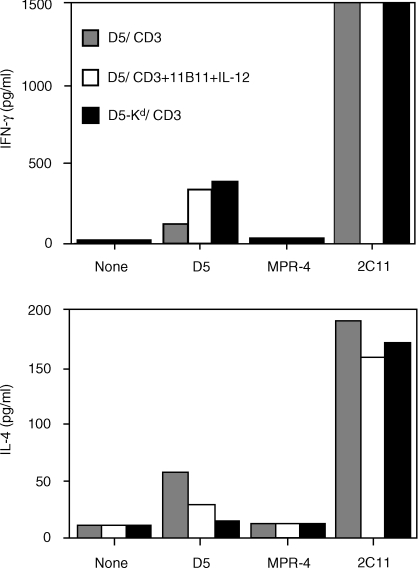
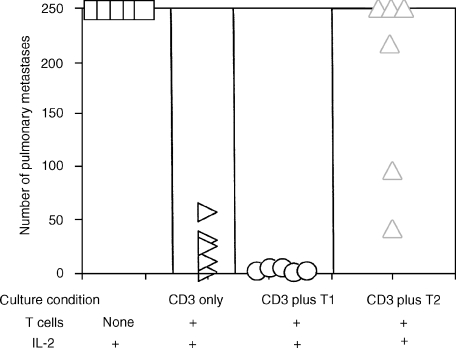
Our results infer that the induction of a tumour-specific T1 response following vaccination will lead to protective immunity whereas a tumour-specific T2 cytokine response will not. As we have shown previously, vaccination with the poorly immunogenic D5 tumour will prime a tumour-specific T2 response by T cells in the TVDLN. Although these cells are specifically primed to the tumour, they lack therapeutic activity in adoptive transfer experiments. Here we investigated whether tumour-primed T cells from D5 TVDLN can be ‘reprogrammed’ to a tumour-specific T1 phenotype. To test this hypothesis, we used a strategy shown by others to polarize T cells toward a T1 cytokine profile.3l-Selectinlow T cells from D5-TVDLN were pooled and half were cultured in CM with anti-CD3 and the rest were cultured with anti-CD3, IL-12 and anti-IL-4. The tumour-specific cytokine response of the resulting effector T cells was then characterized (Fig. 3). In this experiment the l-selectinlow D5 TVDLN exhibited a mixed tumour-specific cytokine profile, expressing both IFN-γ and IL-4. Notably, the TVDLN cultured with IL-12 and anti-IL-4 more than doubled their tumour-specific secretion of IFN-γ and reduced by half their secretion of IL-4. In this experiment additional mice were vaccinated with D5 lipofected with the cDNA encoding the allogeneic major histocompatibility complex (MHC) class I, H-2 Kd. TVDLN from D5-H-2Kd-vaccinated mice were used as a positive control, because we have previously shown that this vaccine would prime T cells with a tumour-specific T1 cytokine response that were therapeutic in adoptive immunotherapy of D5 metastases.1 The tumour-specific cytokine response of D5 TVDLN cultured with IL-12 and anti-IL-4 approached that seen for effector T cells generated from the D5-Kd TVDLN. Thus, by exploiting in vitro culture conditions, it is possible to polarize a tumour-specific T2 response towards a T1 cytokine profile.
Figure 3.
Non-therapeutic TVDLN become therapeutic following a shift from T2 to T1 cytokine profile. l-Selectinlow/− D5 TVDLN were isolated and half were activated with anti-CD3 for 2 days and expanded in 60 IU IL-2/ml for 3 days. The remainder were cultured with anti-CD3 together with a source of IL-12 (100 pg/ml) and anti-IL-4 (11B11). At five days the resulting effector T cells were cultured alone, with the syngeneic but unrelated MPR-4 tumour, D5 tumour cells, or anti-CD3. Eighteen-hour supernatants were assayed for tumour-specific release of IFN-γ and IL-4, and the cells were adoptively transferred to test for therapeutic efficacy (Table 2).
Non-therapeutic TVDLN become therapeutic following a shift to T1 cytokine profile
To test whether repolarization of effector cells from lymph nodes draining the non-immunogenic tumour D5 would confer therapeutic activity, we adoptively transferred repolarized effector cells into mice with D5 pulmonary metastases that had been established for 3 days. Thirteen days following tumour inoculation mice were killed and the number of pulmonary metastases was enumerated in a blinded fashion. Control mice that received only IL-2 developed more than 250 metastases. Effector T cells that exhibited a dominant T2 cytokine response were not therapeutic. In contrast, T cells from the same TVDLN that had been polarized to a T1 cytokine profile prior to adoptive transfer exhibited significant therapeutic efficacy. This clearly demonstrates that the poorly immunogenic D5 tumour is recognized by the host immune system, however, the T2 response that is generated is ineffective at mediating tumour regression.
Therapeutic effector T cells from GKO mice express tumour-specific TNF-α
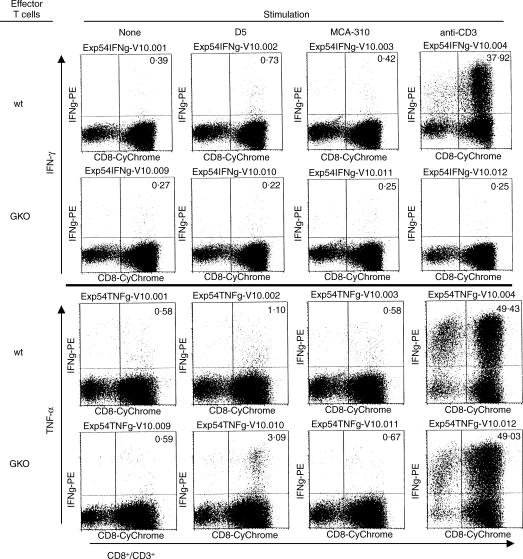
Previously we have shown that GM-CSF-producing D5 melanoma cells (D5-G6) prime highly therapeutic T cells in wt or GKO mice. Effector T cells from wt mice exhibit a T1 cytokine profile, while effector T cells from GKO mice did not produce IFN-γ but also failed to release tumour-specific T2 (IL-4 and IL-10) cytokines. These results have suggested to us that effector T cells from GKO mice might utilize T1 cytokines other than IFN-γ to mediate tumour regression. However, tumour-specific TNF-α, a likely candidate for a compensating T1 cytokine, could not be detected in the supernatants of GKO effector T cells. Therefore, tumour-specific TNF-α expression was examined using intracellular staining. A representative experiment, shown in Fig. 4, compares TVDLN from wt and GKO mice vaccinated with the GM-CSF secreting D5-G6. Effector T cells from wt mice express tumour-specific IFN-γ and TNF-α, while effector T cells from GKO mice express only tumour-specific TNF-α. Consistent with published data, vaccination in GKO mice results in a higher percentage of CD8 T cells producing TNF-α.16 This documents that effector T cells generated in the absence of IFN-γ do not a priori express a T2 cytokine profile and provides a T1-mediated mechanism for GKO effector T cells to mediate tumour regression.
Figure 4.
Effector T cells from both wt and GKO mice express tumour-specific TNF-α. Effector T cells generated from D5-G6-vaccinated wt and GKO mice were stimulated with D5, syngeneic fibrosarcoma MCA 310, anti-CD3, or left alone for 12 hr in the presence of Brefeldin A. Cells were harvested, permeabilized and stained with PE-labelled anti-IFN-γ and -TNF-α mAbs and analysed for intracellular expression of IFN-γ and TNF-α. The number in the top-right quarter indicates the percentage of CD3+ CD8+ T cells producing IFN-γ or TNF-α.
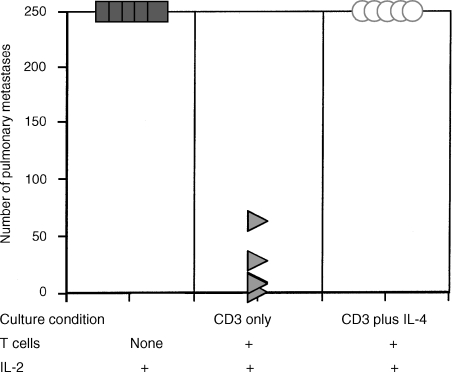
T2 culture condition inhibits generation of therapeutic cells
To examine whether generation of effector T cells can be influenced by T2 culture conditions, TVDLN from wt mice were activated with anti-CD3 in the presence of recombinant IL-4 and anti-IFN-γ antibody for 2 days and subsequently expanded with IL-2 before being adoptively transferred into mice bearing established D5 metastases. As a control, TVDLN were also activated with anti-CD3 alone or together with anti-IL-4 and IL-12 (T1 condition). T cells activated under T2 polarizing conditions were significantly (P < 0·05) less therapeutic compared to either those activated with anti-CD3 or anti-CD3 and a T1 polarizing condition (Fig. 5). A modification of this experiment was performed with D5-G6 TVDLN from GKO mice. GKO T cells were activated with anti-CD3 or with anti-CD3 and IL-4. GKO effector T cells activated under T2 conditions were significantly less therapeutic than GKO effector cells generated with anti-CD3 and IL-2 (Fig. 6). Thus, culturing T cells with therapeutic potential under T2 polarizing conditions was detrimental to the generation of effector T cells with therapeutic efficacy.
Figure 5.
TVDLN with therapeutic potential lose anti-tumour efficacy following culture with IL-4. D5-G6 TVDLN were cultured with anti-CD3 alone, or together with anti-IL-4 and IL-12 (T1 conditions), or anti-IFN-γ and IL-4 (T2 conditions). Effector T cells generated under these three conditions were adoptively transferred into mice bearing established D5 pulmonary metastases and anti-tumour efficacy was evaluated. Control animals received IL-2 only. Effector T cells cultured under T2 conditions were significantly (P < 0·05) less therapeutic than effector cells generated under T1 or T0 conditions.
Figure 6.
GKO TVDLN activated in IL-4 lose anti-tumour efficacy: D5-G6 TVDLN from GKO mice were activated with anti-CD3 or anti-CD3 and IL-4. Effector T cells generated under these conditions were adoptively transferred into mice with 3-day-established intravenous tumour burdens. Control animals received IL-2 only. Effector T cells cultured with IL-4 were significantly (P < 0·05) less therapeutic than effector cells generated with anti-CD3.
Discussion
Standard immunization strategies do not protect animals from a subsequent challenge with even a minimal number of D5 tumour cells. Our explanation for the failure to generate an anti-tumour immune response was that the tumour was non-immunogenic. However, recent data demonstrate that this is clearly not the case. D5 and the parental B16BL6 tumour express a variety of tumour-associated antigens that can be recognized by T cells, including; gp100, MART1, tyrosinase-1 and -2, and tyrosinase-related proteins. While the frequency of T cells that respond to each of these antigens in vaccinated animals has not been reported, it is clear from our studies that a tumour-specific T2 cytokine profile dominates the immune response to the D5 tumour. We have previously shown that gene-modification of D5 to express a class I alloantigen or GM-CSF alters the immune response to the vaccine and primes T cells, so that following in vitro activation and expansion, they exhibit a tumour-specific T1 cytokine profile and can eradicate pulmonary metastases. This led us to postulate that the development of a tumour-specific T1 response was critical for T-cell-mediated tumour regression and the development of protective anti-tumour immunity. The corollary of this was that development of a tumour-specific T2 response would tolerize the anti-tumour immune response.
The induction of tolerance to tumour antigens has been discussed as a particularly potent mechanism to evade a destructive immune response. T cells that are not tolerized within the thymus have the potential to be rendered tolerant by one of three major tolerizing mechanisms. Deletion, because of a lack of co-stimulation,17–21 anergy, in which the T cells are rendered unresponsive by the loss of antigens, MHC molecules and/or MHC-associated molecules,22–24 and ignorance, as a result of low antigenicity or tumour antigens being inaccessible to the immune system through ‘walling-off’.25 Our observations identified immune deviation as an additional mechanism of tolerance that is operational in the D5 tumour model. Immune deviation occurs when responding T cells differentiate away from the functional phenotype required to affect a particular immune response. The phenomenon of immune deviation was first described by Asherson and Stone in experiments immunizing guinea-pigs with soluble or alum-precipitated antigens prior to challenging the animals with the same antigen in CFA.26 They found that the delayed type hypersensitivity response was inhibited in these animals and that the antibody response to immunization had changed from an IgG2 isotype to an IgG1. Later, work by Mossmann and others identified that the character of the immune response was determined by distinct T-cell clones, Th1 and Th2, that were induced during the onset of the immune response.27 Here we show for the first time that the immunogenicity of a tumour correlates to the type of immune response it induces in the draining lymph node. Whereas immunogenic tumours (MCA 304, MCA 309, MPR4) induce a T1 immune response that will lead to protective immunity, weakly and poorly immunogenic tumours (MCA 310, MPR 3, MPR 5) induce a T2 immune response that does not protect the host. The reason for the differential induction of a protective T1 immune response and a non-protective T2 response can only be speculated. However, events in the TVDLN 8 days following vaccination lead to alterations in the LN population such that TVDLN draining the weakly and poorly immunogenic tumours exhibit a dominant T2 cytokine response to polyclonal stimulation with anti-CD3, while the strongly immunogenic tumours exhibit a dominant T1 cytokine profile under the same conditions. Clearly, some profound alterations occur in the lymph nodes where the developing anti-tumour immune response is being shaped.
Many different factors have been shown to influence a developing immune response and the phenotype of resulting effector cells. The presence of T1 or T2 cytokines will drive uncommitted T cells to develop a cytokine profile similar to that of the cytokine milieu during their activation and expansion. These same conditions inhibit the development of T cells with the reciprocal cytokine phenotype.3,8,9 Therefore, the presence of T2 cytokines during the initial interaction between the T cell and tumour antigen in vivo would facilitate the development of a T2 anti-tumour response. This could be caused by secretion of T2 cytokines by tumour cells, by secretion of IL-4 by natural killer (NK) 1.1 T cells, or by IL-6 secreted from APC.28–32 The secretion of IFN-γ by NK cells or IL-12 by APC acts reciprocally and would be expected to activate T1 and inhibit T2 cells.3,31 So far we have not detected secretion of type 1 or type 2 cytokines by D5 or any other of the murine tumours used in these studies (data not shown). Although the D5 tumour expresses message for IL-4, we have been unable to detect IL-4 in supernatants or lysates of the D5 tumour. Therefore, we believe that other mechanisms must be involved in determining the polarization of the induced immune response. The dose of antigen used to sensitize T cells was also shown to affect the type of cytokine response. High concentrations lead to a predominant T1 response in CD4 cells, whereas low doses of antigen promote differentiation of predominantly T2 cells that produce high levels of IL-4 (reviewed in refs 33,34). Since the T-cell cytokine profile was performed on TVDLN generated by injecting 1 × 106 tumour cells each flank, it seems unlikely that the differences in cytokine release patterns would be explained by the antigen dose. Another explanation for the low immunogenicity of these tumours might be the result of decreased expression of MHC class I. However, we found no correlation between the level of MHC class I and the immunogenicity of these tumours (data not shown).
Recently Schnare et al. showed that mice deficient in the MyD88 adapter protein that mediates signal transduction by toll-like receptors (TLRs) have a profound defect in the induction of antigen-specific Th1 but not Th2 responses.35 They concluded that induction of Th1 responses requires Toll-mediated recognition and signalling whereas Th2 responses appear to be independent of Toll function and will occur as the default pathway. Although Toll-like receptors are thought to play a central role in the recognition of microbial components such as lipopolysaccharide, CpG DNA and lipoproteins through TLR2, -4, -6, -5 and -9, TLR4 was also shown to recognize endogenous host-derived products such as heat-shock protein 60 (hsp 60) and fibronectin, both of which might also be expressed by tumours. It can be postulated that immunogenic tumours activate TLR4 through hsp 60, fibronectin, or other as yet undefined factors and will thus induce a T1 cytokine response. In contrast, non-immunogenic tumours may fail to signal through TLR4, resulting in the induction of a T2 immune response as their default cytokine pattern. Recently, different subsets of dendritic cells were identified based on their expression of the CD4 and CD8α homodimers.36 Adoptive transfer of antigen pulsed CD8α– dendritic cells led to a T2 response, whereas antigen-pulsed CD8α+ dendritic cells induced a T1 response.37,38 It is possible that immunogenic tumours attract a subset of dendritic cells that will induce a tumour-specific T1 immune response, while weakly and poorly immunogenic tumours attract dendritic cells promoting T2 responses.
Recently, Th1 effector cells were shown to mediate autoimmune diseases and graft rejection.39,40 Therefore, some have tried to induce tolerance by deviating the predominant Th1 response towards a Th2 response by altering the cytokine milieu, believed to be the most critical parameter in determining the polarization of T cells.41–44
Our results are consistent with this paradigm in that the development of a tumour-specific T2 response against the D5 tumour would be non-destructive. However, an alternative explanation is that these tumour-specific T cells, that are secreting type 2 cytokines, were non-therapeutic because they recognized an antigen, or antigens, that were not ‘tumour-rejection’ antigens. The experiments in Table 2 directly tested whether T cells primed by a poorly immunogenic tumour, where vaccination never provides protective immunity, could exhibit therapeutic efficacy if reprogrammed towards a T1 cytokine response. The strategy employed in these studies, based on well-established work of others, focused on ‘reprogramming’ the cytokine profile of recently primed tumour-specific T cells. It is our assumption that by manipulating the cytokine milieu the recently primed T cells were induced to switch from a T2 to a T1 cytokine profile. However, there is some controversy about whether it is possible to switch fully committed or differentiated T1 or T2 T cells to the alternative phenotype. These experiments cannot exclude the possibility that the changes in the cytokine milieu only blocked the activity of T cells with a T2 phenotype and uncovered another population of tumour-specific T cells exhibiting a T1 cytokine profile.
Table 2.
Culture of D5 l-selectinlow/− TVDLN in IL-12 and anti-IL-4 induces expression of therapeutic activity
| Lung metastases Mean (SEM)‡ | |||||
|---|---|---|---|---|---|
| Tumour vaccine* | T cells | IL-12 + anti-IL-4 | IL-2† | Exp. 1 | Exp. 2 |
| None | None | − | + | 250 | 300 |
| D5 | l-selectinlow | − | + | 159 (49) | 224 (35) |
| D5 | l-selectinlow | + | + | 3 (1)§ | 49 (15)§ |
Mice were vaccinated subcutaneously with D5-G6 tumour cells, and TVDLN were harvested 8 days later. Lymph node cells were stimulated in vitro with anti-CD3 for 2 days and then expanded for 3 days in 60 IU/ml IL-2. Effector cells were harvested and 35 million T cells were adoptively transferred into animals with established 3-day D5 pulmonary metastases.
IL-2 (90 000 IU) was administered daily intraperitoneally for 4 consecutive days following adoptive transfer.
Mice were killed 13 days following intravenous inoculation of tumour and the number of pulmonary metastases was enumerated in a blinded fashion. Results presented for each experiment are the mean of five animals.
Significantly different from all other groups (P < 0·05).
The results of these studies documented that T cells in the lymph nodes draining the poorly immunogenic tumour recognize ‘tumour-rejection’ antigens and that therapeutic activity can be unmasked by polarizing the developing immune response from a T2 to a T1 profile. Since the ability to alter the cytokine profile, and thereby enhance the therapeutic activity of primed TVDLN, has important implications for the development of effective immunotherapy we further investigated this paradigm. Vaccination with the GM-CSF gene-modified B16BL6-D5 tumour, D5-G6, primes tumour-specific T cells with a dominant tumour-specific T1 cytokine response and potent therapeutic activity. Results of the studies in Fig. 5, document that this tumour-destructive activity is significantly reduced by culture conditions that promote the development of a T2 cytokine profile and further strengthens the role for IFN-γ and/or other T1 cytokines in therapeutic anti-tumour immunity.
We have recently shown, that IFN-γ plays an essential role in the induction of active-specific immunity.45 Vaccination of wt mice with D5-G6 induced protective immunity in 90% of the mice, whereas vaccination of GKO mice failed to induce protective immunity. Recently, further evidence for the central role of IFN-γ in the maintenance of immune surveillance came from Shankaran et al. who evaluated the incidence of tumour formation in RAG knockout and STAT-1 knockout immune-deficient mice.46 The spontaneous outgrowth of epithelial tumours was found to be significantly elevated in RAG knockout mice and STAT-1 knockout mice. Evaluation of RAG knockout × STAT-1 knockout mice identified a significantly higher level of spontaneous tumours, indicating that tumour suppressor mechanisms from RAG and STAT-1 knockout mice only partially overlap and that IFN-γ plays an essential role in the immune surveillance of tumours. This is further supported by the observation that IFN-γ controlled both spontaneously arising tumours and chemically induced tumours and negatively regulated the tumorigenicity of tumour cells.47,48 One possible explanation is that IFN-γ can enhance antigen processing and presentation by both the MHC class I and II pathways and thus augment tumour recognition by tumour-specific T cells.49 Although IFN-γ plays a critical role in the induction of active-specific immunity, IFN-γ is not essential for the induction of tumour-specific cells in TVDLN.45 Vaccination of GKO mice primed T cells that, following in vitro activation and expansion, mediated significant regression and cure of GKO animals bearing systemic tumour. While these T cells failed to release IFN-γ, they also did not release T2 cytokines. Leading us to consider that other T1 cytokines, possibly TNF-α and LT-β, were responsible for the therapeutic activity exhibited by GKO T cells.1,45,50 Here we show that therapeutic GKO effector T cells express TNF-α, detectable by intracellular cytokine staining, and identifying a potential T1 cytokine mechanism for effector T cells from GKO mice. These results further strengthen the hypothesis that a tumour-specific T1 cytokine response is critical to therapeutic efficacy. Additional studies from our laboratory suggest that TNF-α is a T1 cytokine that is critical for the therapeutic efficacy of T cells lacking IFN-γ (Poehlein et al. J Immunol, in press).
Our results also suggest that the failure of tumour vaccination to protect animals from a subsequent tumour challenge is a result of the nature of the immune response, not of its absence. To develop effective immune-based treatments for cancer, it will be important to define the molecular mechanisms that determine whether antigen recognition results in apoptosis, anergy, or immune deviation rather than effector or memory cell generation. This knowledge might then be used to develop strategies that circumvent the mechanisms responsible for the induction and maintenance of tolerance in tumour-bearing hosts. Finally, the observation that therapeutic efficacy could be uncovered by skewing the tumour-specific cytokine response to a T1 profile has particular significance to the development and monitoring of cancer vaccine trials. Studies to determine optimal conditions for generating tumour-specific T1 T cells may improve vaccine strategies. Towards this end, we have begun to use intracellular cytokine analysis with CIITA-transduced tumour cells to evaluate the frequency of tumour-specific CD4 and CD8 T-cell responses in D5 and other tumour models.51 Given the interesting report of Perez-Diez and colleagues, showing a correlation between the intensity of the IFN-γ response by reverse transcription-polymerase chain reaction and the anti-tumour effects of vaccination,52 and our work presented here, a strong rationale for engineering vaccination strategies that polarize strong T1 cytokine responses emerges. Furthermore, these results strengthen the importance of monitoring both tumour-specific T1 and T2 cytokine profiles in patients on clinical trials. Recently, we reported that some patients treated by vaccination with autologous tumour and adoptive immunotherapy with TVDLN exhibited both tumour-specific T1 and T2 responses.53 Additional studies by us and others have identified vaccine-specific or tumour-specific T2 responses, predominantly IL-5, in patients where vaccination failed to induce objective responses and in patients with advanced tumours (Dols et al. submitted for publication).54 Future studies will need to evaluate whether these tumour-specific T2 responses can reduce the therapeutic efficacy of vaccine strategies or adoptive immunotherapy strategies with T1 polarized effector T cells.
Acknowledgments
This work was supported in part by NIH (RO1 CA 80964 to B.A.F.) and by grants from the Murdock Charitable Trust and the Chiles Foundation.
References
- 1.Hu HM, Urba WJ, Fox BA. Gene-modified tumor vaccine with therapeutic potential shifts tumor-specific T cell response from a type 2 to a type 1 cytokine profile. J Immunol. 1998;161:3033–41. [PubMed] [Google Scholar]
- 2.Rocken M, Shevach EM. Immune deviation – the third dimension of nondeletional T cell tolerance. Immunol Rev. 1996;149:175–94. doi: 10.1111/j.1600-065x.1996.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 3.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more [see comments] Immunol Today. 1996;17:138–43. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Sad S, Kagi D, Mosmann TR. CD8Tc1 and Tc2 cells secrete distinct cytokine patterns in vitro and in vivo but induce similar inflammatory reactions. J Immunol. 1997;158:4152–61. [PubMed] [Google Scholar]
- 5.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL) -4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–28. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salgame P, Abrams JS, Clayberger C, Goldstein H, Convit J, Modlin RL, Bloom BR. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279–82. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 7.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunology. 1994;12:635–73. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 8.Gollob JA, Kawasaki H, Ritz J. Interferon-gamma and interleukin-4 regulate T cell interleukin-12 responsiveness through the differential modulation of high-affinity interleukin-12 receptor expression. Eur J Immunol. 1997;27:647–52. doi: 10.1002/eji.1830270311. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Lee RK, Nam SY, Podack ER, Bottomly K, Flavell RA. Roles of IL-4 and IFN-gamma in stabilizing the T helper cell type 1 and 2 phenotype. J Immunol. 1997;158:2648–53. [PubMed] [Google Scholar]
- 10.Aruga A, Aruga E, Tanigawa K, Bishop DK, Sondak VK, Chang AE. Type 1 vs Type 2 cytokine release by Vb T cell subpopulations determines in vivo antitumor reactivity: IL-10 mediates a suppressive role. J Immunol. 1997;159:664–73. [PubMed] [Google Scholar]
- 11.Arca MJ, Krauss JC, Aruga A, Cameron MJ, Shu S, Chang AE. Therapeutic efficacy of T cells derived from lymph nodes draining a poorly immunogenic tumor transduced to secrete granulocyte-macrophage colony-stimulating factor. Cancer Gene Ther. 1996;3:39–44. [PubMed] [Google Scholar]
- 12.Thompson TC, Truong LD, Timme TL, Kadmon D, McCune BK, Flanders KC, Scardino PT, Park SH. Transgenic models for the study of prostate cancer. Cancer. 1993;71:1165–71. doi: 10.1002/1097-0142(19930201)71:3+<1165::aid-cncr2820711440>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Shu SY, Chou T, Sakai K. Lymphocytes generated by in vivo priming and in vitro sensitization demonstrate therapeutic efficacy against a murine tumor that lacks apparent immunogenicity. J Immunol. 1989;143:740–8. [PubMed] [Google Scholar]
- 14.Shu S, Krinock RA, Matsumura T, Sussman JJ, Fox BA, Chang AE, Terman DS. Stimulation of tumor-draining lymph node cells with superantigenic staphylococcal toxins leads to the generation of tumor-specific effector T cells. J Immunol. 1994;152:1277–88. [PubMed] [Google Scholar]
- 15.Yoshizawa H, Sakai K, Chang AE, et al. Activation by anti-CD3 of tumor-draining lymph node cells for specific adoptive immunotherapy. Cell Immunol. 1991;134:473–9. doi: 10.1016/0008-8749(91)90318-6. [DOI] [PubMed] [Google Scholar]
- 16.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–7. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 17.Hellstrom KE, Hellstrom I, Chen L, Ashe S, Brady WA, Ledbetter JA, McGowan P, Linsley PS. Can co-stimulated tumor immunity be therapeutically efficacious? Immunol Rev. 1995;145:123–41. doi: 10.1111/j.1600-065x.1995.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in. Cell. 1992;71:1065–8. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Ashe S, Brady WA, Hellstrom I, Hellstrom KE, Ledbetter JA, McGowan P, Linsley PS. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71:1093–102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 20.Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells [see comments] Science. 1993;259:368–70. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 21.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 22.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms. Adv Immunol. 2000;74:181–95. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 23.Trowsdale J, Travers P, Bodmer WF, Patillo RA. Expression of HLA-A-B, and -C and beta 2-microglobulin antigens in human. J Exp Med. 1980;152:11s–7s. [PubMed] [Google Scholar]
- 24.Restifo NP, Esquivel F, Kawakami Y, et al. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265–72. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochsenbein AF, Pinschewer DD, Sierro S, Horvath E, Hengartner H, Zinkernagel RM. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc Natl Acad Sci USA. 2000;97:13263–8. doi: 10.1073/pnas.230417497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asherson GL, Stone SH. Selective and specific inhibition of 24 hour skin reactions in the guinea-pig. I. Immune deviation: description of the phenomenon and the effect of splenectomy. Immunology. 1965;9:205–17. [PMC free article] [PubMed] [Google Scholar]
- 27.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 28.Tamada K, Harada M, Abe K, Li T. Immunosuppressive activity of cloned natural killer (NK1.1+) T cells established from murine tumor-infiltrating lymphocytes. J Immunol. 1997;158:4846–54. [PubMed] [Google Scholar]
- 29.Inoue M, Minami M, Fujii Y, Matsuda H, Shirakura R. Granulocyte colony-stimulating factor and interleukin-6-producing lung cancer cell line, LCAM. J Surg Oncol. 1997;64:347–50. doi: 10.1002/(sici)1096-9098(199704)64:4<347::aid-jso18>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL) -6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–9. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bendelac A, Rivera MN, Park S-H, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–62. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 32.von der Weid T, Beebe AM, Roopenian DC, Coffman RL. Early production of IL-4 and induction of Th2 responses in the lymph node originate from an MHC class I-independent CD4+NK1.1– T cell population. J Immunol. 1996;157:4421–7. [PubMed] [Google Scholar]
- 33.Constant SL, Bottomly K. Induction of Th1 and Th2, CD4+ T cell responses: alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 34.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995;182:1579–84. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–50. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 36.Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maldonado-Lopez R, De Smedt T, et al. CD8alpha+ and CD8alpha– subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–92. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maldonado-Lopez R, De Smedt T, Pajak B, et al. Role of CD8alpha+ and CD8alpha– dendritic cells in the induction of primary immune responses in vivo. J Leukoc Biol. 1999;66:242–6. doi: 10.1002/jlb.66.2.242. [DOI] [PubMed] [Google Scholar]
- 39.Navikas V, Link H. Review: cytokines and the pathogenesis of multiple sclerosis. J Neurosci Res. 1996;45:322–33. doi: 10.1002/(SICI)1097-4547(19960815)45:4<322::AID-JNR1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 40.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 41.Davies JD, Martin G, Phillips J, Marshall SE, Cobbold SP, Waldmann H. T cell regulation in adult transplantation tolerance. J Immunol. 1996;157:529–33. [PubMed] [Google Scholar]
- 42.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance [published erratum appears in Nature 1995 Sep 21; 377 (6546): 257] Nature. 1995;376:177–9. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 43.Maeda H, Takata M, Takahashi S, Ogoshi S, Fujimoto S. Adoptive transfer of a Th2-like cell line prolongs MHC class II antigen disparate skin allograft survival in the mouse. Int Immunol. 1994;6:855–62. doi: 10.1093/intimm/6.6.855. [DOI] [PubMed] [Google Scholar]
- 44.Plain KM, Fava L, Spinelli A, He XY, Chen J, Boyd R, Davidson CL, Hall BM. Induction of tolerance with nondepleting anti-CD4 monoclonal antibodies is associated with down-regulation of TH2 cytokines. Transplantation. 1997;64:1559–67. doi: 10.1097/00007890-199712150-00009. [DOI] [PubMed] [Google Scholar]
- 45.Winter H, Hu HM, McClain K, Urba WJ, Fox BA. Immunotherapy of melanoma: a dichotomy in the requirement for IFN-gamma in vaccine-induced antitumor immunity versus adoptive immunotherapy. J Immunol. 2001;166:7370–80. doi: 10.4049/jimmunol.166.12.7370. [DOI] [PubMed] [Google Scholar]
- 46.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–11. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 47.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system. Proc Natl Acad Sci USA. 1998;95:7556–61. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–56. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 49.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–95. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 50.Dobrzanski MJ, Reome JB, Dutton RW. Immunopotentiating role of IFN-gamma in early and late stages of type 1, CD8 effector cell-mediated tumor rejection. Clin Immunol. 2001;98:70–84. doi: 10.1006/clim.2000.4945. [DOI] [PubMed] [Google Scholar]
- 51.Hu H-M, Poehlein CH, Urba WJ, Fox BA. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res. 2002;62:3914–9. [PubMed] [Google Scholar]
- 52.Perez-Diez A, Spiess PJ, Restifo NP, Matzinger P, Marincola FM. Intensity of the vaccine-elicited immune response determines tumor clearance. J Immunol. 2002;168:338–47. doi: 10.4049/jimmunol.168.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meijer SL, Dols A, Urba WJ, et al. Adoptive cellular therapy with tumor vaccine-draining lymph node lymphocytes after vaccination with HLA-B7/beta2-microglobulin gene-modified autologous tumor cells. J Immunother. 2002;25:359–72. doi: 10.1097/00002371-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Tatsumi T, Kierstead LS, Ranieri E, et al. Disease-associated bias in T helper type 1 (Th1)/Th2, CD4(+) T cell responses against MAGE−6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196:619–28. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]