Abstract
Two phenotypically distinct subpopulations of dendritic cells (SIRPα+ CC81Ag− DC and SIRPα− CC81Ag+ DC) have previously been identified in bovine afferent lymph which show functional differences when assayed in vitro. The purpose of this study was to investigate whether differences in cytokine production between the two subpopulations might occur which could influence the bias of the immune response they stimulate. Qualitative and quantitative polymerase chain reactions were used to detect specific mRNA transcripts and flow cytometry and enzyme-linked immunosorbent assays were used to detect protein production. The SIRPα− CC81Ag+ DC produced considerably more interleukin-12 (IL-12) mRNA transcripts and protein than the SIRPα+ CC81Ag− DC. Conversely, SIRPα+ CC81Ag− DC contained more of both transcripts and protein for IL-1 and of transcripts for IL-6. A small percentage of both subpopulations produced interferon-γ (IFN-γ) as evidenced by cytoplasmic staining. Stimulation of DC by culture with CD40L+ cells increased the production of IL-1, IL-6 and IL-12 but quantitative differences between the subpopulations remained. Production of IL-10 was also evident following culture with CD40L+ cells or lipopolysaccharide primarily by the SIRPα+ CC81Ag− DC. No evidence was found for type 1 IFN production, and hence plasmacytoid DC, by DC in afferent lymph; both subpopulations appear to be myeloid in origin. These different cytokine repertoires of the two subpopulations of ex vivo DC isolated from afferent lymph imply functional differences that could influence the presentation of antigen to T cells and bias of the immune response following vaccination or infection.
Introduction
Dendritic cells (DC) play a unique role in the initiation of immune responses, being the most potent antigen-presenting cells (APC) and the only APC capable of stimulating naïve T cells and initiating primary immune responses. DC are also involved in determining the type of immune response that is initiated, primarily by affecting the cytokine environment in which the T cells respond. In general the production of interleukin-12 (IL-12) by DC has been shown to bias naïve T cells towards a T helper type 1 (Th1) -type [interferon-γ (IFN-γ)-producing] response1–3 whereas the absence of IL-12 and presence of IL-4, IL-6 and IL-10 have all been implicated in skewing naïve T cells toward Th2-type (IL-4-producing) responses.4,5 Whilst the cytokine profile produced by DC may depend to some extent on the cell-type of origin of the DC6–8 this is not fixed. Plasticity in the production of cytokines by DC has been recognized, influenced by such factors as environmental conditions or exposure to microbial products.9–16
DC in afferent lymph (ALDC) are a mixture of cells from various tissue sources migrating to the local lymph node and are potent APC both in vitro and in vivo.17,18 Evidence from a number of studies suggests that ALDC are involved in the transport of antigens to the lymph node where they become the interdigitating DC (IDC) and present to naïve T cells.17–19 They therefore represent a crucial stage of the migration and maturation of DC to be studied in order to understand the initiation of immune responses. Although in vitro models of DC migration and maturation have been developed with murine tissues20,21 there is evidence to suggest that the properties of cultured DC obtained from these systems can be significantly different from DC collected ex vivo from afferent lymph.22 In ruminants it has been possible to obtain fresh ALDC by direct cannulation of the afferent lymphatics of the popliteal node in sheep23 or by pseudoafferent duct cannulation following ablation of superficial lymph nodes in sheep24 and cattle.25 These techniques can provide sufficient cells to allow phenotypic and functional characterization of ex vivo ALDC, unaltered by culture, as they migrate from peripheral tissues to the lymph nodes, thus providing an insight into the mechanisms of in vivo immune responses.
Previous analysis has shown that ALDC draining bovine skin can be divided into two main subpopulations based on their expression of a number of surface antigens.26,27 The major subpopulation expresses the myeloid antigen signal regulatory protein-α (SIRP-α),28 has low intensity or no expression of the integrin CD11a, and is not recognized by the monoclonal antibody (mAb) CC81. Conversely the minor DC subpopulation shows high-intensity expression of CD11a and the antigen recognized by the mAb CC81 (CC81Ag) but does not express SIRP-α. Similar phenotypic subpopulations have been identified in both ovine peripheral lymph (Walsh, Howard and Chaplin, unpublished results) and rat mesenteric lymph,29 suggesting that equivalent groups of DC are likely to be found in afferent lymph across a diverse range of species. Functional studies have shown that the two subpopulations also differ in their ability to stimulate T cells.26,27 Whilst both subpopulations are capable of taking up soluble protein antigen and presenting it to resting memory T cells, SIRP-α+ DC are more effective at stimulating proliferative responses to respiratory syncytial virus (RSV) antigen and allogeneic CD8+ T-cell proliferation. For CD8+ T cells this has been suggested to be the result of a difference in production of IL-1α by the two populations.30
The purpose of the investigation reported here was to examine the cytokine production by the two subpopulations of ex vivo DC obtained from afferent lymph and determine whether there might be differences that would be expected to bias T-cell responses.
Materials and methods
Collection of afferent lymph
Conventionally reared Friesian calves (Bos taurus) were used to provide ALDC, essentially as previously described25 by cannulation of the efferent duct of the pre-scapular lymph node following previous ablation of the node. Cells from the lymph were either used fresh or pelleted, resuspended in fetal calf serum containing 10% dimethylsulphoxide and stored frozen in liquid nitrogen until use. The percentage of ALDC was also enriched in some samples by separation over a density gradient (Histopaque 1083; Sigma-Aldrich Chemical Co. Poole, Dorset, UK) prior to freezing. The comparative studies of cytokine production by fresh and stimulated cells were carried out on paired afferent lymph samples taken from calves 3 or 4 days after cannulation surgery.
Monoclonal antibodies and fluorescence-activated cell sorting (FACS)
DC were distinguished from the other cells in afferent lymph by flow cytometry on the basis of their high forward scatter (FSC) and high-intensity expression of a 210 000-MW antigen currently termed Workshop Cluster 6 (WC6). WC6 antigen was detected with the mAb IL-A53.31 In this study the two major subpopulations of ALDC (SIRP-α+ CC81Ag− and SIRP-α− CC81Ag+) were identified on the basis of their expression of the antigen recognized by the mAb CC81.26 A combination of the fluorescein isothiocyanate (FITC)-labelled, isotype-specific goat anti-mouse immunoglobulin G1 (IgG1) and phycoerythrin (PE) -labelled goat anti-mouse IgG2a mAb (Southern Biotechnology Associates, Birmingham, AL) were used as secondary reagents for the immunofluorescent staining and then the WC6+ CC81Ag− and WC6+ CC81Ag+ subpopulations were isolated using a FACStar-plus (Becton Dickinson, Mountain View, CA) cell sorter. Sorted cell populations were > 99% pure by flow cytometric analysis.
Detection of cytokine RNA transcripts
Extraction of mRNA and synthesis of cDNA
Polyadenylated mRNA was extracted from 5 × 104 FACS-sorted cells from each of the two ALDC populations using the Dynabeads® mRNA Direct Kit [Dynal (UK) Ltd, Merseyside, UK]. Extracted mRNA was eluted in 10 mm Tris–HCl buffer and synthesis of single-stranded cDNA was carried out by use of the Promega Reverse Transcription system (Promega, Madison, WI).
To study the effect of CD40 ligation on the production of cytokines by ALDC the afferent lymph samples were stimulated by culture with transfected Chinese hamster ovary (CHO) cells expressing murine CD40 ligand (CD40L; Line K47, kindly supplied by Dr G. Wohleben, Würzburg University, Germany). Mouse CD40L has previously been reported to recognize bovine CD4026 and is active in bovine B-cell co-stimulation assays (Howard et al., unpublished results). Briefly, CHO cell lines were grown in RPMI-1640 media with the addition of 0·3 μg/ml geneticin (G418, Sigma-Aldrich). Cells were cultured for 2 days, or until they were confluent, at 37° in 5% CO2. They were then removed from the culture flasks by incubating at 37° with phosphate-buffered saline (PBS) containing 0·5% EDTA and 0·025% Trypsin, washed with PBS and added to an equal number of afferent lymph cells suspended in tissue culture medium. Samples were incubated for 4 hr at 37° before FACS immunostaining for cytokines.
RT-PCR for bovine cytokine transcripts
Amplification of cDNA was performed on a programmable thermal cycler (PTC1000™ M.J. Research Inc., Watertown, MA) using Taq DNA Polymerase (Gibco BRL, Paisley, UK). The cytokine-specific primers listed in Table 1 were designed at the institute spanning introns to prevent detection of genomic DNA and based on the published sequences of bovine cytokines. Reactions were carried out in standard polymerase chain reaction (PCR) buffer (10× PCR Buffer, Gibco BRL) with the addition of 1·5 mm MgCl2, 0·2 mm dNTPs and the cytokine-specific primers at 0·4 pmol/μl. The amplification programme consisted of 30–35 cycles of denaturation (94° for 1 min), annealing (55–60° for 1 min) and then extension (72° for 1 min) followed by a final 10-min extension period at 72°. Plasmid DNAs containing full-length sequences for each of the cytokines (confirmed by restriction enzyme digest and sequencing) were used for positive control reactions. PCR products were analysed by gel electrophoresis in 1% agarose gels stained with 0·8 μg/ml ethidium bromide. Where no plasmids with appropriate inserts were available to use as controls in the PCR reactions, cDNA from known positive populations of cells was used. Cloning and sequencing were used to confirm the identity of PCR products from these cells. To allow for differences in the efficiency of extraction of mRNA and conversion to cDNA between the samples initial reverse transcription (RT) PCR reactions for β-actin were conducted in all cases. The quantities of cDNA template used in subsequent reactions were adjusted where necessary to produce product bands for β-actin of approximately the same intensity for each of the cell populations.
Table 1.
Cytokine primer sequences for RT-PCR
| Cytokine | Primer sequences 5′ → 3′ | Annealing temp. | Product size (bp) |
|---|---|---|---|
| β-actin | CCAGACAGCACTGTGTTGGC | 59 | 270 |
| GAGAAGCTGTGCTACGTCGC | |||
| IL-1α | GTAGAGTGCACAGTCAAGGCT | 59 | 481 |
| TACAGCTTCCAGAGTAACGTG | |||
| IL-1β | AGTGAAGTTCAGGCTGCAGCT | 59 | 564 |
| TGACGCACCCGTTCAGTCAAT | |||
| IL-6 | AGGCAGACTACTTCTGACCAC | 59 | 505 |
| CAGCTACTTCATCCGAATAGC | |||
| IL-10 | GCAGCTCAGCACTGCTCTGTT | 59 | 518 |
| CGTTGTCATGTAGGTTTCTAT | |||
| IL-12p40 | GCAGTACACCTGTCACAAAG | 55 | 415 |
| CTACCACGACCTCAATAAGC | |||
| IL-12p35 | CTGTCAGCAACACGCTAC | 55 | 325 |
| CTCCACGTGGTACATCTT | |||
| IL-13 | GCCCTGTGCCTTCTGCTAC | 58 | 220 |
| AGCATCTTCTTGGTCCTTTGG | |||
| IL-18 | ACTTTGGCAAACTTGAACGAAG | 55 | 470 |
| CTAGTTCTTGTTTTGAACAGTGAACAT | |||
| IFN-α | GCAAGTAGCCCAGATGTAGC | 59 | 750 |
| GGTGACAGGTCATTCATCAC | |||
| TNF-α | ACTCAGGTCCTCTTCTCAAGCC | 60 | 464 |
| ATGATCCCAAAGTAGACCTGCC | |||
| TGF-β | GGACTACTACGCCAAGGAGG | 60 | 461 |
| GGTTCATCGCGTGAATGGTG |
TGF-β, transforming growth factor-β.
Taqman® quantitative PCR
Quantitative PCR was carried out using the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). The probes were labelled with the fluorescent reporter 6-carboxy-fluorescein (FAM) and the quencher dye 6-carboxy-tetramethyl-rhodamine (TAMRA). For this study a ready-made mastermix containing the buffer, dNTPs and Taq enzyme was used (Universal Mastermix, Applied Biosystems). The primers and probes used had previously been designed and used at the institute and were based on published sequences for bovine cytokines, details are given in Table 2. Primers were used at 3 × 10−7 molar concentration and the probes were used at 1 × 10−7 molar concentration. Following an initial activation step of 95° for 10 min, samples were run for 40 cycles of 95° for 15 seconds then 60° for 1-min. Results were quantified by comparison with standard curves produced from plasmid DNAs containing full-length sequence inserts for each of the cytokines, the concentrations of which had been determined by spectrophotometry. Expression of the housekeeping gene GAPDH was also measured in each sample as a measure of the efficiency of RNA extraction and conversion to cDNA. GAPDH values for each sample were compared to a standard curve produced from four-fold dilutions of a sample of cDNA synthesized from mRNA extracted from 105 peripheral blood mononuclear cells (PBMC). For this study a 0·5-μl sample of this cDNA was designated as containing 4 arbitrary units of GAPDH. This enabled the cytokine concentrations for each sample to be standardized for the amount of nucleic acid present.
Table 2.
Primer and probe sequences for Taqman® quantitative PCR
| Cytokine | Primer sequence 5′ → 3′ | |
|---|---|---|
| GAPDH | F | CATGTTCCAGTATGATTCCACCC |
| R | GAGCTTCCCGTTCTCTGCC | |
| P | ACGGCAAGTTCAACGGCACAGTCA | |
| IL-1β | F | AAAGCTTCAGGCAGGTGGTG |
| R | TGCGTAGGCACTGTTCCTCA | |
| P | CCGGTCATCGTGGCCATGGAGAAG | |
| IL-6 | F | CAGCTATGAACTCCCGCTTCA |
| R | AGGAGCAGCCCCAGGG | |
| P | AAGCGCCTTCACTCCATTCGCTGTC | |
| IL-10 | F | GGTGATGCCACAGGCTGAG |
| R | AGCTTCTCCCCCAGTGAGTTC | |
| P | CACGGGCCTGACATCAAGGAGCA | |
| IL-12p35 | F | TCCTCCTCATATCCACCCTGG |
| R | AGGCTCCTGCCCAAACTGA | |
| P | TCTCCTCCACCACCTGCCCCAC | |
| IL-12p40 | F | CCAAAGTCACATGCCACAAGG |
| R | CTGTAGTAGCGGTCCCGGG | |
| P | TGCCAACGTCCGCGTGCAA | |
| TNF-α | F | CGGTGGTGGGACTCGTATG |
| R | GCTGGTTGTCTTCCAGCTTCA | |
| P | CAATGCCCTCATGGCCAACGG |
F, forward primer; R, reverse primer; P, fluorescent reporter-labelled probe.
Detection of cytokine protein production
Flow cytometric detection of cytokines
Stored frozen cells were rapidly defrosted and washed once with PBS before staining. Cells were cultured in RPMI-1640 with Glutamax-1 and 25 mm HEPES (Gibco BRL, Life Technologies, Paisley, UK) containing 10% heat-inactivated (56°) fetal calf serum, 50 μg/ml gentamycin and 5 × 10−5 m 2-mercaptoethanol (RPMI media) with the addition of 10 μg/ml Brefeldin-A for 4·5 hr at 37°. Duplicate samples were also stained following the addition of 50 ng/ml phorbol 12-myristate 13-acetate (PMA) and 1 μg/ml ionomycin to the incubation medium and after lipopolysaccharide (LPS) prestimulation by culturing in medium containing 1 µg/ml LPS for 8 hr prior to adding the Brefeldin-A. After incubation the cells were stained with either mAb CC81 or CC149 (SIRP-α) detected with isotype-specific goat anti-mouse PE (Southern Biotechnology), followed by an allophycocyanin-labelled MHC class II mAb (CC158) so that the two populations of DC could be identified. They were then fixed with 1% paraformaldehyde for 10 min, and permeabilized using FACS™ Permeabilizing Solution (Becton Dickinson). Cytokines were detected by staining with specific anti-bovine cytokine mAb for IL-10 (CC322), IL-12 (CC326)32,33 and IFN-γ (anti-bovine IFN-γ FITC, MCA 1783F, Serotec Ltd, Kidlington, UK) and cross-reactive anti-ovine mAb for IL-1α30 and IL-6 (rabbit anti-ovine IL-6, AHP424, Serotec). Permeabilized cells were incubated with the cytokine mAb for 30 min at room temperature. The unlabelled mAb were then detected with isotype-specific FITC-labelled anti-murine mAb (Southern Biotechnology). Samples were analysed on a FACScalibur flow cytometer (Becton Dickinson). The mean fluorescence intensity (MFI) for each cytokine was calculated by subtracting the fluorescence from an identical afferent lymph sample stained with isotype-matched control mAb. Average MFIs for each cytokine were then calculated for CC81Ag+ ALDC and SIRP-α+ ALDC from a minimum of four samples from three different cattle.
Detection of cytokines by enzyme-linked immunosorbent assay (ELISA)
For the ELISAs the two populations of ALDC were first stained and sorted by FACS. Sorted populations were cultured in RPMI media for 12–24 hr at 37° before removing the supernatants for analysis by ELISA. Capture ELISAs were performed for bovine IL-10 and IL-12 as have been previously described.32,33 Essentially the same method was used for tumour necrosis factor-α (TNF-α) using mAb CC327 at 2 μg/ml as the first layer then biotinylated CC328 at 1 μg/ml followed by streptavidin–horseradish peroxidase for detection.
IL-12 bioactivity assay
Supernatants analysed by ELISA were also assessed for IL-12 biological activity. The supernatants were incubated with PBMC suboptimally stimulated with Concanavalin A and IFN-γ production was assayed by ELISA.33
Type I interferon measurement
To investigate the capacity of cell populations to produce type-1 interferons (IFN-αβ) in response to either double-strand RNA or cytopathic virus two sources of cell populations were used. These populations were afferent lymph cells or suspensions of cells from pre-scapular lymph nodes. Both cell preparations were sorted by FACS into two populations, WC6+ high forward scatter and WC6− prior to culture. Culture of the cell populations and analysis of the resulting supernatants for IFN-αβ biological activity were performed as previously described.34 Briefly, triplicate wells of a 96-well plate each containing 5 × 105 cells in media were cultured for 48 hr with either mock antigen, lysate of a primary cell culture of calf testis cells, cytopathic bovine viral diarrhoea virus (cpBVDV), multiplicity of infection of 2 and double-stranded RNA 1 μg/ml.
Results
Qualitative RT-PCR
Initial screening by RT-PCR of the cDNA derived from fresh, unstimulated ALDC revealed the presence of transcripts for a number of cytokines that are reported to be typically produced by DC. The two major ALDC subpopulations, purified by FACS, both consistently produced transcripts for IL-6, IL-12 p35 and p40 subunits, IL-18 and TNF-α. Transcripts for IL-1α and IL-1β were detected in all of the samples derived from sorted SIRP-α+ ALDC but were either absent or only detected at low levels (i.e. a faint product band in agarose gel after 35 cycles of PCR) in sorted CC81Ag+ ALDC samples. Consistent differences in the intensities of IL-6, the IL-12 p40 subunit and TNF-α bands were seen for the two ALDC subpopulations, suggesting quantitative differences in cytokine production. Transcripts for IL-10 were only detected at a low level in one sample from sorted SIRP-α+ ALDC out of a total of 12 fresh ALDC samples examined. No IFN-α, transforming growth factor-β, or IL-13 mRNA was detected in either of the ALDC populations.
Following stimulation of the ALDC by culture with CD40L+ cells for most cytokines there was increased intensity of the product bands compared to the unstimulated samples, implying up-regulation. TNF-α differed in that there seemed to be a decrease in band intensity. In addition IL-10 mRNA production was detected at the end of the culture period particularly by the SIRP-α+ subpopulation. Figure 1 shows some results typical of those obtained. The gels show the products of RT-PCR for IL-1α, IL-6, IL-10, IL-12, IL-18 and TNF-α stained with ethidium bromide and illuminated with UV light. The results for IL-1β were very similar to those for IL-1α (not shown).
Figure 1.
RT-PCR cytokine products from unstimulated and cultured bovine ALDC. UV-illuminated agarose gels stained with ethidium bromide. Lanes 1 and 8, 100-bp DNA ladder; lane 2, unstimulated CC81Ag+ ALDC; lane 3, unstimulated SIRP-α+ ALDC; lane 4, cultured CC81Ag+ ALDC; lane 5, cultured SIRP-α+ ALDC; lane 6, positive control plasmid DNA; lane 7, negative control, no template DNA.
Taqman® quantitative PCR
Quantitative PCR was carried out to measure cytokine transcripts for which the initial RT-PCR results indicated differences in production between the two ALDC subpopulations. A summary of the results for IL-1β, IL-6, IL-10 and IL-12 obtained from three different animals is shown in Table 3. Distinct differences were evident between the quantities of cytokine transcripts for the two populations of ALDC and most of these differences appeared to remain after stimulation by culture with CD40L+ cells.
Table 3.
Quantitative PCR results for cytokine production by fresh and cultured ALDC
| Unstimulated ALDC | Cultured ALDC | ||||
|---|---|---|---|---|---|
| Animal | |||||
| Cytokine | no. | CC81Ag+ | SIRP-α+ | CC81Ag+ | SIRP-α+ |
| IL-1β | |||||
| 7321 | 0·43 | 4·21 | 49·40 | 139·11 | |
| 7152 | 4·97 | 8·19 | 43·50 | 110·9 | |
| 1899 | 8·13 | 19·55 | 131·09 | 244·22 | |
| Mean | 4·51 | 10·65 | 74·66 | 164·74 | |
| Ratio | 2·36 | 2·21 | |||
| IL-6 | |||||
| 7321 | 3·61 | 9·45 | 403·63 | 494·10 | |
| 7152 | 0·77 | 0·99 | 5·34 | 7·46 | |
| 1899 | 3·93 | 9·80 | 30·96 | 70·18 | |
| Mean | 2·77 | 6·75 | 146·64 | 190·58 | |
| Ratio | 2·44 | 1·30 | |||
| IL-10 | |||||
| 7321 | ND | 11·28 | 190·77 | 364·83 | |
| 7152 | ND | ND | 15·26 | 55·11 | |
| 1899 | ND | ND | 13·37 | 304·13 | |
| Mean | – | – | 73·13 | 241·36 | |
| Ratio | 3·30 | ||||
| IL-12 p40 | |||||
| 7321 | 379·04 | 8·65 | 1862·21 | 252·54 | |
| 7152 | 182·09 | 14·75 | 526·70 | 115·44 | |
| 1899 | 175·40 | 6·91 | 360·04 | 45·46 | |
| Mean | 245·51 | 10·10 | 916·32 | 137·81 | |
| Ratio | 24·30 | 6·65 | |||
| IL-12 p35 | |||||
| 7321 | 5·48 | 1·57 | – | – | |
| 7152 | 1·70 | 0·52 | – | – | |
| 1899 | 1·68 | 0·53 | 2·74 | 1·49 | |
| Mean | 2·95 | 0·87 | |||
| Ratio | 3·38 | ||||
Results are given in pg/ml/UnitGAPDH. ND = not detected, for IL-10 limit of detection was 1 pg/ml. Ratio = mean-fold difference in production between the ALDC populations. Cultured ALDC p35 results were only available for one animal.
IL-1β transcripts were evident in all of the ALDC samples examined. In general there was between two- and three-fold greater production by SIRP-α+ ALDC than CC81Ag+ ALDC sorted from the same afferent lymph sample. Culture with CD40L+ cells resulted in about a 16-fold increase in production by both subpopulations. Fresh SIRP-α+ ALDC produced two to three times more transcripts for IL-6 than did CC81Ag+ ALDC. Culture with CD40L+ cells resulted in a marked increase in mRNA and a less pronounced difference between the two subpopulations. In concurrence with the RT-PCR results IL-10 was only detected at levels above background (>1 pg/ml) in one fresh ALDC sample, derived from sorted SIRP-α+ ALDC. Following culture however, both populations of ALDC produced transcripts for this cytokine with SIRP-α+ ALDC producing an average of three-fold more transcripts than CC81Ag+ ALDC. For IL-12 initially only the production of p40 subunit transcripts was measured by quantitative PCR as production of p35 is generally considered to be constitutive and there was no indication of a significant difference in p35 production between the two ALDC populations from the RT-PCR results (Fig. 1). Although both ALDC subsets produced transcripts for p40 it was noticeable that CC81Ag+ ALDC produced particularly high levels, even in the fresh, uncultured state. On average fresh CC81Ag+ ALDC produced 24-fold greater amounts of p40 than SIRP-α+ ALDC. Culture with CD40L+ cells produced a further four-fold increase in p40 production by the CC81Ag+ ALDC and an average 14-fold increase in SIRP-α+ ALDC. Following the detection of such high levels of p40 transcripts IL-12 p35 mRNA production was also assessed by quantitative PCR. The amounts of p35 transcripts detected were very low compared to the results for p40. There did however, appear to be a similar difference between the populations, with CC81Ag+ ALDC producing more p35 transcripts than SIRP-α+ ALDC.
The results for TNF-α were more variable than for the other cytokines and they are not included in Fig. 2. In fresh ALDC samples there were no consistent differences in the number of transcripts present between the two ALDC subpopulations. The effect of culture on TNF-α production was also variable, in some samples production appeared to be down-regulated by culture whereas other cultured samples had increased levels compared to the fresh ALDC.
Figure 2.
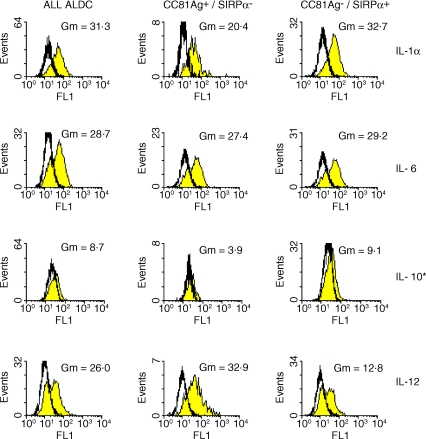
Cytokine production by ALDC detected by flow cytometry. ALDC stained with cytokine-specific mAb following 4·5-hr incubation in media containing Brefeldin-A. Shaded histograms show ALDC stained with cytokine mAb. Open histograms are ALDC stained with isotype-matched control mAb. Gm = geometric mean, the mean fluorescence intensity (MFI) of cytokine mAb stained cells minus control MFI. *indicates that production of IL-10 shown is after 8 hr prestimulation with LPS.
Cytokine detection by flow cytometry
Afferent lymph samples, both with and without prior stimulation, from five different animals were examined by flow cytometry. ALDC were identified and assayed for staining with mAb to IL-1α, IL-6, IL-10 and IL-12 and IFN-γ. Typical results for each cytokine are shown in Figs 2 and 3. As with the PCR results there appeared to be a range in the intensity of staining for cytokines between individual animals, however, the differences in levels of cytokine production that this indicated between the two groups of ALDC were consistent. Paired t-tests were used to compare the production of the cytokines by the two groups of ALDC in each sample. Results are summarized in Table 4.
Figure 3.
Flow cytometry staining for IFN-γ production by ALDC. Dotplots are gated to show only ALDC (distinguished on the basis of high forward scatter (FSC) and high intensity staining for WC6. (a) Unstimulated ALDC. (b) ALDC stimulated with PMA and ionomycin for 4 hr.
Table 4.
Production of cytokines by ALDC measured by mAb staining and flow cytometry
| Stimulated ALDC | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unstimulated | P | LPS | P | PMA/ ionomycin | P | Mixed stimulation‡ | P | |
| IL-1α | ||||||||
| All ALDC | 43·5 ± 12·6 | 42·5 ± 8·1 | 106·3 ± 20·5 | |||||
| CC81Ag+ | 41·0 ± 12·5 | 0·021† | 26·6 ± 6·4 | 0·032† | 83·2 ± 21·8 | 0·041† | ||
| SIRP-α+ | 44·6 ± 12·3 | 46·8 ± 7·5 | 113·9 ± 18·5 | |||||
| IL-6 | ||||||||
| All ALDC | 42·4 ± 7·7 | 47·8 ± 18·0 | 45·9 ± 4·3 | |||||
| CC81Ag+ | 44·1 ± 8·7 | 0·465 | 45·9 ± 18·9 | 0·595 | 51·7 ± 4·8 | 0·004* | ||
| SIRP-α+ | 41·8 ± 7·4 | 49·1 ± 17·3 | 43·0 ± 4·3 | |||||
| IL-12 | ||||||||
| All ALDC | 31·0 ± 3·9 | 31·6 ± 10·5 | 34·8 ± 4·2 | |||||
| CC81Ag+ | 47·4 ± 5·6 | < 0·001* | 46·7 ± 12·2 | 0·012† | 53·1 ± 7·4 | 0·003* | ||
| SIRP-α+ | 27·1 ± 3·9 | 28·7 ± 10·6 | 28·3 ± 4·2 | |||||
| IL-10 | ||||||||
| All ALDC | 1·4 ± 0·3 | 7·8 ± 1·3 | ||||||
| CC81Ag+ | – | 4·5 ± 1·1 | 0·001* | |||||
| SIRP-α+ | – | 8·6 ± 1·5 | ||||||
Results shown are average mean fluorescence intensities above background (MFI ± SEM). All figures are means of a minimum of four results from three different animals.
Paired t-test results
significant at 1% level,
significant at 5% level.
Mean results from ALDC stimulated either with LPS or PMA/ionomycin, both treatments had similar effects.
There was a marked difference for IL-12 staining between the two subpopulations with CC81Ag+ ALDC consistently staining much more intensely for IL-12 p40 than SIRP-α+ ALDC. In several of the samples, staining for IL-12 produced a bimodal histogram (Fig. 2), suggesting that there are subsets of SIRP-α+ ALDC producing different amounts of IL-12.
Both CC81Ag+ and SIRP-α+ ALDC stained with IL-1α mAb. In every sample the MFI for SIRP-α+ ALDC was greater than for the CC81Ag+ ALDC, although the wide individual variation meant that there did not appear to be a significant difference between the average MFIs for each population of unstimulated cells. Following stimulation with either LPS or PMA and ionomycin however, the differences became more distinct. A paired t-test demonstrated a significant difference in production of IL-1α between the two groups of ALDC (P < 0·05).
Although in the unstimulated samples less than 1% of ALDC stained for IFN-γ, following stimulation with PMA and ionomycin significant subpopulations of both CC81Ag+ and SIRP-α+ ALDC stained with mAb to this cytokine (Fig. 3).
There was no convincing staining for IL-10 seen with either population of ALDC although consistent low intensity staining for IL-10 was detected following stimulation of the cells with LPS (Fig. 2) or PMA and ionomycin (result not shown) with higher levels of staining seen in the SIRP-α+ ALDC than the CC81Ag+ ALDC. There was no clear difference in staining for IL-6 between the two populations.
Cytokine detection by ELISA
In total, 18 supernatant samples from FACS-sorted cultured ALDC were used for the ELISAs, nine from CC81Ag+ ALDC and nine from SIRP-α+ ALDC.
IL-12 was detected in all of the samples examined. There was, however, considerable variation between the results for different animals and samples. Despite the variation it was still apparent that from each individual afferent lymph sample the CC81Ag+ ALDC produced higher amounts of IL-12 than SIRPα+ ALDC over the first 12–24 hr of culture. Results are shown in Table 5.
Table 5.
Detection of IL-12 in supernatants of cultured ALDC by ELISA; the number of samples (n) is of each ALDC population
| Mean IL-12 (units/ml/106 cells ± SE) | |||
|---|---|---|---|
| Culture period | No. of samples | CC81Ag+ ALDC | CC81Ag− ALDC |
| 12–24 hr | 4 | 93·5 ± 35·6 | 29·2 ± 18·1 |
| 36–48 hr | 5 | 6·3 ± 2·8 | 6·5 ± 2·9 |
Supernatants containing IL-12 from culture of FACS-sorted CC81Ag+ or SIRP-α+ ALDC both induced significant IFN-γ production by PBMC (measured by ELISA), indicating that the IL-12 produced was biologically active.
TNF-α was detected in four of the supernatant samples from SIRP-α+ ALDC and in one of the CC81Ag+ ALDC samples. There was, however, a wide range in the levels detected. The SIRP-α+ ALDC samples contained between 1·5 U/ml and 53·8 U/ml and the only positive CC81Ag+ ALDC sample contained 31·0 U/ml. IL-10 production was not detected by the ELISA.
Production of IFN-αβ
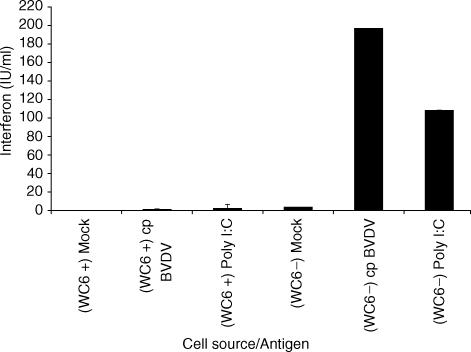
IFN-αβ biological activity was detectable after 48 hr in the supernatants of WC6− cells, isolated from lymph node preparations, when stimulated with either cpBVDV or dsRNA. IFN-αβ biological activity was not detected in supernatants from mock stimulated cultures. In contrast, IFN-αβ biological activity could not be detected in the supernatants of WC6+ cells isolated from lymph node preparations, whether they were stimulated with cpBVDV, dsRNA, or mock antigen. These results are summarized in Fig. 4.
Figure 4.
Interferon-αβ production by sorted pre-scapular lymph node cells. Cell suspensions from whole pre-scapular lymph node were sorted by FACS into two populations, WC6+ and WC6− prior to culture. Triplicate wells each containing 5 × 105 cells were cultured for 48 hr with either mock antigen, cytopathic bovine viral diarrhoea virus (cpBVDV), multiplicity of infection of 2 and double stranded RNA 1 μg/ml (Poly I:C). Values shown for IFN-αβ (international units) for each treatment are the mean of three replicates ± SD.
Similar assays performed on sorted cell preparations from afferent lymph failed to detect IFN-αβ biological activity in either WC6+ or WC6− cell preparations (data not shown).
Discussion
Results from this study provide evidence that ex vivo ALDC from cattle are capable of producing a range of cytokines. These were typical of those produced by DC from other animal species, based primarily on data from in vitro experimental systems. The present study has also shown that there are distinct quantitative differences in cytokine production seen between the two ALDC subpopulations. These differences could have a significant effect on the outcome and bias of immune responses produced to antigens presented by the two different subpopulations.
The most striking difference between the two ALDC subpopulations was in the production of IL-12. This cytokine drives T cells towards Th1-type responses.1–3 On this basis, CC81Ag+ ALDC would be expected to bias the T cells they stimulate more strongly towards a Th1 response than CC81Ag− ALDC. However, following activation and more prolonged periods of culture the difference between the two subpopulations in amounts of IL-12 became less evident. Thus, it is likely that it would be the early interaction of T cells and the ALDC where this differing effect would operate. The bioassay indicated that the IL-12 detected in this study had biological activity. However, the only mAb available do not distinguish between IL-12 p40 and the IL-12 p75 heterodimer. Results from the quantitative PCR suggest that ALDC produce a large excess of p40 compared to p35 and this is similar to a previous report for DC and monocytes.35 The low levels of IL-12 p35 detected in unstimulated ALDC is consistent with the suggestion that DC, unlike most other cell types, do not constitutively express this subunit of the heterodimer.36 The presence of mRNA in ex vivo ALDC that had not been cultured is in accordance with a recent suggestion that in vivo the production of p40 by DC is a T-cell-independent early response to host–pathogen interactions and that it is this early production of p40 that skews T-cell responses towards a Th1-type (IFN-γ-producing) cytokine pattern.37 Production of cytokines by DC in afferent lymph could result in the initiation of T-cell activation before cells reach the draining lymph node. Consistent with this is that clusters of DC and T cells have been reported in sheep lymph.38 The proposal that CC81Ag+ ALDC might bias a Th1-type response is further supported by a recent finding that this ALDC subpopulation also expresses CD26,39 a molecule which has been proposed to play a role in biasing immune responses towards a Th1-type by modifying the specificity of some chemokines.40
Although the observations are consistent with the homogeneous CC81Ag+ ALDC subpopulation producing a strong Th1 bias it is not suggested that the CCAg81− ALDC cannot also affect the T-cell response and induce the synthesis of Type 1 cytokines. Both subpopulations were shown to contain cells that stained for IFN-γ. If this occurred in vivo it could clearly promote a Th1 bias by both subpopulations, adding to a Th1 bias for the CC81Ag+ ALDC. There are reports of IFN-γ production by various subsets of splenic DC in mice following stimulation or culture.41,42
A second cytokine for which distinct differences in synthesis by the two ALDC subpopulations was evident was IL-10. Of note was that mRNA for this cytokine was produced more abundantly by the CC81Ag− ALDC. In mice the presence of IL-6 and IL-10 during T-cell stimulation have both been linked to the generation of Th2-type responses.4,5 Both of these cytokines have been suggested to act by inducing early IL-4 production by naïve T cells, which in turn stimulates the development of a Th2-type response.43 In humans and cattle however, IL-10 appears to have a down-regulatory effect, reducing the proliferation of both Th1- and Th2-type clones.44,45 In humans this effect is accompanied by a reduction in cytokine production by all types of T-cell clones but in cattle there seems to be mainly a reduction in production of IFN-γ. IL-10 production appeared primarily to be a function of the activated SIRP-α+ ALDC and this may imply an anti-inflammatory role for this subpopulation or for a subset of cells within it.
Differences in the production of IL-1α by the two subpopulations of ALDC have already been demonstrated to have a role in their ability to stimulate CD8+ T cells.30 The results presented here confirm that SIRP-α+ ALDC and CC81Ag+ ALDC differ in their ability to produce both this cytokine and also IL-1β. In other species IL-1 has also been proposed to affect the proliferation of Th2-type CD4+ T cells46 however, a similar role in cattle has not yet been identified.
Little difference was evident in TNF-α for the two subpopulations and its role is likely to be DC recruitment and activation.47
Although most of the cytokine results for the three animals in this study show consistent patterns of expression there were considerable differences in the levels of expression of cytokines between individual animals. This could represent true individual variation, or differences in the type or intensity of stimulus the ALDC have been activated by in each cannulation. In the quantitative PCR expression of the housekeeping gene GAPDH was used as an internal standard. Whilst expression of this gene should be consistent for samples derived from the same collection of afferent lymph, previous studies have shown that there can be considerable differences in expression between individuals.48,49
Overall, the results of this study show that both of the subpopulations of ALDC are capable of producing a range of cytokines known to be typically produced by DC in other species and experimental systems. There does however, appear to be distinct quantitative differences in cytokine production between the two subpopulations that could lead to a significant difference in the immune responses generated to antigens presented by them. This could have important consequences with regard to the generation of particular types of immune responses at different tissue sites. In ruminant peripheral lymph and lymph nodes draining the skin SIRP-α+ ALDC are the predominant DC population26,27 but there is evidence to suggest that CC81Ag+ ALDC predominate in mesenteric afferent lymph and in the mesenteric lymph nodes draining the gut39 (Stephens et al. unpublished results). In the rat SIRPα− ALDC (the equivalent of the CC81Ag+ ALDC subpopulation) have already been reported to be the most numerous DC in mesenteric lymph and a possible role has been suggested for these cells in the induction and maintenance of tolerance.29,50
These findings raise questions about the origin and role of the two phenotypically distinct groups of ALDC. Studies in humans and mice have classified DC as myeloid and plasmacytoid or myeloid, plasmacytoid and lymphoid, respectively, depending on their presumed cell lineage, expression of a range of surface molecules and cytokine production.51 Expression of CD45RO26 by both ALDC subsets, rather than CD45RA as seen in human plasmacytoid DC,51 is evidence that these two ALDC populations are probably both myeloid DC. In addition, there was an absence of a cell population in afferent lymph capable of producing large quantities of IFN-αβ in response to dsRNA or cpBVDV. As dsRNA and cpBVDV induce IFN-αβ in a range of cell types34,52 the assays were performed with limited cell numbers so that only cells producing large quantities of IFN-αβ would be detected and synthesis was evident in the WC6− cells from lymph nodes. Absence of cells in the afferent lymph samples that produce significant quantities of IFN-αβ implies that plasmacytoid DC are not present in afferent lymph and, as suggested by Cella et al.,53 enter the lymph nodes from the blood via high endothelial venules.
Whether the ALDC subsets in lymph draining the skin represent myeloid DC populations that are derived from precursors that have differentiated into committed subtypes before emigration from the blood to the skin, or whether they differentiate from a common precursor in the skin in response to the local microenvironment remains unanswered. Nevertheless, the two distinct subpopulations clearly differ in their ability to stimulate T cells and in the range of cytokines they produce before and after stimulation. The phenotypically homogeneous SIRP− CC81Ag+ population would be expected to produce a Th1 bias as a result of early IL-12 and IFN-γ synthesis. The heterogeneous SIRP+ CC81Ag− population produces IL-12 later and cells within this population produce IL-10. The likely explanation is that this phenotypically heterogeneous subpopulation is also functionally heterogeneous and contains subsets that might stimulate or down-regulate the immune response. The very early responses induced by either of these subsets in vivo are likely to be biased according to which population is presenting the antigen and therefore they could potentially be targeted for modulating immune responses to vaccination. Functional studies would be required to establish whether the difference in IL-12 production between the two ALDC populations detected in this study actually leads to a tendency to skew naïve T cells towards a Th1-type response in vivo.
References
- 1.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–92. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–9. [PubMed] [Google Scholar]
- 3.Heufler C, Koch F, Stanzl U, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–68. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 4.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–9. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L, Rich BE, Inobe J, Chen W, Weiner HL. Induction of Th2 cell differentiation in the primary immune response: dendritic cells isolated from adherent cell culture treated with IL-10 prime naive CD4+ T cells to secrete IL-4. Int Immunol. 1998;10:1017–26. doi: 10.1093/intimm/10.8.1017. [DOI] [PubMed] [Google Scholar]
- 6.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–41. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maldonado-Lopez R, De Smedt T, Michel P, et al. CD8alpha+ and CD8alpha– subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–92. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation [see comments] Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 9.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164:4507–12. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 10.Piemonti L, Monti P, Allavena P, Sironi M, Soldini L, Leone BE, Socci C, Di Carlo V. Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol. 1999;162:6473–81. [PubMed] [Google Scholar]
- 11.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol. 1997;27:1229–35. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki A, Kelsall BL. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229–39. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whelan M, Harnett MM, Houston KM, Patel V, Harnett W, Rigley KP. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J Immunol. 2000;164:6453–60. doi: 10.4049/jimmunol.164.12.6453. [DOI] [PubMed] [Google Scholar]
- 14.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid DCs activated by influenza virus and CD40L drive a potent Th1 polarization. Nature Immunol. 2000;1:305–10. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 15.Kalinski P, Smits HH, Schuitemaker JH, Vieira PL, van Eijk M, de Jong EC, Wierenga EA, Kapsenberg ML. IL-4 is a mediator of IL-12p70 induction by human Th2 cells: reversal of polarized Th2 phenotype by dendritic cells. J Immunol. 2000;165:1877–81. doi: 10.4049/jimmunol.165.4.1877. [DOI] [PubMed] [Google Scholar]
- 16.Edwards AD, Manickasingham SP, Sporri R, et al. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J Immunol. 2002;169:3652–60. doi: 10.4049/jimmunol.169.7.3652. [DOI] [PubMed] [Google Scholar]
- 17.McKeever DJ, Awino E, Morrison WI. Afferent lymph veiled cells prime CD4+ T cell responses in vivo. Eur J Immunol. 1992;22:3057–61. doi: 10.1002/eji.1830221205. [DOI] [PubMed] [Google Scholar]
- 18.Knight SC, Balfour BM, O'Brien J, Buttifant L, Sumerska T, Clarke J. Role of veiled cells in lymphocyte activation. Eur J Immunol. 1982;12:1057–60. doi: 10.1002/eji.1830121214. [DOI] [PubMed] [Google Scholar]
- 19.Hendriks HR, Eestermans IL, Hoefsmit EC. Depletion of macrophages and disappearance of postcapillary high endothelial venules in lymph nodes deprived of afferent lymphatic vessels. Cell Tissue Res. 1980;211:375–89. doi: 10.1007/BF00234394. [DOI] [PubMed] [Google Scholar]
- 20.Roake JA, Rao AS, Morris PJ, Larsen CP, Hankins DF, Austyn JM. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J Exp Med. 1995;181:2237–47. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoitzner P, Zanella M, Ortner U, et al. Migration of langerhans cells and dermal dendritic cells in skin organ cultures: augmentation by TNF-alpha and IL-1beta. J Leukoc Biol. 1999;66:462–70. [PubMed] [Google Scholar]
- 22.Liu LM, MacPherson GG. Antigen processing. cultured lymph-borne dendritic cells can process and present native protein antigens. Immunology. 1995;84:241–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Hall JG. Studies of the cells in the afferent and efferent lymph of lymph nodes draining the site of skin homografts. J Exp Med. 1967;125:737–54. doi: 10.1084/jem.125.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopkins J, McConnell I, Bujdoso R, Munro AJ. Studies of MHC class II products on sheep peripheral and efferent lymph cells. In: Miyasaka M, editor. Immunology of the Sheep. Basel: Hoffmann La Roche; 1985. p. 441. [Google Scholar]
- 25.Emery DL, MacHugh ND, Ellis JA. The properties and functional activity of non-lymphoid cells from bovine afferent (peripheral) lymph. Immunology. 1987;62:177–83. [PMC free article] [PubMed] [Google Scholar]
- 26.Howard CJ, Sopp P, Brownlie J, Kwong LS, Parsons KR, Taylor G. Identification of two distinct populations of dendritic cells in afferent lymph that vary in their ability to stimulate T cells. J Immunol. 1997;159:5372–82. [PubMed] [Google Scholar]
- 27.McKeever DJ, MacHugh ND, Goddeeris BM, Awino E, Morrison WI. Bovine afferent lymph veiled cells differ from blood monocytes in phenotype and accessory function. J Immunol. 1991;147:3703–9. [PubMed] [Google Scholar]
- 28.Brooke GP, Parsons KR, Howard CJ. Cloning of two members of the SIRP alpha family of protein tyrosine phosphatase binding proteins in cattle that are expressed on monocytes and a subpopulation of dendritic cells and which mediate binding to CD4 T cells. Eur J Immunol. 1998;28:1–11. doi: 10.1002/(SICI)1521-4141(199801)28:01<1::AID-IMMU1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Zhang M, Jenkins C, MacPherson GG. Dendritic cell heterogeneity in vivo: two functionally different dendritic cell populations in rat intestinal lymph can be distinguished by CD4 expression. J Immunol. 1998;161:1146–55. [PubMed] [Google Scholar]
- 30.Hope JC, Sopp PA, Collins RA, Howard CJ. Differences in the induction of CD8+ T cell responses by sub-populations of dendritic cells from afferent lymph are related to IL-1 alpha secretion. J Leukocyte Biol. 2001;69:271–9. [PubMed] [Google Scholar]
- 31.Howard CJ, Morrison WI, Bensaid A, et al. Summary of workshop findings for leukocyte antigens of cattle. Vet Immunol Immunopathol. 1991;27:21–7. doi: 10.1016/0165-2427(91)90072-k. [DOI] [PubMed] [Google Scholar]
- 32.Kwong LS, Hope JC, Thom ML, Sopp P, Duggan S, Bembridge GP, Howard CJ. Development of an ELISA for bovine IL-10. Vet Immunol Immunopathol. 2002;85:213–23. doi: 10.1016/s0165-2427(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 33.Hope JC, Kwong LS, Entrican G, Wattegedera S, Vordermeier HM, Sopp P, Howard CJ. Development of detection methods for ruminant interleukin (IL)-12. J Immunol Meth. 2002;266:117–26. doi: 10.1016/s0022-1759(02)00113-8. [DOI] [PubMed] [Google Scholar]
- 34.Fray MD, Mann GE, Charleston B. Validation of an Mx/CAT reporter gene assay for the quantification of bovine type-I interferon. J Immunol Meth. 2001;249:235–44. doi: 10.1016/s0022-1759(00)00359-8. [DOI] [PubMed] [Google Scholar]
- 35.Trinchieri G. Proinflammatory and immunoregulatory functions of interleukin-12. Int Rev Immunol. 1998;16:365–96. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]
- 36.Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–62. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 37.Abdi K. IL-12. the role of p40 versus p75. Scand J Immunol. 2002;56:1–11. doi: 10.1046/j.1365-3083.2002.01101.x. [DOI] [PubMed] [Google Scholar]
- 38.Haig DM, Hopkins J, Miller HR. Local immune responses in afferent and efferent lymph. Immunology. 1999;96:155–63. doi: 10.1046/j.1365-2567.1999.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gliddon DR, Howard CJ. CD26 is expressed on a restricted subpopulation of dendritic cells in vivo. Eur J Immunol. 2002;32:1472–81. doi: 10.1002/1521-4141(200205)32:5<1472::AID-IMMU1472>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 40.De Meester I, Korom S, Van Damme J, Scharpe S. CD26, let it cut or cut it down. Immunol Today. 1999;20:367–75. doi: 10.1016/s0167-5699(99)01486-3. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Zhang Y, Yoneyama H, Onai N, Sato T, Matsushima K. Identification of CD8alpha+CD11c– lineage phenotype-negative cells in the spleen as committed precursor of CD8alpha+ dendritic cells. Blood. 2002;100:569–77. doi: 10.1182/blood.v100.2.569. [DOI] [PubMed] [Google Scholar]
- 42.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O'Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol. 2001;166:5448–55. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka T, Hu-Li J, Seder RA, Fazekas de St Groth B, Paul WE. Interleukin 4 suppresses interleukin 2 and interferon gamma production by naive T cells stimulated by accessory cell-dependent receptor engagement. Proc Natl Acad Sci USA. 1993;90:5914–18. doi: 10.1073/pnas.90.13.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–60. [PubMed] [Google Scholar]
- 45.Brown WC, Estes DM. Type 1 and Type 2 responses in cattle and their regulation. In: Schijns MC, editor. Cytokines in Veterinary Medicine. Wallingford, UK: CAB International,; 1997. pp. 15–34. [Google Scholar]
- 46.Weaver CT, Hawrylowicz CM, Unanue ER. T helper cell subsets require the expression of distinct costimulatory signals by antigen-presenting cells. Proc Natl Acad Sci USA. 1988;85:8181–5. doi: 10.1073/pnas.85.21.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joseph SB, Miner KT, Croft M. Augmentation of naive, Th1 and Th2 effector CD4 responses by IL-6, IL-1 and TNF. Eur J Immunol. 1998;28:277–89. doi: 10.1002/(SICI)1521-4141(199801)28:01<277::AID-IMMU277>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 48.Bustin SA, Gyselman VG, Williams NS, Dorudi S. Detection of cytokeratins 19/20 and guanylyl cyclase C in peripheral blood of colorectal cancer patients. Br J Cancer. 1999;79:1813–20. doi: 10.1038/sj.bjc.6990289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–93. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 50.Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes [see comments] J Exp Med. 2000;191:435–44. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–62. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 52.Charleston B, Fray MD, Baigent S, Carr BV, Morrison WI. Establishment of persistent infection with non-cytopathic bovine viral diarrhoea virus in cattle is associated with a failure to induce type I interferon. J General Virol. 2001;82:1893–7. doi: 10.1099/0022-1317-82-8-1893. [DOI] [PubMed] [Google Scholar]
- 53.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon [see comments] Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]