Abstract
Charged droplets, produced by electrostatic dispersion of solutions of amino acids and peptides are driven by a potential difference a countercurrent to a flow of heated nitrogen bath gas. Evaporation of solvent from those droplets increases surface charge density, resulting in subdivision into smaller charged droplets. Each smaller droplet repeats that sequence until the ultimate result is a dispersion of solvent-free solute ions in the bath gas. Surprisingly, mass spectrometric analyses of the final ion-bath gas mixtures showed that the relative abundances of the desolvated ions were substantially higher when the nitrogen bath gas contained vapor of a polar solvent species than when no such solvent vapor was present. Adding solvent vapor to the background bath gas can certainly not increase, but must decrease, the net rate of solvent evaporation from the charged droplets. Consequently, the observed enhancement of ion formation by the presence of solvent vapor in the bath gas constitutes persuasive evidence that the observed solute ions cannot have been produced by the charged residue mechanism originally suggested by Dole et al. [Dole M, et al. (1968) J Chem Phys 49:2240–2249 and Dole M, Rheude A, Mack LL (1970) J Chem Phys 52:4977–4986]. It is therefore concluded that electrospray ions are most likely produced by the ion evaporation mechanism of Iribarne and Thomson [Iribarne JV, Thomson BA (1975) J Chem Phys 64:2287–2294]. Moreover, and probably as important, this observed signal enhancement constitutes a welcome increase in detection sensitivity.
Keywords: electrospray ionization, formation of ions
For the past several decades, the production of gas-phase solute ions by solvent evaporation from charged droplets of solution, as in electrospray ionization (ESI), has enjoyed increasingly widespread use in the detection and identification of the complex organic molecules of interest and importance in biochemical systems (1). Even so, there is still much debate on the mechanism(s) by which those gaseous ions are formed. The two most favored of these mechanisms are embodied in the charged residue model (CRM) originally proposed by Malcolm Dole et al. in 1968 (2) and 1970 (3) and the ion evaporation model (IEM) suggested by Iribarne and Thomson in 1975 (4). The CRM has its roots in a theoretical paper published by Lord Rayleigh (John Williams Strutts) in 1882 (5). In that paper, Rayleigh addressed the question of what would happen as solvent evaporates from a droplet of volatile liquid containing an excess of either anions or cations. He reasoned that the repulsive forces between those excess charges of like sign would cause their associated ions to be situated at equidistant intervals on the surface of the droplet. As the droplet size decreased by evaporation of solvent, those surface ions would get closer and closer together until the integral over the droplet surface of the coulomb repulsion forces between those surface ions would exceed the integral of surface tension of the droplet liquid over that same area. At that point, now frequently referred to as the “Rayleigh limit,” the droplet would increase the available surface area by breaking up into a plurality of smaller offspring droplets. These offspring droplets also would undergo solvent evaporation until they, too, would reach the Rayleigh limit and subdivide into still smaller droplets. Such subdivision of evaporating charged droplets had indeed been observed and reported by Zeleny (6).
Dole et al.'s (2, 3) idea was that a succession of such subdivisions of the original droplets would eventually lead to the formation of “ultimate droplets” so small that each of them would contain only one solute molecule. As the last solvent molecules evaporated from such an ultimate droplet, the residual solute molecule would retain some or all of the charges on that droplet to become a gas-phase solute ion. They also realized that this scenario might make possible the production of gas-phase ions of molecules too large to be ionized by the then-customary procedures based on gas-phase encounters between neutral molecules and sufficiently energetic electrons, photons, or other ions. The large oligomer molecules in which they were interested simply could not be vaporized by the usual methods without undergoing catastrophic thermal decomposition. In 1968 and 1970, Dole et al. (2, 3) published the results of their attempts to produce and mass analyze ions of polystyrene oligomers by means of this ESI technique. For several reasons, not realized until later, the molecular weight values they obtained were at odds with what was known about the probable values for those oligomers. Consequently, their results did not persuade other investigators to follow their lead until some years later. Fortunately, as it turned out, Dole himself did live long enough to see those ideas evolve into what is now sometimes referred to as “The Electrospray Revolution” by which ESI-MS has become one of the most widely practiced analytical techniques now in use.
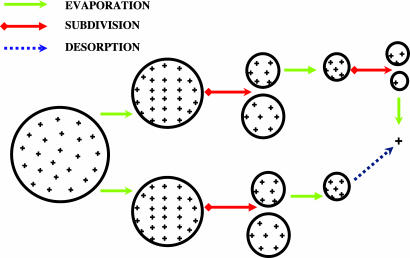
Sometime after Dole et al.'s first two papers (2, 3) on ESI, Iribarne and Thomson (4) had experimented briefly with the technique and in 1975 offered a somewhat different explanation for the possible production of gas-phase ions by evaporation of solvent from charged liquid droplets (4). They argued that before a charged droplet became small enough to contain only one solute molecule, the charge density on its surface would become so high that the resulting field would be sufficiently intense to “push” one or more of those surface ions into the ambient gas, thereby forming gaseous ions of at least some of those solute molecules. Continued evaporation of solvent from successive generations of such charged droplets thus would ultimately result in driving many, if not most, of the surface cations (or anions) on the original droplets into the gas phase. The diagrams in Fig. 1 attempt to illustrate schematically these two possible mechanisms by which nonvolatile solute species in a charged droplet could become free ions in the ambient gas.
Fig. 1.
A schematic representation of the possible pathways for ion formation from a charged liquid droplet. The upper and the lower parts of the diagram illustrate the ion formation mechanisms depicted in the CRM of Dole et al. (2, 3) and the IEM of Iribarne and Thomson (4), respectively. + represents a desolvated solute ion. The major difference between these two models is that the final ion in the latter model is produced by desorption, whereas the ion in the former model is produced by evaporation of solvent comprising the droplet.
In the years after Dole et al.'s papers (2, 3), there were several attempts to develop a satisfactory ionization technique based on these charged droplet scenarios but none of them were very successful. Then in the 1980s, building on the ideas of Dole et al. (2, 3) and Iribarne and Thomson (4), and drawing on our own extensive studies on the free jet expansion of gases from relatively high pressure into vacuum, our group, then at Yale, found the right combination of conditions and demonstrated the successful production and mass analysis of intact solute ions from molecules having a wide range of molecular weights (7, 8). This “success” has spawned much argument and discussion on the mechanisms by which such ions could be formed (9–21). Over the past few years, we have investigated this ion formation mechanism for a number of molecular species having a wide range of compositions and molecular weights, e.g., tetra-alkyl ammonium compounds, amino acids, peptides, proteins, carbohydrates, and some polar synthetic polymers such as polyethylene glycols (PEGs) (12, 13). After careful consideration of all of the results, we conclude that for most, if not all, cases in which ESI is effective, gas-phase solute ions are formed from charged droplets according to the sequence of events described in the IEM of Iribarne and Thomson (4). However, in the case of very large parent species, including PEGs with molecular masses as high as 5,000,000 Da, we believe that the CRM of Dole et al. (2, 3) may comprise the more likely ionization scenario (13). Even so, recent studies by de la Mora (16) suggest that ions of globular proteins (nondenatured) with molecular masses as low as 6,600 Da may also be produced according to the CRM. Indeed, some species seem capable of forming multiply charged ions by either the CRM or the IEM but de la Mora and Gamero-Castrano (17) argue persuasively that smaller species like tetraheptyl ammonium cations are usually formed by the IEM.
By a somewhat different approach, Kebarle et al. (18, 19) have also extensively investigated the mechanisms of ion formation for a variety of solute species including metal atoms and protein molecules. They concluded that metal ions such as Na+, K+, or Cs+ are most likely to be produced by the IEM (18), whereas larger ions of denatured proteins probably are produced by the CRM (19). More recently, Williams and colleagues (20, 21) have studied the formation of highly protonated ions of peptides, proteins, and synthetic polymers when the electrosprayed solutions were doped with compounds having low vapor pressures, e.g., glycerol or m-nitrobenzyl alcohol. The results from such studies led them to conclude that organic ions, with a mass range from 100 to 7,000 Da, were most likely produced by the CRM (21).
In this article, we present experimental results that seem to provide positive proof that the actual mechanisms by which electrospray ions are most often produced from charged droplets is embodied in the IEM of Iribarne and Thomson (4). We hope these results may serve to resolve some of the remaining uncertainties about those mechanisms.
Results and Discussion
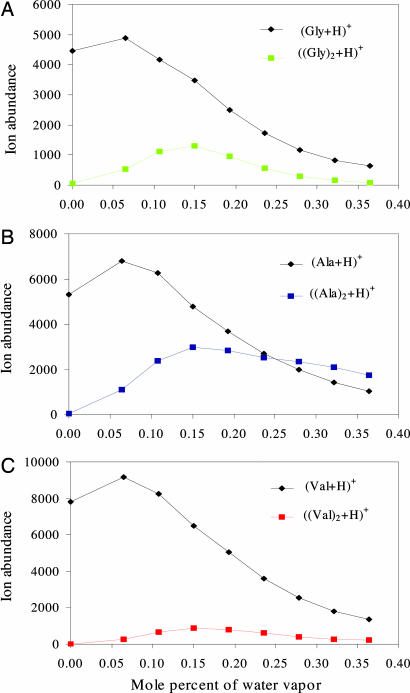
Figs. 2–5 illustrate the relation that we found between the observed abundances of some amino acid ions and the ions of some small peptides (amino acid oligomers), and the concentration of solvent vapor in the bath gas. In all of the graphs shown, the ordinate values of the data points indicate the abundances of the relevant ions, while the abscissa values show the concentration of solvent vapor in the bath gas in terms of mole percent, i.e., moles of vapor per 100 moles of gas (nitrogen plus vapor). The results in Fig. 2 were obtained with glycine (Gly), alanine (Ala), and valine (Val) as the protonated solute species. With no solvent vapor in the bath gas, mass analyses of ions from methanol–water solutions containing amino acids at a concentration of 400 μM showed positive ions comprising mostly singly protonated monomers with comparable abundances. Some singly protonated dimers were also produced but their abundances were substantially <1% of the abundances of singly protonated monomers.
Fig. 2.
The effects of water vapor in the bath gas on the formation of desolvated singly protonated monomers and dimers of glycine (A), alanine (B), and valine (C) from charged liquid droplets. In each case, amino acid solutes were at concentrations of 400 μM in 99.5% methanol–0.5% water solutions containing 0.1% acetic acid. The nozzle and skimmer were at the same potential.
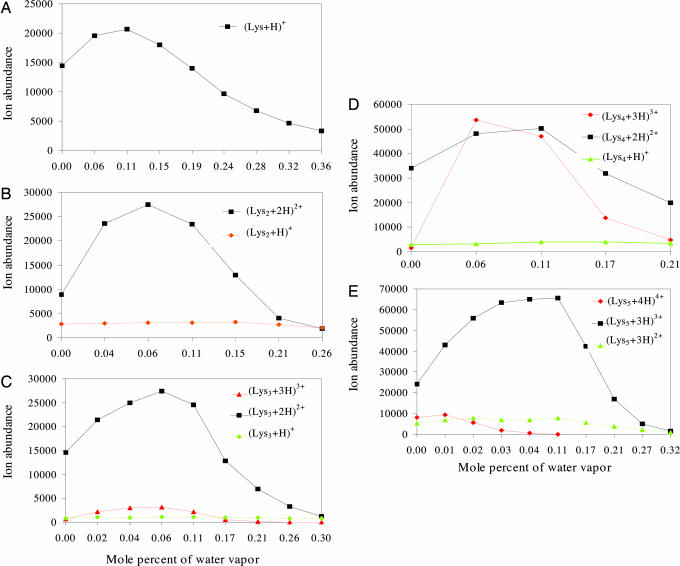
Fig. 3.
The effects of water vapor in the bath gas on the formation of multiply protonated ions of lysine and its oligomers (Kn=2–5). In all cases, the sample solutions comprised analyte at a concentration of 400 μM in solvent comprising 99.5% methanol and 0.5% water containing 0.1% acetic acid.
Fig. 4.
An illustration of the effect of water vapor in the bath gas on the ion formation from charged droplets of solution comprising anIII (A), anII (B), and a mixture of anII and anII (C). Electrospray solution of anII was at a concentration of 200 μM in a mixture of 93% methanol–7% water containing 0.1% acetic acid. The solution of anIII was also at a concentration of 200 μM but in 83% methanol–17% water containing 0.1% acetic acid solution. The admixture solution was composed of 266 μM anII and 133 μM anIII in 90% methanol–10% water solution doped with 1% acetic acid.
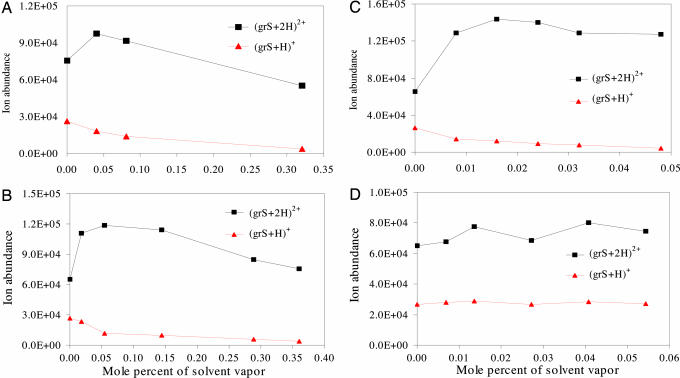
Fig. 5.
An illustration of the effects of different solvent vapors in the bath gas on the production of bare ions from charged droplets of grS solution. The vapors added to the nitrogen bath gas were water (A), methanol (B), butanol (C), and cyclohexane (D). Electrospray solution was prepared from a mixture containing 60% methanol–40% water in 1% acetic acid solution and contained 160 μM grS.
After having adjusted the system so that the ions produced were completely free from solvation, water vapor at known concentrations was deliberately added to the countercurrent flow of heated nitrogen bath gas. Because the presence of water vapor in the bath gas can only decrease the net evaporation rate of solvent from the charged droplets containing solute ions, we had initially expected that such addition of vapor to the bath gas would lead to a decrease in the abundance of bare ions and an increase in the abundance of hydrated ions. Much to our surprise, we found that small amounts of water vapor in the bath gas actually increased the abundances of bare (desolvated) ions, as is shown in Fig. 2. Perhaps even more interesting is the finding, also shown in Fig. 2, that small amounts of water vapor in the bath gas significantly increased the relative abundances of singly protonated dimers of Gly, Ala, and Val. With further increases in the concentration of water vapor, the bare-ion abundance did decrease as originally expected. Not surprisingly, a reduction in the bare ion abundance caused by water vapor in the bath gas was always accompanied by an increase in the abundances of solvated ions. Because the focus of this study was on the effect of solvent vapor in the bath gas (at relatively low concentrations) on the abundances of desolvated solute ions, we save for another day any discussion of the effects of such solvent vapor on the abundances of ions with higher degrees of solvation, a much more complicated subject.
Experiments were also carried out with several molecular species capable of forming ions with multiple charges. The results in Fig. 3 were obtained for lysine (Lys) and its oligomers (“peptides”) containing up to five monomers. Such lysine peptides readily formed ions with several charge states. As was the case for monomers and dimers of amino acids, the abundances of peptide ions first increased and then decreased as the concentration of water vapor in the bath gas increased. The maxima in ion abundance curves shifted slightly toward the region of higher concentrations of vapor in the bath gas as the number of amino acid residues in these oligomers increased from two to five. Except for the quadruply protonated pentalysine ions, the overall abundances of the multiply protonated species were significantly increased by the presence of water vapor, whereas little or no change in abundance occurred for any of the singly protonated lysine oligomers. This finding seems to imply a correlation between the extent of ion abundance increases for a particular species and the charge state of that species.
The results shown in the plots of Fig. 4 were obtained with bioactive angiotensin II (anII) (DRVYIHPF) and angiotensin III (anIII) (RVYIHPF) separately and together. Examination of these results reveals that the presence of water vapor in the bath gas had entirely different effects on the ion abundances of anII and anIII, a somewhat surprising result in view of the fact that the only chemical difference between these two peptides is the extra amino acid residue on the N terminus. With anIII, the abundances observed for the doubly and triply protonated species increased substantially. With anII, increases in abundances were found only for the triply protonated species. On the other hand, the abundances of all charged species of both anII and anIII were increased when they were both present in the electrosprayed solutions. In several experiments, the vapor of water and organic solvents was injected into the nitrogen bath gas. As shown in Fig. 5 A–C, vapors of water, methanol, or butanol in the bath gas enhanced the abundances of doubly protonated ions of gramicidin S (grS) while decreasing the abundances of singly protonated ions. Noteworthy, as shown in Fig. 5D and discussed later, there was little or no effect on ion abundance when the same experiments were repeated with cyclohexane as the vapor added to the bath gas.
Attempts were also made to determine the extent of abundance increases for solute ions having larger molecular weights. Unfortunately, to remove all solvent molecules from such larger ions (before their admixture with bath gas having a known concentration of solvent vapor) required much larger voltage differences between nozzle and skimmer than when the ions to be desolvated were smaller. (The fraction of collision energy that is available for heating a collision partner is inversely proportional to the ratio of the mass of the heavier partner to the mass of the lighter one.) Consequently, it is difficult to determine how much of the resulting increase in ion production was caused by solvent vapor in the bath gas, and how much might be caused by improved ion collection efficiency brought about by the larger voltage difference between nozzle and skimmer.
Thus far, we have shown only a few examples of ion abundance enhancement caused by the presence of solvent vapor in the bath gas. In the routine practice of ESI-MS, it is possible to increase the abundances of solute ions by varying any of several combinations of the governing variables including, among others, the flow rate of bath gas (22), the temperature of the analyte solution (23), the applied voltages (24), the distance from the needle tip to the entrance capillary (25), the pH of solute solution (26), the polarizability of the solvent molecules (26), the temperature of inlet capillary (27), the diameter of the electrospray tip (9), and the gas densities in the region between the capillary exit and skimmer (28). Because all of the changes in ion abundance reported in these experiments occurred only after solvent vapor was added to the nitrogen bath gas, the observed increases in ion abundance must be caused only by the presence of such solvent vapor in that bath gas. Therefore, the question seeking an answer is: “Why and how does the presence of such vapor in the bath gas increase the abundance of solute ions formed by solvent evaporation from charged liquid droplets? Because the time frame was the same for all these experiments, this question becomes: “Why and how does the extent of solute ion formation during evaporation of solvent from a charged droplet increase when solvent vapor is present in the ambient gas?”
To answer this question, we consider what happens to a charged liquid droplet after it leaves the injection needle and is immersed in the nitrogen bath gas. We assume that the temperature of the droplet is the same or very near to that of the ambient gas and that the partial pressure of the liquid vapor in the ambient gas is equal to the vapor pressure of the droplet liquid. That is to say, the system comprising the droplet and its ambient gas is in a state of equilibrium such that the number of solvent molecules evaporating from the droplet surface per unit area per unit time is exactly the same as the number of solvent molecules from the ambient gas that are condensing on that unit area. In this equilibrium state, there is no net transfer over time of molecules from the droplet to the ambient gas or vice versa. We now suppose that the composition of the ambient bath gas is changed by the addition of molecules of a new species maintained at some specified concentration in the bath gas. Because the solubility of nitrogen is negligible and nitrogen molecules are nonpolar, essentially all of the incident nitrogen molecules simply bounce back into the gas phase after colliding with the droplet surface. Conversely, when solvent molecules in the bath gas strike the droplet surface, some of them may bounce back but others may stick by forming noncovalent bonds with surface molecules. The formation of such bonds is an exothermic process. The consequence is a “hot spot” on the liquid surface caused by the enthalpy of condensation released by the adsorption of the incident vapor molecule. That enthalpy of condensation can then be dissipated throughout the droplet by conduction, thereby raising the overall temperature of the droplet by an infinitesimal amount. Alternatively, that enthalpy of condensation can be absorbed locally by helping one or more nearby solvent molecules to evaporate from the droplet surface. Indeed, it is quite likely that such a departing evaporated molecule may even be the same molecule that had just condensed¡ In other words, the arriving vapor molecule had in effect simply engaged in an elastic collision with the liquid droplet. Thus, from a macroscopic overall perspective, there is no net change over time in the distribution of water molecules between surface liquid and the ambient gas, or in the average “temperature” of either the liquid or the vapor, during the nonsticking interactions of the incident molecules with the droplet surface. However, from a microscopic standpoint, there is a very broad thermal distribution in the translational rotational and vibrational kinetic energies of the various species both in the liquid droplet and the contiguous vapor. Also, to be remembered is that the droplet in this scenario is highly charged with some of its surface species being ions. Each such surface ion feels a coulombic repulsive force caused by the presence of the other surface ions of like sign. That force tries to push the ion away from the droplet surface. In sum, at any instant of time, such a surface ion is more likely to leave, i.e., evaporate from, the droplet surface than is any of its neutral neighbors. Consequently, when a solvent molecule from the bath gas condenses on the surface near one of these excess surface anions or cations, its “heat of condensation” may provide the last bit of energy that such a surface ion needs to escape from the surface. The overall result is a net increase in the flux of ions from the droplet surface into the ambient gas caused by condensation on the droplet surface of solvent molecules from the ambient gas. This scenario accounts for the experimental finding that the addition of polar solvents to the background bath gas results in a net flux of desolvated ions from the droplet surface into the ambient gas phase in a greater relative abundance than would be the case for evaporation from the droplet if there were no solvent vapor in the ambient gas. It also accounts for the finding that the abundances of multiply protonated ions leaving the droplet are enhanced to a greater extent than the abundances of singly protonated ions. The former feel more lift and have a greater tendency to depart from the droplet. Therefore, the probability that a nearby surface ion is released is directly proportional to the number of charges on that solute ion. Noteworthy, support for this idea comes from the finding that when the condensable molecules in the ambient gas were of nonpolar cyclohexane, no significant change occurred in the abundance of ions in the gas phase¡ The reason for this difference in behavior is simply that the enthalpy of condensation is much lower for nonpolar cyclohexane than for any polar solvent vapors such as water or alcohol. (The molar enthalpy of condensation for water vapor is six times that for cyclohexane.) Consequently, when a cyclohexane molecule condenses on the surface of a charged droplet, its heat of condensation is not sufficient to release any surface molecule that has an appreciable polarity or polarizability.
Indeed, there is a provocative analogy between the enhancement of ion evaporation from a charged droplet by condensation of solvent molecules on that droplet and the once widely practiced process of “steam distillation.” In that old art, a jet of live steam is introduced below the surface of a liquid mixture containing a desired solute species with a partial vapor pressure so low that to distill it from the mixture, i.e., vaporize it from the surface of a body of that mixture, at a practical rate would require a liquid temperature so high, and for so long a time, that many, if not most, of the components of that mixture, including the desired species, would undergo appreciable thermal decomposition. In the steam distillation alternative, some of the injected steam condenses at the liquid vapor interface, thereby raising the temperature of the immediately adjacent liquid so rapidly that its more volatile components are vaporized almost instantly and get swept out of the liquid by the transfer of momentum from the injected steam to the liquid at that steam–liquid interface. By this stratagem, none of the liquid or vapor is at a high temperature long enough for appreciable thermal decomposition to occur. So it is with the condensation of a neutral polar molecule on the surface of a charged droplet. The enthalpy released during such condensation produces a transient hot spot at which a nearby charged polar molecule (i.e., a surface ion) can be lifted from the surface by the droplet's electric field without any accompanying thermal decomposition. The overall result is the production of free ions in the gas phase of neutral species too fragile to be vaporized without decomposition by the heating that would be required for steady-state thermal vaporization of neutral molecules of that species from an extended surface area of the liquid.
In the Introduction, we outlined the two different scenarios by which free gaseous solute ions might be produced by evaporation of solvent from charged liquid droplets, i.e., the CRM proposed by Dole et al. (2, 3) and the IEM suggested by Iribarne and Thomson (4). For solute ions to be produced by the CRM, the solvent molecules remaining on the ultimate droplets must be completely evaporated. However, as shown in Figs. 2–5, the addition of vapor of polar solvents to the bath gas clearly increased the “yield” of desolvated solute ions from the charged droplets. Because increasing the solvent vapor concentration in the bath gas can not accelerate, but can only retard, the net rate of evaporation of solvent molecules from the droplet, this experimental finding that the addition of polar solvent vapor to the bath gas increased the bare ion abundances seems to constitute incontrovertible evidence that those ions must have been produced by the sequence of events assumed in the IEM (4) rather than the CRM (2, 3).
Furthermore, as is clear from visual examination of Figs. 2–5, the ion abundance increases caused by the addition of solvent vapor to the ambient gas are significant. In some cases (Fig. 2), the addition of solvent vapor to the ambient gas produced substantial signal for solute species that produced no visible peaks at all in the absence of any solvent vapor in the bath gas¡ This approach, namely the addition of solvent vapor to the bath gas, can thus be used to improve the overall ion signal intensities and thus the analytical sensitivity of mass spectrometric analyses.
Conclusions
The results reported here clearly show that adding the vapor of polar solvents to bath gas containing charged droplets of a solution of polar solute species increases the rate at which solute ions desorb from those droplets into the ambient bath gas. The extent of the observed increases correlates directly with the number of charges on the solute ions. The observed increases in ion abundances are presumably caused by condensation of solvent molecules on the surface of the liquid droplets. The condensation enthalpy released by the bonding of solvent molecules to the droplet surface is sufficient, as it were, to “sputter” solute ions from that surface into the ambient gas. The fact that more bare solute ions are produced from the droplets when the surrounding bath gas contains some solvent vapor overwhelmingly indicates that the gas-phase solute ions in ESI are produced according to the sequence of events set forth in the IEM of Iribarne and Thomson (4) rather than by the CRM originally proposed by Dole et al. (2, 3).
Materials and Methods
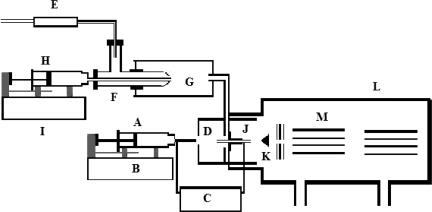
All mass spectrometric analyses were carried out in the positive ion mode with a triple quadrupole mass analyzer (model R30–10; Delsi-Nermag, Argentueil, France) equipped with an electrospray ion source (Analytica, Branford, CT). The essential features of the apparatus used in the present study are shown schematically in Fig. 6 and have been fully set forth in previous reports (29, 30). Even so, for the convenience of the reader there follows a brief description of its operation. Sample solution in syringe A was introduced through a hypodermic needle with a sharpened tip at a desired rate (80–100 μl/hr) by syringe driver B (model 11; Harvard Apparatus, Holliston, MA). A high-voltage dc power supply C maintained a potential difference (≈2.4 kV) between the hypodermic needle and brass capillary J, which served as an opposing counter electrode. This substantial potential difference induced an intense electric field at the hypodermic needle tip and dispersed the emerging liquid into a fine spray of charged droplets. These nascent droplets subsequently underwent solvent evaporation and subdivision as they were drifting toward chamber D against a countercurrent flow of bath gas. The resulting solute ions derived from the droplets of sample solutions were then transferred inside vacuum chamber L containing mass analyzer M. Because the important discovery that gave birth to this article was the finding that the presence of solvent vapor at low concentrations in the bath gas increased the production of bare ions, it is important to show how the composition of that bath gas was controlled. High-purity nitrogen from a standard high-pressure tank equipped with a pressure regulator (data not shown) first entered the outer tube of concentric nebulizer F at a rate determined by flow meter E (Gilmont Instruments, Great Neck, NY) and then emerged through a constricted orifice having a small enough flow area to ensure that the pressure drop across it was always high enough so that the flow velocity was always sonic and independent of the downstream pressure. Known amounts of liquid from syringe H were deliberately injected into spray chamber G via the inner tube of F by syringe driver I (model 100; Kds, Montreal, Canada). The annular flow of nitrogen from the outer tube of F traveling at sonic velocity vaporized the emerging liquid from the inner tube of F. The mixtures of nitrogen and solvent vapor were then transported to D where the electrospray-charged droplets were entering. All parts of the system downstream of G were heated by means of electrically insulated resistance-heating wires that was controlled by a variable transformer (model 116B; Superior Electronics, Phoenix, AZ) (data not shown). Thus, the gas temperature was always well above the dew point of the vapor-laden bath gas. The temperature of the gas heater of the Analytica ion source was set at 180°C.
Fig. 6.
A schematic diagram of the apparatus used in the present investigation. The essential components of the system shown are a syringe containing analyte solution (A), a syringe pump (B), a high-voltage dc power supply (C), a vapor/bath gas chamber (D), a gas flow meter (E), a concentric nebulizer (F), a spray chamber (G), a solvent-containing syringe (H), a pump for delivering precise amounts of liquid solvent to the concentric nebulizer (I), a brass capillary (J), a conical skimmer (K), a vacuum chamber (L), and a quadrupole mass analyzer (M).
anII (DRVYIHPF), anIII (RVYIHPF), grS, lysine oligomers (Kn=2–5), and amino acids were obtained from Sigma–Aldrich (St. Louis, MO). Glacial acetic acid (ACS reagent grade) and methanol (HPLC grade) were from J. T. Baker (Phillipsburg, NJ). Deionized water was from a deionization system (Nanopure, Dubuque, IA) fitted with an organic-free filter (Barnstead, Dubuque, IA). The average acquisition time for each data point in the plots of Figs. 2–5 is 6 min.
Acknowledgments
We thank Prof. David Muddiman (W. M. Keck FT-ICR MS Laboratory, North Carolina State University, Raleigh, NC), Prof. Matthias Mann (Max-Planck Institute for Biochemistry, Martinsried, Germany), Prof. Gary Tepper (Virginia Commonwealth University School of Engineering), Dr. Dongliang Zhan (Philip Morris Research Laboratories, Richmond, VA), and Pavel Kiselev (Virginia Commonwealth University School of Engineering) for their interest, valuable insights, discussions, and comments. This work was supported by the Department of Chemistry of Virginia Commonwealth University and U.S. Army Corps of Engineering/Fluorescence Laboratory Grant W9132V-04-C-0023.
Abbreviations
- ESI
electrospray ionization
- CRM
charged residue model
- IEM
ion evaporation model
- anII
angiotensin II
- anIII
angiotensin III
- grS
gramicidin S
Footnotes
The authors declare no conflict of interest.
References
- 1.Mann M, Aebersold R. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 2.Dole M, Mack LL, Hines R, Alice MB, Ferguson LD, Mobley RC. J Chem Phys. 1968;49:2240–2249. [Google Scholar]
- 3.Dole M, Rheude A, Mack LL. J Chem Phys. 1970;52:4977–4986. [Google Scholar]
- 4.Iribarne JV, Thomson BA. J Chem Phys. 1975;64:2287–2294. [Google Scholar]
- 5.Raleigh JWS. Philos Mag. 1882;14:184. [Google Scholar]
- 6.Zeleny J. Phys Rev. 1917;5:1–6. [Google Scholar]
- 7.Fenn JB, Yamashita M. J Chem Phys. 1984;88:4451–4459. [Google Scholar]
- 8.Fenn JB, Mann M, Meng CK, Wong MG, Whitehouse CM. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- 9.Cole RB, Yan L. Anal Chem. 2003;75:5739–5746. doi: 10.1021/ac0301402. [DOI] [PubMed] [Google Scholar]
- 10.Vekey K, Schlosser G, Drahos L, Takats Z. Anal Chem. 2002;74:6427–6429. doi: 10.1021/ac020547r. [DOI] [PubMed] [Google Scholar]
- 11.Berkel GJV. J Mass Spectrom. 2000;35:773–783. doi: 10.1002/1096-9888(200007)35:7<773::AID-JMS4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Fenn JB, Meng CK. Org Mass Spectrom. 1991;26:542–549. [Google Scholar]
- 13.Fenn JB, Nohmi T. J Am Chem Soc. 1992;114:3241–3246. [Google Scholar]
- 14.Fenn JB. J Am Soc Mass Spectrom. 1993;4:524–535. doi: 10.1016/1044-0305(93)85014-O. [DOI] [PubMed] [Google Scholar]
- 15.Fenn JB, Rosell J, Nohmi T, Shen S, Banks FJ. In: Biochemical and Biotechnical Applications of Electrospray Ionization Mass Spectrometry. Snyder AP, editor. Vol 619. Washington, DC: Am Chem Soc; 1995. pp. 61–80. [Google Scholar]
- 16.de la Mora JF. Anal Chim Acta. 2000;406:93–104. [Google Scholar]
- 17.de la Mora JF, Gamero-Castano M. J Mass Spectrom. 2000;35:790–803. doi: 10.1002/1096-9888(200007)35:7<790::AID-JMS21>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Kebarle P, Tang L. Anal Chem. 1993;65:3654–3668. [Google Scholar]
- 19.Kebarle P. J Mass Spectrom. 2000;35:804–817. doi: 10.1002/1096-9888(200007)35:7<804::AID-JMS22>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 20.Williams ER, Jurchen JC, Lavarone AT. Anal Chem. 2001;73:1455–1460. doi: 10.1021/ac001251t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams ER, Lavarone AT. J Am Chem Soc. 2003;125:2319–2327. doi: 10.1021/ja021202t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith RD, Loo JA, Barinaga CJ, Edmonds CG, Harold RU. J Am Soc Mass Spectrom. 1990;1:53–65. doi: 10.1016/1044-0305(90)80006-9. [DOI] [PubMed] [Google Scholar]
- 23.Kebarle P, Ikonomou MG. J Am Soc Mass Spectrom. 1994;5:791–799. doi: 10.1016/1044-0305(94)87001-2. [DOI] [PubMed] [Google Scholar]
- 24.Kebarle P, Ikonomou MG, Blades A. Anal Chem. 1991;63:1989–1998. [Google Scholar]
- 25.Kebarle P, Ikonomou MG, Blades A. Anal Chem. 1990;62:957–967. [Google Scholar]
- 26.Cole RB, Wang G. J Am Soc Mass Spectrom. 1996;7:1050–1058. doi: 10.1016/1044-0305(96)00051-7. [DOI] [PubMed] [Google Scholar]
- 27.Chait A, Chowdhury SK, Katta V. Rapid Commun Mass Spectrom. 1990;4:81–97. doi: 10.1002/rcm.1290040305. [DOI] [PubMed] [Google Scholar]
- 28.Karas M, Bahr U, Schmidt A. Anal Chem. 2001;73:6040–6046. doi: 10.1021/ac010451h. [DOI] [PubMed] [Google Scholar]
- 29.Fenn JB, Zhan D, Rosell J. J Am Soc Mass Spectrom. 1998;9:1242–1247. [Google Scholar]
- 30.Fenn JB, Zhan D. J Am Soc Mass Spectrom. 2002;219:1–10. [Google Scholar]