Abstract
BNIP3 is a recently described pro-apoptotic member of the Bcl-2 family and in BNIP3 cDNA-transfected cell lines, cell death occurs via a caspase-independent pathway with opening of the mitochondrial permeability transition (PT) pore and rapid loss of mitochondrial transmembrane potential (Δψm). However, its expression or function in physiologic cell types is not known. Our results using the T-cell receptor transgenic mice P14, specific for lymphocyte choreomeningitis virus (LCMV) glycoprotein, show that in contrast to the other Bcl-2 family pro-apoptotic molecules, BNIP3 is transcriptionally highly up-regulated in effector cytotoxic T lymphocytes (CTL). Because CTL have a propensity to undergo activation-induced cell death (AICD) upon restimulation, we tested for other features associated with BNIP3-induced cell death. AICD of CTL was caspase-independent as determined by measuring caspase activation during target cell killing as well as by lack of inhibition with caspase inhibitors. Moreover, similar to BNIP3-induced cell death, CTL apoptosis was associated with increased production of reactive oxygen species and decreased Δψm. Finally, retroviral transduction of BNIP3 antisense RNA diminished AICD in effector CTL. These results suggest that BNIP3 may play an important role in T-cell homeostasis by regulating effector CTL numbers.
Introduction
Activation-induced cell death (AICD) plays an important role in the immune response to limit expansion of activated T cells after the antigen is cleared.1 The decision on the fate of a cell between survival and death is made by the balance between pro- and anti-apoptotic molecules of the Bcl-2 family and their propensity to form homo- and heterodimers.2 The role of Bcl-2 in the survival of memory T cells is well established.3 Among the pro-apoptotic members, Bax and the ‘BH3-only’ protein, Bim, appear to be involved in regulating mature T-cell numbers and Bim also controls thymocyte-negative selection.4–6 However, which pro-apoptotic molecules are involved in the induction of AICD in T cells is not completely understood.
BNIP3 is a recently described pro-apoptotic protein classified in the Bcl-2 family based on limited sequence homology to the Bcl-2 BH3 domain and C-terminal transmembrane domain.7 It may be functionally unique in that, unlike other ‘BH3-only’ members, the C-terminal transmembrane domain rather than the BH3 domain appears to be involved in BNIP3-induced cell death.8 Moreover, in cell lines transfected with BNIP3 cDNA, cell death occurs via a caspase-independent pathway with rapid opening of the mitochondrial permeability transition (PT) pore and profound mitochondrial dysfunction.9 Although BNIP3 mRNA can be detected in multiple organs by Northern blot10 its physiologic function remains unexplored.
We have previously reported that distinct populations of effector and memory cytotoxic T lymphocytes (CTL) can be generated from antigen-primed CD8 T cells in vitro by varying the cytokine milieu.11 We further found by gene chip analysis, that BNIP3 is one of the genes over-expressed in effector CTL compared to naïve and memory CTL (unpublished data). We therefore compared the gene expression of BNIP3 with that of other pro-apoptotic members of the Bcl-2 family in effector CTL by real-time polymerase chain reaction (PCR). We report that only BNIP3 is transcriptionally up-regulated in CTL and that it contributes to CTL AICD.
Materials and methods
Mice
P14 T-cell receptor (TCR) transgenic mice expressing the transgenic TCR specific for the lymphocyte choreomeningitis virus (LCMV) gp33–41 peptide12 were maintained under viral antigen free (VAF) specific pathogen-free conditions as described previously.11 C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME).
Naïve and effector CD8+ T cells
Naïve CD8+ T cells were purified from splenocytes by negative selection using the murine T-cell CD8 subset isolation kit (R & D Systems, Minneapolis, MN) according to the manufacturer's instructions. To generate effector CTL in vitro, splenocytes from P14 mice were stimulated with gp33–41 peptide and cultured in interleukin-2 (IL-2) for 7 days as described previously.11 After 7 days of culture, > 95% of viable cells are CD8+ T cells.11 To derive effector CTL in vivo, C57BL/6 mice were infected with vaccinia virus and 7 days later, CD8+ T cells were isolated from peritoneal exudate cells as described above.
Real-time PCR
Total RNAs extracted from naïve and effector cells using TRIzol reagent (Life Technologies, Gaithesberg, MD) were reverse transcribed using 100 U Superscript reverse transcriptase (RT; Life Technologies) according to the manufacturer's instructions. For every reaction set, one RNA sample was used without RT to provide a negative control in the subsequent PCR reactions. PCR primers for all target genes (derived from the GenBank data base) were designed using the computer program primer express (Applied Biosystems, Foster City, CA) and were synthesized at Operon (Alameida, CA). PCR was performed using the SYBR Green PCR kit (Applied Biosystems) according to the manufacturer's instruction's in the iCycler IQ Real-Time PCR Detection System (Bio-Rad, Hercules, CA). For each PCR assay, standard curves were generated using serial dilutions of the target and β-actin cDNA. The data from the Real-Time PCR machine were exported into a microsoft excel spreadsheet to construct standard curves and to perform statistical analyses. The quantity of target gene or β-actin PCR product was calculated using the corresponding standard curve and the amount of target gene in a given sample was normalized with that of β-actin gene. The normalized quantity of target gene in the RNA sample from naïve CD8+ T cells was used as a calibrator. The normalized target values of the test samples were divided by the calibrator values to obtain relative expression.
Western blot
Cell lysates were analysed by Western blot using human polyclonal anti-BNIP3 antibody (gift of late Dr Arnold Greenberg, University of Manitoba, Winnipeg), monoclonal anti-β-actin antibody and horseradish-peroxidase-conjugated goat anti-rabbit secondary antibody (Amersham Pharmacia Biotech, Piscataway, NJ). The results were visualized using an enhanced chemiluminescence detection system (Amersham) and quantified after scanning, using the nih image 1·63 program.
Caspase activation assay
Caspase activation in target and effector cells was studied using Cytoxilux™ and Phiphilux-G1D2 kits (OncoImmunin, Gaithersburg, MD) according to the manufacturer's instructions. Briefly, 1 × 106 effector cells were incubated for 6 hr with EL-4 target cells unpulsed or pulsed with gp33–41 peptide at an effector to target ratio of 1 : 1 and subsequently incubated with 10 μm of indicated fluorogenic caspase substrates for 45 min at 37°. The cells were then washed in phosphate-buffered saline and stained on ice with anti-mouse CD8-Cy5 and AnnexinV-phycoerythrin (PE) or propidium iodide (PI) and analysed by flow cytometry.
Caspase inhibition
P14 effector CTL were either not stimulated or stimulated with plate-bound αCD3 (5 μg/ml) in the presence or absence of 100 μm zVAD-fmk (Calbiochem, San Diego, CA) for 6 hr before staining with αCD8-Cy5 and AnnexinV-PE or PI. Where appropriate, samples were pre-incubated with 100 μm zVAD-fmk for 1 hr. To determine the activity of zVAD-fmk, NIH 3T3 cells (American Type Culture Collection, Manassas, VA) were treated with 1 μm staurosporine (Sigma, St Louis, MO) for 2 hr and tested for cleavage of the caspase substrate, poly (ADP-ribose) polymerase (PARP) using a monoclonal antibody that is specific for the cleaved form of PARP (Cell Signaling, Beverly, MA) in Western blot analyses.
Reactive oxygen species (ROS) and mitochondrial transmembrane potential (Δψm).
Unstimulated as well as αCD3-stimulated naïve and P14 effector CD8+ T cells were stained with 2 μm dihydroethidium or 40 nm DiOC6 (all from Molecular Probes, Inc.) for 30 min at 37° in Hanks' balanced salt solution, stained with αCD8-Cy5 and examined by flow cytometry.
Retroviral transduction
BNIP3 cDNA was obtained by RT-PCR using effector CD8+ T-cell RNA as template. The primers were designed to incorporate XhoI sites at both ends and a Kozac consensus sequence before the initiation codon. The cDNA was cloned into the retroviral vector, green flurorescent protein-retrovirus (GFP-RV)13 (gift of Ken Murphy, Washington University School of Medicine, St Louis, MO) at the unique XhoI site. Clones containing the insert in the sense as well as antisense orientation (determined by restriction mapping) were selected. To generate viral particles, GFP-RV as well as BNIP3 retroviral constructs were transfected into the packaging cell line, Phoenix Eco (ATCC, Manassas, VA) using Lipofectamine plus reagent kit (Invitrogen, Carlsbad, CA) according to the manufacturer's instruction and the virus-containing supernatant was harvested after 48 hr. For transduction into effector CD8+ T cells, P14 splenocytes were stimulated with gp33–41 peptide and 2 days later, CD8+ T cells were isolated and infected as described previously13 The cells were cultured for 4 more days in IL-2 before the apoptosis assay was performed as described earlier.
RT-PCR
P14 splenocytes were transduced with GFP-RV or GFP-RV-BNIP3 antisense retroviruses as described earlier and 2 days after transduction, GFP+ cells were sorted using a FACSVantage SE cell sorter (BD Biosciences, Mountainview, CA). The sorted cells were nore than 95% pure as determined by flow cytometry (not shown). Total RNA extracted from the sorted cells was reverse transcribed and PCR-amplified using a Perkin-Elmer RT-PCR core kit and BNIP3 or β-actin primers. To obtain semi-quantitative data during the linear phase of amplification, the number of cycles was limited to 25.
Results
BNIP3 is selectively up-regulated in effector CTL
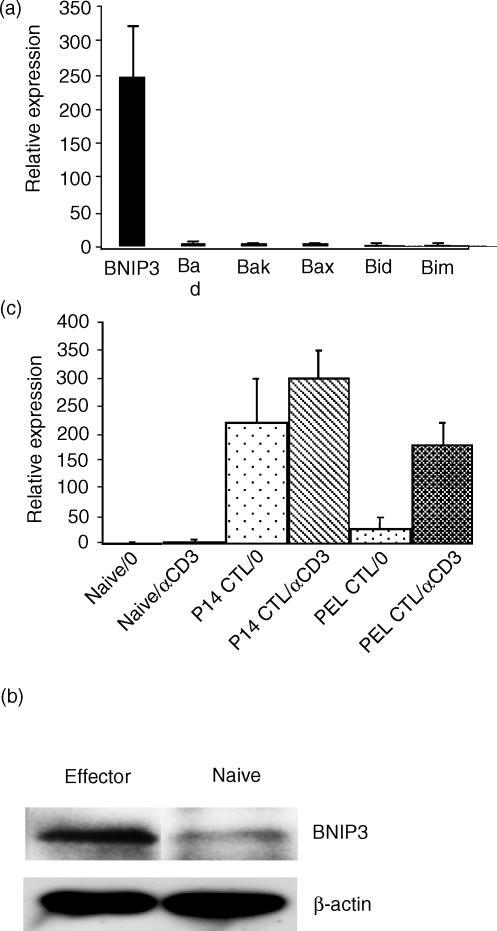
P14 mice express the transgenic TCR specific for the LCMV glycoprotein epitope, gp33–41.12 When stimulated with the cognate peptide and cultured in the presence of 20 ng/ml recombinant IL-2 for 7 days, the transgenic CD8+ T cells differentiate into cells that exhibit all the features of effector CTL, including effector-specific phenotype and high levels of cytotoxicity.11 They are also highly susceptible to AICD upon restimulation. To study the pro-apoptotic molecules that may regulate AICD, we compared the gene expression of BNIP3 and other pro-apoptotic members of the Bcl-2 family in naïve and effector CTL by quantitative real-time PCR. There were no significant differences between naïve and effector cells in the gene expression of Bad, Bak, Bax, Bid, or Bim. In contrast, BNIP3 gene expression was highly increased in effector compared to naïve CD8+ T cells (Fig. 1a). The increase was noticeable from 3 days after stimulation, reached maximal levels by 6 days and did not increase further without restimulation (not shown). Western blot analysis also revealed higher levels of BNIP3 protein expression in day 7 effector cells compared to naïve CD8+ T cells, although the differences were not as pronounced as in gene expression (Fig. 1b). A threefold difference in BNIP3 protein expression could be consistently (in three independent experiments) demonstrated after normalizing to β-actin expression.
Figure 1.
BNIP3 expression increases in effector CD8+ T cells. (a) Splenocytes from P14 TCR transgenic mice were stimulated with gp33–41 peptide and cultured with 20 ng/ml of IL-2. Seven days later, total RNA was extracted from the cultured as well as from naïve CD8+ T cells purified from naïve transgenic mouse splenocytes. BNIP3 and other pro-apoptotic molecule mRNA expression was determined by SYBR Green RT-PCR as described in the Materials and Methods section. Results are expressed as mean expression relative to naïve cells ± SD of three independent experiments. (b) Cell lysates from activated and naïve CD8 T cells were probed for BNIP3 protein expression by Western blot using polyclonal anti-human BNIP3 antibody. Densitometric analysis after normalization to β-actin expression revealed a 2·8-fold increase in BNIP3 protein expression in activated compared to naïve CD8 T cells. (c) Naïve CD8+ T cells, in vitro peptide-activated P14 CD8+ T cells as well as CD8+ T cells isolated from peritoneal exudates obtained 7 days after vaccinia infection (PEL) were examined for BNIP3 mRNA expression by SYBR Green RT-PCR before and 15 hr after stimulation with 5 μg/ml plate-bound αCD3 antibody. Results are expressed as mean ± SD of two independent experiments.
We also tested in vivo-generated effector CTL, isolated from the peritoneal exudates of vaccinia-virus-infected mice for BNIP3 gene expression before and after stimulation with αCD3 antibody. Although BNIP3 RNA was not increased in unstimulated cells, the RNA levels measured 15 hr after stimulation were increased to levels similar to those seen with in vitro-activated CTL (Fig. 1c). These results suggest that unlike the other pro-apoptotic members tested, BNIP3 expression is regulated at a transcriptional level when effector CTL are activated.
CTL do not activate caspases during AICD
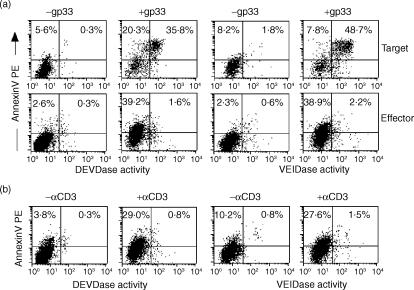
One important feature of BNIP3-induced cell death is that it is independent of caspase activation.9 Following TCR recognition of antigenic peptide-major histocompatibility complex (MHC) class I complexes on target cells, CTL release perforin and granzymes which are known to induce caspase-dependent and independent death of target cells. At the same time, some of the CTL also undergo AICD. Thus, an assay that can simultaneously assess caspase activation in target cells and effector CTL would provide a rigorous internal control to determine if CTL apoptosis is caspase dependent. We took advantage of the recently described technique of using cell-permeable fluorogenic caspase substrates to visualize caspase activation by flow cytometry.14 The fluorogenic substrates are peptides containing recognition and cleavage sequences for individual caspases with covalently linked fluorophores. In their uncleaved form, the fluorescence is quenched because of the formation of intramolecular excitonic dimers. Once the substrates are cleaved by caspases, the dimers are broken down and the substrate now becomes fluorescent. To study caspase activation, we stimulated effector CTL with EL-4 target cells either pulsed or not pulsed with gp33–41 peptide. After 6 hr of incubation, the cells were loaded with the fluorogenic caspase substrates for DEVDase (caspase-3/7) or VEIDase (caspase-6) and incubated for a further 45 min. The cells were then washed and stained with anti-mouse CD8-Cy5 and AnnexinV-PE or PI.
Because the effector CTL populations used are over 95% CD8+ (not shown), we could simultaneously study caspase activation in effector CTL and the target EL-4 cells by simply gating on or gating out the CD8+ populations. While the AnnexinV+ CD8− EL-4 target cells uniformly activated the caspases, as revealed by green substrate fluorescence, the AnnexinV+ CD8+ CTL failed to activate caspases (Fig. 2a). These results suggest that while the CTL-mediated target cell apoptosis activates the caspase pathway, the AICD of CTL is caspase-independent. These results were also confirmed after overnight incubation, ruling out the possibility that caspase activation may be a later event in effector cell apoptosis (not shown). We also used another approach of stimulation with αCD3 antibody to induce AICD. Even in this system, the CTL undergoing apoptosis failed to activate caspases (Fig. 2b). Similar results were also obtained using in vivo-generated effector CTL, isolated from the peritoneal exudates of vaccinia virus-infected mice (not shown).
Figure 2.
AICD of effector CTL is largely caspase-independent. P14 splenocytes were stimulated as described in Fig. 1 and on day 7 post-stimulation, effector cells (1 × 106) were incubated with EL-4 target cells, pulsed or not pulsed with gp33–41 peptide at an effector to target ratio of 1 : 1 (a) or stimulated with 5 μg/ml plate-bound αCD3 antibody (b). Six hours later, the cells were incubated with 10 μm fluorogenic caspase substrates for DEVDase (caspase-3/7) or VEIDase (caspase-6) for 45 min. The cells were then washed and stained with anti-mouse CD8-Cy5 and AnnexinV-PE. All samples were analysed without fixation by flow cytometry. Representative results from one experiment (of three performed with similar results) are shown. In (a) cells were gated on CD8− (target cells, top panel) or CD8+ population (effector cells, bottom panel) for analysis. Quadrant statistics are indicated as a percentage of gated cells.
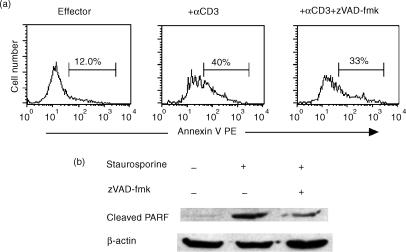
The Pan-caspase inhibitor zVAD-fmk, does not inhibit CTL AICD
To confirm that AICD in effector CTL is caspase-independent, we looked at the effect of a pan-caspase inhibitor on AICD. For these studies, effector CTL were stimulated with αCD3 and cultured in the presence or absence of zVAD-fmk for 6 hr before staining with anti-mouse CD8-Cy5 and AnnexinV-PE. Compared to untreated controls, the AnnexinV+ CD8+ T-cell numbers were not significantly diminished in the inhibitor-treated cultures (Fig. 3a). To confirm that zVAD-fmk was active, we treated NIH 3T3 cells with staurosporine and evaluated the cells for the cleavage of the caspase substrate, PARP using a monoclonal antibody that specifically recognizes cleaved PARP in Western blot analyses. Compared to staurosporine-untreated control cells, a prominent cleaved PARP band was apparent in the staurosporine-treated cells and this band was substantially reduced after treatment with zVAD-fmk, indicating that zVAD-fmk used was indeed active (Fig. 3b).
Figure 3.
The pan-caspase inhibitor zVAD-fmk, does not significantly inhibit CTL apoptosis. A. P14 splenocytes, stimulated as in Fig. 1 were restimulated with 5 μg/ml of plate-bound αCD3 antibody for 6 hr in the presence or absence of 100 μm zVAD-fmk. The cells were then stained with αCD8 Cy5 and AnnexinV-PE and analysed by flow cytometry after gating on CD8+ cells. (b) NIH3T3 cells were either cultured without treatment or treated with 1 μm Staurosporine for 2 hr in the presence or absence of 100 μm zVAD-fmk. The cells were then lysed and the lysates analyzed by Western blot for caspase 3 substrate, PARP cleavage using a monoclonal antibody that recognizes cleaved but not native PARP.
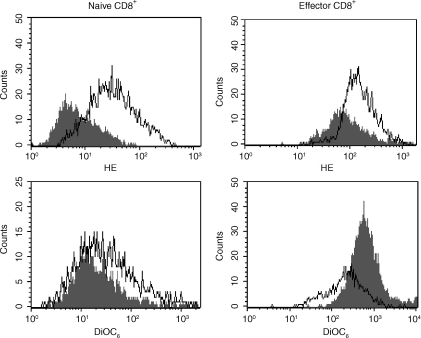
CTL AICD is associated with increased ROS generation and decreased mitochondrial transmembrane potential (Δψm)
Apart from being caspase independent, BNIP3-induced cell death is also characterized by increased ROS production and by a rapid loss of Δψm.9 Thus, we tested naïve and effector CD8+ T cells for ROS production and Δψm before and after stimulation with αCD3. ROS production was measured by staining with dihydroethidium (HE), a dye that reacts with superoxide anion, and Δψm was assessed by staining with the cationic dye, DiOC6, which accumulates in mitochondria that have an intact membrane potential. As shown in Fig. 4, compared to naïve cells, effector CD8+ T cells exhibited significantly higher HE staining and this difference was further accentuated after αCD3 stimulation, indicating that ROS production is increased in effector cells. Δψm decreased, as measured by DiOC6 staining, in effector but not in naive CD8+ T cells following stimulation. The observable increase in Δψm in unstimulated effector cells compared to naïve cells probably represents the fact the effector cells contain more mitochondria because of their larger size and increased metabolism.
Figure 4.
Increased production of ROS and loss of Δψm in effector CTL. Naïve and P14 effector CD8+ T cells obtained as in Fig. 1 were untreated or treated with 5 μg/ml of plate-bound αCD3 antibody and after incubation overnight, were stained with 2 μm dihydroethidium (HE) or 40 nm DiOC6 and analysed by flow cytometry to determine ROS production and Δψm, respectively. Overlay histograms of CD8-gated unstimulated (filled) and stimulated (open) cells are shown. Results are representative of two independent experiments.
Abrogation of BNIP3 expression reduces CTL apoptosis
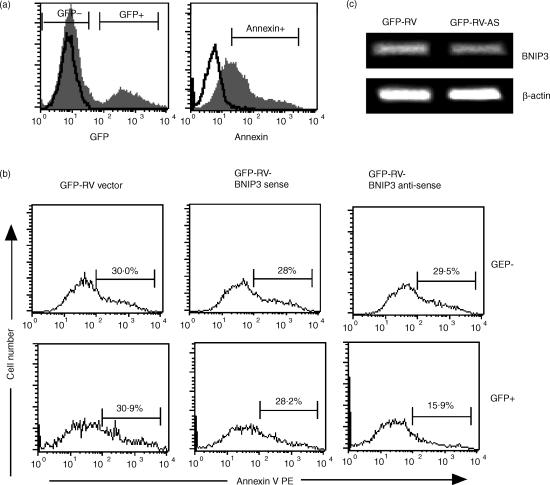
Next we sought to test directly the role of BNIP3 in AICD by targeting BNIP3 mRNA in effector CTL. For this purpose, we derived retroviral vectors encoding BNIP3 RNA in the sense or antisense orientation. P14 CD8+ T cells were activated with gp33–41 peptide and 2 days later were infected with retroviruses expressing GFP alone or along with sense or antisense BNIP3 RNA. The cells were cultured in the presence of IL-2 for 4 more days, following which AICD was induced by αCD3 stimulation. Because dead cells rapidly lose GFP expression precluding analysis of BNIP3 antisense effects in our system, we used AnnexinV positivity in the live cell gate as a readout. Although BNIP3-transfected 293 T cells were found not to externalize phosphatidylserine9 other cell types become AnnexinV-positive following BNIP3-induced cell death.15
Because not all CD8 T cells are infected (we obtained 12–20% infection as evidenced by GFP fluorescence), the uninfected GFP− CD8+ T cells provide a good internal control for variability in the induction of apoptosis. Thus, we analysed the GFP− and the GFP+ cell populations (Fig. 5a) separately for AnnexinV staining. In the GFP− cells, AnnexinV positivity was comparable between all three experimental groups, indicating that αCD3 treatment induced similar levels of apoptosis (Fig. 5b top panel). In the GFP+ group, similar levels of AnnexinV staining were seen between the cells transduced with retroviral vector alone or with retrovirus expressing BNIP3 sense RNA. In contrast, AnnexinV positivity was reduced by nearly 50% when BNIP3 antisense RNA was expressed (Fig. 5b, bottom panel), indicating that BNIP3 antisense RNA specifically inhibited CTL apoptosis. We did not observe an increase in apoptosis using BNIP3 sense RNA-expressing retrovirus, which may be because BNIP3 mRNA is already highly expressed in effector cells and a further increase in BNIP3 RNA may not lead to increased protein levels. Unfortunately we could not test the BNIP3 protein levels in transduced cells because the only available antibody that recognizes mouse BNIP3 is not suitable for intracellular staining (not shown) and the low numbers of transduced cells precluded analysis by Western blot. However, we were able to demonstrate a 2·3-fold reduction in BNIP3 gene expression (after normalizing to β-actin expression) by RT-PCR using sorted GFP+ cells after transduction with BNIP3 antisense retroviral construct (Fig. 5C).
Figure 5.
Retroviral transduction of antisense BNIP3 RNA diminishes effector CTL apoptosis. P14 splenocytes were stimulated with gp33–41 peptide and 2 days later, were infected with retroviruses expressing GFP alone (GFP-RV) or expressing GFP along with sense or antisense BNIP3 RNA. The cells were cultured for 4 days in the presence of 10 ng/ml IL-2, stimulated with 5 μg/ml plate-bound αCD3 for 6 hr and stained with CD8-Cy5 and AnnexinV-PE and examined by flow cytometry. In (a), gates set to analyse GFP and AnnexinV positivity are shown using GFP-RV transfected (filled) or untransfected (open) P14 cells and annexin-stained (filled) and isotype control antibody-stained (open) P14 effectors stimulated with αCD3. (b) shows AnnexinV staining on retrovirally nontransduced (GFP−, top panel) and transduced (GFP+, bottom panel) CD8-gated cells. The percentage of AnnexinV+ cells is indicated. (c) shows RT-PCR analysis for BNIP3 gene expression using sorted GFP-RV and GFP-RV-BNIP3 antisense retrovirally transduced P14 cells. Densitometric analysis after normalization to β-actin expression revealed a 2·3-fold decrease in BNIP3 gene expression in GFP-RV BNIP3 antisense transduced cells compared to GFP-RV vector transduced cells.
Discussion
So far, BNIP3 has been studied only by transfection in cell lines and its expression or function in particular cell types is not known. Our results, for the first time, show that BNIP3 is expressed in CTL, is transcriptionally regulated, and may play an important role in regulating effector CTL numbers. A number of observations support such a role for BNIP3: BNIP3 mRNA and protein are up-regulated in effector CTL; CTL apoptosis, like BNIP3-induced cell death, occurs in a caspase-independent manner; similar to BNIP3-induced cell death, CTL apoptosis is associated with increased ROS production and decreased Δψm; and targeting BNIP3 mRNA renders CTL less susceptible to AICD.
The Bcl2 family of pro-apoptotic molecules is thought to be regulated mostly at a post-translational level through processes such as subcellular translocation, dimerization, phosphorylation or protein cleavage (reviewed in ref. 16) BNIP3 appears to be unique in that its mRNA is highly up-regulated in effector CTL compared to naïve CD8+ T cells. In the cultured P14 CTL, the BNIP3 gene was highly expressed even before αCD3 restimulation, and increased further with stimulation (Fig. 1a). However, in CTL obtained from vaccinia virus-infected mice, basal level BNIP3 gene expression was not increased, but it rapidly rose after restimulation with αCD3 antibody (Fig. 1c). The reason for this discrepancy is not apparent and may indicate that high-level BNIP3 expression delivers apoptotic signals and such cells may be rapidly cleared by macrophages in vivo. Alternatively, BNIP3 may be more involved in the cell death induced by cytokines such as high doses of IL-2, a situation not generally found in vivo. However, this is unlikely considering the fact that in vivo-generated CTL rapidly up-regulated BNIP3 expression following restimulation in the absence of IL-2 (Fig. 1c). We are currently generating BNIP3 transgenic mice to address this issue more fully.
Our results that AICD in effector CTL is caspase-independent also suggest that Fas and tumour necrosis factor (TNF) may not be involved in the induction of AICD in CTL. A number of studies have addressed the role of Fas and TNF in the elimination of mature T cells and have come to differing conclusions. In mice lacking Fas or Fas ligand, activated T cells with abnormal phenotype accumulate leading to a lymphoproliferative disease.17 Moreover, superantigen-, peptide- as well as αCD3-induced deletion of CD4+ T cells is impaired in these mice.18,19 An important role for both Fas and TNF in peripheral deletion of CD4+ T cells has also been shown using an MHC-class-II-restricted TCR transgenic system.20 However, while Fas and TNF appear to be important in mature CD4+ T-cell apoptosis, many studies suggest that they may not play an equally important role in the elimination of CD8+ T cells. CD8+ T-cell down-regulation after acute viral infection occurred normally in the absence of Fas.21,22 Similarly, peptide antigen-induced deletion of CD8+ T cells was not impaired in Fas-deficient mice in two different TCR transgenic models.23,24 Although some studies suggested that TNF may be involved in mature CD8+ T cell apoptosis25–27 two recent studies directly addressing the role of Fas and TNF using mice deficient for either or both molecules came to the conclusion that neither Fas nor TNF is required for the down-sizing of CD8+ T cell pool after viral infection.28,29 Our results are consistent with these studies and indicate that the death pathways used in the elimination of CD8+ T cells may not use Fas, TNF or other molecules causing caspase activation and support a role for BNIP3 in the process.
Two other pro-apoptotic members of the Bcl-2 family, Bax and Bim, have been shown to play an important role in T-cell physiology. Expression of a Bax transgene leads to a reduction in mature T-cell numbers and conversely, Bax-deficient mice display increased thymocyte and peripheral T-cell numbers.4,30 Deficiency of the ‘BH3-only’ member Bim also leads to an increase in thymocyte and mature T-cell numbers. Bim-deficient T cells are resistant to some, but not other forms of apoptotic stimuli.6 Bim−/− mice are also defective in downsizing superantigen-induced T-cell expansion.31 Moreover, Bim−/− mice are severely impaired in thymocyte-negative selection.6 Together with our findings, these results suggest that different pro-apoptotic members of the Bcl-2 family may regulate T-cell apoptosis under different circumstances.
The signals responsible for BNIP3 induction in CTL are not clear. A number of recent reports suggest that hypoxic stress can induce up-regulation of BNIP3 mRNA and protein levels in cultured cells and tumours.32–34 In fact, the BNIP3 promoter contains a functional hypoxia-inducing factor (HIF)-1-responsive element (HRE) and is potently activated by both hypoxia and forced expression of HIF-1α.32 Hypoxia is also known to induce ROS generation.35 Our results, as well as those of others, suggest that effector T cells generate enhanced amounts of ROS which increases their susceptibility to AICD (Fig. 4, and ref. 36). Moreover, cell death occurs in a caspase-independent manner with early loss of Δψm and can be prevented by antioxidants.36 These features also characterize BNIP3-induced cell death.9 Moreover, one of the genes we found over expressed in effector cells is HIF-1 (unpublished data). Thus, oxidative stress in effector cells might induce BNIP3 gene expression through the HIF/ROS pathway, leading to an increased susceptibility to AICD. Whatever the mechanisms involved, our results suggest that BNIP3 is involved in regulating CTL survival and therefore BNIP3 may provide a target to induce or interfere with the apoptotic pathway and CTL numbers.
Acknowledgments
We thank Zhang Dong for assistance with the quantitative PCR and Premlata Shankar for critically reviewing the manuscript. This work was supported by NIH grant AI46566 to M.N.
References
- 1.Van Parijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280:243–8. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 2.Oltvai ZN, Korsmeyer SJ. Checkpoints of dueling dimers foil death wishes. Cell. 1994;79:189–92. doi: 10.1016/0092-8674(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 3.Cory S, Harris AW, Strasser A. Insights from transgenic mice regarding the role of bcl-2 in normal and neoplastic lymphoid cells. Philos Trans R Soc Lond B Biol Sci. 1994;345:289–95. doi: 10.1098/rstb.1994.0108. [DOI] [PubMed] [Google Scholar]
- 4.Brady HJ, Gil-Gomez G, Kirberg J, Berns AJ. Bax alpha perturbs T cell development and affects cell cycle entry of T cells. EMBO J. 1996;15:6991–7001. [PMC free article] [PubMed] [Google Scholar]
- 5.Bouillet P, Purton JF, Godfrey DI, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–6. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 6.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–8. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 7.Yasuda M, Theodorakis P, Subramanian T, Chinnadurai G. Adenovirus E1B-19K/BCL-2 interacting protein BNIP3 contains a BH3 domain and a mitochondrial targeting sequence. J Biol Chem. 1998;273:12415–21. doi: 10.1074/jbc.273.20.12415. [DOI] [PubMed] [Google Scholar]
- 8.Ray R, Chen G, Vande Velde C, Cizeau J, Park JH, Reed JC, Gietz RD, Greenberg AH. BNIP3 heterodimerizes with Bcl-2/Bcl-X (L) and induces cell death independent of a Bcl-2 homology 3 (BH3) domain at both mitochondrial and nonmitochondrial sites. J Biol Chem. 2000;275:1439–48. doi: 10.1074/jbc.275.2.1439. [DOI] [PubMed] [Google Scholar]
- 9.Vande Velde C, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, Hakem R, Greenberg AH. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol. 2000;20:5454–68. doi: 10.1128/mcb.20.15.5454-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G, Ray R, Dubik D, et al. The E1B 19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein that activates apoptosis. J Exp Med. 1997;186:1975–83. doi: 10.1084/jem.186.12.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manjunath N, Shankar P, Wan J, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–8. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–61. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 13.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4- independent mechanism. Immunity. 1998;9:745–55. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Chahroudi A, Silvestri G, et al. Visualization and quantification of T cell-mediated cytotoxicity using cell-permeable fluorogenic caspase substrates. Nat Med. 2002;8:185–9. doi: 10.1038/nm0202-185. [DOI] [PubMed] [Google Scholar]
- 15.Mizutani A, Furukawa T, Adachi Y, Ikehara S, Taketani S. A zinc-finger protein, PLAGL2, induces the expression of a proapoptotic protein Nip3, leading to cellular apoptosis. J Biol Chem. 2002;277:15851–8. doi: 10.1074/jbc.M111431200. [DOI] [PubMed] [Google Scholar]
- 16.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 17.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 18.Scott DE, Kisch WJ, Steinberg AD. Studies of T cell deletion and T cell anergy following in vivo administration of SEB to normal and lupus-prone mice. J Immunol. 1993;150:664–72. [PubMed] [Google Scholar]
- 19.Singer GG, Abbas AK. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–71. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 20.Sytwu HK, Liblau RS, McDevitt HO. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity. 1996;5:17–30. doi: 10.1016/s1074-7613(00)80306-4. [DOI] [PubMed] [Google Scholar]
- 21.Ehl S, Hoffmann-Rohrer U, Nagata S, Hengartner H, Zinkernagel R. Different susceptibility of cytotoxic T cells to CD95 (Fas/Apo-1) ligand-mediated cell death after activation in vitro versus in vivo. J Immunol. 1996;156:2357–60. [PubMed] [Google Scholar]
- 22.Lohman BL, Razvi ES, Welsh RM. T-lymphocyte downregulation after acute viral infection is not dependent on CD95 (Fas) receptor–ligand interactions. J Virol. 1996;70:8199–203. doi: 10.1128/jvi.70.11.8199-8203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehal WZ, Crispe IN. TCR ligation on CD8+ T cells creates double-negative cells in vivo. J Immunol. 1998;161:1686–93. [PubMed] [Google Scholar]
- 24.Zimmermann C, Rawiel M, Blaser C, Kaufmann M, Pircher H. Homeostatic regulation of CD8+ T cells after antigen challenge in the absence of Fas (CD95) Eur J Immunol. 1996;26:2903–10. doi: 10.1002/eji.1830261215. [DOI] [PubMed] [Google Scholar]
- 25.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–51. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 26.Alexander-Miller MA, Leggatt GR, Sarin A, Berzofsky JA. Role of antigen, CD8, and cytotoxic T lymphocyte (CTL) avidity in high dose antigen induction of apoptosis of effector CTL. J Exp Med. 1996;184:485–92. doi: 10.1084/jem.184.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speiser DE, Sebzda E, Ohteki T, Bachmann MF, Pfeffer K, Mak TW, Ohashi PS. Tumor necrosis factor receptor p55 mediates deletion of peripheral cytotoxic T lymphocytes in vivo. Eur J Immunol. 1996;26:3055–60. doi: 10.1002/eji.1830261235. [DOI] [PubMed] [Google Scholar]
- 28.Reich A, Korner H, Sedgwick JD, Pircher H. Immune down-regulation and peripheral deletion of CD8 T cells does not require TNF receptor–ligand interactions nor CD95 (Fas, APO-1) Eur J Immunol. 2000;30:678–82. doi: 10.1002/1521-4141(200002)30:2<678::AID-IMMU678>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen LT, McKall-Faienza K, Zakarian A, Speiser DE, Mak TW, Ohashi PS. TNF receptor 1 (TNFR1) and CD95 are not required for T cell deletion after virus infection but contribute to peptide-induced deletion under limited conditions. Eur J Immunol. 2000;30:683–8. doi: 10.1002/1521-4141(200002)30:2<683::AID-IMMU683>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–9. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 31.Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759–67. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 32.Bruick RK. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci USA. 2000;97:9082–7. doi: 10.1073/pnas.97.16.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo K, Searfoss G, Krolikowski D, et al. Hypoxia induces the expression of the pro-apoptotic gene BNIP3. Cell Death Differ. 2001;8:367–76. doi: 10.1038/sj.cdd.4400810. [DOI] [PubMed] [Google Scholar]
- 34.Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. 2001;61:6669–73. [PubMed] [Google Scholar]
- 35.Steiner DR, Gonzalez NC, Wood JG. Leukotriene B (4) promotes reactive oxidant generation and leukocyte adherence during acute hypoxia. J Appl Physiol. 2001;91:1160–7. doi: 10.1152/jappl.2001.91.3.1160. [DOI] [PubMed] [Google Scholar]
- 36.Hildeman DA, Mitchell T, Teague TK, Henson P, Day BJ, Kappler J, Marrack PC. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735–44. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]