Abstract
Cytotoxic T-cell responses are thought to play a significant role in the host defence against mycobacterial infections. Little is understood about such responses of cattle to Mycobacterium bovis, the causative agent of bovine tuberculosis. The work described in this report demonstrates the activity of cytotoxic cells during experimental infection of cattle with M. bovis. The cytotoxic cells were found to have the ability to specifically lyse macrophages infected with M. bovis and were detected in peripheral blood lymphocytes after in vitro re-exposure to M. bovis. Cytotoxic activity was detected 4 weeks after experimental infection with M. bovis; a similar level of activity was maintained during the infection and it was mediated by both WC1+γδ and CD8+ T cells. In addition, inhibition of the growth of M. bovis within infected macrophages was detected when they were exposed to cultures containing M. bovis-specific cytotoxic cells. The ability to detect cytotoxic cells after infection of cattle with M. bovis will allow their activity to be measured during vaccination trials. Correlation of cytotoxic activity with disease outcome may aid in the design of new vaccines and vaccination strategies.
Introduction
Tuberculosis presents an important health problem to humans and cattle and is an economic problem in countries where bovine tuberculosis is endemic. In order to develop a more successful vaccine it is important to understand the protective immune response of the host against the causative agent. In recent years, small animal models, particularly mice, have been used to determine which components of the immune system are involved in protective immunity against mycobacteria. Studies in humans infected with Mycobacterium tuberculosis have shown that many of the results obtained using experimental infection of mice also apply to humans. It is well established that cell-mediated responses are important in protection, and several subpopulations of T cells appear to be involved. Protective immunity to mycobacteria is considered to be mediated predominantly by T helper (Th)1 CD4+ and CD8+ T-cell secretion of interferon-γ (IFN-γ)1,2 but other T-cell populations, such as γδ T cells3 and natural killer (NK) T cells,4 are probably also involved. Results from vaccine trials, using experimental challenge of cattle with M. bovis, have shown that vaccines which stimulate fast and strong IFN-γ responses following infection are protective, whereas those which stimulate antibody responses are not.5 Therefore, the Th1 paradigm holds for bovine tuberculosis and all the major T-cell subsets appear to be involved in the response to infection.6–10
IFN-γ and other Th1 cytokines upregulate the antimicrobial function of macrophages (Mφ) to kill the bacilli which they harbour.11 Bactericidal and bacteriostatic functions of activated Mφs can be demonstrated in vitro but it is clear that virulent mycobacteria are hard to kill. There may be mechanisms, other than those involving Mφs, that are engaged in the killing of virulent mycobacteria. Cytotoxic T lymphocytes (CTL) have the ability to kill human12 and mouse Mφs13 infected with M. tuberculosis. Bovine CTL have been shown to kill cells infected with other intracellular pathogens.14,15 At least in vitro, the direct killing of mycobacteria by CTL has been demonstrated16 and the involvement of molecules, such as granulysin, in the cytotoxic granules of CTL, have been implicated in this killing.17,18 CTL may also have a role in more efficient antigen presentation, so that intracellular mycobacteria released from infected cells can be taken up by more proficient antigen-presenting cells.
Studies with putative DNA and subunit vaccines support the idea that CTL should be considered in the design of new-generation vaccines for tuberculosis.19–21 Protection can be mediated by the adoptive transfer of T-cell clones, specific for M. tuberculosis hsp65, in the mouse, and the most protective clones not only produced high levels of IFN-γ when stimulated with mycobacterial antigens, but also displayed the highest cytotoxic activity.22 Vaccines that stimulate CTL responses may be useful as both prophylactic and immunotherapeutic agents.23,24
T-cell clones from M. bovis-infected cattle can cause the release of mycobacteria from infected Mφs,25 but CTL activity has not been measured by more conventional methods. In this report, a cytotoxicity assay, based on the release of 51chromate (51Cr) was used to follow cytotoxic responses in cattle experimentally infected with M. bovis.
Materials and methods
Animals
Friesian-cross female calves (5–6 months old) were obtained from tuberculosis-free accredited herds from an area of New Zealand where both farmed and feral animals are free of tuberculosis. Prior to the start of the experiments, the cattle tested negative for reactivity to purified protein derivative from M. bovis (bovine PPD) in the whole-blood IFN-γ assay.26 The cattle were grazed on pasture in an isolation unit, and all procedures involving the use of animals had the approval of the Wallaceville Research Centre Animal Ethics Committee (Upper Hutt, New Zealand).
Mycobacteria
A virulent M. bovis strain, WAg 202 (previously designated 83/6235), used in previous cattle studies,27 was used to infect cattle, stimulate CTL activity in vitro and infect Mφs. It was originally isolated from a tuberculous lesion of a brushtail possum (Trichosurus vulpecula). M. bovis was grown to mid-log phase in Tween albumin Dubos base broth (Difco Laboratories, Detroit, Michigan) supplemented with 0·006% (vol/vol) alkalinized oleic acid, 0·5% (wt/vol) albumin fraction V and 0·25% (wt/vol) glucose. The numbers of bacteria were estimated by the degree of turbidity. Dilutions were made, in Tween albumin broth, to obtain the appropriate doses for inoculating cattle, and the remainder were frozen and stored at −20° in 1-ml aliquots for in vitro infections. The number of colony-forming units (CFU) inoculated into cattle was determined retrospectively by plating 10-fold dilutions onto Middlebrook 7H11 agar (Difco) supplemented with 0·5% (wt/vol) glucose and 1% (wt/vol) sodium pyruvate; the numerical value thus obtained was used to calculate the number of CFU in frozen stocks that were used for in vitro infection of cells.
M. bovis infection of calves
Animals were infected with 5 × 103 CFU M. bovis by the intratracheal route, and killed and necropsied 17–21 weeks after inoculation, as described previously.27M. bovis was cultured from the lungs or lymph nodes of all animals.
Generation of CTL in vitro
Cattle peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood by centrifugation at 1400 g for 35 min over Lymphoprep (density 1·0777) (Nycomed, Oslo, Norway). PBMC were washed three times with phosphate-buffered saline (PBS) and cells were resuspended in RPMI-1640 (Gibco, Grand Island, NY) supplemented with 5% heat-inactivated fetal calf serum (FCS; Gibco), 2 mm glutamine (Sigma, St Louis, MO), 1 mm non-essential amino acids (Gibco), 100 U/ml penicillin G (Sigma), 0·05 mm 2-mercaptoethanol (Sigma) and 110 mg/l sodium pyruvate (Sigma) (tissue culture medium). To generate CTL, PBMC were cultured, at a density of 3 × 106/ml, in 10 ml of tissue culture medium in T25 flasks (Nunc, Roskilde, Denmark) containing 6 × 105 CFU M. bovis, unless stated otherwise. In some experiments, M. bovis-infected Mφs or bovine PPD (300 µg/ml; CSL Ltd, Parkville, Victoria, Australia) were used to stimulate CTL in vitro. Infected Mφs, for in vitro stimulation of CTL, were prepared as follows. PBMC were cultured at 3 × 106/ml for 4 hr, non-adherent cells were removed by careful washing (three times) with tissue culture medium at 37°, and adherent cells were infected with M. bovis at a multiplicity of infection (m.o.i.) of 2 : 1. After 18 hr, the cells were washed and fresh PBMC (at 3 × 106/ml) were added. Cultures were incubated in humidified 5% CO2 in air for 7 days.
Cytotoxicity assay
The method follows one described previously, with modifications.13 Mφ target cells were prepared by culturing 1 × 106 PBMC in tissue culture medium in round-bottomed 96-well plates (Nunc) and then, after an 18-hr incubation, non-adherent cells were removed. After a further 5 days of culture, target cells were either infected with M. bovis (at an m.o.i. of 2 : 1) or uninfected, and then labelled with 3 µCi/well of 51Cr (Amersham, Bucks., UK) overnight (18 hr). Prior to use as targets, they were washed three times with prewarmed (37°) tissue culture medium. Effector cells from PBMC stimulated in vitro were centrifuged over Lymphoprep to remove dead cells and washed three times. In vitro-stimulated or fresh PBMC were serially diluted and added to the infected and uninfected target cells. After 18 hr, supernatants were removed to measure 51Cr release. Any remaining 51Cr in the targets was released by lysis with 5% Triton-X-100 (Sigma) so that the total uptake could be calculated. Target cells incubated without CTL effectors were included to measure the spontaneous release of 51Cr. Mycobacteria were killed by adding glutaraldehyde (2% final concentration). Radioactivity was determined using Optiphase scintillant in a Trilux liquid scintillation counter (Wallac, Turku, Finland). Specific lysis, in counts per minute (c.p.m.) was calculated as follows:
 |
The standard error of the mean (SEM) of triplicate determinations did not usually exceed 4·5% specific lysis.
IFN-γ assay
Heparinized blood, in 1·5-ml aliquots, was dispensed into a 24-well plate within 4 hr of collection, and 100 µl of bovine PPD (final concentration 20 µg/ml) or 100 µl of PBS was added. The whole-blood cultures were incubated at 37° for 18 hr and the IFN-γ levels in the plasma supernatants were measured, as described previously,26 using the BOVIGAM sandwich enzyme-linked immunosorbent assay (ELISA) kit (CSL Ltd). The amount of IFN-γ was calculated from a standard curve prepared with recombinant bovine IFN-γ.28
Phenotying of CTL
T-cell subset depletion was carried out using the Mini-Macs system (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). For CD8+ depletion, CTL effector cells were harvested and incubated with antibody specific for CD8 (CC63; Serotec, Oxford, UK) at 4° followed by goat anti-mouse immunoglobulin G (IgG) magnetic beads and passed through a Mini Macs column, according to the manufacturers' instructions. WC1+γδ effector cells were similarly depleted using WC1-specific antibody (CC15; Serotec) and the Mini-Macs system. Depleted cells were washed twice in tissue culture medium prior to being assayed for CTL activity. Depletion of the specific subset was >95%. In one experiment, CD8+ depletion was carried out using complement-mediated lysis following antibody treatment. CTL effector cells were harvested and incubated with antibody specific for CD8 (CC63; Serotec) at 37° for 30 min followed by guinea-pig complement (Behring Diagnostics GmbH, Marburg, Germany) at 37° for 30 min. Cells were centrifuged over Lymphoprep and washed twice in tissue culture medium before being assayed for CTL activity.
Mycobacteria growth-inhibition assay
PBMC that had been restimulated in vitro were incubated with M. bovis-infected Mφs for 5 days. Growth of M. bovis within the Mφs was determined, as described previously, using uptake of [3H]uracil.29 Briefly, Mφ cultures were lysed with 0·1% saponin (Sigma), pulsed with 3·7 × 104 Bq [3H]uracil (specific activity 1·85 TBq/mmol; Amersham) per well and harvested 24 hr later. Mycobacteria were heat killed and the incorporated radioactivity was counted in a Trilux liquid scintillation counter (Wallac). Growth inhibition was expressed as follows:
Statistical analysis
To determine significant differences, the results from the CTL and IFN-γ assays were log10 transformed to achieve homogeneity of variance and were then analysed by analysis of variance (anova).
Results
Cytotoxic activity of PBMC from experimentally infected cattle
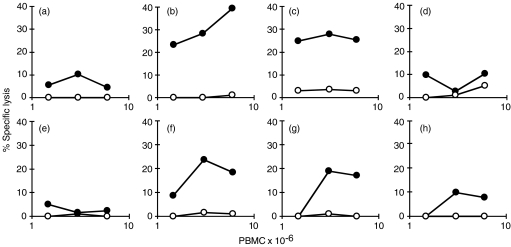
To determine conditions whereby specific cytotoxic cells could be generated, the PBMC of two experimentally infected cattle were restimulated in vitro under various conditions. After 7 days of culture they were tested for the ability to lyse their own M. bovis-infected or uninfected Mφs by the 51Cr-release assay. PBMC from one animal, cultured for 7 days without restimulation, did not display cytotoxic activity (Fig. 1a). PBMC that were cultured for 7 days with live M. bovis at 6 × 105 or 3 × 106 CFU per 3 × 107 PBMC, specifically lysed M. bovis-infected Mφs (Fig. 1b, 1c, respectively). When the dose of M. bovis was increased to 1·5 × 107 CFU, M. bovis cytotoxic activity was almost undetectable (Fig. 1d). The other animal was used to compare the ability of bovine PPD, live M. bovis (6 × 105 CFU) and M. bovis-infected or -uninfected Mφs to stimulate CTL in vitro from PBMC. PPD did not stimulate CTL activity (Fig. 1e). Adding 6 × 105 CFU M. bovis directly to PBMC cultures (Fig. 1g), gave almost the same response as adding Mφs previously infected with M. bovis (Fig. 1f). When uninfected Mφs were used there was a barely detectable response (Fig. 1h). Similar numbers of viable lymphocytes were recovered from each culture and comparable levels of IFN-γ were detectable in cultures that had been re-exposed to mycobacterial antigens (data not shown). In subsequent experiments, 6 × 105 CFU M. bovis were used for in vitro restimulation of PBMC to generate CTL.
Figure 1.
Lysis of Mycobacterium bovis-infected macrophages (Mφs) by cytotoxic cells. Peripheral blood mononuclear cells (PBMC) from two calves (no. 1 and no. 2), experimentally infected with M. bovis 11 weeks previously, were restimulated in vitro and assayed for cytotoxic T-lymphocyte (CTL) activity on M. bovis-infected (•) or uninfected (○) Mφs. PBMC from calf no. 1 were cultured alone (a), with 6 × 105 colony-forming units (CFU) of M. bovis (b), with 3 × 106 CFU of M. bovis (c), or with 1·5 × 107 CFU of M. bovis (d) PBMC from calf no. 2 were cultured with bovine purified protein derivative (PPD) (e), with 6 × 105 CFU of M. bovis (f), or with M. bovis-infected Mφs (g) or uninfected Mφs(h).
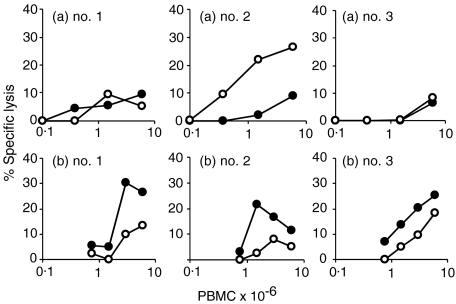
Effector activity of PBMC after in vitro restimulation with M. bovis may reflect activity derived from non-effector CTL precursors or memory cells present in the blood. In order to measure CTL effector-cell activity in fresh blood, PBMC from the two calves used in the first experiment, and an additional experimentally infected calf, were assayed for cytotoxicity on infected or uninfected Mφs without prior in vitro restimulation. For comparison, PBMC, from the same blood sample, were restimulated in vitro with live M. bovis for 7 days (Fig. 2). There was minimal cytotoxic activity in fresh PBMC against M. bovis-infected Mφs (<10% specific lysis; Fig. 2a) but fresh PBMC from one calf (Fig. 2a, no. 2) contained cytotoxic cells that were able to lyse uninfected Mφs. M. bovis-specific cytotoxic activity was detected in all three animals after in vitro stimulation (Fig. 2b).
Figure 2.
Comparison of cytotoxic activity in fresh and cultured peripheral blood mononuclear cells (PBMC). Freshly isolated PBMC from three calves (no. 1, no. 2 and no. 3), experimentally infected with Mycobacterium bovis 13 weeks previously, were assayed for cytotoxic activity (a) or were restimulated in vitro with M. bovis for 7 days prior to assay (b). The mean percentage-specific lysis of M. bovis-infected macrophages (Mφs) (•) or uninfected Mφs (○) of triplicate determinations are shown.
A further experiment was carried out to follow the generation of CTL activity during the course of experimental infection with M. bovis. PBMC were prepared immediately before infection and at various time-points after infection, restimulated in vitro and assayed for cytotoxic activity. The zero time-point, immediately prior to infection, where cytotoxic activity was ≤5% specific lysis, using either infected or uninfected target cells, acted as the negative control. Cytotoxic activity was detected at 4 weeks after challenge, and responses, although not significantly different during the course of infection, were significantly different at 4, 8 and 13 weeks postchallenge compared with 0 weeks (P < 0·05; Fig. 3a). For comparison, whole-blood IFN-γ responses to bovine PPD during the course of infection are shown (Fig. 3b). IFN-γ responses peaked 4 weeks after infection; the mean values at 5, 10 and 17 weeks postchallenge were significantly different from those at 0 weeks (P < 0·05) and there was a significant decrease between weeks 5 and 17 postinfection (P < 0·05).
Figure 3.
Cytotoxic activity and interferon-γ (IFN-γ) responses during experimental infection with Mycobacterium bovis. Six calves (no. 4, no. 5, no. 6, no. 7, no. 8 and no. 9) were experimentally infected with M. bovis; during the course of the infection, peripheral blood mononuclear cells (PBMC) were assayed for in vitro cytotoxic responses to M. bovis[mean response ± standard error of the mean (SEM); Fig. 3a), or plasma from whole blood stimulated with bovine purified protein derivative (PPD) was assayed for IFN-γ (mean response ± SEM; Fig. 3b). Cytotoxic activity generated from 6 × 106 PBMC was determined in triplicate using M. bovis-infected (•) or -uninfected (○) macrophage targets. *Significantly different from 0 weeks (preinfection; P < 0·05); †significantly different from week 17 (P < 0·05).
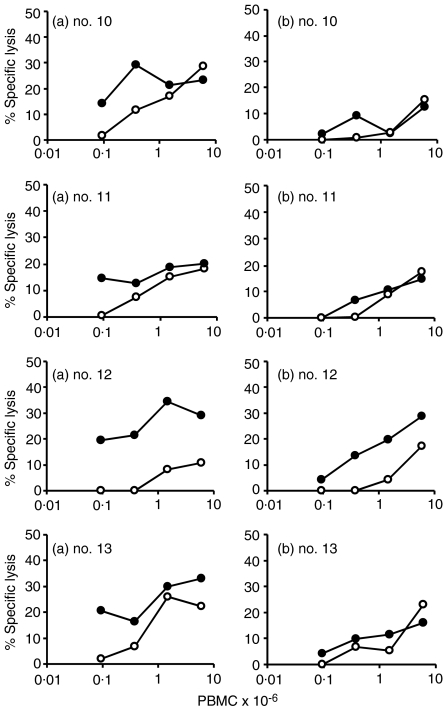
WC1+γδ and CD8+ T-cell depletion
The effect of depleting WC1+γδ T cells (using Mini Macs columns) from CTL cultures of PBMC, taken from four calves 13 weeks following an experimental infection with M. bovis, was determined. The results are shown in Fig. 4. Untreated cultures displayed good CTL activity (Fig. 4a), whereas after γδ depletion the activity was reduced in all four animals. This was particularly evident as effector cells were diluted out to lower concentrations (<105 PBMC). For comparison, PBMC, taken from two of the calves (no. 10 and no. 11), 2 weeks previously at 11 weeks after infection, were stimulated in vitro and depleted of CD8+ T cells in a similar manner. Another calf (no. 14) had CD8+ T cells depleted by antibody and complement treatment 11 weeks after infection (Table 1). There was reduced CTL activity in all three animals after CD8+ T-cell depletion compared with non-depleted controls.
Figure 4.
Effect of WC1+γδ depletion of effector cells on cytotoxic activity. Cytotoxic effector cells generated in vitro from four calves (no. 10, no. 11, no. 12 and no. 13), experimentally infected with Mycobacterium bovis 13 weeks previously, were assayed for cytotoxic activity (a) or treated to remove γδ cells prior to assay (b). The mean percentage-specific lysis from triplicate assays, using M. bovis-infected (•) or uninfected (○) macrophage targets are shown.
Table 1.
Effect of depleting CD8+ T cells from the effector population
| Animal number | Method of anti-CD8+ depletion | Untreated | Anti-CD8 depleted |
|---|---|---|---|
| 10 | Mini-Macs column | 9·4 ± 0·7 | 6·3 ± 1·7 |
| 11 | Mini-Macs column | 16·4 ± 4·5 | 7·8 ± 1·4 |
| 14 | Anti-CD8 antibody+ complement | 22·5 + 2·0 | 9·4 ± 2·3 |
Mean (±SEM) percentage-specific lysis of Mycobacterium bovis-infected macrophages by 3 × 106 peripheral blood mononuclear cells restimulated in vitro from calves infected with M. bovis 11 weeks previously.
Growth inhibition of mycobacteria
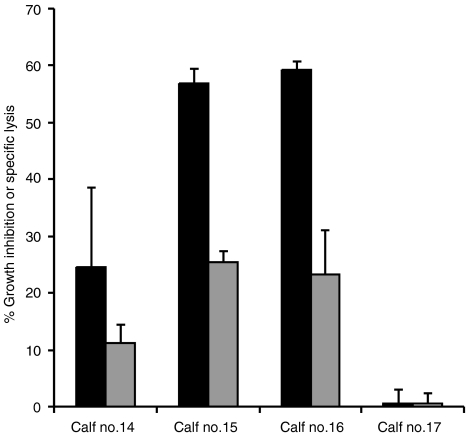
To determine whether cell cultures with cytotoxic activity could also inhibit the growth of mycobacteria, PBMC from four animals were stimulated in vitro with M. bovis to generate CTL activity and tested after 24 hr and 6 days for mycobacterial growth-inhibitory properties on M. bovis-infected Mφs. CTL activity of the cultured cells was determined. There was no inhibition of growth of M. bovis after 24 hr of incubation (data not shown) but this was detected in three of four animals when CTL cultures were incubated with infected Mφs for 6 days. The same three animals (no. 14, no. 15, and no. 16) gave a CTL response, and the culture that displayed the highest level of growth-inhibiting activity also gave the strongest CTL response (Fig. 5). All four animals had macroscopic lesions in the lung and lymph nodes, and the lesioned samples were culture positive for M. bovis.
Figure 5.
Comparison of effector function. Effector cells from 6 × 106 peripheral blood mononuclear cells (PBMC) of four calves (no. 14, no. 15, no. 16 and no. 17), experimentally infected with Mycobacterium bovis 20 weeks previously, were assayed for their ability to inhibit the growth of M. bovis within infected macrophages (Mφs) (black bars) or to lyse M. bovis-infected Mφs (grey bars). Results are shown as mean percentage growth inhibition and mean percentage specific lysis (± standard error of the mean).
Discussion
It is clear, from studies using mouse models of tuberculosis and from human patients, that cytotoxic cells are generated during infections with M. tuberculosis.12,28 In this work, we show that cytotoxic cells, which specifically lyse M. bovis-infected Mφs, contribute to the immune response during an experimental M. bovis infection of cattle. The cytotoxic response was maintained as the disease progressed, and cytotoxic activity could be assigned to WC1+γδ and CD8+ T cells. Although CD4+ T cells can also function as CTL, they were not studied in this work. This is the first report of such cytotoxic activity in cattle infected with bovine tuberculosis.
The method used to evaluate the killing of mycobacteria-infected Mφs is based on an assay used in human PBMC30 and mice.13 The specific CTL responses were weak, like those detected during M. tuberculosis infections of mice.28 They could be detected when PBMC were exposed to lower doses of M. bovis (6 × 105 or 3 × 106 CFU) in vitro, but not when a higher dose (1·5 × 107 CFU) was used. Mycobacteria are known to possess agents that modify immune responses, and the balance of the concentration of activating and inhibitory molecules may be important in stimulating CTL activity. M. bovis-infected Mφs also stimulated in vitro CTL responses but, as expected, bovine PPD did not, and M. bovis-specific CTL activity could not be detected in fresh blood. The responses varied from animal to animal. This variation could have been caused, in part, by previous exposure of the animals to environmental mycobacteria or as a result of using an outbred population of animals. The responses were maintained during the course of the experimental infection and did not decline as did the IFN-γ responses. When animals were killed at 21 weeks postinfection, mediastinal lymph node cells displayed CTL activity after in vitro stimulation with M. bovis (data not shown). The dose–response relationship between the concentration of effector cells and the percentage-specific lysis did not always follow the characteristic relationship generally observed in 51Cr-release assays, suggesting that more than one population of cells was involved. Depletion studies showed that both WC1+γδ T cells and CD8+ T cells were involved in cytotoxic activity.
The importance of cytotoxic responses in protective immunity to tuberculosis is not clear. All the major T-cell subsets – CD4+, CD8+ and γδ– have been shown to kill Mφs expressing mycobacterial antigens.7,31,32 In this work, the focus has been on CD8+ and γδ T cells, as their function in protective immunity to mycobacterial infections is not so well defined. Depletion studies showed that both WC1+γδ and CD8+ T cells had the capacity, when re-exposed to M. bovis in vitro, to kill infected Mφs during experimental infections with M. bovis. WC1+γδ T-cell numbers increase in the blood of cattle early during infection, with other T-cell subsets increasing in number at later time-points.6 In this work, the depletion of WC1+γδ T cells in vitro at 13 weeks postinfection resulted in reduced CTL activity, and CD8+ T-cell depletion at a similar time-point (11 weeks after infection) also resulted in reduced CTL activity; however, it is possible that different CTL populations are generated at different time-points during the course of infection. CD8+ T cells recognize conventional protein mycobacterial antigens, whereas WC1+γδ T cells have been shown in cattle, as have γδ T cells in other species, to respond to both protein and non-protein phosphate-rich antigens of mycobacteria.33γδ T cells from M. bovis-infected cattle have been shown to exert immunomodulatory effects on αβ T cells, inhibiting their proliferation,10 and it is possible that they could even kill them. γδ T cells from humans can be autoreactive and lyse autologous lymphocytes.34 Some indication of self-reactivity was detected in PBMC directly after isolation from blood, but the cells responsible for this activity were not investigated in this study. After in vitro restimulation, cytolytic cells lysed both uninfected, as well as infected, Mφs, an observation that may reflect the cross-reactivity between mycobacterial and self-antigens, for example heat-shock proteins. This type of non-specific lysis has long been established as a feature of CTL responses to intracellular pathogens and has been attributed to conventional CTL, NK cells and lymphokine-activated killer cells.35,36
Cattle have high numbers of circulating γδ T cells compared to mice and humans, and these cells may play a more significant role in bovine tuberculosis. However, depletion of γδ T cells in both mice3 and cattle37 does not appear to markedly change the course of disease. CD8+ T cells have been shown to release mycobacteria from Mφs.25 One proposed concept is that the released mycobacteria are taken up by more efficient antigen-presenting cells, but CTL have also been shown to reduce the viability of intracellular and extracellular M. tuberculosis.16–18 In this report, we have shown that the cytotoxic activity of lymphocytes from M. bovis-infected cattle appears to correlate with the ability of these lymphocytes to inhibit the number of mycobacteria within infected Mφs. This inhibitory effect was observed after 6 days of incubation, suggesting that it was not mediated by IFN-γ.38 However, it is yet to be proven whether some other mediator, for example tumour necrosis factor-α (TNF-α), is involved.
Vaccination of cattle remains an option for the control of bovine tuberculosis,39 but a better vaccine or vaccination strategy is required. Many of the experimental problems associated with the development of protective vaccination against bovine and human tuberculosis are similar, and bovine tuberculosis models are being viewed as a suitable model for evaluating human tuberculosis vaccines and vaccination strategies.40 Vaccine trials in cattle provide an opportunity to follow immune responses after vaccination and correlate them with disease or protection.5,41,42 Whole-blood IFN-γ, interleukin-2 and antibody responses have been studied in previous work and future work is aimed at following cytotoxic responses in the same way.
Acknowledgments
We thank Denise Keen for technical assistance and the New Zealand Ministry of Agriculture and Forestry for financial support.
References
- 1.Orme IM, Roberts AD, Griffin JP, Abrams JS. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–25. [PubMed] [Google Scholar]
- 2.Serbina NV, Flynn JL. Early emergence of CD8+ T cells primed for production of type 1 cytokines in the lungs of Mycobacterium tuberculosis-infected mice. Infect Immun. 1999;67:3980–8. doi: 10.1128/iai.67.8.3980-3988.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An anti-inflammatory role for gamma delta T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–21. [PubMed] [Google Scholar]
- 4.Yoneda T, Ellner JJ. CD4+ T cell and natural killer cell-dependent killing of Mycobacterium tuberculosis by human monocytes. Am J Respir Crit Care Med. 1998;158:395–403. doi: 10.1164/ajrccm.158.2.9707102. [DOI] [PubMed] [Google Scholar]
- 5.Wedlock DN, Vesosky B, Skinner MA, de Lisle GW, Orme IM, Buddle BM. Vaccination of cattle with Mycobacterium bovis culture filtrate proteins and interleukin-2 for protection against bovine tuberculosis. Infect Immun. 2000;68:5809–15. doi: 10.1128/iai.68.10.5809-5815.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollock JM, Pollock DA, Campbell DG, Girvin RM, Crockard AD, Neill SD, Mackie DP. Dynamic changes in circulating and antigen-responsive T cell subpopulations post-Mycobacterium bovis infection in cattle. Immunology. 1996;87:236–41. doi: 10.1046/j.1365-2567.1996.457538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liebana E, Girvin RM, Welsh M, Neill SD, Pollock JM. Generation of CD8+ T-cell responses to Mycobacterium bovis and mycobacterial antigen in experimental bovine tuberculosis. Infect Immun. 1999;67:1034–44. doi: 10.1128/iai.67.3.1034-1044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waters WR, Palmer MV, Pesch BA, Olsen SC, Wannemuehler MJ, Whipple DL. Lymphocyte subset proliferative responses of Mycobacterium bovis-infected cattle to purified protein derivative. Vet Immunol Immunopathol. 2000;77:257–73. doi: 10.1016/s0165-2427(00)00245-2. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy JP, Bryson DG, Gutiérrez Cancela MM, Forster F, Pollock JM, Neill SD. Lymphocyte subtypes in experimentally induced early-stage bovine tuberculosis lesions. J Comp Path. 2001;124:46–51. doi: 10.1053/jcpa.2000.0427. [DOI] [PubMed] [Google Scholar]
- 10.Rhodes SG, Hewinson RG, Vordermeier HM. Antigen recognition and immunomodulation by gamma delta T cells in bovine tuberculosis. J Immunol. 2001;166:5604–10. doi: 10.4049/jimmunol.166.9.5604. [DOI] [PubMed] [Google Scholar]
- 11.Flesch IE, Kaufmann SH. Mechanisms involved in mycobacterial growth inhibition by gamma-interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect Immun. 1991;59:3213–8. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalvani A, Brookes R, Wilkinson RJ, et al. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1998;95:270–5. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skinner MA, Yuan S, Prestidge R, Chuk D, Watson JD, Tan PLJ. Immunization with heat-killed Mycobacterium vaccae stimulates CD8+ cytotoxic T cells specific for macrophages infected with Mycobacterium tuberculosis. Infect Immun. 1997;65:4525–30. doi: 10.1128/iai.65.11.4525-4530.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaddum RM, Cook RS, Thomas LH, Taylor G. Primary cytotoxic T-cell responses to bovine respiratory syncytial virus in calves. Immunology. 1996;88:421–7. doi: 10.1046/j.1365-2567.1996.d01-667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison WI, Taracha EL, McKeever DJ. Theileriosis: progress towards vaccine development through understanding immune responses to the parasite. Vet Parasitol. 1995;57:177–87. doi: 10.1016/0304-4017(94)03119-h. [DOI] [PubMed] [Google Scholar]
- 16.Stenger S, Mazzaccaro RJ, Uyemura K, et al. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–7. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 17.Silva CL, Lowrie DB. Identification and characterization of murine cytotoxic T cells that kill Mycobacterium tuberculosis. Infect Immun. 2000;68:3269–74. doi: 10.1128/iai.68.6.3269-3274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dieli F, Troye-Blomberg M, Ivanyi J, et al. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vgamma9/Vdelta2 T lymphocytes. J Infect Dis. 2001;184:1082–5. doi: 10.1086/323600. [DOI] [PubMed] [Google Scholar]
- 19.Denis O, Tanghe A, Palfliet K, et al. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect Immun. 1998;66:1527–33. doi: 10.1128/iai.66.4.1527-1533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith SM, Brookes R, Klein MR, et al. Human CD8+ CTL specific for the mycobacterial major secreted antigen 85A. J Immunol. 2000;165:7088–95. doi: 10.4049/jimmunol.165.12.7088. [DOI] [PubMed] [Google Scholar]
- 21.Coler RN, Campos-Neto A, Ovendale P, et al. Vaccination with the T cell antigen Mtb 8.4 protects against challenge with Mycobacterium tuberculosis. J Immunol. 2001;166:6227–35. doi: 10.4049/jimmunol.166.10.6227. [DOI] [PubMed] [Google Scholar]
- 22.Silva CL, Silva MF, Pietro RC, Lowrie DB. Characterization of T cells that confer a high degree of protective immunity against tuberculosis in mice after vaccination with tumor cells expressing mycobacterial hsp65. Infect Immun. 1996;64:2400–7. doi: 10.1128/iai.64.7.2400-2407.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowrie DB, Silva CL, Colston MJ, Ragno S, Tascon RE. Protection against tuberculosis by a plasmid DNA vaccine. Vaccine. 1997;15:834–8. doi: 10.1016/s0264-410x(97)00073-x. [DOI] [PubMed] [Google Scholar]
- 24.Lowrie DB, Tascon RE, Bonato VL, et al. Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400:269–71. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- 25.Liebana E, Aranaz A, Aldwell FE, McNair J, Neill SD, Smyth AJ, Pollock JM. Cellular interactions in bovine tuberculosis: release of active mycobacteria from infected macrophages by antigen-stimulated T cells. Immunology. 2000;99:23–9. doi: 10.1046/j.1365-2567.2000.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothel JS, Jones SL, Corner LA, Cox JC, Wood PR. A sandwich enzyme immunoassay for bovine interferon-gamma and its use for the detection of tuberculosis in cattle. Aust Vet J. 1990;67:134–7. doi: 10.1111/j.1751-0813.1990.tb07730.x. [DOI] [PubMed] [Google Scholar]
- 27.Buddle BM, de Lisle GW, Pfeffer A, Aldwell FE. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine. 1995;13:1123–30. doi: 10.1016/0264-410x(94)00055-r. [DOI] [PubMed] [Google Scholar]
- 28.Wedlock DN, Aldwell FE, Collins DM, de Lisle GW, Wilson T, Buddle BM. Immune responses induced in cattle by virulent and attenuated Mycobacterium bovis strains: correlation of delayed-type hypersensitivity with ability of strains to grow in macrophages. Infect Immun. 1999;67:2172–7. doi: 10.1128/iai.67.5.2172-2177.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldwell FE, Wedlock DN, Buddle BM. Bacterial metabolism, cytokine mRNA transcription and viability of bovine alveolar macrophages infected with Mycobacterium bovis BCG or virulent M. bovis. Immunol Cell Biol. 1996;74:45–51. doi: 10.1038/icb.1996.6. [DOI] [PubMed] [Google Scholar]
- 30.Kumararatne DS, Pithie AS, Drysdale P, et al. Specific lysis of mycobacterial antigen-bearing macrophages by class II MHC-restricted polyclonal T cell lines in healthy donors or patients with tuberculosis. Clin Exp Immunol. 1990;80:314–23. doi: 10.1111/j.1365-2249.1990.tb03287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orme IM, Miller ES, Roberts AD, et al. T lymphocytes mediating protection and cellular cytolysis during the course of Mycobacterium tuberculosis infection. Evidence for different kinetics and recognition of a wide spectrum of protein antigens. J Immunol. 1992;148:189–96. [PubMed] [Google Scholar]
- 32.Tsukaguchi K, Balaji KN, Boom WH. CD4+ alpha beta T cell and gamma delta T cell responses to Mycobacterium tuberculosis: similarities and differences in antigen recognition, cytotoxic effector function, and cytokine production. J Immunol. 1995;154:1786–96. [PubMed] [Google Scholar]
- 33.Welsh MD, Kennedy HE, Smyth AJ, Girvin RM, Andersen P, Pollock JM. Responses of bovine WC1+ gamma delta T cells to protein and nonprotein antigens of Mycobacterium bovis. Infect Immun. 2002;70:6114–20. doi: 10.1128/IAI.70.11.6114-6120.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halary F, Peyrat MA, Champagne E, et al. Control of self-reactive cytotoxic T lymphocytes expressing gamma delta T cell receptors by natural killer inhibitory receptors. Eur J Immunol. 1997;27:2812–21. doi: 10.1002/eji.1830271111. [DOI] [PubMed] [Google Scholar]
- 35.Skinner MA, Finberg RW, Ertl HCJ. Regulation of cytotoxic lymphocyte precursors. II. The effect of interleukin-2 and interferon-γ on the apparent specificity of effector cells. Cell Immunol. 1986;100:239–46. doi: 10.1016/0008-8749(86)90023-7. [DOI] [PubMed] [Google Scholar]
- 36.Kaleab B, Ottenoff T, Converse P, Halapi E, Tadesse G, Rottenberg M, Kiessling R. Mycobacterial-induced cytotoxic T cells as well as nonspecific killer cells derived from healthy individuals and leprosy patients. Eur J Immunol. 1990;20:2651–9. doi: 10.1002/eji.1830201219. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy HE, Welsh MD, Bryson DG, Cassidy JP, Forster FI, Howard CJ, Collins RA, Pollock JM. Modulation of immune responses to Mycobacterium bovis in cattle depleted of WC1+ gamma delta T cells. Infect Immun. 2002;70:1488–500. doi: 10.1128/IAI.70.3.1488-1500.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato K, Akaki T, Tomioka H. Differential potentiation of anti-mycobacterial activity and reactive nitrogen intermediate-producing ability of murine peritoneal macrophages activated by interferon-gamma (IFN-gamma) and tumour necrosis factor-alpha (TNF-alpha) Clin Exp Immunol. 1998;112:63–8. doi: 10.1046/j.1365-2249.1998.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skinner MA, Wedlock DN, Buddle BM. Vaccination of animals against Mycobacterium bovis. Rev Sci Tech Off Int Epiz. 2002;20:112–32. doi: 10.20506/rst.20.1.1276. [DOI] [PubMed] [Google Scholar]
- 40.Hewinson RG, Vordermeier HM, Buddle BM. Use of the bovine model of tuberculosis for the development of improved vaccines and diagnostics. Tuberculosis. 2003;83:119–30. doi: 10.1016/s1472-9792(02)00062-8. [DOI] [PubMed] [Google Scholar]
- 41.Buddle BM, Keen D, Thomson A, et al. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res Vet Sci. 1995;59:10–6. doi: 10.1016/0034-5288(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 42.Buddle BM, Skinner MA, Wedlock DN, Collins DM, de Lisle GW. New generation vaccines and delivery systems for control of bovine tuberculosis in cattle and wildlife. Vet Immunol Immunopathol. 2002;87:177–85. doi: 10.1016/s0165-2427(02)00049-1. [DOI] [PubMed] [Google Scholar]