Abstract
Interleukin-12 p70 (IL-12p70) is a key cytokine produced by dendritic cells (DC) able to drive the development of T helper type 1 (Th1) lymphocytes. We showed that thymic and other fibroblasts strongly inhibit IL-12p70 production by splenic DC stimulated by lipopolysaccharide plus either anti-CD40 or interferon-γ (IFN-γ) and by purified splenic DC stimulated by Pansorbin plus IFN-γ. This IL-12p70 inhibitory activity is secreted in the conditioned medium of primary fibroblasts and fibroblast cell lines but not by haematopoietic cell lines. As IL-10 was the unique factor able to inhibit IL-12p70 produced by cultured splenic DC, we showed that a neutralizing antibody to IL-10 did not suppress the IL-12p70 inhibitory activity of thymic fibroblast-conditioned medium (FCM). This FCM potently inhibits the maturation and expression of major histocompatibility complex class II and co-stimulatory molecules induced by stimulation of spleen-derived DC. While thymic FCM suppressed the IL-12p70 expression by stimulated spleen-derived DC, tumour necrosis factor-α production is not affected. This inhibitory activity is able to down-regulate the IL-12p35 subunit transcription and expression, resulting in the impaired assembly of IL-12p70 heterodimer. As fibroblasts are present in the tissue microenvironment and are active players in the establishment of an immune response, the nature and role of the fibroblastic inhibitory activity remain to be established.
Introduction
Dendritic cells (DC) are the main professional antigen-presenting cells with the unique capacity to induce the primary T-cell immune response.1 Bioactive interleukin-12 p70 (IL-12p70) is a major T helper type 1 (Th1) -driving cytokine produced by DC interacting with T helper cells. The IL-12p70 molecule is a heterodimer of 70 000 MW comprising two disulphide-linked p40 and p35 subunits, encoded by separate genes. Most cell types express IL-12p35 constitutively whereas p40 is inducible and restricted to a subset of haematopoietic cells, namely B cells, macrophages, granulocytes and DC.2 Optimal production of the bioactive IL-12p70 heterodimer by DC in vivo and in vitro requires two signals provided by an initial microbial stimulus which up-regulates the expression of CD40 on DC and a T-cell-derived stimulus produced by CD40 ligand expressed on interacting activated T cells.3,4 IL-12p70 production by DC is modulated by environmental factors acting during the development and final maturation of DC.5 A number of inhibitory factors of the IL-12p70 production by DC have been described mostly by using the human monocyte-derived cultured DC. Among them, IL-10 is the most potent, acting on several types of human and murine DC.6–9 Other factors like prostaglandin E2 (PGE2)10–12 and type I interferons which are secreted by stromal fibroblasts were also reported to inhibit IL-12p70 production by mouse splenic leucocytes,13 human monocyte–macrophages14 and human monocyte-derived DC.15 In a previous study, we observed that thymic DC can be obtained in culture and developed in tight interaction with thymic fibroblasts present in the culture16 whereas DC derived in vitro from splenic progenitors proliferate actively in the supernatant culture medium.17 We showed that thymic DC were unable to produce IL-12p70 whereas splenic DC secrete high levels of IL-12p70 in response to the same appropriate stimuli.16 In this report, we demonstrate that thymic and other fibroblasts inhibited the production of IL-12p70 by cultured derived and ex vivo purified splenic DC. This fibroblastic inhibitory activity (FIA) is diffusible and different from IL-10 and type I interferons. The FIA does not inhibit tumour necrosis factor-α (TNF-α) production by stimulated DC and acts by down-regulating the transcription and expression of the IL-12p35 gene, resulting in the impaired production of bioactive IL-12p70.
Materials and methods
Mice
Female BALB/c (H-2d) mice (6–10-week-old) were used in all experiments and were purchased from IFFA-CREDO (L'arbresle, France).
DC culture
Splenic DC were derived from BALB/c myeloid progenitors contained in total splenocytes cultured in Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% fetal calf serum (FCS), granulocyte–macrophage colony-stimulating factor (GM-CSF) and Flt-3 ligand (Flt-3L) as previously described.17
DC purification
BALB/c mice were injected intraperitoneally each day with 10 μg of recombinant human Flt-3L (human Chinese Hamster Ovary cell-derived, produced and kindly provided by Immunex, Seattle, WA) for 9 consecutive days. Splenic DC were purified as previously described.18 Briefly, spleen cells were digested with collagenase and further dissociated in Ca2+ Mg2+-free medium; the low-density cells containing DC were then separated on a Nycodenz gradient (Nycomed, Oslo, Norway). The low-density cells were then incubated with biotinylated anti-CD11c and streptavidin magnetic microbeads (Miltenyi Biotec, Paris, France), and separated over a MidiMacs column. When further separation of CD11c+ CD8+ DC was needed, low-density spleen cells were enriched for CD11c expression and then separated according to CD8α labelling using a Multisort anti-FITC kit (Miltenyi Biotec). Then, 1 × 106 cells/ml were cultured overnight with 20% fibroblast-conditioned medium (FCM) or plated on confluent thymic monolayers of fibroblasts and then stimulated for 24 hr with Pansorbin (20 μg/ml) + murine interferon-γ (IFN-γ; 20 ng/ml).
Biological characterization of the FIA
The current assay to test the FIA is based on its capacity to inhibit the IL-12p70 production of day 12 cultured splenic DC. At this time of the culture, a mixture of immature (50–60%) and mature (25–30%) DC are characterized by fluorescence-activated cell sorting (FACS) analysis. A contaminant cell population represented by macrophages is depleted by 1-hr plastic adhesion in fresh culture medium immediately before testing FIA. A fixed number of macrophage-depleted day 12 cultured DC (5 × 105/ml complete culture medium containing 10 μl/ml GM-CSF and 25 ng/ml Flt-3L) was mixed with 20% FCM or was plated over a confluent monolayer of fibroblasts for 24 hr. DC were then stimulated to produce IL-12 by lipopolysaccharide (LPS; 1 μg/ml) + anti-CD40 (1 μg/ml) (DS-1), LPS (1 μg/ml) + IFN-γ (20 ng/ml) (DS-2) or Pansorbin (20 μg/ml) + IFN-γ (20 ng/ml) (PSB-2) during a further 24-hr incubation. The cell-free culture supernatant was then collected and frozen to −20° until enzyme-linked immunosorbent assay (ELISA) for measurement of IL-12p70.
Cultures of primary fibroblasts
Thymic fibroblasts were isolated from total thymocyte cultures after 2 weeks of incubation as previously described.16 The cells present in the supernatant were removed and adherent layers containing macrophages and fibroblasts were dissociated after 10 min at 37° with 0·02% ethylenediaminetetraacetic acid solution (Sigma, Saint Louis, MO) allowing fibroblasts to detach rapidly whereas most macrophages remained adherent. Fibroblasts were further purified after labelling of the adherent cell suspension with biotinylated anti-CD11b (M1/70 from Pharmingen, San Diego, CA) and CD11c followed by streptavidin microbeads. Thymic fibroblasts were recovered in the negative fraction by using magnetic sorting (Miltenyi Biotec). Ten thousand fibroblasts were plated per well (four-well plates, Nunclon, Glostrup, Denmark) and allowed to reach confluency by 3–4 days in culture (IMDM + 10% FCS). Fibroblast monolayers were then irradiated with 20 grays (CEA, Saclay, France, Cobalt source γ) and used within 3 weeks post-irradiation after washing with complete culture medium just before DC plating.
Bone marrow and splenic fibroblasts were isolated from total cells cultured for 2–3 weeks in IMDM + 10% FCS, grown until confluency and γ-irradiated as described above for thymic fibroblasts.
Muscular fibroblasts were grown from total crushed BALB/c mouse triceps. Confluent layers of fibroblasts were obtained after 2–3 weeks of culture as described19 and γ-irradiated.
Conditioned media from thymic, bone marrow, splenic and muscular fibroblasts were collected every 4 days of culture for 1 month, starting from γ-irradiation.
Cell lines
MS-520 and ST-221 murine bone marrow fibroblastic cell lines were grown in four-well plates (Nunclon) until confluency and γ-irradiated as described above.
Non-fibroblastic cell lines were purchased from the American Type Culture Collection (Rockville, MD), namely EL-4 (T-cell lymphoma, ref. TIB-39), M1 (myeloblast, ref. TIB-192) and WEHI-3B (myelomonocyte, ref. TIB-68). Conditioned medium was collected every 4 days when cells were at saturating densities.
Cytokine ELISA assays
Culture supernatants were assayed for IL-12p70 and TNF-α by using ELISA commercial kits developed by PharMingen (San Diego, USA). Duplicate ELISA measurements were made on duplicate spleen-derived DC culture wells.
Real-time polymerase chain reaction (PCR)
Day 12 cultured splenic DC (1 × 106/ml) or ex vivo purified splenic DC (1 × 106/ml) were cultured, or not, on MS-5 fibroblasts or in the presence of 20% thymic FCM for 20 hr. Then, cultures were stimulated for 9 hr either with DS-1 (day 12 cultured DC) or with PSB-2 (ex vivo purified DC). Total cellular RNA was then extracted from purified and cultured splenic DC by using the RNeasy Protect Mini kit (QIAGEN, Courtaboeuf, France) according to the manufacturer's instructions. Total RNA (0·5–1 μg) was then reverse-transcribed using Superscript II RNase H– kit according to the manufacturer's instructions (Gibco-BRL, Life Technologies, Pontoise, France).
Real-time PCR was conducted on a LightCycler System (Roche Diagnostics, Meylan, France) in a 20-μl final volume containing: 2 μl cDNA, 1× LightCycler-DNA master SYBRGreen I, 3 mm MgCl2, 0·5 μm each of sense and antisense primers, 0·2 μl Taq polymerase, 0·07 μmTaqStart Antibody (Clontech, Heidelberg, Germany). After a 2-min denaturation step at 95°, the reactions were cycled 35–40 times for 5 seconds at 95°, 10 seconds at 60°, and 15 seconds at 72°. The oligonucleotides used were: IL-12p35 sense, 5′-CACGCTACCTCCTCTTTTTG-3′, IL-12p35 antisense 5′-CAGCAGTGCAGGAATAATGTT-3′; IL-12p40 sense, 5′-AAACCAGACCCGCCCAAGAAC-3′, IL-12p40 antisense 5′-AAAAAGCCAACCAAGCAGAAGACAG-3′; hypoxanthine–guanine phospho ribosyl transferase (HPRT) sense, 5′-GGTTAAGCAGTACAGCCCCAAAAT-3′, HPRT antisense, 5′-ATAGGCACATAGTGCAAATCAAAAGTC-3′. Product specificity was determined by melting curve analysis as described in the LightCycler handbook, visualization of PCR products on agarose gels and sequencing. The amount of transcripts in each sample is given as the number of copies determined with an internal standard curve and normalized against number of HPRT copies found in each sample.
Reagents
LPS (Escherichia coli 026:B6) and PGE2 were purchased from Sigma. Pansorbin (Gram-negative Staphylococcus aureus Cowan 1 strain) was purchased from Calbiochem (La Jolla, CA); murine recombinant IL-10, IL-4, IFN-γ, transforming growth factor-β1 (TGF-β1) and vascular endothelial growth factor (VEGF) were obtained from Peprotech Inc. (Rocky Hill, NJ). Recombinant mouse IFN-β and a neutralizing polyclonal sheep anti-mouse IFN-α/β were obtained from BioSource (Nivellas, Belgium). Anti-CD40 antibody (clone 3/23), neutralizing monoclonal antimurine IL-10 and control mouse immunoglobulin G1 (IgG1) were purchased from Pharmingen.
The cell surface phenotype of cultured and purified DC was studied using a rapid standardized procedure as described previously.16 The following monoclonal antibodies biotinylated or conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE) or Cy-chrome (CY) were purchased from Pharmingen: 25.9.17-FITC [major histocompatibility complex (MHC) -class II anti I-Ab] that cross-reacts with I-Ad class II antigen of BALB/c mouse, HL3-PE (anti-CD11c), 3/23-FITC (anti-CD40) and 53-6.7-Cy (anti-CD8α). RMPP-2-PE (anti-CD80, B7-1) and RMPP-1-PE (anti-CD86, B7-2) were obtained from Caltag (Burlingame, CA).
Results
Thymic fibroblasts inhibit IL-12p70 production by cultured and ex vivo purified splenic DC
The secretion of IL-12p70 by DC is not constitutive and can be induced by the combination of bacterial and T-lymphocyte-dependent stimuli. Previous studies using human or murine cultured DC showed that LPS + anti-CD40 (double stimulation 1 or DS-1) or LPS + IFN-γ (DS-2) are very efficient stimuli to induce high level of IL-12p70 secretion by DC.3,4,16
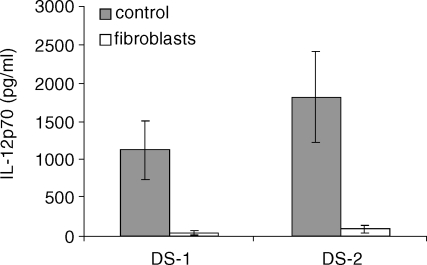
Cultured splenic DC secrete high levels of IL-12p70, reaching a mean of 1126 ± 380 pg/ml in 11 experiments when stimulated with LPS + anti-CD40 and 1819 ± 592 pg/ml in seven experiments with LPS + IFN-γ (Fig. 1). When DC are plated during 24 hr on confluent monolayers of thymic fibroblasts and further stimulated for 24 hr, the level of IL-12p70 is strongly reduced to a mean of 42 ± 33 pg/ml with DS-1 and 95 ± 48 pg/ml with DS-2. As shown in Fig. 1, the level of IL-12p70 secreted by DC using these two types of stimulation may vary between experiments but the relative percentages of IL-12p70 inhibition by fibroblasts were similar using DS-1 or DS-2. Most experiments were conducted using both types of stimulation but for the sake of clarity, we choose to show the experiments with the highest level of IL-12p70 secreted by DC.
Figure 1.
IL-12p70 production by splenic derived DC is suppressed by co-culture on thymic fibroblasts; 5 × 105 day 12 splenic DC/ml were cultured for 24 hr on thymic fibroblasts monolayers and further stimulated by LPS + anti-CD40 (DS-1) or LPS + IFN-γ (DS-2) for 24 hr. Means ± SD of 11 experiments with DS-1 and seven experiments with DS-2 are depicted.
We then used ex vivo purified splenic DC from Flt-3L-treated animals. In contrast with cultured splenic DC, ex vivo purified splenic DC respond poorly to LPS stimulation using DS-1 or DS-2 suggesting that splenic cultured DC are composed of different DC subtypes which are not equivalent to ex vivo splenic purified DC. Hence, we used Pansorbin + IFN-γ (PSB-2) as previously described.18 IL-12p70 production by total CD11c+ or CD11c+ CD8α+ purified DC (1 × 106 cells/ml) was studied in three experiments. Table 1 shows a strong inhibition (from 76 to 96%) of the amount of IL-12p70 in DC supernatants after a 24-hr co-culture on thymic confluent fibroblasts, with PSB-2.
Table 1.
Inhibitory effect of thymic fibroblasts on IL-12p70 production by ex vivo purified splenic DC; stimulation with PSB-2
Results are expressed in pg/ml IL-12p70 in culture supernatants; 1 × 106 CD11c+ CD8+ splenic purified DC (Expt 1 and 2) or total CD11c+ cells (Expt 3) per ml were co-cultured or not on thymic fibroblast monolayers for 24 hr and then stimulated with PSB-2 for a further 24 hr.
The FIA is diffusible in conditioned medium of primary fibroblasts and fibroblastic cell lines
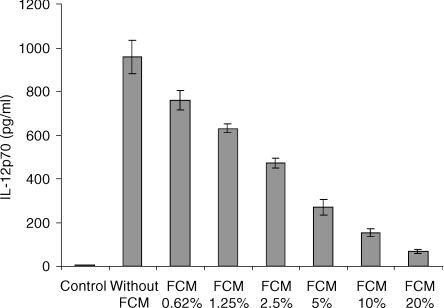
Conditioned medium from confluent thymic fibroblasts was harvested every 4 days for 3 weeks and pooled, and its IL-12p70 inhibitory activity was measured in a semi-standardized assay using day 12 splenic cultured DC stimulated with DS-1. A dose–response study of the IL-12p70 inhibitory activity was performed by incorporating 0·625% to 20% of thymic FCM in DC culture medium (Fig. 2). Depending on the experiments, the 50% IL-12p70 inhibitory activity was obtained when 4–7% of FCM was present in the DC culture. To challenge the specificity of thymic fibroblasts, we looked at the inhibitory activity of other sources of fibroblasts. Confluent monolayers of primary fibroblasts were prepared from the spleen, bone marrow and muscle of BALB/c mice and FCM was harvested in the same conditions as thymus. Tables 2 and 3 showed that splenic and muscular FCM have a strong IL-12p70 inhibitory activity similar to that of thymic FCM (78–93%) whereas bone marrow FCM was weaker (54% to 61%). Conditioned medium from fibroblastic cell lines derived from mouse bone marrow like MS-5 and ST-2 exhibited strong IL-12p70 inhibitory activity similar to those of primary thymic fibroblasts. However, it is to be stressed that the number of confluent MS-5 or ST-2 cells per culture well was 10 times higher than those of thymic primary fibroblasts contained in the same culture well (1·5 × 104 thymic fibroblasts/well/0·5 ml versus 1·6 × 105 MS-5 cells/well/0·5 ml). This means that on a per cell basis, FCM produced by primary fibroblasts could be 10 times more potent than those of fibroblastic cell lines. Conditioned medium obtained from haematopoietic non-fibroblastic cell lines, such as EL-4, M1 and WEHI-3, at saturating densities (1 × 106/ml) were unable to inhibit IL-12p70 secretion of DC or exhibit a weak inhibitory activity (8–12%) at a concentration of 20% in DC culture medium.
Figure 2.
Thymic fibroblast-conditioned medium (FCM) contains a potent inhibitory activity of IL-12p70 production by splenic derived DC; 5 × 105 day 12 splenic derived DC/ml were cultured for 24 hr in the presence of different concentrations of FCM and then stimulated for a further 24 hr with DS-1. Means ± SD are obtained from duplicate culture wells.
Table 2.
Effect of FCM (20%) prepared from primary fibroblasts (four experiments) on the capacity of production of cultured splenic DC to secrete IL-12p70
| Thymic FCM | Splenic FCM | Bone marrow FCM | Muscle FCM |
|---|---|---|---|
| 78% | 81% | 54% | nd |
| 93% | nd | nd | 91% |
| 89% | 84% | nd | 81% |
| 91% | 78% | 61% | 82% |
Values are percentage inhibition of IL-12p70 produced by control cultures stimulated with DS-1 or DS-2.
nd, not determined.
Table 3.
Effect of CM (20%) prepared from fibroblastic or non-fibroblastic cell lines (three experiments) on the capacity of production of cultured splenic DC to secrete IL-12p70
| Fibroblastic cell lines | Non-fibroblastic cell lines | ||||
|---|---|---|---|---|---|
| Thymic FMC | MS-5 | ST-2 | EL-4 | M1 | WEHI-3 |
| 83% | 72% | nd | 8% | nd | 12% |
| 79% | 71% | 81% | 6% | 2% | 0% |
| 85% | nd | 87% | nd | 5% | 17% |
Values are percentage inhibition of IL-12p70 produced by control cultures stimulated with DS-1 or DS-2.
nd, not determined.
The FIA is different from other known IL-12p70 inhibitory molecules
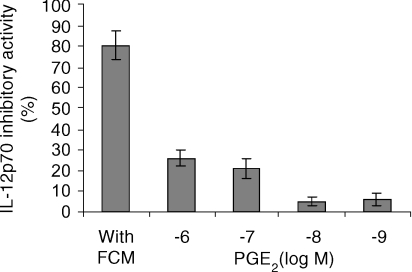
Using our splenic cultured DC system, we tested the activity of molecules known to inhibit IL-12p70 production by DC. Numerous factors were described to have IL-12p70 inhibitory activity, most of them being tested in the human monocyte-derived DC culture system. We chose to test factors known to be secreted by fibroblasts and IL-10 as the prototypic IL-12p70 inhibitory molecule active in vivo and in vitro. The dose–response of VEGF, TGF-β1, IFN-β and IL-10 tested separately showed that only IL-10 had a strong inhibitory activity on the secretion of IL-12p70 by splenic cultured DC stimulated with DS-2 (Fig. 3a). Depending on the experiments, 1–5 ng/ml of IL-10 in the culture medium completely suppressed IL-12p70 secretion of 5 × 105 DC stimulated by DS-2 (Fig. 3b). We evaluated the concentration of IL-10 in thymic FCM which was found to be always lower than 200 pg/ml. As we used 20% FCM in 1-ml DC culture medium, the highest final concentration of IL-10 would be 40 pg/ml, which is not inhibitory in the dose–response curve. Nevertheless, the effect of a neutralizing anti-IL-10 antibody was tested against the dose response of a pool of thymic FCM in our day 12 spleen-derived DC culture system stimulated with DS-2 (Fig. 3c). This anti-IL-10 antibody was unable to neutralize the IL-12p70 inhibitory activity of FCM whereas it completely counteracted the activity of 5 ng/ml IL-10 in the same DC culture system. These results were reproducible and confirmed several times using different pools of thymic FCM and stimulation with DS-1. Despite the lack of activity of IFN-β in our IL-12p70 inhibitory assay, we looked at the effect of a polyclonal neutralizing anti-mouse IFN-αβ on FCM. Again, it was unable to counteract the IL-12p70 inhibitory activity of thymic FCM (data not shown). The effect of several other molecules produced by fibroblasts and described to inhibit IL-12p70 secretion by DC or monocyte–macrophages was studied. The dose–response activity of PGE2 (10 × 10−6 m to 10 × 10−8m), IL-6 (1–40 ng/ml), TNF-α (1–25 ng/ml), IL-1β (1–25 ng/ml) and the chemokines RANTES (1–100 ng/ml) and MCP-1 (1–100 ng/ml) were tested separately for their capacity to inhibit IL-12p70 production by cultured splenic DC stimulated by DS-1 or DS-2. None of these molecules was able to inhibit IL-12p70 production except PGE2 which reduced by 26% ± 4% (mean ± SD of three experiments) the production of IL-12p70 at micromolar concentration (Fig. 4). Therefore, the inhibitory activity present in FCM seems to be different from the already identified IL-12p70 inhibitory molecules.
Figure 3.
The IL-12p70 inhibitory activity contained in FCM is different from IL-10. (a) Dose–response inhibition of IL-12p70 production by splenic derived DC stimulated with DS-2 obtained in the presence of IL-10, TGF-β1, IFN-β and VEGF. (b) The inhibitory effect of IL-10 on the production of IL-12p70 by day 12 splenic derived DC is efficiently counteracted by a neutralizing anti-IL-10 antibody. (c) The inhibitory effect of thymic FCM on the production of IL-12p70 by day 12 splenic derived DC is not suppressed by a neutralizing anti-IL-10 antibody. Day 12 splenic derived DC were cultured for 24 hr in the presence of increased concentrations of the different inhibitory molecules and then stimulated with DS-2 for further 24 hr of incubation. Anti-IL-10 monoclonal antibody (5 μg/ml) or the corresponding isotype (IgG1; 5 μg/ml) were added to the cultures in (b) and (c) during the first 24 hr of incubation at the same time as IL-10 and FCM. Splenic DC cultures were then stimulated with DS-2 for a further 24 hr.
Figure 4.
Dose–response curve (10−6−10−9 m) of PGE2 inhibitory activity of IL-12p70 production by murine splenic derived DC: comparison with FCM (20%). Results are expressed as percentage inhibition of IL-12p70 produced by control DC stimulated with DS-1 or DS-2. 5 × 105 day 12 splenic derived DC/ml were cultured for 24 hr in the presence of 20% of FCM or with different concentrations of PGE-2 and then stimulated for a further 24 hr with DS-1 or DS-2. Means ± SD are obtained from duplicate culture wells.
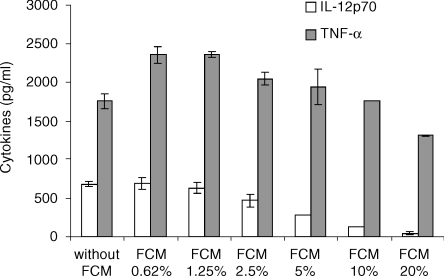
Thymic FCM does not inhibit the production of TNF-α by cultured splenic DC
The specificity of FIA for IL-12p70 production by DC was challenged by studying its effect on another cytokine produced by DC like TNF-α. The dose–response from three different pools of thymic FCM were studied using the same population of day 12 spleen-derived DC. A typical representative dose–response study is depicted in Fig. 5 showing that a moderate inhibitory effect (19 ± 8% in the three experiments) on TNF-α production is observed by using 20% FCM whereas a concentration of 10% is no more inhibitory. Conversely, this 10% concentration of thymic FCM reduces by 77 ± 6% the level of IL-12p70 produced by control spleen-derived DC stimulated with DS-1.
Figure 5.
Thymic FCM does not inhibit TNF-α production by day 12 splenic derived DC; 5 × 105 day 12 SDC/ml were cultured in the presence of increased concentrations of thymic FCM for 24 hr and then stimulated with DS-1 for a further 24 hr. ELISA measurements of TNF-α and IL-12p70 were performed on these supernatants. Means ± SD represent values from duplicate culture wells in one experiment representative of three.
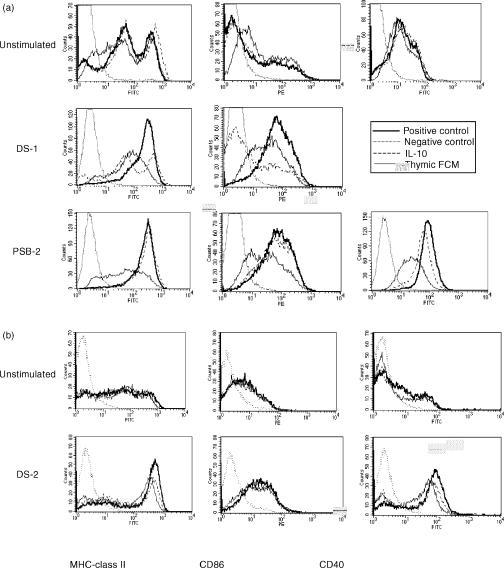
Thymic FCM induces an impaired ability of DC to up-regulate the expression of MHC-class II and co-stimulatory molecules in response to DS-1, DS-2 and PSB-2 stimulations.
The addition of 20% FCM for 24 hr to day 12 splenic DC did not modify their number nor their viability. After a further 24 hr of stimulation with DS-1 or PSB-2, it was obvious that the cells exposed to FCM were larger and did not exhibit characteristic cytoplasmic extensions, suggesting an inhibition of maturation. Hence, the phenotypic expression of MHC-class II, CD40 and CD86 was studied on day 12 spleen-derived DC incubated or not for 24 hr with IL-10 (10 ng/ml) or 20% thymic FCM and further stimulated with DS-1 or PSB-2 for 24 hr (Fig. 6a). The FACS profile of unstimulated DC was not modified by the presence of IL-10 or FCM. After DS-1 stimulation, MHC-class II and CD86 expressions were highly up-regulated in control cells. CD40 expression could not be studied due to the use of anti-CD40 agonist monoclonal antibody for stimulation. The presence of IL-10 prevented the increased level of MHC-class II and strongly inhibited CD86 expression. The effect of FCM (20%) also resulted in a severe inhibition of the expression of MHC-class II whereas CD86 was less inhibited than with IL-10. Concerning PSB-2 stimulation of control cells, a large increase in MHC-class II, CD86 and CD40 expression was observed. IL-10 did not inhibit the increased expression of MHC-class II, CD86 whereas CD40 was slightly inhibited. This contrasted with the severe inhibition of MHC-class II and CD40 by thymic FCM exposure, whereas CD86 was less inhibited. Another set of experiments was carried out using DS-2 stimulation (Fig. 6b) allowing the study of CD40 expression. The expression of MHC-class II and CD40 was also strongly inhibited by FCM and more slightly by IL-10, whereas CD86 was marginally decreased by both inhibitors. The simultaneous measurements of IL-12p70 in these cultures showed a similar level of inhibition produced by IL-10 and FCM after DS-1, DS-2, or PSB-2 stimulations (data not shown). This suggested that modifications of DC phenotype are not essential to the inhibition of IL-12p70 production.
Figure 6.
Phenotype analysis of splenic derived DC cultured in the absence or the presence of IL-10 and thymic FCM. Day 12 splenic DC were incubated for 24 hr with or without IL-10 (10 ng/ml) or thymic FCM (20%) and then stimulated with DS-1 and PSB-2 (a) or DS-2 (b) for a further 24 hr. The cells were analysed by flow cytometry for the surface expression of the indicated antigens. Dotted line represents negative control; thick bold line represents positive control; a dashed line represents incubation with IL-10 and the bold line represents incubation with FCM.
FCM and MS-5 fibroblasts inhibit the transcription of IL-12p35 gene
The inhibition of the transcriptional activity of IL-12p40 and IL-12p35 genes was studied using ex vivo purified splenic DC and day 12 splenic DC in culture, submitted to the inhibitory activity of FCM or MS-5 fibroblasts. DC suspensions were cultured for 20 hr in the presence or absence of the inhibitor and then stimulated for 9 hr with PSB-2 for ex vivo purified splenic DC or with DS-1 for day 12 splenic cultured DC. After RNA extraction, real-time quantitative PCR was performed on DC cultured or not with FCM or MS-5 fibroblasts. The concentration of bioactive IL-12p70 and IL-12p40 proteins was simultaneously measured in the culture supernatants by using ELISA. Using DS-1 stimulation of day 12 cultured splenic DC, Fig. 7(a) shows that the transcriptional activity of IL-12p35 gene was strongly inhibited by MS-5 fibroblasts (one experiment representative of two). A concomitant drastic decrease of the bioactive IL-12p70 protein was observed in the supernatant of DC cultured on MS-5. However, neither the transcriptional activity of the IL-12p40 gene nor the secretion of the IL-12p40 protein were affected by culturing DC on MS-5 fibroblasts. Similar results Fig. 7(b) were obtained with day 12 cultured splenic DC stimulated in the presence of 20% thymic FCM. Figure 7(c) showed a strong inhibition of the transcriptional activity of both IL-12p40 and IL-12p35 genes by ex vivo purified splenic DC cultured on MS-5 fibroblasts. Simultaneous measurements of IL-12 proteins showed a clear inhibition of the bioactive IL-12p70 heterodimer (75%) whereas the IL-12p40 subunit was slightly inhibited (31%) by MS-5 fibroblasts. Taken together, these results suggested that the FIA acted essentially by down-regulating the transcription and expression of IL-12p35 gene which prevented the assembly of bioactive IL-12p70 heterodimer.
Figure 7.
Regulatory effects of MS-5 fibroblasts or thymic FCM on the transcriptional activity of IL-12p40 and p35 genes and IL-12 protein production by DC. Each figure is composed of histograms showing the IL-12p35 or p40 copies number per 1000 HPRT, and of symbols (×) showing the level of IL-12p70 (pg/ml) or IL-12p40 proteins (ng/ml). Day 12 cultured splenic DC (1 × 106/ml) were cultured or not on MS-5 fibroblasts monolayers (a) or in the presence of 20% thymic FCM (b) for 20 hr and then stimulated with DS-1 for a further 9 hr. (c) Ex vivo purified splenic CD11c+ cells (1 × 106/ml) were cultured on MS-5 fibroblast monolayers for 20 hr and then stimulated for a further 9 hr with PSB-2. Messenger RNA were extracted, reverse transcribed and amplified by real-time PCR using primers specific for IL-12p40, p35 and HPRT. IL-12p40 and p35 mRNA levels were quantified and normalized against HPRT mRNA using an internal standard curve of HPRT. Simultaneous measurements of IL-12p70 and IL-12p40 proteins were performed on culture supernatants using ELISA.
Discussion
In this study, we showed that thymic murine fibroblasts are potent inhibitors of IL-12p70 production by spleen-derived cultured DC and by DC purified from spleen. This IL-12p70 inhibitory activity is produced by thymic fibroblasts in conditioned medium. This activity is not specific to thymic fibroblasts as other primary fibroblasts prepared from bone marrow, spleen and muscle also secreted this inhibitory activity as well as fibroblastic cell lines established from bone marrow like MS-5 and ST-2. Interestingly, this FIA seems to be specific for IL-12p70 as thymic FCM tested simultaneously for TNF-α and IL-12p70 inhibitory activity in day 12 splenic DC culture system did not inhibit the production of TNF-α while IL-12p70 was severely impaired. As several molecules have been reported to inhibit IL-12p70 production by murine or human DC, we investigated the effect of several of them reported to be produced by murine fibroblasts.22 Using a semi-standardized IL-12p70 inhibitory assay based on day 12 spleen-derived DC, we found that IL-10 was the unique factor able to strongly inhibit IL-12p70. The other factors tested were IFN-β, VEGF, TGF-β1, IL-6, TNF-α, IL-1β, PGE2 and the chemokines RANTES and MCP-1 which are secreted by human fibroblasts.23 We found that only PGE2 at micromolar concentration slightly inhibits (less than 30%) the production of IL-12p70 by spleen-derived DC. Our data seem to conflict with those of several authors reporting an IL-12p70 inhibitory activity of the molecules tested in this paper. Concerning PGE2, all the data were obtained in the human monocyte-derived DC culture system. Kalinski et al. showed that PGE2 does not induce final DC maturation by itself but synergizes with low doses of IL-1β and TNF-α, allowing their effectiveness at 100-fold lower concentrations.24 We did not test PGE2 in the presence of low doses of TNF-α. However, we found that DS-1 and DS-2 stimulations induce the secretion of high levels (1–2 ng/ml) of TNF-α by DC in the culture medium which has been shown to be a level sufficient to induce DC maturation and IL-12p70 inhibition.24 This observation contrasts with our data showing that FCM inhibits the maturation of our murine derived splenic DC. Recently, these authors showed that PGE2 in the presence of TNF-α is a selective inducer of IL-12p40 production and an inhibitor of bioactive IL-12p70 heterodimers.12 Furthermore, in the same paper, they showed that PGE2 inhibits the production of both IL-12p70 and IL-12p40 proteins by monocyte-derived DC stimulated with the ‘classical inducers’ CD40 ligand or LPS. These results pointed out that the regulation of IL-12p40 and p35 subunits is extremely flexible depending on the presence and combination of various stimuli present in the DC environment. However, conflicting results were produced by Rieser et al.25 also using monocyte-derived DC, who showed that PGE2 alone stimulated the production of a low amount of IL-12p70 and synergized with TNF-α to induce high levels of IL-12p70 secretion. Concerning the regulatory effects of type I interferons on IL-12p70 production, a few studies were performed using in vitro and in vivo murine system. Cousens et al. reported that IFN-α and IFN-β inhibited the production of IL-12p70 by mouse splenic leucocytes in culture stimulated by Staphylococcus aureus Cowan strain.13 They also showed that endogenous IFN-α/β induced by a viral infection inhibited IL-12p70 production after LPS stimulation in vivo. Most studies of the role of type I IFNs on IL-12p70 production by DC have been conducted using cultured human monocyte-derived DC or differentiated from CD34+ progenitors. Several studies showed a direct (IL-10-independent) inhibitory activity of type I IFNs on the level of IL-12p70 secreted by human monocyte-derived DC and an inhibition of their differentiation.26 However, some conflicting data exist showing that type I IFNs induce the maturation of human monocyte-derived DC which produce high amounts of IL-15 responsible of the development of Th1-biased immune responses.27 Luft et al. generated human DC from CD34+ progenitor cells and showed that IFN-α/β enhance their terminal differentiation and maturation.28 A study on CD11c+ CD1a+ and CD11c– CD1a– blood DC precursors showed that IFN-α enhanced the maturation of CD11c+ precursors and their IL-10 production while it acted only as a survival factor for CD11c– DC precursor cells.29 Our results using murine splenic derived DC showed that IFN-β did not inhibit their IL-12p70 production and that a neutralizing polyclonal antibody to IFN-α/β was unable to suppress the IL-12p70 inhibitory activity of thymic FCM. A recent paper reported that the chemokines RANTES and MCP-1 inhibited IL-12p70 production by human monocytes while they did not suppress IL-12p70 production by monocyte-derived DC.30 We confirmed that these two chemokines did not inhibit IL-12p70 by murine splenic derived DC (data not shown). Finally, IL-10 was the unique factor exhibiting a strong inhibitory activity of IL-12p70 production by our splenic derived DC culture system confirming data previously obtained in murine and human DC culture systems. To ensure that IL-10 was not the molecule responsible for the IL-12p70 inhibitory activity of thymic FCM, we measured the level of this cytokine which was too low to be inhibitory. Furthermore, we showed that a neutralizing anti-IL-10 monoclonal antibody was unable to get rid of the IL-12p70 inhibitory activity of thymic FCM. Then, we examined the effect of thymic FCM on the phenotype of splenic derived DC and showed that it inhibits the increased expression of MHC-class II and co-stimulatory molecules when DC are stimulated by DS-1 and PSB-2. A similar inhibition of the activation of splenic derived DC was observed with IL-10 using DS-1 stimulation whereas IL-10 did not modify the activated phenotype of DC induced by PSB-2. Simultaneous measurements of IL-12p70 production showed a similar degree of inhibition by IL-10 and thymic FCM using DS-1 and PSB-2, suggesting that the modifications of DC phenotype are not essential to the production of IL-12p70. Finally, we examined the molecular basis of the IL-12p70 inhibitory activity secreted by fibroblasts by studying the transcriptional levels of IL-12p40 and IL-12p35 genes in DC cultured on MS-5 fibroblasts and in the presence of thymic FCM. A strong inhibition of the IL-12p35 mRNA was observed in day 12 cultured splenic DC stimulated with DS-1 and cultured on MS-5 fibroblasts or in the presence of thymic FCM, which resulted in the almost complete inhibition of the production of bioactive IL-12p70 heterodimer. This contrasted with the absence of inhibition of IL-12p40 mRNA transcripts and protein secretion in the same experimental conditions. In one experiment conducted on ex vivo purified splenic DC cultured on MS-5 fibroblasts and stimulated with PSB-2, we observed the inhibition of both IL-12p40 and IL-12p35 mRNA. However, at the protein level, the production of IL-12p70 was more inhibited than those of IL-12p40 protein subunit. These results suggested that the FIA essentially acts by down-regulating the IL-12p35 subunit production, resulting in the impaired association of bioactive IL-12p70 heterodimer. This is consistent with the observation of Snidjers et al. who showed that the level of IL-12p70 production in monocytes stimulated by LPS and cytokines, is determined by the level of IL-12p35 expression.31 In conclusion, we showed that fibroblasts can modulate negatively the production of IL-12p70 by splenic derived DC, most of them being immature. The functional characterization of this factor showed that it is different from already known IL-12p70 inhibitory factors. Functional DC modulation by tissue-specific factors has been reported by numerous investigators (reviewed in ref. 5) which might take place during the local development of DC precursors or may affect local populations of resting immature DC. Fibroblasts represent a cellular component of tissue microenvironment disseminated throughout the body. They respond to diverse stimuli like wounds, infection, allergens and tumoral development by releasing cytokines, chemokines and by the expression of various surface molecules. FIA may play an important role in the initiation of the immune response by inhibiting IL-12 production by DC, thereby preventing establishment of Th1 lymphocyte response. It remains to be seen how and when FIA is produced in vivo and if its production is constitutive or more probably induced by stimuli which take place during the establishment of immune response.
Acknowledgments
This work was supported by Institutional grants from INSERM (Institut National de la Santé et de la Recherche Médicale), CEA (Commissariat à l'Energie Atomique) and in part by ARC (Association pour la Recherche sur le Cancer). A. R. is supported by a fellowship from the Ministère de l'Enseignement Supérieur et de la Recherche and from ARC. We thank the Immunex Corporation (Dr Lyman) for the generous gift of recombinant human Flt3-ligand produced in Chinese hamster ovary cells, and F. Cretin and colleagues for critical reading of the manuscript.
Abbreviations
- DC
dendritic cells
- DS-1
double stimulation-1
- FCM
fibroblast-conditioned medium
- FIA
fibroblast inhibitory activity
- IL-12
interleukin-12
- HPRT
hypoxanthine–guanine phospho ribosyl transferase
- PSB
pansorbin
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 3.Snijders A, Kalinski P, Hilkens CM, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998;10:1593–8. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 4.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 6.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol. 1997;27:1229–35. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 7.Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F. Interleukin-10 prevents the generation of dendritic cells from human peripheral blood mononuclear cells cultured with interleukin-4 and granulocyte/macrophage-colony-stimulating factor. Eur J Immunol. 1997;27:756–62. doi: 10.1002/eji.1830270326. [DOI] [PubMed] [Google Scholar]
- 8.Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, Mantovani A. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol. 1998;28:359–69. doi: 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–8. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 10.van der Pouw Kraan TC, Boeije LC, Smeenk RJ, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–9. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 12.Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E (2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97:3466–9. doi: 10.1182/blood.v97.11.3466. [DOI] [PubMed] [Google Scholar]
- 13.Cousens SN, Vynnycky E, Zeidler M, Will RG, Smith PG. Predicting the CJD epidemic in humans. Nature. 1997;385:197–8. doi: 10.1038/385197a0. [DOI] [PubMed] [Google Scholar]
- 14.Byrnes AA, Ma X, Cuomo P, et al. Type I interferons and IL-12: convergence and cross-regulation among mediators of cellular immunity. Eur J Immunol. 2001;31:2026–34. doi: 10.1002/1521-4141(200107)31:7<2026::aid-immu2026>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 15.McRae BL, Semnani RT, Hayes MP, van Seventer GA. Type I IFNs inhibit human dendritic cell IL-12 production and Th1 cell development. J Immunol. 1998;160:4298–304. [PubMed] [Google Scholar]
- 16.Martinon-Ego C, Berthier R, Cretin F, Collin V, Laharie AM, Marche PN. Murine dendritic cells derived from myeloid progenitors of the thymus are unable to produce bioactive IL-12p70. J Immunol. 2001;166:5008–17. doi: 10.4049/jimmunol.166.8.5008. [DOI] [PubMed] [Google Scholar]
- 17.Berthier R, Martinon-Ego C, Laharie AM, Marche PN. A two-step culture method starting with early growth factors permits enhanced production of functional dendritic cells from murine splenocytes. J Immunol Methods. 2000;239:95–107. doi: 10.1016/s0022-1759(00)00186-1. [DOI] [PubMed] [Google Scholar]
- 18.Maldonado-Lopez R, Maliszewski C, Urbain J, Moser M. Cytokines regulate the capacity of CD8alpha(+) and CD8alpha(−) dendritic cells to prime Th1/Th2 cells in vivo. J Immunol. 2001;167:4345–50. doi: 10.4049/jimmunol.167.8.4345. [DOI] [PubMed] [Google Scholar]
- 19.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 20.Itoh K, Tezuka H, Sakoda H, Konno M, Nagata K, Uchiyama T, Uchino H, Mori KJ. Reproducible establishment of hemopoietic supportive stromal cell lines from murine bone marrow. Exp Hematol. 1989;17:145–53. [PubMed] [Google Scholar]
- 21.Nishikawa S, Ogawa M, Kunisada T, Kodama H. B lymphopoiesis on stromal cell clone: stromal cell clones acting on different stages of B cell differentiation. Eur J Immunol. 1988;18:1767–71. doi: 10.1002/eji.1830181117. [DOI] [PubMed] [Google Scholar]
- 22.Gimble JM, Pietrangeli C, Henley A, et al. Characterization of murine bone marrow and spleen-derived stromal cells: analysis of leukocyte marker and growth factor mRNA transcript levels. Blood. 1989;74:303–11. [PubMed] [Google Scholar]
- 23.Brouty-Boye D, Pottin-Clemenceau C, Doucet C, Jasmin C, Azzarone B. Chemokines and CD40 expression in human fibroblasts. Eur J Immunol. 2000;30:914–9. doi: 10.1002/1521-4141(200003)30:3<914::AID-IMMU914>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 24.Kalinski P, Schuitemaker JH, Hilkens CM, Kapsenberg ML. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J Immunol. 1998;161:2804–9. [PubMed] [Google Scholar]
- 25.RieSer C, Bock G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–8. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McRae BL, Nagai T, Semnani RT, van Seventer JM, van Seventer GA. Interferon-alpha and -beta inhibit the in vitro differentiation of immunocompetent human dendritic cells from CD14 (+) precursors. Blood. 2000;96:210–7. [PubMed] [Google Scholar]
- 27.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–88. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–53. [PubMed] [Google Scholar]
- 29.Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961–9. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- 30.Braun MC, Lahey E, Kelsall BL. Selective suppression of IL-12 production by chemoattractants. J Immunol. 2000;164:3009–17. doi: 10.4049/jimmunol.164.6.3009. [DOI] [PubMed] [Google Scholar]
- 31.Snijders A, Hilkens CM, van der Pouw Kraan TC, Engel M, Aarden LA, Kapsenberg ML. Regulation of bioactive IL-12 production in lipopolysaccharide- stimulated human monocytes is determined by the expression of the p35 subunit. J Immunol. 1996;156:1207–12. [PubMed] [Google Scholar]