Abstract
Presumably because of the selective pressure exerted by the immune system, many viruses have evolved proteins that interfere with antigen presentation by major histocompatibility complex (MHC) class I molecules. These viruses utilize a whole variety of ingenious strategies to inhibit the MHC class I pathway. Viral proteins have been characterized that exploit bottlenecks in the MHC class I pathway, such as peptide translocation by the transporter associated with antigen processing. Alternatively, viral proteins can cause the degradation or mislocalization of MHC class I molecules. This is often achieved by the subversion of the host cell's own protein degradation and trafficking pathways. As a consequence elucidation of how these viral proteins act to subvert host cell function will continue to give important insights not only into virus–host interactions but also the function and mechanism of cellular pathways.
Introduction
Viruses are obligate intracellular parasites, which hijack the host cell's biosynthetic machinery to enable the translation of viral proteins and the replication of the viral genome. The major histocompatibility (MHC) class I antigen presentation pathway plays an important role in alerting the immune system to virally infected cells. MHC class I molecules are expressed on the cell surface of all nucleated cells and present peptide fragments derived from intracellular proteins. These peptides are normally derived from the cell's own ‘house-keeping’ proteins but in a virally infected cell, peptides derived from viral proteins may also be presented. Virus specific cytotoxic T lymphocytes (CTL) monitor cell surface MHC class I molecules for peptides derived from viral proteins and eliminate infected cells.
Given the role that the MHC class I antigen presentation pathway plays in the detection of virally infected cells by CTLs, it is not surprising that many viruses have evolved proteins that interfere with this pathway. This review will discuss recent findings on some of the diverse array of mechanisms employed by human viruses to inhibit the MHC class I pathway in order to escape CTL lysis. Examples of proteins that interfere with the MHC class I pathway are encoded by adenoviruses and retroviruses.1 These include the adenovirus E3/19K and the human immunodeficiency virus-1 (HIV-1) Nef gene products, which are discussed herein. However, much of this review focuses on the strategies employed by members of the herpesvirus family to inhibit the MHC class I pathway. Herpesviruses establish persistent lifelong infections in immunocompetent hosts; in fact most if not all, herpesviruses encode proteins that inhibit MHC class I antigen presentation and these proteins play an important role in allowing the virus to evade detection by CTLs.1–3 This is perhaps exemplified by the human cytomegalovirus (HCMV), where the unique short region of the viral genome encodes at least five proteins (US2, US3, US6, US10 and US11) that inhibit the MHC class I pathway.1,4 Indeed, HCMV inhibition of the MHC class I presentation pathway illustrates the intimate relationship that can exist between a virus and the infected host.
An overview of the mhc class i antigen presentation pathway
MHC class I molecules are heterodimers of a heavy chain, a 45 000 MW type I integral membrane glycoprotein, and β2-microglobulin (β2M) a 12 000 MW soluble protein.5 The extracellular region of the heavy chain folds into three domains (α1, α2 and α3), with β2M contributing a fourth domain. The α1 and α2 domains form the peptide-binding site: this is a groove on the upper surface of the MHC class I molecule, which binds antigenic peptides of 8–10 amino acids in length. Surprisingly, the majority of peptides presented by MHC class I molecules are not derived from the turnover of ‘old’ proteins. Instead most of the peptides presented by MHC class I molecules are derived from defective ribosomal translation products, which are degraded by the multisubunit proteasome complex in the cytosol (Fig. 1).6–8 This allows the MHC class I pathway to sample proteins immediately after synthesis hence rapidly alerting CTLs to the presence of a virus in an infected cell.
Figure 1.
The MHC class I antigen presentation pathway. (1) Proteins are proteolytically processed in the cytosol by the proteasome. (2) Peptides generated by the proteasome are translocated into the ER lumen by TAP. (3) MHC class I molecules (heavy chain and associated β2M) fold and assemble in the ER lumen with the aid of the ER chaperones calnexin, calreticulum and ERP57. (4) The MHC class I molecule in a complex with calreticulum and ERP57 associates with TAP and tapasin facilitates peptide binding. (5) Peptide loaded MHC class I molecules dissociate from TAP and are transported through the secretory pathway to the plasma membrane.
MHC class I molecules fold and assemble within the endoplasmic reticulum (ER) lumen and peptide binding is an integral part of the assembly process (Fig. 1). As a consequence it is necessary to translocate peptides from the cytosol into the ER lumen, this function is performed by the transporter associated with antigen processing (TAP).9,10 TAP also acts as a scaffold for the final stage of MHC class I assembly, i.e. peptide binding. ER resident chaperones facilitate the folding of nascent MHC class I molecules and the MHC class I molecule (heavy chain and β2M) binds to TAP in a complex with the chaperones calreticulum and ERP57.11,12 Critical to this interaction is tapasin, which acts as a bridging molecule between the MHC class I/chaperone complex and TAP. Tapasin does not simply link nascent MHC class I molecules to TAP, but is also required to facilitate binding of high affinity peptides to the MHC class I molecule.13 After peptide loading, MHC class I molecules dissociate from TAP and cluster at export sites on the ER membrane where they are selectively recruited into cargo vesicles for transport to the Golgi apparatus.14 MHC class I molecules then traffic through the Golgi apparatus to the plasma membrane.
Turning off the tap
TAP is a heterodimer; the two subunits TAP1 and TAP2 each have an N-terminal membrane domain and a C-terminal nucleotide-binding domain (NBD).9,10 The membrane domains of TAP form the peptide-binding site and presumably a pore through which peptides are translocated, whereas ATP hydrolysis by the NBDs energizes peptide translocation by the membrane domains. TAP represents an obvious target for viral inhibition, because the vast majority of peptides presented by MHC class I molecules are generated in the cytosol and require translocation across the ER membrane. This bottleneck in the MHC class I pathway is exploited by the herpes simplex virus (HSV) ICP47 and the HCMV US6 gene products (Fig. 2). The ICP47 gene product is a small cytosolic protein of 88 amino acids of which only residues 3–34 are necessary to inhibit TAP function.15–18 ICP47 inhibits peptide binding to TAP, but does not affect ATP binding.19,20 With an affinity for TAP of 10–1000-fold greater than most peptides, ICP47 acts as a competitive inhibitor of peptide binding to TAP and is thought to bind directly to the peptide-binding site.19,20 Yet ICP47 does not behave like a normal peptide as it is not translocated across the membrane and it remains associated with TAP. Furthermore, whereas peptide binding by TAP stimulates ATP hydrolysis and causes a conformational rearrangement of TAP, these events are inhibited by ICP47 binding.21,22
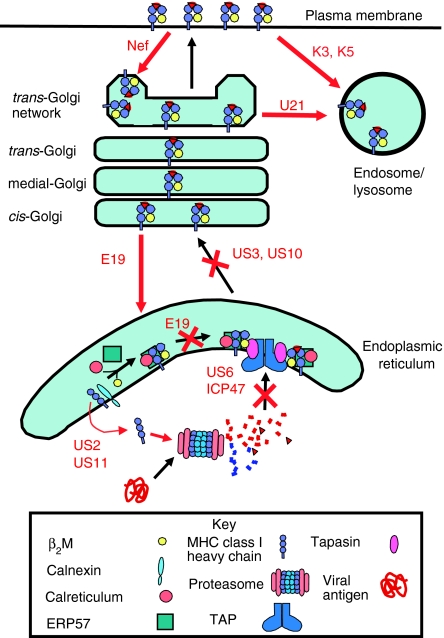
Figure 2.
Viral inhibition of the MHC class I antigen presentation pathway. Viruses have evolved proteins that inhibit the MHC class I pathway at virtually every step. US2 and US11 dislocate the MHC class I heavy chain into the cytosol where it is degraded by the proteasome. ICP47 and US6 inhibit peptide translocation by TAP. E19 inhibits MHC class I association with TAP, E19 also inhibits MHC class I trafficking by retrieving MHC class I molecules from the cis-Golgi. Similarly US3 and US10 inhibit the ER export of class I molecules. U21 diverts MHC class I molecules to the lysosome. Nef down-regulates MHC class I molecules from the plasma membrane sequestering them in the TGN. Finally K3 and K5 down-regulate MHC class I molecules and sort them into the late endocytic pathway where they are degraded.
US6 is an ER-localized, 21 000 MW type I integral membrane glycoprotein. Despite sharing the same target as ICP47, US6 uses a different mechanism to inhibit TAP. US6 interacts with TAP and inhibits peptide translocation, but unlike ICP47 it has no effect upon peptide binding.23–25 Instead, US6 prevents the conformational rearrangement of TAP that is normally associated with peptide binding, as evidenced by chemical cross-linking experiments and studies of the lateral mobility of TAP.8,26 Furthermore US6 inhibits ATP binding by TAP and more specifically by the TAP1 NBD.26,27 ATP is absolutely required for peptide translocation by TAP and by inhibiting ATP binding US6 effectively starves TAP of its energy source. The ER luminal domain of US6 is sufficient to inhibit both ATP binding and peptide translocation by TAP.25,26 As a consequence the inhibition of ATP binding cannot be a direct effect because the NBDs are on the opposite side of the ER membrane to the US6 luminal domain. Therefore by interacting presumably with the ER lumen exposed-loops of the TAP membrane domains, US6 must exert an indirect conformational effect upon the TAP1 NBD.
Throwing the mhc class i heavy chain out with the cellular garbage
The folding and assembly of nascent secretory proteins in the ER lumen is scrutinized by the ER quality control apparatus. Misfolded proteins are exported out of the ER back into the cytosol, where they are degraded by the proteasome. This process is known as ER-associated protein degradation (ERAD).28 HCMV encodes two related proteins, US2 and US11, that co-opt this host cell ‘garbage’ removal pathway to promote degradation of the MHC class I heavy chain and hence inhibit MHC class I antigen presentation (Fig. 2). Furthermore, degradation of the heavy chain by US2 and US11 has been used as a tool to study the ERAD pathway in mammalian cells.
US2 and US11 are ER resident type I integral membrane glycoproteins that share 21% sequence identity with each other. Expression of either protein causes rapid degradation of newly synthesized MHC class I heavy chains.29,30 The heavy chain is dislocated into the cytosol by retrotranslocation through the Sec61p ER protein translocon.31 Once in the cytosol, heavy chain is deglycosylated and then degraded by the proteasome.29,30 The US2 luminal domain interacts with the MHC class I heavy chain and binds to the heavy chain between the peptide binding site and the α3 domain.32,33 However, whereas the ERAD pathway is normally associated with the degradation of misfolded proteins, binding of the US2 luminal domain has no obvious effect upon heavy chain conformation.33 How do US2 and US11 divert the heavy chain into the ERAD pathway? The MHC class I heavy chain's cytosolic tail is required for dislocation into the cytosol mediated either by US2 or US11.34 Therefore, one plausible model is that US2 and US11 bind to the heavy chain via their luminal domains and recruit host cell proteins that extract the polypeptide from the ER membrane by ‘pulling’ on the cytosolic tail of the heavy chain.
What is known about the extraction of the MHC class I heavy chain from the ER membrane and which proteins other than Sec61p are involved? In US2- and US11-mediated dislocation the heavy chain is ubiquitinated as it enters the cytosol.35 Ubiquitin is a small 76 residue protein that is conjugated to the ε-amino group of lysines and can also form polyubiquitin chains attached to a specific protein. The process of ubiquitination involves the sequential action of three enzymes, a ubiquitin-activating enzyme (E1), a ubiquitin-conjugating enzyme (E2) and a ubiquitin ligase (E3) that is responsible for substrate recognition. Although polyubiquitination targets proteins for degradation by the proteasome, the addition of ubiquitin to the heavy chain is also required for its dislocation into the cytosol by US11.36,37 However, ubiquitination cannot act as a signal to initiate MHC class I heavy chain dislocation across the ER membrane, as the mutagenesis of the lysine residues in the heavy chain cytosolic tail has little effect upon degradation.35 Instead, the ER luminal domains of the heavy chain must be principle sites of ubiquitination and this may serve to prevent the heavy chain from ‘slipping’ back into the ER during retrotranslocation across the membrane.
In addition to ubiquitin the ATPases associated with a variety of cellular activities (AAA) family member p97 is involved in the dislocation of the MHC class I heavy chain across the ER membrane.38 The p97 ATPase is associated with the cytosolic face of the ER membrane and binds to MHC class I heavy chains in cells that express either US2 or US11.38,39 Expression of an ATPase-deficient mutant of p97 causes the accumulation of ubiquitinated heavy chains in the membrane, which are sensitive to protease digestion suggesting that they are only partially dislocated.38 This demonstrates that the ATPase activity of p97 is required to extract the heavy chain from the membrane and perhaps provides the mechanical force to pull the heavy chain into the cytosol. How the p97 ATPase is recruited is unclear as although p97 can interact with ubiquitin via the adaptor proteins Ufd1p and Npl4p, not all p97 associated heavy chains are ubiquitinated.38,40
Hold-ups and diversions in mhc class i traffic to the plasma membrane
The adenovirus gene product E3/19K (E19) is a type I integral membrane protein that interacts with MHC class I molecules in the ER and prevents their transport to the plasma membrane.41,42 E19 uses two distinct mechanisms to inhibit MHC class I antigen presentation (Fig. 2). The cytosolic tail of E19 contains a dilysine motif that inhibits MHC class I trafficking by acting as an ER retrieval motif.43,44 The dilysine motif was also found to be present in the cytosolic tails of many ER resident membrane proteins.45 Proteins carrying this motif are recognized by the coatamer protein complex (COP1) that is involved in the retrieval of proteins from the Golgi apparatus to the ER.45 However, an E19 mutant that lacks this ER retrieval motif can still inhibit MHC class I transport. This is because E19 binds to TAP and prevents TAP/MHC class I association.46 E19 thus appears to be acting as a tapasin mimic and thus would be predicted to interfere with peptide binding to MHC class I molecules.
The HCMV US3 gene product is an ER-resident type I integral membrane protein that inhibits the export of peptide-loaded MHC class I molecules from the ER (Fig. 2).47,48 However, US3 only interacts transiently with MHC class I molecules and US3 subsequently traffics to the lysosome where it is degraded.49 Therefore, if US3 expression is blocked, MHC molecules can eventually leave the ER and transit normally to the plasma membrane. Unlike E19, the US3 cytosolic tail has no known ER retrieval motifs and is not required for either the association with or the retention of class I molecules.50 However, both the transmembrane domain and luminal domain of US3 are required to retain MHC class I molecules in the ER.50,51 How US3 retains MHC class I molecules in the ER is poorly understood. US3 may interact with an ER resident protein to retain MHC class I molecules. Alternatively, US3 may interfere with the recruitment of peptide-loaded MHC class I molecules to sites on the ER membrane where proteins are recruited into vesicles for transport to the Golgi. In addition to US3, the HCMV has recently been shown to express another protein US10, which affects MHC class I export from the ER. US10 is an ER-localized integral membrane glycoprotein that retards but does not block the transport of MHC class I molecules from the ER (Fig. 2).4,52 Again, although US10 is predicted to be a type I integral membrane protein, the cytosolic domain does not contain any obvious ER localization signals. Further work will be required to determine how the interaction of MHC class I molecules with US10 can retard ER export.
The human herpesvirus 7 U21 gene product employs a different strategy to prevent MHC class I molecules from reaching the plasma membrane. U21 is a 60 000 MW type I integral membrane glycoprotein that rather than causing ER retention, binds to MHC class I molecules in the ER and targets them to the lysosome (Fig. 2).53 Once in the lysosome both U21 and the MHC class I molecule are degraded. How U21 is sorted to the lysosome is unclear as although a number of lysosomal sorting sequences have been identified, U21 has no known motif in its cytosolic domain.54
Mhc class i down-regulation: internalization and degradation
Arrival at their final destination (plasma membrane) does not protect MHC class I molecules from viral interference. The Kaposi's sarcoma-associated herpesvirus (KSHV) encodes two related proteins, K3 and K5, that share 40% sequence identity. Expression of either protein causes the rapid down-regulation of MHC class I molecules from the plasma membrane by clathrin-dependent endocytosis (Fig. 2).55,56 The internalized MHC class I molecules are sorted into an acidic endocytic compartment where they are degraded by acidic proteases.56,57 In addition, K5 can down-regulate the costimulatory molecules intracellular adhesion molecule-1 and B7.2.58,59 Indeed, a cellular homologue of K3 and K5 has been identified whose function appears to be to regulate cell surface expression of B7.2.60 This suggests that K3 and K5 have both evolved from a gene ‘hijacked’ by KSHV from the host genome.
K3 and K5 are integral membrane proteins with two transmembrane domains, a short luminal segment and both the N- and C-termini are cytosolically orientated.61 Crucially, K3 and K5 have an N-terminal plant homeodomain motif (PHD) motif. The PHD motif shares structural and sequence homology with the RING finger domain, that is found in a subset of E3 ubiquitin ligases.62,63 Indeed, K3 and K5 are novel E3 ubiquitin ligases and the expression of either protein promotes ubiquitination of MHC class I molecules.64,65 Both K3 and K5 have been localized to the ER by immunofluorescence microscopy (E. Hewitt and P. Lehner, unpublished data).56 However, there is no evidence for an association between K3 and MHC class I molecules in the ER. Instead K3 interacts with and promotes the ubiquitination of MHC class I molecules after progression past the medial-Golgi compartment along the secretory pathway.65 This indicates that a proportion of K3 (and presumably K5) must be localized in either the late secretory pathway or at the plasma membrane. Why K3 does not ubiquitinate MHC class I molecules in the ER is unknown. One possibility is that ubiquitination by K3 may require accessory proteins such as a host cell E2 ubiquitin-conjugating enzyme, which is present or associated with the late or distal secretory pathway.
Ubiquitination by K3 and K5 does not appear to target MHC class I molecules for proteasomal degradation. Instead ubiquitination is necessary for internalization from the cell surface and subsequent sorting into the late endosomal pathway. Mutagenesis of conserved residues in the PHD motif prevents both ubiquitination and down-regulation of MHC class I molecules.65,66 However, the interaction between K3 and MHC class I molecules was unaffected indicating that association in the absence of ubiquitination is insufficient to promote MHC class I down-regulation.65,66 Furthermore, lysine substitution in the cytosolic tail of the MHC class I heavy chain prevents internalization and degradation of the MHC class I molecule by K3 and K5.64,65 These findings suggest the heavy chain's cytosolic tail is the site of K3 and K5-induced ubiquitination.
How does ubiquitination cause class I down-regulation? Studies in both yeast and mammalian cells indicate that ubiquitin can function as an endosomal-sorting motif. The addition of ubiquitin to specific lysines in the cytosolic domains of membrane proteins promotes their internalization from the plasma membrane.67,68 Ubiquitination also acts as a signal to sort proteins into the late endocytic pathway for degradation. A key step in this process is the formation of the multivesicular body (MVB). MVBs are formed when the endosomal membrane invaginates forming internal vesicles into which proteins destined for lysosomal degradation are sorted.69 In the yeast Saccharomyces cerevisiae Vps23p regulates the recruitment of ubiquitinated proteins into the internal vesicles in MVBs.70 The mammalian homologue of Vps23p is the tumour susceptibility gene 101 (TSG101), which is also involved in late endosomal protein sorting and binds to ubiquitin, suggesting conservation of this pathway from yeast to mammals.71,72 K3 and K5 may also utilize this MVB pathway to degrade MHC class I molecules as TSG101 is required for the down-regulation and degradation of MHC class I molecules by K3.65
Mhc class i down-regulation: internalization and sequestration
HIV-1 Nef is a 27 000 MW myristoylated protein that performs multiple functions in the infection of host cells, including down-regulation of CD4 and MHC class I.73 Nef utilizes different pathways in the down-regulation of CD4 and MHC class I. CD4 is internalized by classic clathrin-mediated endocytosis and routed to the lysosome for degradation.74 In contrast Nef mediated internalization of MHC class I molecules is clathrin-independent and class I molecules are sequestered in the trans-Golgi network (TGN) (Fig. 2).75–77 In the absence of Nef MHC class I molecules are constitutively internalized and recycled back to the plasma membrane via a pathway that is regulated by the small GTPase, ADP-ribosylation factor 6 (ARF6).78 Elegant studies by Blagoveshchenskaya et al.78 demonstrate that Nef accelerates the ARF6-dependent internalization of MHC class I molecules. Taken together with previous mutational analyses of the Nef sequence a role for three motifs in MHC class I down-regulation is highlighted: 62EEEE65, 72PXXP75 (where X can be any amino acid) and Met20 within an amphiphatic α-helix.77,79,80 These motifs are not functionally equivalent but act sequentially to promote MHC class I down-regulation by subverting the ARF6 pathway.78
Mutation of either 62EEEE65 or 72PXXP75 prevents the internalization of MHC class I molecules by Nef.77–80 In contrast, mutation of Met20 does not affect Nef-induced internalization, but the MHC class I molecules instead of being sequestered in the TGN recycle back to the plasma membrane.78 The 62EEEE65 motif interacts with phosphofurin acidic cluster sorting protein-1 (PACS-1) which is involved in the retrieval of furin from the plasma membrane to the TGN.79 PACS-1 sorts Nef to the TGN, but does not directly facilitate the sequestration of MHC class I molecules in the TGN.78,79 This is because whereas mutation of 72PXXP75 prevents class I internalization it does not affect the TGN sorting of Nef. Instead the sorting of Nef to the TGN appears to be necessary to activate MHC class I internalization in a 72PXXP75-dependent manner. Nef interacts with phosphatidylinositide 3-kinase (PI3K) and this may be facilitated by the 72PXXP75 motif, which functions as an SH3 domain binding site.81,82 Indeed, inhibitors of PI3K block Nef mediated internalization of MHC class I, whereas a fusion between PI3K and Nef negates the effect of mutating the 72PXXP75 motif.78 Furthermore, PI3K activity is required to recruit both ARF6 and the ARF nucleotide binding site opener (ARNO) to plasma membrane ruffles. ARNO is an ARF6 guanine nucleotide exchange factor which activates ARF6 by increasing GTP loading, hence activating the ARF6-dependent internalization of MHC class I.78 Internalized MHC class I molecules are subsequently sequestered in the TGN, although further studies are required to understand how internalized MHC class I molecules are sorted to the TGN and how the Nef Met20 motif facilitates this event.
Acknowledgments
I thank Drs Mark Harris, Vas Ponnambalam and Adrian Whitehouse for comments on the manuscript.
References
- 1.Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu Rev Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson PG, May JS, Smith XG, Marques S, Adler H, Koszinowski UH, Simas JP, Efstathiou S. K3-mediated evasion of CD8 (+) T cells aids amplification of a latent gamma-herpesvirus. Nat Immunol. 2002;3:733–40. doi: 10.1038/ni818. [DOI] [PubMed] [Google Scholar]
- 3.Krmpotic A, Messerle M, Crnkovic-Mertens I, Polic B, Jonjic S, Koszinowski UH. The immunoevasive function encoded by the mouse cytomegalovirus gene m152 protects the virus against T cell control in vivo. J Exp Med. 1999;190:1285–96. doi: 10.1084/jem.190.9.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furman MH, Dey N, Tortorella D, Ploegh HL. The human cytomegalovirus US10 gene product delays trafficking of major histocompatibility complex class I molecules. J Virol. 2002;76:11753–6. doi: 10.1128/JVI.76.22.11753-11756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones EY. MHC class I and class II structures. Curr Opin Immunol. 1997;9:75–9. doi: 10.1016/s0952-7915(97)80162-8. [DOI] [PubMed] [Google Scholar]
- 6.Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–4. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 7.Princiotta MF, Finzi D, Qian SB, Gibbs J, Schuchmann S, Buttgereit F, Bennink JR, Yewdell JW. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 2003;18:343–54. doi: 10.1016/s1074-7613(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 8.Reits EA, Vos JC, Gromme M, Neefjes J. The major substrates for TAP in vivo are derived from newly synthesized proteins. Nature. 2000;404:774–8. doi: 10.1038/35008103. [DOI] [PubMed] [Google Scholar]
- 9.Abele R, Tampe R. Function of the transport complex TAP in cellular immune recognition. Biochim Biophys Acta. 1999;1461:405–19. doi: 10.1016/s0005-2736(99)00171-6. [DOI] [PubMed] [Google Scholar]
- 10.van Endert PM, Saveanu L, Hewitt EW, Lehner P. Powering the peptide pump: TAP crosstalk with energetic nucleotides. Trends Biochem Sci. 2002;27:454–61. doi: 10.1016/s0968-0004(02)02090-x. [DOI] [PubMed] [Google Scholar]
- 11.Pamer E, Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Annu Rev Immunol. 1998;16:323–58. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 12.Antoniou AN, Powis SJ, Elliott T. Assembly and export of MHC class I peptide ligands. Curr Opin Immunol. 2003;15:75–81. doi: 10.1016/s0952-7915(02)00010-9. [DOI] [PubMed] [Google Scholar]
- 13.Williams AP, Peh CA, Purcell AW, McCluskey J, Elliott T. Optimization of the MHC class I peptide cargo is dependent on tapasin. Immunity. 2002;16:509–20. doi: 10.1016/s1074-7613(02)00304-7. [DOI] [PubMed] [Google Scholar]
- 14.Spiliotis ET, Manley H, Osorio M, Zuniga MC, Edidin M. Selective export of MHC class I molecules from the ER after their dissociation from TAP. Immunity. 2000;13:841–51. doi: 10.1016/s1074-7613(00)00081-9. [DOI] [PubMed] [Google Scholar]
- 15.Fruh K, Ahn K, Djaballah H, Sempe P, van Endert PM, Tampe R, Peterson PA, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–8. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 16.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–5. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 17.Galocha B, Hill A, Barnett BC, et al. The active site of ICP47, a herpes simplex virus-encoded inhibitor of the major histocompatibility complex (MHC)-encoded peptide transporter associated with antigen processing (TAP), maps to the NH2-terminal 35 residues. J Exp Med. 1997;185:1565–72. doi: 10.1084/jem.185.9.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann L, Kraas W, Uebel S, Jung G, Tampe R. The active domain of the herpes simplex virus protein ICP47: a potent inhibitor of the transporter associated with antigen processing. J Mol Biol. 1997;272:484–92. doi: 10.1006/jmbi.1997.1282. [DOI] [PubMed] [Google Scholar]
- 19.Tomazin R, Hill AB, Jugovic P, York I, van Endert P, Ploegh HL, Andrews DW, Johnson DC. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 1996;15:3256–66. [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn K, Meyer TH, Uebel S, et al. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 1996;15:3247–55. [PMC free article] [PubMed] [Google Scholar]
- 21.Gorbulev S, Abele R, Tampe R. Allosteric crosstalk between peptide-binding, transport, and ATP hydrolysis of the ABC transporter TAP. Proc Natl Acad Sci U S A. 2001;98:3732–7. doi: 10.1073/pnas.061467898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacaille VG, Androlewicz MJ. Herpes simplex virus inhibitor ICP47 destabilizes the transporter associated with antigen processing (TAP) heterodimer. J Biol Chem. 1998;273:17386–90. doi: 10.1074/jbc.273.28.17386. [DOI] [PubMed] [Google Scholar]
- 23.Lehner PJ, Karttunen JT, Wilkinson GW, Cresswell P. The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proc Natl Acad Sci U S A. 1997;94:6904–9. doi: 10.1073/pnas.94.13.6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hengel H, Koopmann JO, Flohr T, Muranyi W, Goulmy E, Hammerling GJ, Koszinowski UH, Momburg F. A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity. 1997;6:623–32. doi: 10.1016/s1074-7613(00)80350-7. [DOI] [PubMed] [Google Scholar]
- 25.Ahn K, Gruhler A, Galocha B, et al. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 1997;6:613–21. doi: 10.1016/s1074-7613(00)80349-0. [DOI] [PubMed] [Google Scholar]
- 26.Hewitt EW, Gupta SS, Lehner PJ. The human cytomegalovirus gene product US6 inhibits ATP binding by TAP. EMBO J. 2001;20:387–96. doi: 10.1093/emboj/20.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyritsis C, Gorbulev S, Hutschenreiter S, Pawlitschko K, Abele R, Tampe R. Molecular mechanism and structural aspects of transporter associated with antigen processing inhibition by the cytomegalovirus protein US6. J Biol Chem. 2001;276:48031–9. doi: 10.1074/jbc.M108528200. [DOI] [PubMed] [Google Scholar]
- 28.Hampton RY. ER-associated degradation in protein quality control and cellular regulation. Curr Opin Cell Biol. 2002;14:476–82. doi: 10.1016/s0955-0674(02)00358-7. [DOI] [PubMed] [Google Scholar]
- 29.Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, Ploegh HL. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–8. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 30.Wiertz EJ, Jones TR, Sun L, Bogyo M, Geuze HJ, Ploegh HL. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell. 1996;84:769–79. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 32.Gewurz BE, Wang EW, Tortorella D, Schust DJ, Ploegh HL. Human cytomegalovirus US2 endoplasmic reticulum-lumenal domain dictates association with major histocompatibility complex class I in a locus-specific manner. J Virol. 2001;75:5197–204. doi: 10.1128/JVI.75.11.5197-5204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gewurz BE, Gaudet R, Tortorella D, Wang EW, Ploegh HL, Wiley DC. Antigen presentation subverted. Structure of the human cytomegalovirus protein US2 bound to the class I molecule HLA-A2. Proc Natl Acad Sci U S A. 2001;98:6794–9. doi: 10.1073/pnas.121172898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Story CM, Furman MH, Ploegh HL. The cytosolic tail of class I MHC heavy chain is required for its dislocation by the human cytomegalovirus US2 and US11 gene products. Proc Natl Acad Sci U S A. 1999;96:8516–21. doi: 10.1073/pnas.96.15.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shamu CE, Story CM, Rapoport TA, Ploegh HL. The pathway of US11-dependent degradation of MHC class I heavy chains involves a ubiquitin-conjugated intermediate. J Cell Biol. 1999;147:45–58. doi: 10.1083/jcb.147.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shamu CE, Flierman D, Ploegh HL, Rapoport TA, Chau V. Polyubiquitination is required for US11-dependent movement of MHC class I heavy chain from endoplasmic reticulum into cytosol. Mol Biol Cell. 2001;12:2546–55. doi: 10.1091/mbc.12.8.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kikkert M, Hassink G, Barel M, Hirsch C, van der Wal FJ, Wiertz E. Ubiquitination is essential for human cytomegalovirus US11-mediated dislocation of MHC class I molecules from the endoplasmic reticulum to the cytosol. Biochem J. 2001;358:369–77. doi: 10.1042/0264-6021:3580369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye Y, Meyer HH, Rapoport TA. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature. 2001;414:652–6. doi: 10.1038/414652a. [DOI] [PubMed] [Google Scholar]
- 39.Chevalier MS, Johnson DC. Human cytomegalovirus US3 chimeras containing US2 cytosolic residues acquire major histocompatibility class I and II protein degradation properties. J Virol. 2003;77:4731–8. doi: 10.1128/JVI.77.8.4731-4738.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer HH, Wang Y, Warren G. Direct binding of ubiquitin conjugates by the mammalian p97 adaptor complexes, p47 and Ufd1-Npl4. EMBO J. 2002;21:5645–52. doi: 10.1093/emboj/cdf579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersson M, Paabo S, Nilsson T, Peterson PA. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985;43:215–22. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- 42.Cox JH, Yewdell JW, Eisenlohr LC, Johnson PR, Bennink JR. Antigen presentation requires transport of MHC class I molecules from the endoplasmic reticulum. Science. 1990;247:715–8. doi: 10.1126/science.2137259. [DOI] [PubMed] [Google Scholar]
- 43.Jackson MR, Nilsson T, Peterson PA. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990;9:3153–62. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gabathuler R, Kvist S. The endoplasmic reticulum retention signal of the E3/19K protein of adenovirus type 2 consists of three separate amino acid segments at the carboxy terminus. J Cell Biol. 1990;111:1803–10. doi: 10.1083/jcb.111.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teasdale RD, Jackson MR. Signal-mediated sorting of membrane proteins between the endoplasmic reticulum and the golgi apparatus. Annu Rev Cell Dev Biol. 1996;12:27–54. doi: 10.1146/annurev.cellbio.12.1.27. [DOI] [PubMed] [Google Scholar]
- 46.Bennett EM, Bennink JR, Yewdell JW, Brodsky FM. Cutting edge: adenovirus E19 has two mechanisms for affecting class I MHC expression. J Immunol. 1999;162:5049–52. [PubMed] [Google Scholar]
- 47.Jones TR, Wiertz EJ, Sun L, Fish KN, Nelson JA, Ploegh HL. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc Natl Acad Sci U S A. 1996;93:11327–33. doi: 10.1073/pnas.93.21.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahn K, Angulo A, Ghazal P, Peterson PA, Yang Y, Fruh K. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc Natl Acad Sci U S A. 1996;93:10990–5. doi: 10.1073/pnas.93.20.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gruhler A, Peterson PA, Fruh K. Human cytomegalovirus immediate early glycoprotein US3 retains MHC class I molecules by transient association. Traffic. 2000;1:318–25. doi: 10.1034/j.1600-0854.2000.010405.x. [DOI] [PubMed] [Google Scholar]
- 50.Lee S, Yoon J, Park B, et al. Structural and functional dissection of human cytomegalovirus US3 in binding major histocompatibility complex class I molecules. J Virol. 2000;74:11262–9. doi: 10.1128/jvi.74.23.11262-11269.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S, Park B, Ahn K. Determinant for endoplasmic reticulum retention in the luminal domain of the human cytomegalovirus US3 glycoprotein. J Virol. 2003;77:2147–56. doi: 10.1128/JVI.77.3.2147-2156.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huber MT, Tomazin R, Wisner T, Boname J, Johnson DC. Human cytomegalovirus US7, US8, US9, and US10 are cytoplasmic glycoproteins, not found at cell surfaces, and US9 does not mediate cell-to-cell spread. J Virol. 2002;76:5748–58. doi: 10.1128/JVI.76.11.5748-5758.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hudson AW, Howley PM, Ploegh HL. A human herpesvirus 7 glycoprotein, U21, diverts major histocompatibility complex class I molecules to lysosomes. J Virol. 2001;75:12347–58. doi: 10.1128/JVI.75.24.12347-12358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 55.Ishido S, Wang C, Lee BS, Cohen GB, Jung JU. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J Virol. 2000;74:5300–9. doi: 10.1128/jvi.74.11.5300-5309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coscoy L, Ganem D. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc Natl Acad Sci U S A. 2000;97:8051–6. doi: 10.1073/pnas.140129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lorenzo ME, Jung JU, Ploegh HL. Kaposi's sarcoma-associated herpesvirus K3 utilizes the ubiquitin-proteasome system in routing class major histocompatibility complexes to late endocytic compartments. J Virol. 2002;76:5522–31. doi: 10.1128/JVI.76.11.5522-5531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishido S, Choi JK, Lee BS, Wang C, DeMaria M, Johnson RP, Cohen GB, Jung JU. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi's sarcoma-associated herpesvirus K5 protein. Immunity. 2000;13:365–74. doi: 10.1016/s1074-7613(00)00036-4. [DOI] [PubMed] [Google Scholar]
- 59.Coscoy L, Ganem D. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J Clin Invest. 2001;107:1599–606. doi: 10.1172/JCI12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goto E, Ishido S, Sato Y, Ohgimoto S, Ohgimoto K, Nagano-Fujii M, Hotta H. c-MIR, a human E3 ubiquitin ligase, is a functional homolog of herpesvirus proteins MIR1 and MIR2 and has similar activity. J Biol Chem. 2003;278:14657–68. doi: 10.1074/jbc.M211285200. [DOI] [PubMed] [Google Scholar]
- 61.Sanchez DJ, Coscoy L, Ganem D. Functional organization of MIR2, a novel viral regulator of selective endocytosis. J Biol Chem. 2002;277:6124–30. doi: 10.1074/jbc.M110265200. [DOI] [PubMed] [Google Scholar]
- 62.Capili AD, Schultz DC, Rauscher IF, Borden KL. Solution structure of the PHD domain from the KAP-1 corepressor: structural determinants for PHD, RING and LIM zinc-binding domains. EMBO J. 2001;20:165–77. doi: 10.1093/emboj/20.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joazeiro CA, Weissman AM. RING finger proteins. mediators of ubiquitin ligase activity. Cell. 2000;102:549–52. doi: 10.1016/s0092-8674(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 64.Coscoy L, Sanchez DJ, Ganem D. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J Cell Biol. 2001;155:1265–73. doi: 10.1083/jcb.200111010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hewitt EW, Duncan L, Mufti D, Baker J, Stevenson PG, Lehner PJ. Ubiquitylation of MHC class I by the K3 viral protein signals internalization and TSG101-dependent degradation. EMBO J. 2002;21:2418–29. doi: 10.1093/emboj/21.10.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Means RE, Ishido S, Alvarez X, Jung JU. Multiple endocytic trafficking pathways of MHC class I molecules induced by a Herpesvirus protein. EMBO J. 2002;21:1638–49. doi: 10.1093/emboj/21.7.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hicke L. Getting' down with ubiquitin. turning off cell-surface receptors, transporters and channels. Trends Cell Biol. 1999;9:107–12. doi: 10.1016/s0962-8924(98)01491-3. [DOI] [PubMed] [Google Scholar]
- 68.Pickart CM. Ubiquitin enters the new millennium. Mol Cell. 2001;8:499–504. doi: 10.1016/s1097-2765(01)00347-1. [DOI] [PubMed] [Google Scholar]
- 69.Piper RC, Luzio JP. Late endosomes. sorting and partitioning in multivesicular bodies. Traffic. 2001;2:612–21. doi: 10.1034/j.1600-0854.2001.20904.x. [DOI] [PubMed] [Google Scholar]
- 70.Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–55. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 71.Babst M, Odorizzi G, Estepa EJ, Emr SD. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 2000;1:248–58. doi: 10.1034/j.1600-0854.2000.010307.x. [DOI] [PubMed] [Google Scholar]
- 72.Bishop N, Horman A, Woodman P. Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein–ubiquitin conjugates. J Cell Biol. 2002;157:91–101. doi: 10.1083/jcb.200112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arora VK, Fredericksen BL, Garcia JV. Nef. agent of cell subversion. Microbes Infect. 2002;4:189–99. doi: 10.1016/s1286-4579(01)01527-1. [DOI] [PubMed] [Google Scholar]
- 74.Marsh M, Pelchen-Matthews A. Endocytosis in viral replication. Traffic. 2000;1:525–32. doi: 10.1034/j.1600-0854.2000.010701.x. [DOI] [PubMed] [Google Scholar]
- 75.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–42. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 76.Le Gall S, Buseyne F, Trocha A, Walker BD, Heard JM, Schwartz O. Distinct trafficking pathways mediate Nef-induced and clathrin-dependent major histocompatibility complex class I down-regulation. J Virol. 2000;74:9256–66. doi: 10.1128/jvi.74.19.9256-9266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greenberg ME, Iafrate AJ, Skowronski J. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 1998;17:2777–89. doi: 10.1093/emboj/17.10.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blagoveshchenskaya AD, Thomas L, Feliciangeli SF, Hung CH, Thomas G. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell. 2002;111:853–66. doi: 10.1016/s0092-8674(02)01162-5. [DOI] [PubMed] [Google Scholar]
- 79.Piguet V, Wan L, Borel C, Mangasarian A, Demaurex N, Thomas G, Trono D. HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat Cell Biol. 2000;2:163–7. doi: 10.1038/35004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mangasarian A, Piguet V, Wang JK, Chen YL, Trono D. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J Virol. 1999;73:1964–73. doi: 10.1128/jvi.73.3.1964-1973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Linnemann T, Zheng YH, Mandic R, Peterlin BM. Interaction between Nef and phosphatidylinositol-3-kinase leads to activation of p21-activated kinase and increased production of HIV. Virology. 2002;294:246–55. doi: 10.1006/viro.2002.1365. [DOI] [PubMed] [Google Scholar]
- 82.Geyer M, Fackler OT, Peterlin BM. Structure-function relationships in HIV-1 Nef. EMBO Rep. 2001;2:580–5. doi: 10.1093/embo-reports/kve141. [DOI] [PMC free article] [PubMed] [Google Scholar]