Abstract
An important factor in the establishment of ocular immune privilege is the dynamic down regulation of T helper 1 (Th1) immune responses that occurs in response to antigens delivered intraocularly; a phenomenon that has been termed anterior chamber-associated immune deviation (ACAID). ACAID is characterized by the generation of splenic regulatory cells that inhibit the expression of delayed-type hypersensitivity. Previous studies have shown that antigens introduced into the anterior chamber of the eye induce the generation of a CD4+ T-cell population that suppress the induction of Th1 immune responses and the appearance of a second population of CD8+ T regulatory cells that suppresses the expression of Th1 inflammatory responses (= efferent suppressor cells). Experiments described here characterized the function of the CD4+ ACAID suppressor cell population and its effect on the generation of CD8+ efferent suppressor cells that inhibit the expression of DTH in situ. Both in vivo and in vitro experiments demonstrated that CD4+ T cells are required for the generation of CD8+ efferent suppressor cells. CD4+ T cells do not require cell contact with CD8+ T cells; instead they produce soluble IL-10 that is sufficient for the generation of ACAID suppressor cells. Finally, the CD4+ afferent T suppressor cells are not natural killer T cells, but do express the CD25 cell surface marker.
Introduction
The immune system utilizes a host of mechanisms to fight invading pathogens, often resulting in inflammation that inflicts irreparable damage to innocent bystander cells. Certain sites in the body are endowed with immune privilege that provides protection from this non-specific tissue damage. Immune privilege of the eye protects delicate ocular tissues from inflammatory damage that can lead to blindness. The immune privilege within the eye is maintained by multiple mechanisms including immunosuppressive factors and neuropeptides within the aqueous humour of the anterior chamber.1 Fas ligand expression on the corneal endothelium2,3 and limited expression of major histocompatibility complex (MHC) class I and class II molecules on ocular tissues also protect the eye for immune-mediated injury.4–7 Finally, a dynamic regulatory mechanism, termed anterior chamber-associated immune deviation (ACAID), contributes to ocular immune privilege. ACAID is characterized by a systemic antigen-specific down-regulation of delayed-type hypersensitivity (DTH) and a shift in antibody production away from complement-fixing isotypes.8,9
ACAID is induced when antigen is introduced into the anterior chamber of the eye, and cells. These regulatory cells are referred to as ‘efferent suppressor cells’.10 results in the production of two independent suppressor cell populations.11–14 One suppressor cell population consists of CD4+ T cells that inhibit the induction of DTH responses, but do not affect the expression of DTH if hosts that have already been immunized. These cells prevent the induction phase of the immune response and are termed ‘afferent suppressor cells’.10,15 A second suppressor cell population is comprised of CD8+ T cells that inhibit the expression of DTH responses by previously sensitized T cells. The generation of ACAID suppressor cells has proven to be quite complex and involves many different immune cells and cytokines. For example, B cells,16,17γδ T cells18,19 and natural killer (NK) T cells20–23 play critical roles in ACAID. Cytokines such as interleukin-10 (IL-10)24–26 and transforming growth factor-β (TGF-β)27,28 also contribute to the induction and expression of ACAID. CD4+ T cells have also been described in ACAID mice. CD4+ T cells isolated from the spleens of ACAID-induced mice have been shown to suppress the induction of a primary immune response.10 Li et al. further showed that the CD4+ T cells from ACAID-induced animals expressed increased IL-10 and decreased interferon-γ (IFN-γ) compared to CD4+ T cells from non-ACAID animals.26
Kosiewicz et al. utilized a class-II restricted OVA peptide to analyse the induction of CD8+ ACAID suppressor cells.29 Because class II peptides present to CD4+ T cells, it was hypothesized that CD4+ T cells played a role in the generation of the CD8+ ACAID suppressor cells. Other investigations have described immunosuppression initiated by CD4+ T cells to antigens introduced at non-ocular sites. One system describes the suppression of DTH responses to Candida albicans mannan by CD8+ T cells.30 Another has described antigen-non-specific CD8+ T cells capable of suppressing DTH responses to sheep red blood cells. In both systems, CD4+ T cells are needed to generate the CD8+ suppressor cells.30,31
The importance of CD4+ afferent suppressor cells in the generation of CD8+ efferent suppressor cells has been demonstrated in other models of tolerance.30–33 The present study examined the role CD4+ afferent T suppressor cells in the generation of CD8+ efferent suppressor cells of ACAID. Furthermore, the phenotype and function of the CD4+ T cells was examined. Finally, experiments addressed if T helper 2 (Th2) cytokines, produced by the CD4+ T cells, played an essential role in generating the CD8+ efferent ACAID suppressor cells.
Materials and methods
Animals and antibodies
C57BL/6, CD4 knockout (KO) mice (B6.129S2-CD4tm1Mak), IL-4 knockout mice (B6.129P2-IL-4tmCgn), and IL-10 knockout mice (B6.129P2-IL-10tmCgn) were obtained from The Jackson Laboratory (Bar Harbor, ME). All animal studies were approved by the Institutional Review Board of the University of Texas South-western Medical Center at Dallas. Anti-CD8 antibody was protein A purified from YTS169.5 hybridoma provided by Dr Michael Bennett (University of Texas South-western Medical Center at Dallas). Fluoroscein isothiocyanate (FITC) anti-2B4 and biotin anti-Ly49c antibodies were used for NK+ cell separation (Pharmingen, San Diego, CA). Anti-CD25 and anti-CD4 antibodies were used in CD25+ T-cell separation (Pharmingen). Finally, anti-IL-10 antibody was purified from JESS2A5.11 hybridoma kindly provided by Dr J. Abrams (DNAX, Palo Alto, CA).
Isolation of peritoneal exudate cells
Naïve C57BL/6 mice were injected intraperitoneally (i.p.) with 2 ml of aged 3% thioglycollate (Sigma Chemical Co., St. Louis, MO). After 3–5 days the animals were killed and their peritoneal cavity was washed twice with Hanks' balanced salt solution (HBSS) to isolate peritoneal exudate cells. These cells were then plated on Primaria Petri dishes (Becton Dickinson Labware, Franklin Lakes, NJ) and cultured for 1·5 hr at 37° and 5% CO2. The non-adherent cells were removed by gentle swirling with cold HBSS, and adherent cells were removed with a rubber cell scraper (Becton Dickinson). The adherent cells were incubated overnight under the appropriate conditions (described below).
Subcutaneous inoculations
Mice were immunized by subcutaneous (s.c.) injection of ovalbumin (OVA; 250 µg) or hen egg lysozyme (HEL; 250 µg) in phosphate-buffered saline (PBS) emulsified 1 : 1 in complete Freund's adjuvant (CFA; Sigma). Each animal received a total volume of 200 µl.
Generation of ACAID antigen-presenting cells (APC)
Peritoneal exudate cells were collected (described above). Adherent cells were cultured overnight (2 × 106 cells/ml) in complete RPMI medium supplemented with 10 mg/ml OVA (Sigma) or 10 mg/ml HEL (Sigma) and 2 ng/ml human TGF-β2 (R & D Systems, Minneapolis, MN).
In vitro ACAID culture
ACAID APC (generated as described above) were collected and washed with HBSS. ACAID APC (5 × 106) were added to a large Petri dish (Falcon 3003). Separately, naïve spleens or CD4 KO spleens were harvested and erythrocytes lysed in Tris-buffered NH4Cl. Whole splenocytes (50–100 × 106) were added to the large Petri dishes containing ACAID APC. The whole splenocyte population contained naïve spleen cells, CD4 KO spleen cells, or CD4 KO spleen cells combined with purified CD4+ T cells. Some cultures were treated with either 20 µg/ml anti-IL-10 antibody or with 10 ng/ml rmIL-10 (Genzyme, Cambridge, MA). A modified in vitro culture system utilized a transwell separating ACAID APC (5 × 106) and splenocytes in the upper chamber (50 × 106) from splenocytes in the lower chamber (50 × 106). In vitro ACAID cultures were incubated at 37° and 5% CO2 for 5–7 days. The cells were then collected and used in a local adoptive transfer (LAT) assay (described below).
Generation of ACAID regulatory cells by intracameral (i.c.) injection
ACAID was induced as described previously using microinjection of antigen into the anterior chamber of the eye.24 Briefly, mice were anaesthetized with 0·133 mg/kg ketamine hydrochloride (Fort Dodge Laboratories, Fort Dodge, IA) and 0·006 mg/kg xylazine (Bayer Corporation, Shawnee Mission, KS) given i.p. A glass micropipette (approximately 80 µm diameter) was fitted onto a sterile infant feeding tube (no. 5 French, Professional Medical Products Inc., Greenwood, SC) and mounted onto a 0·1-ml Hamilton (Hamilton Co. Inc., Whittier, CA) syringe. A Hamilton automatic dispensing apparatus was used to inject 5 µl of the 20 mg/ml OVA (Sigma) in PBS (100 µg OVA) into the anterior chamber. Seven days after the i.c. injection animals were s.c.. immunized with OVA in CFA (Sigma). Spleen cells containing the regulatory cells were collected on day 14 and used in the LAT assay (described below).
LAT assay
The LAT assay to test for ACAID suppressor cells has been described elsewhere and detects ACAID efferent suppressor cells that are generated either in vitro15 or in vivo.10 ACAID efferent suppressor cells, whether generated in vitro or in vivo, express the CD8 surface marker.10,15 Regulatory cells were generated using the in vitro ACAID model, or by i.c. injection of OVA. The regulatory cells were suspended at 5 × 107 cells/ml in 10 mg/ml OVA or HEL in PBS. Immune cells were generated by s.c. immunizing naïve C57BL/6 mice on day 0 with OVA (250 µg) or HEL (250 µg) in PBS emulsified in CFA. Splenocytes were collected on day 14, erythrocytes lysed, and the splenocytes were passed over nylon wool to enrich for T cells. The immune T cells collected from the nylon wool column were suspended at 5 × 107 cells/ml in 10 mg/ml of OVA or HEL. The immune and regulatory cell populations were then mixed 1 : 1. Both ears of naïve C57BL/6 mice were measured with a Mitutoyo engineer's micrometer immediately prior to challenge. The left ear pinna of naïve C57BL/6 mice was injected with 20 µl (1 × 106) of the mixed cell population. The opposite ear was injected with 10 mg/ml of OVA or HEL as a negative control. Ear swelling was measured 24 hr later to measure DTH. Results were expressed as: specific-ear swelling = (24-hr measurement − 0-hr measurement) for experimental ear − (24-hr measurement − 0-hr measurement) for negative control ear.
Depletion and enrichment of T-cell populations
For CD4+ enrichment, splenocytes were collected and erythrocytes were lysed. The splenocytes were enriched for T cells by passing the cells over a nylon wool column. This enriched population was then incubated with anti-CD8 antibody and incubated on ice for 30 min The cells were washed three times with HBSS. The cells were then incubated with Low-Tox baby rabbit complement (1 : 10; Accurate Chemical, Westbury NY) for 30 min at 37° in 5% CO2. Alternatively, CD4+ T cells or CD8+ T cells were isolated/depleted using miniMACS columns (Miltenyi Biotec, Bergisch-Gladbach, Germany) according to the manufacturer's instructions. Briefly, splenocytes were isolated and erythrocytes lysed before incubating the cells with either CD4-specific microbeads or CD8-specific microbeads (10 µl beads/107 cells) in 0·5% fetal calf serum in PBS for 15 min in the refrigerator. The cells were washed with 0·5% bovine serum albumin (BSA) in PBS followed by magnetic separation using miniMACS columns (Mittenyi Biotec, Bergisch-Gladbach, Germany) as described by the manufacturer. The retained cells were eluted from the column and shown by flow cytometric analysis to be enriched CD4+ T cells (94% positive) or CD8+ T cells (90% positive). The depleted cell populations were shown to be depleted of CD4+ T cells (<1% positive) or CD8+ T cells (<1% positive). CD25+ T cells were also isolated using miniMACS columns (Miltenyi Biotec) according to the manufacturer's instructions, and as described previously.34 Briefly, splenocytes were incubated with FITC anti-CD25 antibody (15 µg/108 cells) min on ice then washed. The cells were washed with 0·5% BSA in PBS and then incubated with anti-FITC microbeads (Miltenyi Biotec) for 15 min in the refrigerator. The cells were washed with 0·5% BSA in PBS followed by magnetic separation using MS+ columns as described by the manufacturer. The retained cells were eluted from the column and shown by flow cytometric analysis to be enriched CD25+ T cells (83% positive for CD25). The flow-through cells (CD25− cells) were analysed and shown that CD25+ expression fell by 70% (from 0·49% to 0·15%). The CD25− T cells were subsequently incubated with anti-CD4+ microbeads (Miltenyi Biotec) for 15 min in the refrigerator. These cells were washed with 0·5% BSA in PBS and then run over MS+ columns to isolate CD25− CD4+ T cells. When Finally, NK cells were separated from whole splenocytes. Briefly, splenocytes from naïve C57BL/6 mice were isolated and erythrocytes lysed. The spleen cells were incubated with FITC anti-2B4 antibody, Biotin anti-Ly49C antibody, and magnetic beads specific for mouse NK cells (Miltenyi Biotec) in the refrigerator for 15 min then washed twice. Subsequently the cells were incubated with anti-FITC and streptavidin magnetic beads for 15 min in the refrigerator. After two washes, the cells were applied to MS+ columns and the NK cells were retained on the column, separating them from the NK− cell population. The NK− population was stained with Cy-TCRβ and analysed by flow cytometry. The NK T-cell population fell by 77% (from 1·3% in normal spleen cells to 0·3% in the depleted spleen cell population). The NK− cell population was then treated with anti-CD4+ microbeads (Miltenyi Biotec), incubated, washed, and run over a MS+ column as described above. The cells retained on the column were the desired NK−CD4+ T cells.
Statistics
The student's t-test was used to evaluate the significance of these experiments. P-values less than 0·05 were considered significant. Each group contained at least five animals per group.
Results
Antigen-specific down-regulation of DTH is one of the hallmarks of ACAID
The expression of DTH is inhibited by CD8+ ACAID regulatory T cells that act at the efferent arm of the immune response.10 Two different protocols were used to reveal the presence of the ACAID afferent and efferent suppressor cells. In the first protocol, antigen was injected i.c. into naïve C57BL/6 mice. These mice were immunized s.c. 7 days after the i.c. injection. Spleens were collected 14 days after i.c. injection and tested for the presence of ACAID suppressor cells. A LAT assay was used to assess the presence of regulatory cells that inhibit the expression of DTH responses in situ. In this assay, local adoptive transfer of ACAID suppressor cells with immune cells to the ears of naïve mice results in a suppressed ear swelling response (i.e. DTH). By definition, the LAT assay detects efferent suppressor cells. ACAID efferent suppressor cells, whether they are generated in vitro or in vivo, are CD8+ T cells.10,15 By contrast, the presence of positive DTH occurs if ACAID suppressor cells are not present. As shown in Fig. 1(a) spleen cells from i.c.-injected mice were capable of inhibiting the expression of DTH.
Figure 1.
Generation of ACAID suppressor cells. (a) Suppressor cells were generated in vivo by i.c. injection of OVA into BALB/c mice. Spleen cells from i.c.-primed animals were tested for suppressor activity in the LAT assay. (b) Suppressor cells were generated in the in vitro ACAID spleen cell culture tested in the LAT assay. Positive control animals received immune cells combined with naïve cells, while negative control animals received naïve cells only. Ear swelling was measured 24 hr after injection of OVA into the ear pinnae. All results are reported as mean ear swelling ± SE. (a) *P = 0·02 for positive versus negative controls. **P = 0·03 for ACAID group versus positive control. (b) *P = 0·0007 for positive versus negative controls. **P = 0·002 for ACAID group versus positive control. Each group represents at least five animals.
One model of ACAID proposes that antigens introduced into the anterior chamber are taken up by antigen presenting cells that leave the eye and travel to the spleen where they present antigen in a manner that induces the generation of ACAID suppressor cells.11,35–37 This paradigm led to the development of an in vitro ACAID culture model, which mimics the unique antigen processing that is believed to occur in the anterior chamber of the eye. The in vitro ACAID culture utilizes antigen presenting cells (i.e. macrophages) that are pulsed with antigen in the presence of the ocular cytokine, TGF-β.36 When these ACAID-inducing APC are injected intravenously, they induce an antigen-specific down-regulation of DTH that displays the characteristics of ACAID.11,16,24,35–38 The capacity to generate ACAID suppressor cells in vitro was confirmed using the two in vitro culture systems and the previously described LAT assay (Fig. 1b).
CD4+ T cells are needed to generate ACAID suppressor cells
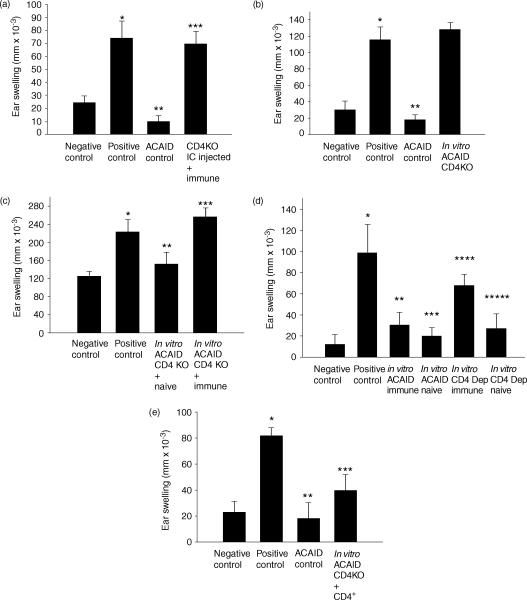
ACAID was induced by conventional i.c. injection of antigen, as well as by the in vitro ACAID culture system, to determine if CD4+ T cells were needed to generate ACAID suppressor cells. First, soluble OVA was injected i.c. into CD4 KO mice on day 0. Seven days later, the i.c.-injected mice were s.c. immunized with OVA in CFA. On day 14 spleens were isolated from the i.c.-injected mice and tested for the presence of ACAID suppressor cells in the LAT assay. Spleen cells from i.c.-injected mice were combined with OVA immune cells and coinjected with OVA into the ear pinnae of naïve mice. The presence of suppressor cells was demonstrated when the DTH responses, which are normally displayed by immune cells, were suppressed by the presence of i.c.-induced regulatory cells. The results in Fig. 2(a) indicate that normal mice injected i.c. with OVA were able to generate ACAID suppressor cells. By contrast, CD4 KO mice were unable to develop ACAID suppressor cells and instead, expressed normal DTH responses.
Figure 2.
CD4+ T cells are needed to generate ACAID suppressor cells. (a) ACAID was induced in vivo by i.c. injection of OVA into CD4 KO mice or normal C57BL/6 mice (ACAID control). i.c.-injected animals were s.c. immunized with OVA in CFA on day 7. Spleen cells were collected on day 14 and tested for suppressor cells in a LAT assay. (b) ACAID regulatory cells were produced in vitro and tested for the presence of suppressor cells in a LAT assay. (c) ACAID was initiated in an in vitro ACAID culture in which OVA-induced ACAID APC were combined with normal C57BL/6 spleen cells or CD4 KO spleen cells. Cultured spleen cells were collected and combined with naïve spleen cells and tested for the presence of immune cells in a LAT assay. (d) OVA-induced ACAID APC (5 × 106 cells) were combined with normal C57BL/6 spleen cells or CD4+ T-cell depleted spleen cells (50–100 × 106 cells). Cultured spleen cells were collected and combined with OVA immune cells and tested for the presence of suppressor cells in a LAT assay. (e) ACAID was initiated in an in vitro using CD4 KO spleen cells that were reconstituted with normal CD4+ T cells. Cultured spleen cells were tested for the presence of suppressor cells in a LAT assay. Positive control animals received immune cells combined with naïve cells, while negative control animals received naïve cells only. Ear swelling was measured 24 h after injecting OVA into the ear pinna. All results are reported as mean ear swelling ± SE. (a) *P = 0·004 for positive versus negative controls. **P = 0·0002 for ACAID versus positive controls. ***P = 0·0008 for i.c. CD4 KO versus ACAID control. (b) *P = 0·001 for positive versus negative controls. **P = 0·0001 for ACAID versus positive controls. ***P = 0·0001 for CD4 KO versus ACAID control. (c) *P = 0·02 for positive versus negative controls. **P = 0·02 for negative control versus CD4 KO + immune. ***P = 0·05 for positive control versus CD4 KO + naïve. (d) *P = 0·008 for positive versus negative controls. **P = 0·04 for ACAID immune versus positive control. ***P = 0·01 for ACAID naïve versus positive control. ****P = 0·03 for CD4 depleted immune versus ACAID immune. *****P = 0·03 for CD4 Depleted naïve versus CD4 depleted immune. (e) *P = 0·0007 for negative versus positive control. **P = 0·002 for ACAID versus positive control. ***P = 0·01 for positive control versus CD4 KO + CD4. Each group represents at least five animals.
The in vitro ACAID culture system was also used to ascertain a potential role of CD4+ T cells in ACAID suppressor cell production. ACAID APC were generated in vitro and incubated with spleen cells from either normal C57BL/6 mice or CD4 KO mice. Five to 7 days later, cell suspensions from the in vitro culture were tested in the LAT assay for the presence of ACAID suppressor cells. That is, cultured cells were combined with OVA immune cells and coinjected with antigen into the ears of normal C57BL/6 mice. The results of the ear swelling assay indicate that ACAID suppressor cells were present in the ACAID cultures from normal mice, but not from CD4 KO mice (Fig. 2b).
The in vitro ACAID cultures were tested to ensure that the positive ear swelling found with cells generated in ACAID cultures using CD4 KO cells was due to the absence of CD4+ regulatory T cells, and not an artefact of the in vitro culture system itself or due to capricious swelling produced by a cell population unique to CD4 KO mice. That is, one might argue that CD4 KO mice develop a cell population that non-specifically produces ear swelling when injected into the ears of naïve mice. Accordingly, CD4 KO spleen cells were coinjected with normal spleen cells instead of immune cells to determine if cells from CD4 KO mice would produce generalized swelling. The results summarized in Fig. 2(c) indicate that neither the in vitro ACAID culture system nor cells from the CD4 KO mice produced capricious ear swelling. Thus, the positive results in the previous LAT assay were due to the absence of suppressor cells in the cultures using CD4 KO spleen cells. To further ensure that the failure to develop ACAID suppressor cells was caused by the absence of CD4+ T cells and not by other defects of the CD4 KO mice, the in vitro ACAID culture was performed using normal C57BL/6 spleens depleted of CD4+ T cells. This would rule out spurious results produced by spleen cell populations that might be unique to the CD4 KO mouse. In this experiment ACAID APC were cultured with either normal C57BL/6 spleen cells or with CD4-depleted C57BL/6 spleen cells. The cultures were then tested for the presence of ACAID suppressor cells in the LAT assay. As shown in Fig. 2(d), when C57BL/6 spleens were depleted of CD4+ T cells, no ACAID suppressors were generated. Similar to the CD4 KO in vitro cultures, these CD4 T cell-depleted cultures did not produce positive ear swelling when coinjected with naïve spleen cells. Thus, the positive DTH responses were caused by a lack of CD4+ T cells and were not an artefact of the in vitro ACAID culture system.
Finally, experiments verified that the absence of CD4+ T cells was responsible for the inability to produce ACAID suppressor cells. ACAID APC were cultured with CD4 KO spleen cells that were reconstituted with CD4+ T cells from normal C57BL/6 donors. These cultures were tested for the presence of ACAID suppressor cells in the LAT assay. The results shown in Fig. 2(e) demonstrate that the addition of CD4+ T cells restored the capacity of CD4 KO spleen cultures to generate ACAID suppressor cells.
Generation of ACAID suppressor cells does not require contact between CD4+ T cells and CD8+ T cells
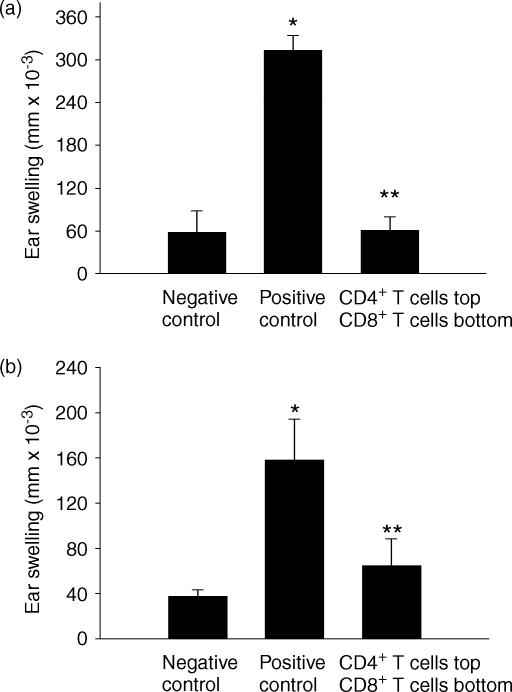
In vitro experiments were performed to determine if cell contact between CD4+ T cells and CD8+ T cells is needed for the generation of ACAID suppressor cells. A modified in vitro ACAID culture system utilized a transwell insert with a semipermeable membrane that permitted the free movement of macromolecules between the two culture chambers, but prevented the migration of lymphocytes. The membrane insert separated the CD4+ T cells and CD8+ T cells. ACAID APC and CD4+ spleen cells (CD8 KO donors) were placed in the top culture chamber, and CD8+ spleen cells (CD4 KO donors) were placed in the bottom culture chamber. The in vitro ACAID transwell cultures were incubated for 5–7 days and the spleen cells in the bottom chambers (i.e. CD8+ T cells) were assessed for ACAID suppressor activity in a LAT assay. The results demonstrate that the CD8+ T cells inhibited DTH responses (Fig. 3a). Thus, direct cell contact between the CD4+ spleen cells and CD8+ spleen cells is not needed for the generation of ACAID suppressor cells.
Figure 3.
CD4+ T cells and CD8+ T cells do not need cell/cell contact to generate ACAID suppressor cells. A modified in vitro ACAID culture separated spleen cell populations using a transwell. (a) The top chamber contained ACAID APC with spleen cells from CD8 KO mice. The bottom chamber contained spleen cells from CD4 KO mice. (b) The top chamber contained ACAID APC with C57BL/6 spleen cells depleted of CD8+ T cells. The bottom chamber contained C57BL/6 spleen cells depleted of CD4+ T cells. The bottom chambers were tested for the presence of suppressor cells in the LAT assay. Positive control animals received immune cells combined with naïve cells, while negative control animals received naïve cells only. Ear swelling was measured 24 hr after OVA injection into the ear pinna. All results are reported as mean ear swelling ± SE. (a) *P = 0·0001 for positive versus negative controls. **P = 0·0001 for the transwell versus positive control. (b) *P = 0·003 positive versus negative controls. **P = 0·008 for the transwell versus positive control. Each group represents at least five animals.
To eliminate concerns regarding the use of KO mice, additional experiments were performed with C57BL/6 spleen cells that were depleted of the appropriate T-cell population. The in vitro transwell culture was performed as before. However, in this experiment the top chamber contained normal C57BL/6 spleen cells depleted of CD8+ T cells, while the bottom chamber contained normal C57BL/6 spleen cells depleted of CD4+ T cells. As shown in Fig. 3(b), depleting the selected population of T cells maintained the ability to produce suppressor cells. This further supports the result demonstrating that CD4+ T cells and CD8+ T cells do not require direct contact for the production of ACAID suppressor cells.
CD4+ T cells must produce IL-10 for the generation of ACAID suppressor cells
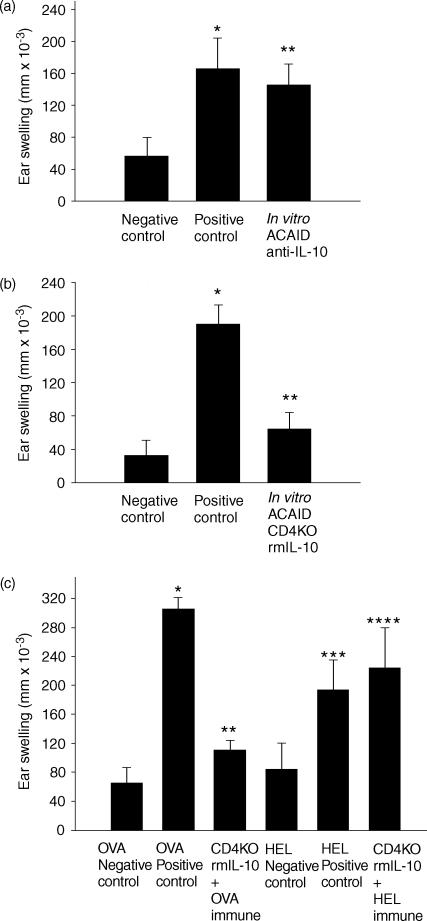
Because cell contact between CD4+ and CD8+ T cells was not needed for the generation of ACAID efferent suppressor cells, cytokines were explored as potential factors required in the ACAID environment. Because animals possessing the ACAID phenotype display a Th2-like profile26,39 IL-4 and IL-10 were examined as potential contributors by the CD4+ T cells for the production of ACAID suppressor cells. The in vitro CD4 KO ACAID culture was utilized. ACAID APC were combined with CD4 KO spleen cells that were reconstituted with CD4+ T cells. As shown above, CD4 KO ACAID spleen cells reconstituted with normal C57BL/6 CD4+ T cells are able to generate ACAID efferent suppressor cells (Fig. 2e). However, as shown in Fig. 4(a), CD4 KO spleen cultures reconstituted with CD4+ T cells from IL-10 KO mice are unable to generate ACAID suppressor cell activity. By contrast, in vitro ACAID cultures using spleen cells from CD4 KO that were reconstituted with CD4+ T cells from IL-4 KO mice were able to generate efferent suppressor cells (Fig. 4b).
Figure 4.
CD4+ T cells must produce IL-10 for the generation of efferent suppressor cells. An in vitro ACAID culture system combined OVA induced ACAID APC with CD4 KO spleen cells reconstituted with CD4+ T cells from (a) IL-10 KO mice or (b) IL-4 KO mice. The putative suppressor populations were tested in a LAT assay. Positive control animals received immune cells combined with naïve cells, while negative control animals received naïve cells only. Ear swelling was measured 24 hr after intradermal injection into the ear pinna. All results are reported as mean ear swelling ± SE. (a) *P = 0·003 for positive versus negative controls. **P = 0·003 for ACAID versus positive controls. ***P = 0·003 for CD4 KO + IL-10 KO CD4 versus ACAID control. (b) *P = 0·02 for positive versus negative controls. **P = 0·02 for ACAID versus positive controls. ***P = 0·003 for CD4 KO + IL-4 KO CD4 versus positive control. Each group represents at least five animals.
IL-10 is necessary and sufficient for the generation of ACAID suppressor cells
In order to verify the role of IL-10 in generating ACAID efferent suppressor cells, two approaches were used. The first approach confirmed the role of IL-10 in the induction of ACAID suppressor cells using cells from wild-type mice instead of IL-10 KO mice. The in vitro ACAID culture combined ACAID APC's with normal C57BL/6 spleens in the presence or absence of neutralizing anti-IL-10 antibody. These cultures were then tested for the presence of DTH efferent suppressor cells in the LAT assay. The results demonstrated that ACAID suppressor cells were not generated in the presence of anti-IL-10 antibody (Fig. 5a). The second approach determined if exogenous IL-10 could restore efferent suppressor cell production in the in vitro ACAID cultures containing CD4KO spleen cells. OVA-pulsed ACAID APC were added to the in vitro ACAID cultures containing CD4 KO spleens in the presence of recombinant murine IL-10. The development of ACAID suppressor cells was assessed using the LAT assay. The results of a typical experiment are shown in Fig. 5(b), and clearly demonstrate that exogenous IL-10 was able to compensate for the absence of CD4+ T cells. Additional experiments confirmed that in the absence of CD4+ T cells, exogenous IL-10 was able to generate antigen-specific ACAID suppressor cells (Fig. 5c). In the latter experiment, the in vitro ACAID cultures contained OVA-pulsed ACAID APC's combined with CD4 KO spleen cells in the presence of exogenous IL-10. These cell cultures were tested for their ability to suppress DTH responses to HEL immune cells. The results clearly demonstrate that the ACAID suppressor cells generated in the presence of IL-10 suppressed DTH in an antigen-specific manner.
Figure 5.
IL-10 is necessary and sufficient for the generation of ACAID efferent suppressor cells. (a) In vitro spleen cell cultures combined OVA-induced ACAID APC with spleen cells from normal C57BL/6 mice cultured in the presence of anti-IL-10 antibody. The suppression of OVA-specific DTH was tested in a LAT assay. (b) In vitro ACAID cultures combined OVA-induced ACAID APC with spleen cells from CD4 KO mice in the presence of rmIL-10 cytokine. The suppression of OVA-specific DTH was tested in a LAT assay. (c) In vitro ACAID cultures combined OVA-induced ACAID APC's with spleen cells from normal C57BL/6 cultured in the presence of anti-IL-10 antibody. These cultures were tested for the ability to suppress HEL-specific immune responses. Positive control animals received immune cells combined with naïve cells, while negative control animals received naïve cells only. Ear swelling was measured 24 hr after intradermal injection into the ear pinna. All results are reported as mean ear swelling ± SE. *P = 0·03 for positive versus negative controls. **P = 0·0002 in vitro ACAID + anti-IL-10 versus negative control. (b) *P = 0·0007 for positive versus negative controls. **P = 0·001 for CD4 KO + rmIL-10 versus positive controls. (c) *P = 0·0001 for OVA positive versus OVA negative. **P = 0·0002 for OVA positive versus CD4 KO + IL-10 OVA immune. ***P = 0·03 for HEL positive versus HEL negative. ****P = 0·03 for HEL negative versus CD4 KO + IL-10 HEL immune. Each group represents at least five animals.
ACAID CD4+ T cells required for DTH suppressor cell production are not NK T cells
IL-10 producing NK T cells play an important role in the induction of ACAID.22 The in vitro ACAID culture system was used to determine if the CD4+ afferent ACAID T suppressor cells were NK T cells. Spleen cell suspensions from CD4 KO donors were added to in vitro ACAID cultures in the presence of OVA pulsed ACAID APC. The CD4 KO in vitro ACAID cultures were reconstituted with wild-type CD4+ T-cell suspensions that were either untreated, or depleted of NK cells prior to their addition to the in vitro cultures. NK cells were labelled with anti-2B4, -Ly49C, and -DX5 and depleted using magnetic bead separation. These ACAID cultures were then tested in a LAT assay for presence of suppressor cells. Results indicated that CD4+ T-cell suspensions depleted of NK cells suppressor cells were able to induce the generation of efferent suppressor cells in the in vitro ACAID culture system (Fig. 6a).
Figure 6.
The CD4+ T cells needed for ACAID efferent suppressor cell production are not NKT cells. (a) In vitro ACAID cultures combined OVA-induced ACAID APC with CD4 KO spleen cells that were reconstituted with normal C57BL/6 CD4+ T cells, or CD4+ T cells depleted of NK cells. Cultures were tested for efferent suppressor cell activity in the LAT assay. (b) In vitro ACAID culture combined OVA-induced ACAID APC with normal C57BL/6 spleen cells (containing both CD4+ T cells and CD8+ T cells) or normal C57BL/6 spleen cells depleted of NK cells. The spleen cell culture was tested for suppressor cell activity using a LAT assay. Positive control animals received immune cells combined with naïve cells, while negative control animals received naïve cells only. Ear swelling was measured 24 hr after OVA injection into the ear pinna. All results are reported as mean ear swelling ± SE. (a) *P = 0·003 for positive versus negative controls. **P = 0·003 for CD4 KO + CD4 versus positive controls. ***P = 0·01 for CD4 KO + CD4+ NK− T cells versus positive control. (B) *P = 0·02 for positive versus negative control. **P = 0·008 for ACAID versus positive control. ***P = 0·0007 for positive control versus NK depleted + naïve. ****P = 0·006 for ACAID versus NK depleted + immune. Each group represents at least five animals.
An additional in vitro culture was performed to confirm that the depletion protocol used was sufficient to remove the NK T cells. In this experiment, ACAID APC were combined with normal C57BL/6 spleen cell cultures containing both CD4+ and CD8+ cell populations. The spleen cell cultures were either untreated, or depleted of NK cells using the same protocol as above. As show in Fig. 6(b), when normal spleen cell cultures were depleted of NK cells, ACAID suppressor cells were not produced. Therefore, the NK cell depletion protocol did indeed remove the NK T-cell population that is necessary for the generation of ACAID. However, the requisite NK T-cell population does not express the CD4 surface determinant.
CD25+ T cells are required for the production of ACAID suppressor cells
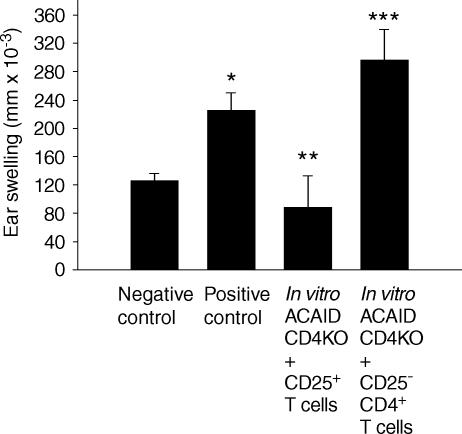
A growing body of evidence indicates that CD4+ regulatory T cells express the IL-2 receptor, CD25.33 The role of CD25+ T cells in the development of ACAID suppressor cells was tested using the in vitro ACAID cultures containing spleen cells from CD4 KO donors. ACAID APC were combined with CD4 KO spleen cell suspensions that were reconstituted with CD4+ T cells depleted of CD25+ cells. The CD25+ T cells were labelled with anti-CD25 conjugated with FITC, and isolated with anti-FITC magnetic beads. The results show that depletion of CD25+ T cells prevented the production of ACAID suppressor cells (Fig. 7a). Likewise, CD4 KO spleen cell cultures reconstituted with CD25+-enriched T cells supported the generation of ACAID suppressor cells indicating that CD25+ T cells are required to produce suppressor cells in the in vitro ACAID culture.
Figure 7.
CD25+ T cells are required to generate ACAID efferent suppressor cells. The in vitro ACAID cultures combined OVA-induced ACAID APC with spleen cells from CD4 KO mice reconstituted with CD4+ T cells depleted of CD25+ T cells, or T cells that were enriched for CD25+ T cells. The ACAID spleen cell cultures were tested for suppressor cell activity in a LAT assay. Positive control animals received immune cells combined with naïve cells, while negative control animals received naïve cells only. Ear swelling was measured 24 hr after OVA injection into the ear pinna. All results are reported as mean ear swelling ± SE. *P = 0·02 for positive versus negative controls. **P = 0·02 for CD4 KO + CD25+ T cells versus positive control. ***P = 0·01 for CD4 KO + CD25−CD4+ versus negative control. Each group represents at least five animals.
Discussion
Unlike most other sites, the eye cannot tolerate inflammatory responses. DTH lesions are especially damaging to the eye because of the extensive collateral injury that is inflicted on innocent bystander cells that have very limited or no regenerative properties. ACAID is one of the safeguard mechanisms that protect the eye from immune-mediated inflammation. In the 25 years since its description, ACAID has been intensely studied and found to be a highly complicated system involving an intricate array of immune cell populations.
From time to time the question arises as to whether ACAID is simply a form of intravenously induced immune deviation. However, there is compelling evidence that these two forms of immune deviation are distinctly different.9 ACAID requires the participation of various cytokines, molecules, and cell populations that are not necessary for the development of intravenously induced immune deviation; these include: (a) IL-10;22 (b) NK T cells;23 (c) B cells;16,40,41 and (d) β2-microglobulin.14 By contrast, intravenously induced immune deviation requires IL-4, while ACAID does not.39 Moreover, intravenously induced immune deviation generates CD8+ afferent regulatory T cells, while ACAID afferent regulatory cells are CD4+.10 Although intravenously induced immune deviation and ACAID are both characterized by a deficiency in IFN-γ production26,39,42 the development of efferent suppressor cells sets ACAID apart from intravenous immune deviation and suggests that ACAID involves more than the mere down regulation of IFN-γ.10 Therefore, the present study examined the role of CD4+ T cells in the generation of ACAID efferent suppressor cells, as this is a crucial distinction between ACAID and other forms of immune tolerance.
The most widely embraced model of ACAID proposes that constituents of aqueous humour, namely TGF-β, alter the behaviour of ocular APC, rendering them tolerogenic.36,43 The rationale for using macrophages in the in vitro model for generating ACAID-inducing APC is based on the following observations: (a) significant numbers of MHC class II negative, F4/80+ macrophages are found in the iris/ciliary body and induce ACAID when isolated, pulsed with antigen, and injected into naïve mice;35 (b) MHC class II-negative, F4/80+ macrophages are found in the bloodstream 24 hr after i.c. injection of antigen and induce ACAID when injected intravenously into naïve recipients;11,13,44 and (c) although other APC are found in the anterior segment of the eye, they are MHC class II positive and there is no evidence that they can induce ACAID.45,46 Moreover, ACAID is abrogated if either MHC class II-positive Langerhans cells are induced to migrate into the cornea47 or if MHC class II-positive dendritic cells are coinjected with antigen into the anterior chamber of the eye.48 Based on these previous findings and the fact that the in vitro ACAID APC culture system has been used extensively by us and others to analyse ACAID,10,11,24,36–38,40 we used macrophages to generate and characterize CD4+ ACAID suppressor cells.
Following the processing of ocular antigens, ACAID APC migrate from the eye to the spleen where they induce the generation of antigen-specific suppressor cells.11–13,43 CD4+ T cells isolated from the spleens of ACAID-induced animals are capable of down-regulating T-cell proliferation responses to specific antigens, while CD8+ T cells isolated from the spleens of ACAID-induced mice display the ability to suppress expression of a DTH response.10 Recently, splenic B cells have been shown to play a role in the generation of CD8+ ACAID suppressor cells.16,17,40 Although the exact role of the B cell in ACAID is not known, it is hypothesized that splenic B cells acquire antigen from ACAID APC and present the antigen to CD8+ suppressor cells.
In addition to B cells, γδ T cells are involved in generating ACAID suppressor cells.18,19 However, the exact role of the γδ T cells has not been elucidated. NK T cells are also necessary for the generation of ACAID suppressor cells.21–23 NK T cells are thought to migrate from the thymus to the spleen.23 Recruitment of the NK T cells to the spleen requires the chemokine macrophage inhibitory protein-2. Once in the spleen, the NK T cells produce IL-10 and promote the generation of ACAID suppressor cells.
Another key player in the evolution of ACAID is the CD4+ T cell. CD4+ T cells were first recognized in ACAID for their role as cells that were capable of inhibiting T cell proliferative responses to specific antigen.10 Li et al. later showed that anterior chamber inoculation of antigen induced the development of CD4+ T cells that preferentially produced increased amounts of IL-10, but decreased amounts of IFN-γ.26 Kosiewicz et al. also showed that CD8+ suppressor cells are produced by a class II-restricted peptide, thereby suggesting an ancillary role for CD4+ T cells in the induction of ACAID.29
Results from in vitro and in vivo experiments indicate that CD4+ T cells are in fact involved in the generation of ACAID regulatory T cells that are capable of suppressing the expression of a DTH response. ACAID cannot be induced in CD4 KO hosts or in the in vitro ACAID culture system using spleen cells from CD4 KO donors, or with CD4 depleted spleen cells. However, ACAID suppressor cell production can be restored when CD4-deficient spleen cell cultures are reconstituted with wild-type CD4+ T cells, but only if the CD4+ T cells are capable of producing IL-10. Interestingly, CD4+ T cells defective in IL-4 production can successfully induce ACAID suppressor cells in vitro. This is consistent with previous studies that demonstrate that there is an increased production of IL-10, but not IL-4, during ACAID.26
Previous in vivo and in vitro studies have clearly demonstrated that IL-10 is necessary for the development of ACAID.22,24,26,39,49 IL-10 production is crucial in the early stages of ACAID as ocular APC from IL-10 KO mice cannot induce ACAID, either in vitro or in vivo.24 IL-10 is also needed in the splenic stage of ACAID. Sonoda and coworkers have shown that IL-10 producing NKT cells are needed for the splenic stage of ACAID.22 The results shown here also indicate that splenic CD4+ T cells must produce IL-10 for the generation of ACAID efferent suppressor cells. Although the NK T cells and the CD4+ T cells that are induced in ACAID are potentially the same population, depletion studies indicate otherwise. That is, CD4+ T cells depleted of NK cells retain their capacity to induce the development of ACAID suppressor cells. This finding is consistent with those of Wang et al. who showed that the thymus-derived NK T cells involved in ACAID were CD4− CD8− T cells.44
A growing body of evidence suggests that CD4+ T cells are important regulators of immune responses.33 Under some conditions, CD4+ T cells produce the immunomodulatory cytokines IL-10 and TGF-β and inhibit lymphoproliferative responses to alloantigens.50,51 Additionally, CD4+ T cells have been shown to influence the development of CD8+ suppressor T cells that inhibit DTH responses.30,31 Finally, emerging evidence also indicates that the CD25 molecule is expressed on a wide array of CD4+ regulatory cells.33,34,52–55 CD25 is a component of the IL-2R that is transiently expressed on activated CD4+ T cells.56 Some studies have indicated that CD25+ T cells produce high levels of TGF-β and IL-10.34,53 These findings would support the results presented here. However, other studies have suggested that CD25+ T cells require cell contact and are cytokine-independent, which is not consistent with the results reported here.34,55 These earlier studies focused on the role of CD25+ CD4+ T cells in suppressing other CD4+ T cells; while studies here focused on the role of CD4+ T cells in generating suppressor T cells. The results reported here demonstrate that reconstitution of CD4 KO spleens with CD25− CD4+ T cells does not generate ACAID suppressor cells. Therefore, the CD4+ T cells involved in generating suppressor cells must express CD25.
The present findings support previous reports indicating that CD4+ T cells are not only present in ACAID, but they are also required for the development of suppressor cells capable of down-regulating DTH responses. The CD4+ afferent suppressor T cells are not NK T cells, but CD25+ expression is needed. Results showing direct cell contact between CD4+ T cells and CD8+ suppressor T cells support a role of a soluble cytokine, IL-10, in this system. Numerous studies have demonstrated that ancillary cells are also needed for the generation of ACAID. Unravelling how NK T cells, B cells, and γδ T cells participate in the development of ACAID remains a daunting, but exciting task.
Acknowledgments
This work was supported by NIH grants EY05631, EY07641, and an unrestricted grant from Research to Prevent Blindness, Inc., New York, NY. We would like to thank Ms. Christina Hay for her technical assistance with these experiments.
Abbreviations
- ACAID
anterior chamber-associated immune deviation
- DTH
delayed-type hypersensivity
- i.c.
intracamerally
- LAT
local adoptive transfer
- OVA
ovalbumin
References
- 1.Streilein JW. Immune privilege as the result of local tissue barriers and immunosuppressive microenvironments. Curr Opin Immunol. 1993;5:428–32. doi: 10.1016/0952-7915(93)90064-y. [DOI] [PubMed] [Google Scholar]
- 2.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–92. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 3.Griffith TSYuX, Herndon JM, Green DR, Ferguson TA. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 4.Pepose JS, Nestor MS, Gardner KM, Foos RY, Pettit TH. Composition of cellular infiltrates in rejected human corneal allografts. Graefes Arch Clin Exp Ophthalmol. 1985;222:128–33. doi: 10.1007/BF02173536. [DOI] [PubMed] [Google Scholar]
- 5.Whitsett CF, Stulting RD. The distribution of HLA antigens on human corneal tissue. Invest Ophthalmol Vis Sci. 1984;25:519–24. [PubMed] [Google Scholar]
- 6.Treseler PA, Foulks GN, Sanfilippo F. The relative immunogenicity of corneal epithelium, stroma, and endothelium. The role of major histocompatibility complex antigens. Transplantation. 1986;41:229–34. doi: 10.1097/00007890-198602000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Fujikawa LS, Colvin RB, Bhan AK, Fuller TC, Foster S. Expression of HLA-A/B/C and -DR locus antigens on epithelial, stromal, and endothelial cells from the human cornea. Cornea. 1982;1:213–22. [Google Scholar]
- 8.Niederkorn JY, Streilein JW. Alloantigens placed into the anterior chamber of the eye induce specific suppression of delayed-type hypersensitivity but normal cytotoxic T lymphocyte and helper T lymphocyte responses. J Immunol. 1983;131:2670–4. [PubMed] [Google Scholar]
- 9.Niederkorn JY. Immune Privilege in the Anterior Chamber of the Eye. Crit Rev Immunol. 2002;22:13–46. [PubMed] [Google Scholar]
- 10.Wilbanks GA, Streilein JW. Characterization of suppressor cells in anterior chamber-associated immune deviation (ACAID) induced by soluble antigen. Evidence of two functionally and phenotypically distinct T-suppressor cell populations. Immunology. 1990;71:383–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Wilbanks GA, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID). 1. Evidence that an antigen-specific, ACAID- inducing, cell-associated signal exists in the peripheral blood. J Immunol. 1991;146:2610–7. [PubMed] [Google Scholar]
- 12.Wilbanks GA, Mammolenti M, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID). II. Eye-derived cells participate in generating blood-borne signals that induce ACAID. J Immunol. 1991;146:3018–24. [PubMed] [Google Scholar]
- 13.Streilein JW, Okamoto S, Hara Y, Kosiewicz M, Ksander B. Blood-borne signals that induce anterior chamber-associated immune deviation after intracameral injection of antigen. Invest Ophthalmol Vis Sci. 1997;38:2245–54. [PubMed] [Google Scholar]
- 14.Hara Y, Okamoto S, Rouse B, Streilein JW. Evidence that peritoneal exudate cells cultured with eye-derived fluids are the proximate antigen-presenting cells in immune deviation of the ocular type. J Immunol. 1993;151:5162–71. [PubMed] [Google Scholar]
- 15.Kezuka T, Streilein JW. Analysis of in vivo regulatory properties of T cells activated in vitro by TGFbeta2-treated antigen presenting cells. Invest Ophthalmol Vis Sci. 2000;41:1410–21. [PubMed] [Google Scholar]
- 16.D'Orazio TJ, Niederkorn JY. Splenic B cells are required for tolerogenic antigen presentation in the induction of anterior chamber-associated immune deviation (ACAID) Immunology. 1998;95:47–55. doi: 10.1046/j.1365-2567.1998.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niederkorn JY, Mayhew E. Role of splenic B cells in the immune privilege of the anterior chamber of the eye. Eur J Immunol. 1995;25:2783–7. doi: 10.1002/eji.1830251011. [DOI] [PubMed] [Google Scholar]
- 18.Skelsey ME, Mellon J, Niederkorn JY. Gamma delta T cells are needed for ocular immune privilege and corneal graft survival. J Immunol. 2001;166:4327–33. doi: 10.4049/jimmunol.166.7.4327. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Kapp JA. gammadelta T cells are critical for the induction of anterior chamber- associated immune deviation. Immunology. 2001;104:142–8. doi: 10.1046/j.0019-2805.2001.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Goldschneider I, Foss D, Wu DY, O'Rourke J, Cone RE. Direct thymic involvement in anterior chamber-associated immune deviation: evidence for a nondeletional mechanism of centrally induced tolerance to extrathymic antigens in adult mice. J Immunol. 1997;158:2150–5. [PubMed] [Google Scholar]
- 21.Sonoda KH, Exley M, Snapper S, Balk SP, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 1999;190:1215–26. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonoda KH, Faunce DE, Taniguchi M, Exley M, Balk S, Stein-Streilein J. NK T cell-derived IL-10 is essential for the differentiation of antigen-specific T regulatory cells in systemic tolerance. J Immunol. 2001;166:42–50. doi: 10.4049/jimmunol.166.1.42. [DOI] [PubMed] [Google Scholar]
- 23.Faunce DE, Sonoda KH, Stein-Streilein J. MIP-2 recruits NKT cells to the spleen during tolerance induction. J Immunol. 2001;166:313–21. doi: 10.4049/jimmunol.166.1.313. [DOI] [PubMed] [Google Scholar]
- 24.D'Orazio TJ, Niederkorn JY. A novel role for TGF-beta and IL-10 in the induction of immune privilege. J Immunol. 1998;160:2089–98. [PubMed] [Google Scholar]
- 25.D'Orazio TJ, Niederkorn JY. The nature of antigen in the eye has a profound effect on the cytokine milieu and resultant immune response. Eur J Immunol. 1998;28:1544–53. doi: 10.1002/(SICI)1521-4141(199805)28:05<1544::AID-IMMU1544>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 26.Li XY, D'Orazio LT, Niederkorn JY. Role of Th1 and Th2 cells in anterior chamber-associated immune deviation. Immunology. 1996;89:34–40. doi: 10.1046/j.1365-2567.1996.d01-714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991;32:2201–11. [PubMed] [Google Scholar]
- 28.Granstein RD, Staszewski R, Knisely TL, Zeira E, Nazareno R, Latina M, Albert DM. Aqueous humor contains transforming growth factor-beta and a small (less than 3500 daltons) inhibitor of thymocyte proliferation. J Immunol. 1990;144:3021–7. [PubMed] [Google Scholar]
- 29.Kosiewicz MM, Streilein JW. Intraocular injection of class II-restricted peptide induces an unexpected population of CD8 regulatory cells. J Immunol. 1996;157:1905–12. [PubMed] [Google Scholar]
- 30.Li SP, Lee SI, Wang Y, Domer JE. Candida albicans mannan-specific, delayed hypersensitivity down- regulatory CD8+ cells are genetically restricted effectors and their production requires CD4 and I-A expression. Int Arch Allergy Immunol. 1996;109:334–43. doi: 10.1159/000237260. [DOI] [PubMed] [Google Scholar]
- 31.Negoro T, Iinuma F, Watanabe M. Cellular induction mechanism of CD8+ suppressor T cells by DMBA and TPA: formation of CD4+ suppressor–inducer T cells. Cell Immunol. 1996;167:216–23. doi: 10.1006/cimm.1996.0029. [DOI] [PubMed] [Google Scholar]
- 32.Ke Y, Pearce K, Lake JP, Ziegler HK, Kapp JA. Gamma delta T lymphocytes regulate the induction and maintenance of oral tolerance. J Immunol. 1997;158:3610–8. [PubMed] [Google Scholar]
- 33.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–22. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 34.Thornton AM, Shevach EM. CD4+ CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williamson JS, Bradley D, Streilein JW. Immunoregulatory properties of bone marrow-derived cells in the iris and ciliary body. Immunology. 1989;67:96–102. [PMC free article] [PubMed] [Google Scholar]
- 36.Wilbanks GA, Streilein JW. Fluids from immune privileged sites endow macrophages with the capacity to induce antigen-specific immune deviation via a mechanism involving transforming growth factor-beta. Eur J Immunol. 1992;22:1031–6. doi: 10.1002/eji.1830220423. [DOI] [PubMed] [Google Scholar]
- 37.Wilbanks GA, Strelien JW. Macrophages capable of inducing anterior chamber associated immune deviation demonstrate spleen-seeking migratory properties. Reg Immunol. 1992;4:130–7. [PubMed] [Google Scholar]
- 38.Sohn JH, Bora PS, Suk HJ, Molina H, Kaplan HJ, Bora NS. Tolerance is dependent on complement C3 fragment iC3b binding to antigen-presenting cells. Nat Med. 2003;9:206–12. doi: 10.1038/nm814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kosiewicz MM, Alard P, Streilein JW. Alterations in cytokine production following intraocular injection of soluble protein antigen. impairment in IFN-gamma and induction of TGF-beta and IL-4 production. J Immunol. 1998;161:5382–90. [PubMed] [Google Scholar]
- 40.D'Orazio TJ, Mayhew E, Niederkorn JY. Ocular immune privilege promoted by the presentation of peptide on tolerogenic B cells in the spleen. II. Evidence for presentation by Qa-1. J Immunol. 2001;166:26–32. doi: 10.4049/jimmunol.166.1.26. [DOI] [PubMed] [Google Scholar]
- 41.Vella AT, Scherer MT, Schultz L, Kappler JW, Marrack P. B cells are not essential for peripheral T-cell tolerance. Proc Natl Acad Sci U S A. 1996;93:951–5. doi: 10.1073/pnas.93.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Josien R, Cuturi MC, Douillard P, Heslan M, Heslan JM, Soulillou JP. Recombinant IFN-gamma abrogates allograft tolerance induced by donor-specific blood transfusion by restoring alloantibody production. Eur J Immunol. 1999;29:317–26. doi: 10.1002/(SICI)1521-4141(199901)29:01<317::AID-IMMU317>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 43.Wilbanks GA, Mammolenti M, Streilein JW. Studies on the induction of anterior chamber-associated immune deviation (ACAID). III. Induction of ACAID depends upon intraocular transforming growth factor-beta. Eur J Immunol. 1992;22:165–73. doi: 10.1002/eji.1830220125. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Goldschneider I, O'Rourke J, Cone RE. Blood mononuclear cells induce regulatory NK T thymocytes in anterior chamber-associated immune deviation. J Leukoc Biol. 2001;69:741–6. [PubMed] [Google Scholar]
- 45.Jiang HR, Lumsden L, Forrester JV. Macrophages and dendritic cells in IRBP-induced experimental autoimmune uveoretinitis in B10RIII mice. Invest Ophthalmol Vis Sci. 1999;40:3177–85. [PubMed] [Google Scholar]
- 46.McMenamin PG, Holthouse I, Holt PG. Class II major histocompatibility complex (Ia) antigen-bearing dendritic cells within the iris and ciliary body of the rat eye. distribution, phenotype and relation to retinal microglia. Immunology. 1992;77:385–93. [PMC free article] [PubMed] [Google Scholar]
- 47.Williamson JS, DiMarco S, Streilein JW. Immunobiology of Langerhans cells on the ocular surface. I. Langerhans cells within the central cornea interfere with induction of anterior chamber associated immune deviation. Invest Ophthalmol Vis Sci. 1987;28:1527–32. [PubMed] [Google Scholar]
- 48.Benson JL, Niederkorn JY. The presence of donor-derived class II-positive cells abolishes immune privilege in the anterior chamber of the eye. Transplantation. 1991;51:834–8. doi: 10.1097/00007890-199104000-00018. [DOI] [PubMed] [Google Scholar]
- 49.Gao Y, Herndon JM, Zhang H, Griffith TS, Ferguson TA. Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J Exp Med. 1998;188:887–96. doi: 10.1084/jem.188.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 51.Zeller JC, Panoskaltsis-Mortari A, Murphy WJ, Ruscetti FW, Narula S, Roncarolo MG, Blazar BR. Induction of CD4+ T cell alloantigen-specific hyporesponsiveness by IL-10 and TGF-beta. J Immunol. 1999;163:3684–91. [PubMed] [Google Scholar]
- 52.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–96. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–18. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 54.Hara M, Kingsley CI, Niimi M, et al. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–96. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 55.Thornton AM, Shevach EM. Suppressor effector function of CD4+ CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 56.Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]