Abstract
A diverse range of infectious organisms, including mycobacteria, have been reported to induce cell death in vivo and in vitro. Although morphological features of apoptosis have been identified in leprosy lesions, it has not yet been determined whether Mycobacterium leprae modulates programmed cell death. For that purpose, peripheral blood mononuclear cells obtained from leprosy patients were stimulated with different concentrations of this pathogen. Following analysis by flow cytometry on 7AAD/CD14+ cells, it was observed that M. leprae induced apoptosis of monocyte-derived macrophages in a dose-dependent manner in both leprosy patients and healthy individuals, but still with lower efficiency as compared to M. tuberculosis. Expression of tumour necrosis factor-α (TNF-α), Bax-α, Bak mRNA and TNF-α protein was also detected in these cultures; in addition, an enhancement in the rate of apoptotic cells (and of TNF-α release) was noted when interferon-γ was added to the wells. On the other hand, incubation of the cells with pentoxifylline impaired mycobacterium-induced cell death, the secretion of TNF-α, and gene expression in vitro. In addition, diminished bacterial entry decreased both TNF-α levels and the death of CD14+ cells, albeit to a different extent. When investigating leprosy reactions, an enhanced rate of spontaneous apoptosis was detected as compared to the unreactive lepromatous patients. The results demonstrated that M. leprae can lead to apoptosis of macrophages through a mechanism that could be at least partially related to the expression of pro-apoptotic members of the Bcl-2 protein family and of TNF-α. Moreover, while phagocytosis may be necessary, it seems not to be crucial to the induction of cell death by the mycobacteria.
Introduction
Apoptosis is a genetically regulated form of cell death that plays an essential role in a variety of biological events, such as morphogenesis, and in pathologies like cancer, human immunondeficiency virus (HIV) infection, and others. Proteins from the Bcl-2 family are capable of determining the life or death of a cell by controlling the release of mitochondrial apoptogenic factors. This family consists of anti-apoptotic members, such as Bcl-2 itself and Bcl-xL, and pro-apoptotic members, such as Bad, Bax and Bak.1 The activity of these proteins appears to be regulated, at least in part, by the formation of homo- and heterocomplexes. It has been shown that, in a number of systems, the Bcl-2 : Bax ratio dictates cell susceptibility to apoptosis.2
The monocyte/macrophage lineage consists of effector cells involved in controlling mycobacterial infection. It has been reported that monocytes are highly susceptible to apoptosis upon culture, and that lipopolysaccharide (LPS) can prolong cell survival by preventing caspase 3 activation.3 On the other hand, an enhanced rate of macrophage apoptosis has been observed after infection with a number of pathogens, including Salmonella,4Shigella5 and mycobacteria, such as M. tuberculosis (MTB)6,7M. avium8 and M. bovis bacillus Calmette–Guèrin (BCG).9 Depending on the infectious agent, phagocytosis has been described as being intimately related to the induction of cell death.5,10 Even so, the role played by host cell apoptosis in the outcome of mycobacterial infection is still not well understood. In human monocytes, however, it has been shown to limit the growth of BCG11 and MTB12 and therefore could function as a defence mechanism of the host. Mycobacterium-induced macrophage apoptosis seems to be under the control of cytokines, and it has also been demonstrated to down-regulate Bcl-2 in vitro in mononuclear cells.9 Accordingly, tumour necrosis factor-α (TNF-α), which is essential for protection against mycobacteria13 appears to be involved in the induction of cell death both in vivo and in vitro.6 Moreover, interleukin-10 (IL-10) has been suggested to attenuate apoptosis by counteracting the TNF-α function.14
Leprosy is a chronic infectious disease encompassing a spectrum of clinical forms related to each patient's immune response. The presence of cell-mediated immunity (CMI) associated with T helper type 1 (Th1) cytokines [IL-2, interferon-γ (IFN-γ)] production has been observed in tuberculoid leprosy (BT/TT), in which disease is localized with few bacilli in the lesions, in contrast to the lepromatous pole (LL). In addition, acute inflammatory reactional episodes (called type I or reversal reaction, RR, and type II or erythema nodosum leprosum, ENL) may also occur during the natural course of the disease. It is widely accepted that TNF-α has a pivotal role in the pathogenesis of these inflammatory episodes and in the development of tissue and nerve damage in leprosy.15,16 Cree et al.17 for example, have demonstrated that morphological changes consistent with apoptosis have taken place in the inflammatory infiltrate of leprosy patients as well as of patients with tuberculosis and sarcoidosis. More recently, the mononuclear blood cells of leprosy patients have been described to exhibit elevated spontaneous apoptosis in vitro as compared to controls.18
Although mycobacteria have been reported as inducing apoptosis, the influence of M. leprae (the aetiological agent of leprosy) is still unclear. It has been hypothesized that, in leprosy, an enhanced rate of macrophage cell death parallels the induction of reactions and an enhanced production of TNF-α. It is demonstrated herein that M. leprae induced apoptosis in monocyte-derived macrophages in a dose-dependent manner while maintaining a low efficiency rate as compared to MTB. An increased production of TNF-α in such cultures, the expression of pro-apoptotic members of the Bcl-2 family and a relationship with bacterial phagocytosis is also presented.
Materials and methods
Studied population
A total of 20 leprosy patients in attendance at the Leprosy Out-Patient Unit, Leprosy Laboratory, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil, were enrolled in the study. Patients [eight lepromatous (LL) and 12 borderline lepromatous (BL)] were diagnosed according to the Ridley and Jopling classification19 and were treated throughout the study with multi-drug therapy (MDT) as recommended by the World Health Organization. All patients (16 males and four females) were multibacillary (systemic bacterial load, Bacillary index (BI) > 0) with a mean age (± SD) of 41 ± 20·7. Five patients experienced a type II reaction or ENL during the course of MDT. After their informed consent, venous blood was withdrawn from all individuals and the assays were performed as described below.
Reagents and antigens
LPS from Salmonella minnesota Re 595, pentoxifylline (PTX), cytochalasin D and diacetate fluorescein were purchased from Sigma Chemical Co. (St Louis, MO). RPMI-1640 medium, fetal calf serum (FCS), antibiotics and l-glutamine were obtained from Gibco BRL (Gibco Laboratories, Gaithersburg, MD). Recombinant human interferon gamma (rIFN-γ) was obtained from Calbiochem (San Diego, CA). Irradiated armadillo-derived whole M. leprae (2 × 109 bacteria/mg) was kindly provided by Dr P. Brennan (Department of Microbiology, Colorado State University, Fort Collins, CO) and BCG was provided by Dr M. C. Pessolani (Leprosy Laboratory, FIOCRUZ). MTB (1 × 108 bacteria/ml), H37Rv, gamma-irradiated, was kindly donated by Dr R. J. Shattock (Division of Infectious Diseases, St George's Hospital Medical School, London, UK). All reagents and media used for the in vitro cultures were shown to contain < 0·1 U/ml endotoxin as measured using the Limulus amoebocyte lysate assay (Whittaker Bioproducts, Walkersville, MD).
Cell isolation and culture condition
Heparinized venous blood was withdrawn from all individuals and peripheral blood mononuclear cells (PBMC) were isolated under endotoxin-free conditions by Ficoll–Hypaque (Pharmacia Fine Chemicals, Piscataway, NJ) density centrifugation. Cells were washed three times in phosphate-buffered saline (PBS; Gibco) and viability was estimated by trypan blue dye exclusion. PBMC were immediately suspended at 1 × 106 cells/ml in RPMI-1640 medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mm l-glutamine, 10% FCS (complete medium), and subsequently cultured (1 ml) in Teflon beakers (Thomas Scientific, Swedesboro, NJ), at 37°. For in vitro stimulation, M. leprae was added to the cultures at 1, 10 and 20 μg/ml (2, 20 and 40 bacteria : cell ratio, respectively). Live BCG and H37Rv MTB were used at a 1 : 1 or 10 : 1 bacteria per cell, and LPS at 1 μg/ml. In some experiments, either 5 μg/ml cytochalasin D, 100 U/ml rIFN-γ, or 25 μg/ml PTX was added to the cultures 1 hr prior to mycobacterial stimulation. For evaluation of cytokine secretion, supernatants were harvested after 2 days of culture and stored at − 20° until further use.
Cell morphology and viability
For morphological evaluation, PBMC (1 × 106), cultured onto coverslips in 24-well plates (Costar Corporation, Cambridge, MA), in the presence or absence of M. leprae (20 μg/ml) or LPS, were harvested after different periods, washed three times with PBS, fixed with methanol, and stained with May–Grünwald–Giemsa stain (Merck, Rahway, NJ). For evaluation of viability, cells were washed, incubated with ethidium bromide (4 μg/ml; Sigma) and diacetate fluorescein (20 μg/ml) for 20 min at room temperature. Thereafter, the samples were applied onto glass slides for examination by fluorescence microscopy (Nikon Microphot System; Nikon Corp., Melville, NY). A total of 100 cells were counted on each slide and scored as live (green staining) or dead (red staining).
Cytokine determination
Concentration of TNF-α in cell-free culture supernatants was determined by using a commercial specific enzyme-linked immunosorbent assay (ELISA), using specific pairs of monoclonal antibodies (R&D Systems Inc., Minneapolis, MN) and processed according to the manufacturer's specifications. Cytokine levels are expressed as pg/ml of protein. The detection limit of the assay was 4 pg/ml.
7-Amino actinomycin D (7-AAD) staining
PBMC, cultured in the Teflon beakers for 2 days in the presence or absence of the bacteria, were washed in PBS containing 3% FCS and 0·1% sodium azide, and incubated with anti-CD14 monoclonal antibody (Becton & Dickison, San Jose, CA) for 30 min at 4°. After additional washing, the cells were incubated for 40 min with 10 μg/ml 7-AAD (Sigma) at 4° in the dark. Samples stained with 7-AAD and the surface marker were analysed by an EPICS XL-MCL Flow Cytometer with a single argon laser at 488 nm (Coulter Corp., Hialeah, FL). Orange fluorescence was measured with a 575-nm filter and the red fluorescence from 7-AAD was filtered through a 670-nm long pass filter. More than 10 000 events were analysed for each sample. Cells without 7-AAD labelling were considered viable, while apototic and dead cells showed low and high 7-AAD staining, respectively.20 In order to evaluate spontaneous apoptosis, freshly isolated unstimulated and non-cultured PBMC were directly stained with 7-AAD as described above.
DNA electrophoresis
A total of 5 × 106 PBMC cultured as above were harvested, washed in PBS, and lysed with 0·2% Triton X-100 in TE [10 mm Tris–HCl (pH 7·4) 1 mm ethylenediaminetetraacetic acid] buffer. Following centrifugation, DNA was extracted with phenol/chloroform/isoamyl alcohol. After precipitation with isopropanol in the presence of 0·5 m NaCl, it was washed in 70% ethanol, air-dried, and suspended in TE buffer. DNA was then subjected to electrophoresis in 1% agarose gel followed by ethidium bromide (1 μg/ml) staining and visualized by UV transillumination.
RNA isolation and cDNA synthesis
For analysis of mRNA expression, 2 × 106 PBMC suspended in complete RPMI medium were stimulated with M. leprae and/or PTX and cultured in the Teflon beakers for 20 hr, after which time the supernatants were harvested, the cells were immediately suspended in 1 ml Trizol™ (Gibco BRL), and RNA was extracted according to the manufacturer's instructions. One microgramme of total RNA was reverse transcribed into cDNA21 and samples were stored at − 20° until further use.
Polymerase chain reaction (PCR) condition
Cytokine-specific oligonucleotide primer pair sequences for GAPDH (5′-CCACCCATGGCAAATTCCATGGCA-3′ and 5′-TCTAGACGGCAGGTCAGGTCCACC-3′) and TNF-α primers22 were obtained at the GENEBANK and synthesized (Gibco BRL). Bax-α (5′-GTTTCATCCAGGATCGAGCAG-3′ and 5′-CTTCCAGATGGTGAGCGAGG-3′) and Bak (5′-GCCCA GGACACAGAGGAGGTTTTC-3′ and 5′-AAACTGGCCCAACAGAACCACACC-3′) primers were purchased from R & D Systems. The primers were RNA specific in that they were designed within different exons spaced by a long intron. PCR reaction in a total volume of 25 μl was performed as detailed elsewhere21 and the samples were amplified in a DNA thermocycler 480 (Perkin Elmer Cetus, Emeryville, CA) for 25 (GAPDH), 27 (TNF-α), and 35 (Bax and Bak) cycles of denaturation at 94° for 45 seconds, annealing at 60° for 45 seconds, and extension at 72° for 90 seconds. The PCR amplified products were resolved by electrophoresis on 1·7% agarose gels and visualized by ethidium bromide staining. Specificity of the amplified bands was validated by their predicted size [GAPDH, 600 base pairs (bp); TNF-α, 355 bp; Bax-α, 482 bp; Bak, 528 bp]. Densitometer analysis (Video Documenting System; Amersham-Pharmacia, Piscataway, NJ) was assessed directly on the PCR products on the agarose gels. Amplification of GAPDH was used as an internal control. Each experiment included a negative control to which no cDNA was added.
Statistical analysis
Results are reported as pooled data from the entire series of experiments and for each group of individuals, and described as mean ± SEM. For data comparison, the Kruskal–Wallis test (Statsoft Inc., 1995; STATISTICA for Windows, Computer Program Manual, Tulsa OK) was used. The statistical significance level adopted was P < 0·05.
Results
M. leprae induces cell death in human PBMC in vitro
Mononuclear cells (PBMC) isolated from lepromatous leprosy patients and cultured for 1–3 days in the presence or absence M. leprae (20 μg/ml) were stained with Wright–Giemsa stain. The adherent cells had an altered morphology, with decreased or vacuolated cytoplasm and densely condensed nuclei. These features were not observed when cells were stimulated with LPS (1 μg/ml). For further quantification of the amount of dead cells, PBMC stimulated under the same conditions were harvested and stained with ethidium bromide and fluorescein diacetate. A reduction in cell viability was observed throughout the culture period, when the rate of viable cells (90%), detected in the non-stimulated cultures after 2–3 days, was reduced to 58% on average, when M. leprae, but not LPS (not shown), was added to the wells. To confirm that the morphological changes observed here were related to the occurrence of apoptosis, the monocyte/macrophage population was assessed by flow cytometry following double staining of the PBMC with 7-AAD and anti-CD14 monoclonal antibody.
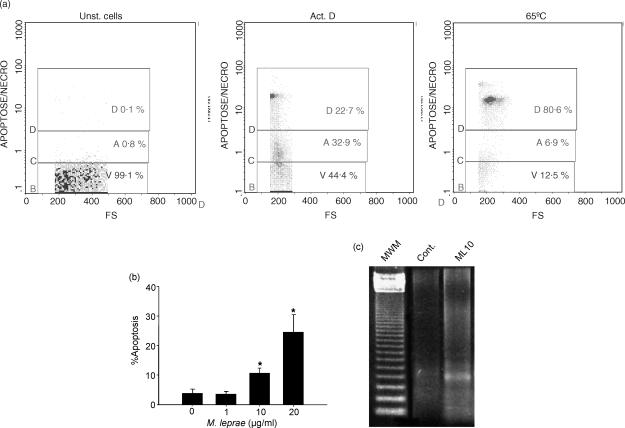
Standardization of the 7-AAD method was established by using PBMC either heated for 10 min at 65°, to induce cell death, or cultured in the presence of actinomycin D (ActD, 5 μg/ml), known to induce macrophage apoptosis. Figure 1(a) shows one representative experiment in which the fluorescence intensity of 7-AAD versus forward scatter was used to define the dead cell population (D, induced by heating), and the early apoptotic cells (A, induced by treatment with ActD). Untreated cells were used as controls. Negligible 7-AAD incorporation was seen in the live cells; V, viable), while 7-AADdim was observed in the early apoptotic cells (A) and 7-AADbright in the dead cells (D; necrosis).
Figure 1.
Mycobacterium leprae induces cell death in monocyte-derived macrophages of leprosy patients. (a) Flow cytometry histograms demonstrate standardization of 7-AAD staining from one representative experiment. Unstimulated PBMC (Unst. cells, left panel), PBMC stimulated with Act.D for 20 hr (middle panel), or heated at 65° for 10 min (right panel) were used to set the gates for viable (V), apoptotic (A, 7-AADdim staining), and dead cells (D, 7-AADbright), respectively. Negligible 7-AAD incorporation was seen in the live cells. (b) Evaluation of apoptosis was assessed by flow cytometry after dual staining with 7-AAD and anti-CD14 antibody in cultures stimulated or not with M. leprae 1, 10, or 20 μg/ml for 2 days. Values represent mean percentage apoptosis ± SEM of five different experiments. *Significant differences (P < 0·05) when compared to unstimulated cultures or cultures stimulated with M. leprae 1 μg/ml. (c) Agarose gel electrophoresis showing fragmented DNA in cultures stimulated or not (Cont) with M. leprae 10 μg/ml. The 123 bp DNA ladder was used as the standard molecular weight marker (MWM).
After adding M. leprae to the cultures, it was shown that mycobacterium-induced apoptosis occurred in a dose-dependent manner. In the non-stimulated wells, 5·3 ± 2·4% (mean ± SEM) apoptotic CD14+ cells were detected after 2 days; when stimulated with 1 μg/ml M. leprae (2 : 1 bacteria : cell ratio), 3·6 ± 0·5% of cells were apoptotic, whereas with 10 μg/ml or 20 μg/ml of this pathogen, it was 10·7 ± 1·4% and 24·6 ± 5·8%, respectively (Fig. 1b; P = 0·04). Similar data were observed when cells from normal healthy individuals (n = 2) were assayed (rate of apoptosis in the non-stimulated wells was 3·4 ± 1% and in response to M. leprae 20 μg/ml = 18·6 ± 3·4%). In addition, fragmented DNA, a hallmark of apoptosis, was observed when cultures were analysed by agarose gel electrophoresis (Fig. 1c).
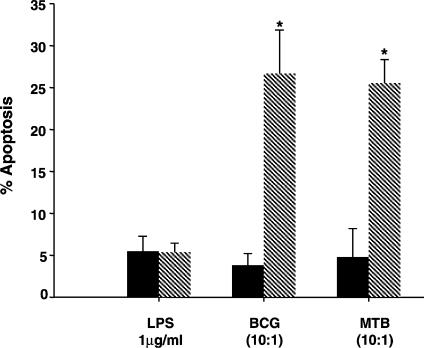
In contrast, cells stimulated with LPS, although presenting high TNF-α levels in culture supernatants (mean TNF-α in unstimulated wells 11·5 ± 6·2 pg/ml, and in response to LPS 2928 ± 480 pg/ml, P = 0·003), they showed 5·4 ± 1·1% apoptotic macrophages as compared to 5·5 ± 1·8% apoptosis detected in the unstimulated cultures, confirming that LPS indeed did not induce macrophage apoptosis in the present conditions (Fig. 2). PBMC were also stimulated either with BCG or M. tuberculosis H37Rv, which had been previously reported to induce cell death both in vivo and in vitro. When live BCG (10 : 1 bacteria : cell ratio) was added to the wells, 26·7 ± 5·2% apoptotic CD14+ cells were noted as compared to the 3·8 ± 1·4% found in the control cultures (Fig. 2; P < 0·05), and to the 5·4 ± 2·2% when cells were stimulated with dead BCG. After the addition of MTB (10 : 1) an average of 25·5 ± 2·8% apoptotic cells was detected as opposed to 4·8 ± 3·4% in the unstimulated wells, and 5·5 ± 2·8% when MTB 1 : 1 was employed.
Figure 2.
BCG and MTB, but not LPS, induce apoptosis in macrophages from leprosy patients in vitro. The rate of apoptosis (%) was assessed by flow cytometry in CD14+ cells following staining of PBMC cultures with 7-AAD and anti-CD14 antibody. Cells were stimulated (hatched bars) or not (solid bars) either with LPS 1 μg/ml, or live BCG, or heat-killed MTB (10 : 1 bacteria : cell ratio), as indicated in the figure. Results represent mean ± SEM of five individual experiments. *Significant differences (P < 0·05) when compared to the unstimulated cultures.
Pre-incubation of monocytes with IFN-γ or pentoxifylline modulates M. leprae-induced apoptosis in vitro
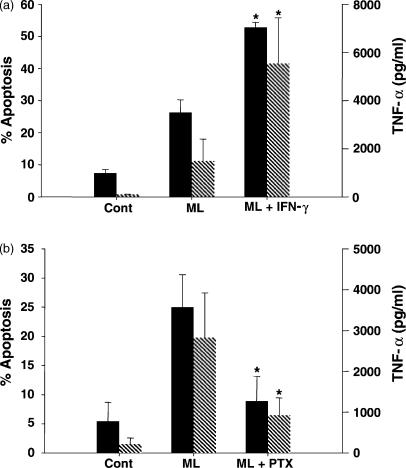
In order to determine whether, IFN-γ, a cytokine known to enhance the microbial activity of macrophages, interferes with macrophage apoptosis, the cytokine was added to the cultures 1 hr prior to M. leprae stimulation. The rate of CD14+ apoptotic cells in the stimulated PBMC (26·3 ± 3·9%) was enhanced to 52·8 ± 1·6% in the presence of IFN-γ (Fig. 3a; P < 0·03). No major effect was observed in the rate of apoptosis (3 ± 1%) when unstimulated cells were treated with IFN-γ alone. As aforementioned23 IFN-γ also increased M. leprae-induced TNF-α release (Fig. 3a); TNF-α values were raised from 1494 ± 908 pg/ml to 5541 ± 1897 pg/ml when cells received M. leprae and IFN-γ (P = 0·012). No enhancement in TNF-α was induced by IFN-γ itself (not shown).
Figure 3.
Modulation of Mycobacterium leprae-induced apoptosis and TNF-α levels in monocyte-derived macrophages obtained from leprosy patients in vitro. (a) Flow cytometry analysis (%) of apoptosis (solid bars) was evaluated in the PBMC cultured for 2 days and stimulated or not (Cont) with M. leprae (ML) 20 μg/ml, in the presence or absence of IFN-γ 100 U/ml (a), after dual staining with 7-AAD and anti-CD14 antibody. The rate of cell death and TNF-α values (hatched bars), measured in these same culture supernatants by ELISA, showed (*) significant differences when IFN-γ was added to the wells (P < 0·05). Results are presented as mean ± SEM of five and three individual experiments, respectively. (b) Following stimulation of the PBMC with M. leprae in the presence or absence of PTX, apoptosis and TNF-α release were evaluated as described. *Significant differences when compared to the M. leprae-stimulated cultures. Results are mean ± SEM of four different experiments. Background values for apoptosis and TNF-α detected in the PBMC cultured with PTX alone were 7·4 ± 5·5% and 85·7 ± 25·8 pg/ml.
In another set of experiments, PTX, a drug known to ameliorate the inflammatory manifestations of ENL and to reduce TNF-α synthesis,21,24 was added to the wells. Bacterium-induced apoptosis of CD14+ cells was 22·1 ± 6·6% as compared to the 8·9 ± 4·2% detected in the PBMC stimulated in the presence of PTX (Fig. 3b; P = 0·02). Accordingly, determination of TNF-α in the stimulated wells (2826 ± 1098 pg/ml) was significantly reduced (P = 0·04) in the presence of the drug (926 ± 424 pg/ml). PTX had no effect on the apoptosis or on TNF-α levels when added alone to the cultures. Mean percentage inhibition of PTX on M. leprae-induced apoptosis and TNF-α synthesis was 53·8 ± 6·9% and 70 ± 10·4%, respectively.
M. leprae-induced apoptosis in monocytes is dependent on bacterial phagocytosis
To determine whether entry of the bacteria into the cell was a required step in the induction of apoptosis in this system, cells were pretreated with cytochalasin D, an actin polymerization inhibitor, and then infected. The uptake of M. leprae by the monocytes was monitored by fluorescent microscopy. Cytochalasin D inhibited > 95% of M. leprae infection (not shown) and also reduced apoptosis of CD14+ cells (mean percentage reduction 49·4 ± 4·3%), suggesting that phagocytosis may play a role in the induction of apoptosis by the mycobacterium (Table 1). In addition, TNF-α production induced by M. leprae (935 ± 213 pg/ml) was significantly affected (P = 0·025) by treatment of the cells with cytochalasin D (156 ± 30 pg/ml; % inhibition 80·8 ± 4·3%). By itself, cytochalasin D had no effect either on the rate of apoptosis or on the amount of TNF-α detected in vitro (Table 1).
Table 1.
Effect of cytochalasin D treatment on apoptosis and TNF-α secretion induced by Mycobacterium leprae in vitro
| Culture conditions | % Apoptosis | TNF-α (pg/ml) |
|---|---|---|
| Unstimulated PBMC | 2·9 ± 1·9 | 7·9 ± 6·5 |
| M. leprae (20 μg/ml) | 22·4 ± 11·3 | 1000 ± 176·3 |
| Cytochalasin D (1 μm) | 5·0 ± 0·5 | 8·2 ± 4·6 |
| M. leprae + cytochalasin D | 10·8 ± 4·8 | 177·5 ± 17* |
PBMC (1 × 106) were cultured as described, when cytochalasin D was added to the wells 1 hr prior to M. leprae stimulation. After 2 days of culture, the rate of apoptosis and levels of TNF-α were determined. Values represent mean ± SEM of three individuals experiments.
Significant differences when compared to the M. leprae-stimulated cultures (P = 0·025).
M. leprae induces the expression of Bax and Bak genes in the PBMC cultures
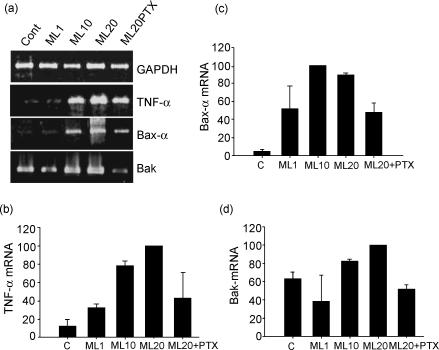
In order to investigate whether M. leprae up-regulates the expression of pro-apoptotic genes of the Bcl-2 family, mRNA expression of Bax-α, Bak, and TNF-α was assessed in the stimulated (1, 10, or 20 μg/ml M. leprae) PBMC cultures by reverse transcription (RT) -PCR. Figure 4(a) shows that, after 20 hr, the mycobacterium induced TNF-α and Bax-α mRNA in a dose-dependent manner. A densitometric analysis of the bands in the gels (Fig. 4b,c), showed enhanced mRNA stimulation (TNF-α and Bax-α) following M. leprae 10 or 20 μg/ml. Mean rate (± SEM) of gene expression for Bax-α was 100 and 89·4 ± 1·2%, respectively. Evaluation of Bak (Fig. 4d) showed induction of message above the background that was more pronounced with M. leprae 20 μg/ml. A correlation between gene expression and functional data was also observed in the cultures treated with PTX. No effect of PTX itself was observed in the overall gene expression in the PBMC cultures in vitro. Along with the inhibition of TNF-α secretion and apoptosis (Fig. 3b), a diminished expression of Bax-α/Bak and TNF-α genes was noted in the M. leprae-stimulated wells (Fig. 4). Relative amounts of message for Bak were 51·8 ± 4·5%. The average percentage reduction in the relative amount of mRNA was 72·5 ± 22·5% for TNF-α and 46 ± 12·1% for Bax-α. As related to Bak mRNA, the inhibitory effect of PTX was detected in three of the four patients tested.
Figure 4.
Mycobacterium leprae induces the expression of pro-apoptotic genes in PBMC cultures of leprosy patients. Expression of TNF-α, Bax-α and Bak mRNA was assayed in vitro by RT-PCR. (a) PBMC were stimulated or not with M. leprae 1, 10 and 20 μg/ml for 20 hr. Additionally, PBMC were cultured in the presence of M. leprae (20 μg/ml) and PTX (25 μg/ml), when RNA was extracted and processed as described in the Materials and methods. Following PCR amplification, specific bands were visualized in the agarose gels; transcripts were analysed by densitometric scanner and the relative amount of message was determined semi-quantitatively by using GAPDH as an internal reference. Expression of (b) TNF-α, (c) Bax-α mRNA, and to a lesser extent (d) Bak was increased with higher amounts of bacteria. On the other hand, incubation of cells with PTX decreased the amount of message for such genes. Results are percentage relative to the most intense band (assigned the value of 100). Data represent mean ± SEM of three different experiments.
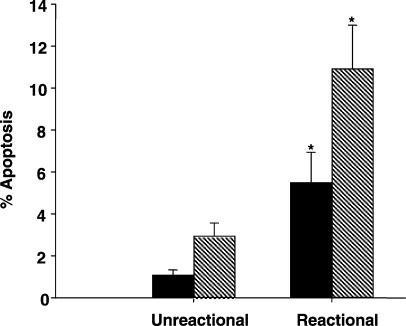
Patients undergoing leprosy reaction show enhanced spontaneous apoptosis
An important role for TNF-α and IFN-γ in the pathogenesis of reactions has been described.22,23 To determine whether monocytes are actively involved in the spontaneous (ex vivo) apoptosis described in leprosy18 six unreactive lepromatous and four reactional patients (ENL) were assayed in this study. The mean rate of spontaneous apoptosis (Fig. 5) evaluated in the PBMC (non-cultured and non-stimulated cells) of unreactive patients was 1·1 ± 0·2% as compared to 5·5 ± 1·4% for the ENL group (P = 0·02). When CD14+ cells were assessed within this population, a significant variation (P = 0·01) between the two groups of patients was also noted (2·9 ± 0·6% versus 10·9 ± 2·1%, respectively).
Figure 5.
Leprosy patients with reaction show enhanced spontaneous apoptosis ex vivo. Evaluation of spontaneous apoptosis was assayed in PBMC (solid bars) cultures and CD14+ cells (hatched bars) obtained from leprosy patients with (n = 4) or without (n = 6) reaction (ENL). The rate of apoptosis was evaluated through 7-AAD staining and percentage of apoptotic cells was determined by flow cytometry analysis. *Significant differences when compared to unreactional patients.
Discussion
The method of 7AAD staining was described initially by Schmidt et al.25 It is a sensitive method for quantification of cells undergoing apoptosis that permits the simultaneous evaluation of dual-colour cell surface immunofluorescence, so that apoptosis within subpopulations can be assessed; it also permits paraformaldehyde fixation of the stained samples. The use of the 7-AAD staining in T-cell lines20 was validated by cell sorting, followed by analysis with a variety of established methods, including TUNEL, DNA agarose electrophoresis, morphological assessment and time-lapse photography. 7-AAD was also shown26 to give similar results when compared to different DNA-binding dyes (propidium iodide, acridine orange and Hoechst 33342). Moreover, assessment of intracellular staining and detection of proteins, including cytokines and Bcl-2, in the apoptotic cells from a complex population, such as PBMC, has been performed also in HIV-infected donors.27,28
In the present study, apoptosis of macrophages was evaluated in cultured PBMC by dual staining of the cells with 7-AAD and anti-CD14 antibody. Although lymphocytes were the most frequently used cell population for this staining procedure, in our hands, the method was successfully used since it reproduced previously published results such as the non-apoptotic effect of LPS in human monocytes, as well as the pro-apoptotic effect of MTB and BCG. In addition, it was demonstrated herein that, in vitro, M. leprae induces cell death in monocyte-derived macrophages of leprosy patients in a dose-dependent manner. High levels of TNF-α were also released in these cultures, which correlated with previous results.29 Accordingly, the lower efficiency of M. leprae (40 : 1 bacteria per cell) to induce apoptosis when compared to MTB (10 : 1) seems to parallel its diminished ability to induce TNF-α.29
It has been widely reported that TNF-α is involved in mycobacterium-related cell death6,30 and that the addition of PTX or anti-TNF-α antibody reduces the apoptosis caused by M. tuberculosis6 and M. avium.31 In our own results, PTX decreased TNF-α mRNA, protein levels, and the rate of apoptosis in vitro (Fig. 3b). In addition, during the reactive states of leprosy, where high amounts of TNF-α and IFN-γ have been detected16,32 a higher rate of spontaneous apoptosis was observed when compared to the unreactive patients. Similarly, Gupta and co-workers18 have demonstrated that anti-TNF-α antibody could block apoptosis of leprosy patients' PBMC with or without reaction. Indeed, thalidomide, a drug used to treat ENL and known to inhibit TNF-α production by human monocytes,33 has also been seen to interfere with the M. leprae-induced cell death.34 Albeit, a similar rate of response has been detected in purified macrophage cultures (not shown), by using PBMC, the participation of additional pathways other than TNF-α in triggering the apoptosis phenomenon, such as the CD95 and CD95L, cannot be ruled out. A role for T cells in the amplification and/or induction of macrophage apoptosis in such cultures shall also be considered. In that sense, it was previously demonstrated that cell–cell contact (T cell–monocytes) can trigger higher rates of apoptosis in vitro.35
On the other hand, in cultures stimulated with LPS, in spite of exhibiting high TNF-α, it did not lead to apoptosis. It has been proposed that the activation process confers a survival signal essential for monocyte function.36 Perera and Waldmann have demonstrated that activation of monocytes, either with LPS or soluble lysates of M. leprae, imparts a selective desensitization to apoptosis signalling and that the expression of caspase 8 is sharply diminished. A dual capacity to induce both activation and apoptosis appears to be a common property of the TNF-R (TNF receptor) superfamily, as in the case of TNF-R1 and Fas.37 In some situations, activated monocytes can then synthesize copious quantities of TNF-α without any obvious self-injury, indicating that activation inhibits the assembly of the cell death machinery. Whether or not the two mechanisms are triggered together or separately by the bacterium remains unknown.
It has been suggested that apoptosis may in fact act as a mechanism for interdicting mycobacterial growth. BCG-infected macrophages have been shown to undergo apoptosis following addition of ATP, which resulted in the killing of intracellular bacteria.11 Likewise, addition of Fas ligand induced apoptosis of infected human macrophages and reduced the viability of intracellular MTB.12 On the other hand, Santucci et al.38 did not observe any differences in mycobacterial viability even when high macrophage mortality was detected. One cannot exclude the hypothesis that induction of apoptosis of M. leprae-infected cells can render the macrophage environment more hostile to the bacteria, limiting their replication. Nevertheless, lower concentrations (1 : 1) of MTB39 or BCG40 have been previously reported to prevent apoptosis in mononuclear phagocytes. Similar data were obtained in our system where low amounts of M. leprae did not induce significant levels of cell death. Such results raise the question of whether mycobacterial infection may lead to the selection of a population of cells able to harbour a large number of bacilli.
The pathogenesis of M. leprae infection is characterized, at one end of the spectrum, by impaired CMI. As such, the anergic scenario observed in lepromatous leprosy may suddenly be reversed during the course of the reactional episodes, where a shift of cytokine production towards a Th1 phenotype appears to take place.22,41 Hence, the reactivation of the immune response in conjunction with enhanced IFN-γ production may be directly correlated to macrophage activation and to the decreased bacterial load seen during reaction.42 In the current experiments, addition of IFN-γ to the PBMC cultures significantly increased the rate of M. leprae-induced apoptosis in vitro and of TNF release (Fig. 3). It is thus feasible to speculate that apoptosis may be contributing to the clearance of M. leprae during the reactional episodes. Similarly, addition of IFN-γ to Coxiella burnetii-infected THP-1 cells impaired bacterial survival and enhanced cell apoptosis.43 Both events were shown to be dependent on TNF-α.
A relationship between phagocytosis and apoptosis has also been suggested, as previously described for Shigella flexneri and Legionella pneumophilla.5,10 Inhibition of the entry of M. leprae into the cell impaired apoptosis of macrophages (Table 1) and also decreased TNF-α levels. Nevertheless, inhibition of apoptosis (49·4%) did not parallel the reduction in the rate of infection (> 95%) or the extent of inhibition on TNF-α release (80·8%). Alternatively, other cytokines and/or additional apoptotic signalling pathways may be operative. In this same vein, the hypothesis that soluble molecules from the bacteria might trigger apoptosis by way of different pathways than the one utilized by the intracellular mycobacteria, is of relevance.
It is known that mitochondria play an important role in the regulation of programmed cell death in that it might be determining the life or death of a cell by controlling the release of mitochondrial apoptogenic factors One of the unique features of such proteins from the Bcl-2 family is the heterodimerization between pro- and anti-apoptotic proteins. Bax has been shown to homodimerize as well as heterodimerize with Bcl-2. In excessive amounts, bax counteracts the ability of bcl-2 to repress cell death.2 When using RT-PCR, it was observed that M. leprae (10 or 20 μg/ml) increased the expression of both Bax-α and Bak (20 μg/ml) mRNA. Moreover, inhibition of TNF-α parallels the decreased expression of such genes and impairment in the apoptosis phenomenon. These results suggest that M. leprae is capable of activating gene expression of the pro-apoptotic members of the Bcl-2 family, which, in turn, can lead to the mitochondrial release of pro-apoptotic factors by way of a mechanism that may be at least partially dependent on TNF-α.
In summary, the present study demonstrates an involvement of M. leprae in the induction of monocyte-derived macrophage apoptosis in vitro. It is then feasible to envisage a scenario where increased production of IFN-γ and TNF-α observed in vivo could modulate enhanced apoptosis activity induced by the mycobacterium. The role of programmed cell death in the pathogenesis of leprosy reactions can contribute to the resolution of the inflammatory lesions as well as to reduction in the bacterial load. Therefore, the diverse profiles characteristic of the immune response and the cytokines produced throughout the spectrum of the clinical forms of the disease most likely directly influence the rate of apoptosis in vivo.
Acknowledgments
We would like to thank Drs J. A. C. Nery and A. M. Sales as the attending physicians of the Leprosy Out-Patient Unit, FIOCRUZ, and Dr A. Miranda for the histological evaluation of the patients' biopsies. Thanks are also due to J. Grevan for English revision of the text, and to D. M. Vieira and E. R. Leite for efficient technical assistance. This work was supported by the World Health Organization, TDR, UNDP/WORLD BANK/WHO, grant ID 970063. M. O. Hernandez, D. S. Carvalho and J. S. Sales were supported by CNPq, Brazil.
References
- 1.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–6. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Oitval ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homologue, Bax, that accelerates programmed cell death. Cell. 1993;74:609–19. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 3.Fahy RJ, Doseff AI, Wewers MD. Spontaneous human monocytes apoptosis utilizes a caspase-3-dependent pathway that is blocked by endotoxin and is independent of caspase-1. J Immunol. 1999;163:1755–62. [PubMed] [Google Scholar]
- 4.Arai T, Hiromatsu K, Nishimura H, Kimura Y, Kobayashi N, Ishida H, Nimura Y, Yoshikai Y. Endogenous interleukin 10 prevents apoptosis in macrophages during Salmonella infection. Bioch Biophys Res Comm. 1995;213:600–7. doi: 10.1006/bbrc.1995.2174. [DOI] [PubMed] [Google Scholar]
- 5.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–9. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 6.Keane J, Balcewicz-Sablinska MK, Remond HG, Chupp G, Meek BB, Fenton MJ, Kornfeld H. Infection by Mycobacterium tuberculosis promotes human alveolar macrophages apoptosis. Infect Immun. 1997;65:298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plácido R, Mancino G, Amendola A, et al. Apoptosis of human monocytes/macrophages in Mycobacterium tuberculosis infection. J Pathol. 1997;181:31–8. doi: 10.1002/(SICI)1096-9896(199701)181:1<31::AID-PATH722>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Gan H, Newman GW, Remond HG. Plasminogen activator inhibitor type 2 prevents programmed cell death of human macrophages infected with Mycobacterium avium, serovar 4. J Immunol. 1995;155:1304–15. [PubMed] [Google Scholar]
- 9.Klingler K, Tchou-Wong KM, Brandli O, Aston C, Kim R, Chi C, Rom WN. Effects of mycobacteria on regulation of apoptosis in mononuclear phagocytes. Infect Immun. 1997;65:5272–8. doi: 10.1128/iai.65.12.5272-5278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hägele S, Hacker J, Brand BC. Legionella pneumophila kills human phagocytes but not protozoan host cells by inducing apoptotic cell death. FMES Microb Lett. 1998;169:51–8. doi: 10.1111/j.1574-6968.1998.tb13298.x. [DOI] [PubMed] [Google Scholar]
- 11.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular Bacillus Calmette-Guérin. J Exp Med. 1994;180:1499–509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oddo M, Renno T, Attinger A, Bakker T, Macdonald HR, Meylan PRA. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J Immunol. 1998;160:5448–54. [PubMed] [Google Scholar]
- 13.Keane J, Gershon S, Wise P, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Miles Braun M. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N Engl J Med. 2001;345:1098–103. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 14.Balcewicz-Sablinska MK, Gan H, Remold HG. Interleukin 10 produced by macrophages inoculated with Mycobacterium avium attenuates mycobacteria-induced apoptosis by reduction of TNFα activity. J Infect Dis. 1999;180:1230–7. doi: 10.1086/315011. [DOI] [PubMed] [Google Scholar]
- 15.Sarno EN, Sampaio EP. The role of inflammatory cytokines in tissue injury of leprosy. Int J Lepr. 1996;64:S69–S74. [PubMed] [Google Scholar]
- 16.Khanolkar-Young S, Rayment N, Brickell PM, Katz DR, Vinayakumar S, Colston MJ, Lockwood DNJ. Tumor necrosis factor-alpha (TNFα) synthesis is associated with the skin and peripheral nerve pathology of leprosy reversal reaction. Clin Exp Immunol. 1995;99:196–202. doi: 10.1111/j.1365-2249.1995.tb05532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cree IA, Nurbhai S, Milne G, Beck JS. Cell death in granulomata. The role of apoptosis. J Clin Pathol. 1987;40:1314–19. doi: 10.1136/jcp.40.11.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta A, Sharma VK, Vohra H, Ganguly NK. Inhibition of apoptosis by ionomicin and zinc in peripheral blood mononuclear cells (PBMC) of leprosy patients. Clin Exp Immunol. 1999;117:56–62. doi: 10.1046/j.1365-2249.1999.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridley DS, Jopling WH. Classification of leprosy according to immunity: a five group system. Int J Lepr. 1966;34:255–73. [PubMed] [Google Scholar]
- 20.Philpott NJ, Turner AJC, Scopes J, Westby M, Marsh JCW, Gordon-Smith EC, Dalgleish AG, Gibson FM. The use of 7-amino actinomycin D in identifying apoptosis: simplicity of use and broad spectrum of application compared with other techniques. Blood. 1996;87:2244–51. [PubMed] [Google Scholar]
- 21.Sampaio EP, Moraes MO, Nery JAC, Santos AR, Matos HC, Sarno EN. Pentoxifylline decreases in vivo and in vitro tumor necrosis factor-α (TNF-α) production in lepromatous leprosy patients with erythema nodosum leprosum. Clin Exp Immunol. 1998;111:300–8. doi: 10.1046/j.1365-2249.1998.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moraes MO, Sarno EN, Almeida AS, Saraiva BCC, Martins RC, Nery JAC, Sampaio EP. Cytokine mRNA expression in leprosy reaction. A possible role for IFNγ and IL-12 in reactions (RR and ENL) Scand J Immunol. 1999;50:541–9. doi: 10.1046/j.1365-3083.1999.00622.x. [DOI] [PubMed] [Google Scholar]
- 23.Sampaio EP, Moreira AL, Sarno EN, Malta AM, Kaplan G. Prolonged treatment with recombinant interferon γ induces erythema nodosum leprosum in lepromatous leprosy patients. J Exp Med. 1992;175:1729–37. doi: 10.1084/jem.175.6.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doherty GM, Jensen JC, Alexander HR, Buresh CM, Norton JA. Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery. 1991;110:192–8. [PubMed] [Google Scholar]
- 25.Schmid I, Uittenbogaart CH, Keld B, Giorgi JV. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J Immunol Meth. 1994;170:145–57. doi: 10.1016/0022-1759(94)90390-5. [DOI] [PubMed] [Google Scholar]
- 26.Telford WG, King LE, Fraker PJ. Comparative evaluation of several DNA binding dyes in the detection of apoptosis-associated chromatin degradation by flow cytometry. Cytometry. 1992;13:137–43. doi: 10.1002/cyto.990130205. [DOI] [PubMed] [Google Scholar]
- 27.Ledru E, Lecoeur H, Garcia S, Debord T, Gougeon ML. Differential susceptibility to activation-induced apoptosis among peripheral Th1 subsets: correlation with Bcl-2 expression and consequences for AIDS pathogenesis. J Immunol. 1998;160:3194–206. [PubMed] [Google Scholar]
- 28.Lecoeur H, Ledru E, Gougeon ML. A cytofluorometric method for the simultaneous detection of both intracellular and surface antigens of apoptotic peripheral lymphocytes. J Immunol Meth. 1998;217:11–26. doi: 10.1016/s0022-1759(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 29.Sampaio EP, Oliveira RB, Warwick-Davies J, Faria Neto RB, Griffin GE, Shattock RJ. T cell-monocyte contact enhances tumor necrosis factor-alpha production in response to Mycobacterium leprae. J Infect Dis. 2000;182:1463–72. doi: 10.1086/315902. [DOI] [PubMed] [Google Scholar]
- 30.Balcewicz-Sablinska MK, Keane J, Kornfeld H, Remond HG. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2 resulting in inactivation of TNFα. J Immunol. 1998;161:2636–41. [PubMed] [Google Scholar]
- 31.Bermudez LE, Parker A, Goodman JR. Growth within macrophages increases the efficiency of Mycobacterium avium in invading other macrophages by a complement receptor-independent pathway. Infect Immun. 1997;65:1916–25. doi: 10.1128/iai.65.5.1916-1925.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarno EN, Grau GE, Vieira LM, Nery JAC. Serum levels of tumor necrosis factor-alpha and interleukin 1β during leprosy reactional states. Clin Exp Immunol. 1991;84:103–8. [PMC free article] [PubMed] [Google Scholar]
- 33.Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor α production by stimulated human monocytes. J Exp Med. 1991;173:699–703. doi: 10.1084/jem.173.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sampaio EP, Hernandez MO, Carvalho DS, Sarno EN. Management of erythema nodosum leprosum by thalidomide: thalidomide analogues inhibit M. leprae-induced TNFα production in vitro. Biomed Pharmacother. 2002;56:13–19. doi: 10.1016/s0753-3322(01)00147-0. [DOI] [PubMed] [Google Scholar]
- 35.Richardson B, Buckmaster T, Kern DF, Johnson KJ. Evidence that macrophages are programmed to die after activating autologous, cloned, antigen-specific, CD4+ T cells. Eur J Immunol. 1993;23:1450–5. doi: 10.1002/eji.1830230708. [DOI] [PubMed] [Google Scholar]
- 36.Perera LP, Waldmann TA. Activation of human monocytes induces differential resistance to apoptosis with rapid down regulation of caspase-8/FLICE. Proc Natl Acad Sci USA. 1998;95:14308–13. doi: 10.1073/pnas.95.24.14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pimentel-Muinos FX, Seed B. Regulated commitment of TNF receptor signaling: a molecular switch for death or activation. Immunity. 1999;11:783–93. doi: 10.1016/s1074-7613(00)80152-1. [DOI] [PubMed] [Google Scholar]
- 38.Santucci MB, Amicosante M, Cicconi R, et al. Mycobacterium tuberculosis-induced apoptosis in monocytes/macrophages: early membrane modification and intracellular mycobacterial viability. J Infect Dis. 2000;181:1506–9. doi: 10.1086/315371. [DOI] [PubMed] [Google Scholar]
- 39.Durrbaum-Landmann I, Gercken J, Flad HD, Ernst M. Effect of in vitro infection of human monocytes with low number of Mycobacterium tuberculosis bacteria on monocytes apoptosis. Infect Immun. 1996;64:5384–9. doi: 10.1128/iai.64.12.5384-5389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kremer L, Estaquier J, Brandt E, Ameisen JC, Locht C. Mycobacterium bovis Bacillus Calmette–Guérin infection prevents apoptosis of resting human monocytes. Eur J Immunol. 1997;27:2450–6. doi: 10.1002/eji.1830270945. [DOI] [PubMed] [Google Scholar]
- 41.Verhagen CE, Wierenga EA, Buffing AAM, Chand MA, Faber WR, Das PK. Reversal reaction in borderline leprosy is associated with a polarized shift to type 1-like Mycobacterium leprae T cell reactivity in lesional skin: a follow up study. J Immunol. 1997;159:4474–83. [PubMed] [Google Scholar]
- 42.Nery JA, Vieira LMM, de Matos HJ, Gallo MEN, Sarno EN. Reactional states in multibacillary Hansen disease patients during multidrug therapy. Rev Inst Med Trop São Paulo. 1998;40:363–70. doi: 10.1590/s0036-46651998000600005. [DOI] [PubMed] [Google Scholar]
- 43.Dellacasagrande J, Capo C, Raoult D, Mege JL. IFNγ-mediated control of Coxiella burnetii survival in monocytes: the role of cell apoptosis and TNF. J Immunol. 1999;162:2259–65. [PubMed] [Google Scholar]