Abstract
Plesiadapiforms are central to studies of the origin and evolution of primates and other euarchontan mammals (tree shrews and flying lemurs). We report results from a comprehensive cladistic analysis using cranial, postcranial, and dental evidence including data from recently discovered Paleocene plesiadapiform skeletons (Ignacius clarkforkensis sp. nov.; Dryomomys szalayi, gen. et sp. nov.), and the most plesiomorphic extant tree shrew, Ptilocercus lowii. Our results, based on the fossil record, unambiguously place plesiadapiforms with Euprimates and indicate that the divergence of Primates (sensu lato) from other euarchontans likely occurred before or just after the Cretaceous/Tertiary boundary (65 Mya), notably later than logistical model and molecular estimates. Anatomical features associated with specialized pedal grasping (including a nail on the hallux) and a petrosal bulla likely evolved in the common ancestor of Plesiadapoidea and Euprimates (Euprimateformes) by 62 Mya in either Asia or North America. Our results are consistent with those from recent molecular analyses that group Dermoptera with Scandentia. We find no evidence to support the hypothesis that any plesiadapiforms were mitten-gliders or closely related to Dermoptera.
Keywords: Euarchonta, phylogeny, Paromomyidae, Micromomyidae, Paleogene
The origin of Primates represents the first clear step in the divergence of humans from the rest of Mammalia, yet our understanding of this important period in evolutionary history remains limited. The systematic relationships of Paleocene–Eocene plesiadapiforms, which have been considered the ancestors of either Euprimates (primates of “modern aspect” or crown-clade primates) (1, 2) or of Dermoptera (3, 4) continue to be debated. Clarifying the position of plesiadapiforms is central to understanding the broader relationships among euarchontan mammals (Primates, Scandentia, Dermoptera), and to testing adaptive hypotheses of primate origins (5, 6) by using direct evidence from the fossil record.
Plesiadapiforms are among the most diverse and well sampled Paleogene mammal groups, with >120 species classified into 11 or 12 families from the Paleocene and Eocene of North America, Europe, Asia, and possibly Africa (7, 8). The plesiadapiform dental record is extremely diverse, suggesting correlated diversity in diet and behavior; however, comparatively little is known about the cranial or postcranial morphology of plesiadapiforms [see supporting information (SI) Text, Part 1]. Well preserved crania have been documented for only three families: Plesiadapidae, Microsyopidae, and Paromomyidae (1, 9–11). Postcrania are known from a taxonomically limited sample of North American and European plesiadapids (1), from a sample of North American paromomyids and micromomyids (3, 4, 12) the identification and associations of which are still controversial (13, 14), from a recently published North American carpolestid skeleton (15, 16), and from a few other isolated and questionably identified elements (7, 17, 18). Following the suggestion that paromomyid and micromomyid plesiadapiforms were mitten-gliders closely related to modern dermopterans (3, 4, 12), interpretations of locomotor modes from this limited postcranial sample have played a central role in evolutionary arguments about the group. Anatomical observations of an exceptionally well preserved cranium of a paromomyid (10) seemed to independently support a plesiadapiform–dermopteran link, leading to the widespread acceptance of this phylogenetic hypothesis. The evidence supporting this interpretation has been questioned (7, 9, 13, 15, 16, 19, 20), but no previous study has evaluated the plesiadapiform–dermopteran link by using cranial, postcranial, and dental evidence, including new data on the most plesiomorphic tree shrew, Ptilocercus, and from recently discovered plesiadapiform skeletons. Here, we describe two new Paleocene plesiadapiform species and demonstrate that, when viewed in an appropriate phylogenetic context, anatomical diversity among plesiadapiforms documents a gradual acquisition of traits leading to the first appearance of Euprimates. This Paleocene record of primate evolution allows a direct test of adaptive scenarios for the origin of Primates and Euprimates and provides details about the impressive adaptive radiation that occurred at the base of our own clade.
Systematic Paleontology
Order Primates Linnaeus, 1758. Family Paromomyidae, Simpson, 1940. Genus Ignacius, Matthew and Granger, 1921. Ignacius clarkforkensis, Sp. Nov.
Etymology.
For the Clarks Fork of the Yellowstone River and for the Clarks Fork Basin in which the holotype was discovered.
Holotype.
University of Michigan Museum of Paleontology (UM) 108210, upper and lower dentitions with right I1-M3, I1-C1, P3-M3; left P4-M3, C1-M3; and a partial skeleton (Figs. 1 and 2).
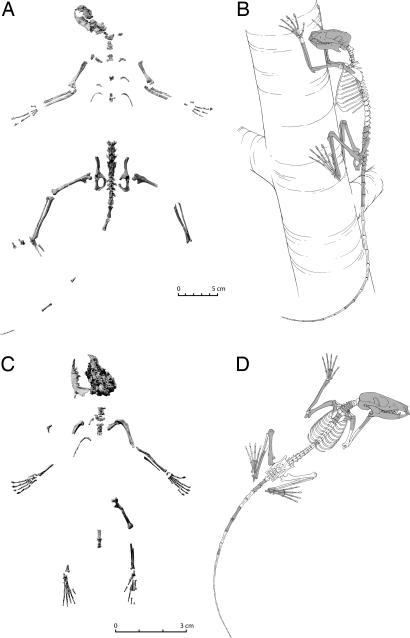
Fig. 1.
Paleocene plesiadapiform skeletons and reconstructions. (A and B) Composite skeleton (A) and reconstruction (B) of paromomyid I. clarkforkensis based on UM specimens 108210 and 82606 (C and D). Skeleton (C) and reconstruction (D) of micromomyid D. szalayi based on UM 41870. Bones not shaded in gray in B and D were not recovered. Documentations of dental–postcranial skeleton associations are outlined in SI Text, Part 1, and its referenced figures.
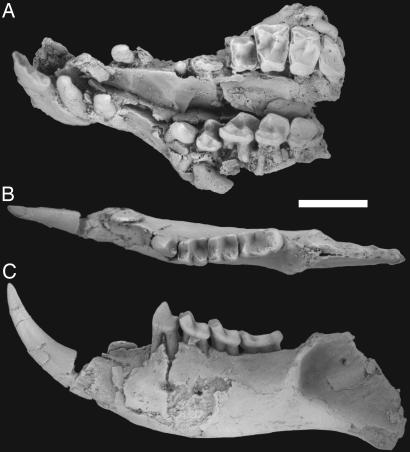
Fig. 2.
Dentition (Holotype: UM 108210) of I. clarkforkensis. Occlusal (A) view of the rostrum and occlusal (B) and buccal (C) views of the left dentary. (Scale bar: 5 mm.)
Horizon and locality.
Type specimen prepared from a limestone nodule from UM locality SC-62, Clarks Fork Basin, northwestern Wyoming; lower Willwood Formation, middle Clarkforkian [uppermost Plesiadapis cookei range zone, late Paleocene (between 55.7 and 55.4 Mya (21)].
Diagnosis.
Largest species of Ignacius. Further differs from all other species in having a single-rooted P2. P4 is wider relative to its length than that of Ignacius frugivorus and longer relative to its width than that of Ignacius graybullianus. Further differs from I. frugivorus in having upper molars with more obliquely oriented postparacone and premetacone cristae, and from Ignacius fremontensis in lacking P3. Further differs from I. graybullianus in having a smaller metacone relative to the paracone on P4 and having a larger P4 relative to M1. For hypodigm, description, and metrics, see SI Text, Part 1, and its referenced figures and tables.
Family Micromomyidae.
Szalay, 1974.
Dryomomys, Gen. Nov. Type Species. Dryomomys szalayi, Sp. Nov. Etymology.
Dryas, Greek, a wood nymph, Root omomys, Greek (masculine), “shoulder mouse,” parallels use in Micromomys. Named in allusion to the arboreal locomotion characteristic of the Micromomyidae.
Diagnosis.
As for the type species.
Dryomomys szalayi Sp. Nov.
Etymology.
Named for Frederick S. Szalay, who described the micromomyids Micromomys and Tinimomys, in recognition of his many contributions to the study of primate evolutionary morphology.
Holotype.
UM 41870, skull and dentary with all tooth positions preserved; and a partial skeleton (Figs. 1 and 3).
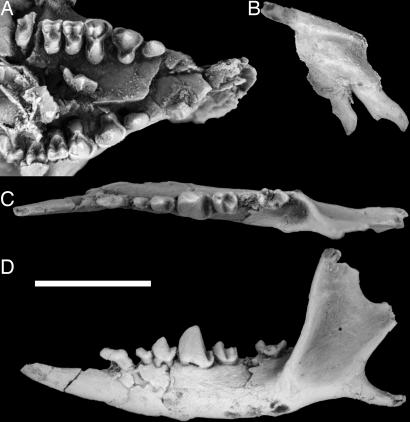
Fig. 3.
Dentition of D. szalayi (Holotype: UM 41870). Occlusal (A) view of the rostrum, lingual (B) view of the left premaxilla with I1–2 and occlusal (C) and buccal (D) views of the left dentary. (Scale bar: 5 mm.)
Horizon and locality.
Type and only specimen prepared from a limestone nodule from UM locality SC-327, Clarks Fork Basin, northwestern Wyoming; lower Willwood Formation, late Clarkforkian [Phenacodus–Ectocion acme zone, latest Paleocene, between 55.3 and 55.0 Mya (21)].
Diagnosis.
Differs from all other micromomyids in having P3 lingually expanded with a strong protocone and a small posterolingual basin bounded by the preprotocrista and postprotocingulum. Further differs from other micromomyids in having a very wide (making the overall proportions more transverse) and inflated P4 relative to the molars, with a broad and long lingually dipping, conical-shaped protocone lobe starting at the lingual margin of the crown and culminating in a large protocone that is closely situated to both the paracone and metacone. Further differs from Micromomys in having no diastema between I1-C1 and in having a single-rooted P2 and a lower crowned P3 with a long, more open talonid. Further differs from Tinimomys in having a C1, lacking a metacone on P3, lacking a lingually continuous cingulum on P4–M2 with no pericone or distinct hypocone, and having a P4 that is higher-crowned with a narrower talonid. Further differs from Tinimomys and Chalicomomys in having a small diastema between C1–P2. For description and metrics, see SI Text, Part 1, and its referenced figures and tables.
Plesiadapiform Skeletons
A number of plesiadapiform skeletons have been found in Clarkforkian age [55.7–55.0 Mya (21)] freshwater limestones from the base of the Willwood Formation in the Clarks Fork Basin (15, 22). Many of these specimens have well documented postcranial-dental associations (see SI Text, Part 1, and its referenced figures and tables). This collection demonstrates a diversity of anatomical form and positional behaviors that mirrors the diversity in dental characteristics.
All known plesiadapiforms have features suggesting that they were committed arborealists, adapted in part for locomotion on large-diameter tree trunks like extant claw-climbing callitrichine primates (18). These features include (i) a humerus with tuberosities that do not extend above the head, a spherical capitulum, and an extended entepicondyle, suggesting mobile shoulder and elbow joints, and the capacity for powerful flexion of the fingers during grasping; (ii) an innominate with a shallow, yet cranially buttressed, elliptical acetabulum, suggesting a mobile hip joint capable of a great range of abduction and lateral rotation during orthograde postures on vertical supports; (iii) a femur with a distally positioned and medially extended lesser trochanter, a posteroproximally extended articular surface of the head, and a shallow patellar groove, suggesting habitual flexion and abduction of the thighs, and infrequent full, forceful extension of the knee; (iv) an astragalus with an elliptical head, short neck, confluent sustentacular and navicular facets, and a shallowly grooved trochlea, suggesting lower ankle joint mobility for inversion of the foot and infrequent exposure to large sagittal loads (as experienced in cursorial locomotion); (v) a calcaneum with a short shaft distal to the ectal facet and a cuboid facet oriented perpendicular to the long axis of the tuberosity, suggesting mobility at the transverse tarsal joint and infrequent cursorial or leaping locomotion; and (vi) terminal phalanges II–V that are mediolaterally compressed and dorsoventrally high, suggesting use in claw-clinging and climbing on large-diameter, vertical supports in which high tensile, sagittal loads were experienced.
Despite detailed similarities among known plesiadapiforms, each also has many unique postcranial characteristics. Plesiadapis differs from all other plesiadapiforms known in functionally significant details of claw, hand, humerus, and scapula morphology. For example, unlike other plesiadapiforms, Plesiadapis has relatively short fingers and extremely long hook-like claws suggesting that, whereas clinging and climbing on large-diameter substrates was an important component of its locomotor repertoire, the ability to grasp small-diameter supports was less than that of other plesiadapiforms (15).
The plesiadapoid Carpolestes simpsoni (15) differs from Plesiadapis in having relatively much shorter claws, longer fingers and toes, and relatively shorter metacarpals and metatarsals. Carpolestes is unique among known plesiadapiforms in having a foot with a divergent, opposable hallux with a nail like that of euprimates, indicating that it was better adapted for grasping small diameter supports in a powerful and precise manner (15). Carpolestes likely spent relatively little time on large-diameter supports and, instead, most frequently occupied a small-branch niche where grasping is more useful than claw-clinging, and bridging is more effective than bounding. Arboreal didelphid marsupials [e.g., Caluromys (6)] represent an appropriate extant model for C. simpsoni (15), especially regarding the grasping mechanism of Carpolestes, which is similar to that of Caluromys (15) and, despite a recent claim to the contrary (23), distinctly different from that of Ptilocercus and noncarpolestid plesiadapiforms (19).
One important component of the hypothesized plesiadapiform–dermopteran link was the conclusion that paromomyids and micromomyids were “mitten-gliders” like modern dermopterans (3, 4, 12). Gliding is expressed structurally in dermopterans by (i) elongate intermediate phalanges and metacarpals that support an interdigital patagium; (ii) a suite of adaptations for gliding that are convergently shared with nondermopteran gliders (e.g., Glaucomys) (24); and (iii) adaptations for quadrupedal suspension. New specimens reveal that paromomyids lack all of these features, and micromomyid plesiadapiforms lack all mitten-gliding traits and many critical gliding and suspensory features (25) (see SI Text, Part 2, and its referenced figures and tables). Instead, paromomyids are most appropriately described as “callitrichine-like” because of their (i) similar body size of 100–500 g; (ii) locomotor repertoire that likely included arboreal bounding, grasping and foraging on small-diameter supports, and frequent clinging and foraging on large-diameter supports (Fig. 1B); and (iii) dental specializations for a diet of exudates (3, 12, 26). Morphological differences from other plesiadapiforms that reflect more active, agile arboreality in paromomyids include (i) lumbar vertebrae with prominent, narrow spinous and transverse processes, suggesting a more sagittally flexible back; (ii) an innominate with broad iliac blades, an ex-tensive pubic symphysis, and a relatively long ischium, suggesting larger areas of origin for hip and thigh muscles; and (iii) a femur with a greater trochanter that extends slightly cranially beyond the femoral head, suggesting powerful extension of the thigh by the lesser gluteal muscles, as required in bounding gaits. The association between vertical claw-clinging and exudate-feeding is well established (12, 27). Such a habitus has been postulated as the primitive plesiadapiform condition (18) and applies specifically to paromomyids.
Micromomyids, the smallest (30–40 g) plesiadapiforms for which postcrania are known (Fig. 1 C and D), are unique among plesiadapiforms in having (i) a radius with a mediolaterally flattened and flaring proximal end, a deeply cupped distal end, and a prominent proximal tubercle associated with insertion of pronator teres; (ii) a fibula with a flaring proximal end; and (iii) an astragalus with a body that is more symmetrical, more deeply grooved, and, on its plantar aspect, has a relatively large groove for the tendon of the flexor digitorum fibularis. The flaring fibula and large groove on the astragalus suggest that flexor digitorum fibularis was large and that micromomyids could powerfully flex their toes. Furthermore, the pronator teres tubercle and expanded fibula suggest the presence of large pronator and peroneal muscles for resisting supination and inversion, respectively, as would be necessary in underbranch postures. Micromomyids are best analogized with Ptilocercus, an arboreal tree shrew, based on similarities in body size and the morphology of the radius and astragalus.
Phylogenetic Analysis
New cranial specimens of C. simpsoni (28) and D. szalayi and new data on paromomyids (9, 20) demonstrate that plesiadapiforms are also characterized by a previously unappreciated diversity of cranial form (see SI Text, Part 4). Variation among scandentians has rarely been considered in studies of mammalian phylogenetics, with Tupaia typically being used to represent the clade (4), even though Ptilocercus is widely considered to be the most plesiomorphic living tree shrew (17, 19, 29, 30). Our phylogenetic analysis takes these sources of variability into account by including data from all well preserved plesiadapiform cranial specimens and from both ptilocercid (Ptilocercus lowii) and tupaiid (Tupaia glis) tree shrews (see SI Text, Part 3).
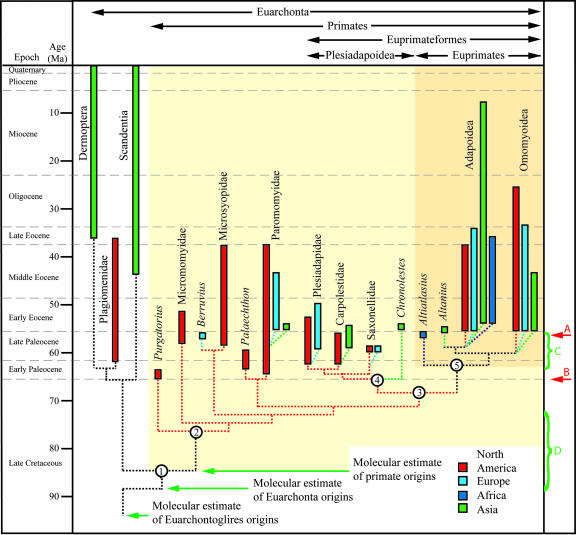
Our cladistic analysis yielded a single-most-parsimonious cladogram (Fig. 4) that supports a monophyletic clade including plesiadapiforms and Euprimates (Primates, sensu lato). “Plesiadapiformes” is paraphyletic, but Plesiadapoidea (Chronolestes, Saxonellidae, Carpolestidae, and Plesiadapidae) is monophyletic and is the sister group to Euprimates. We propose the name Euprimateformes for the plesiadapoid–euprimate clade. Euprimateformes can be formally defined under the guidelines of the Phylocode (34) as the clade stemming from the most recent common ancestor of C. simpsoni and Homo sapiens. Traits likely characterizing the common ancestor of this group include P3 protocone present [character 27(0)], P4 parastylar lobe small and not projecting [character 46(1)], M1–2 trigonids swollen basally [59(1)], M1–2 protocone positioned centrally on the tooth [76(0)], preparacrista on M1–2 straight [82(1)], petrosal bulla [83(1)], and plantodistal process on the entocuneiform strongly reduced to absent [138(1)]. Other traits likely associated with this node include nail present on at least one digit [129(1); DELTRAN] and metatarsal I torsion present [173(1); ACCTRAN].
Fig. 4.
Phylogeny of euarchontans based on a single-most-parsimonious cladogram (tree length = 555, consistency index = 0.548, retention index = 0.523). Divergences of sister taxa are shown schematically, with no implied knowledge of the exact timing of cladogenesis. Node 1 is Euarchonta. Decay indices and bootstrap percentages are as follows (respectively): Node 2 (Primates) = 4, 67%; Node 3 (Euprimateformes) = 3, 30%; Node 4 (Plesiadapoidea) = 2, 52%; Node 5 (Euprimates) = 1, 42%; Scandentia + Dermoptera = 5, 64%. When the analysis is run with all characters unordered, the topology of the resulting single-most-parsimonious cladogram is identical to the one presented here. See SI Text, Part 5, for a list of apomorphies supporting each node. Molecular estimate of primate origins from Springer et al. (31). Logistical model estimate of euprimate origins (D) from Tavaré et al. (32) and probability estimate of euprimate origins (C) from Gingerich and Uhen (33). Molecular divergence estimates (31) as well as the logistical model estimate (32) are notably older than the first occurrences of Euprimates (A) and Primates (B) documented in the fossil record. Results from our analysis suggest that Primates (light tan area) originated in the latest Cretaceous or earliest Paleocene of North America, Euprimateformes originated in the early Paleocene of either Asia or North America, and Euprimates (dark tan rectangle) originated in the Late Paleocene of Asia, Africa, or North America.
Previous morphological studies have documented support for a link between Dermoptera and Chiroptera in Volitantia (35). When we included bats in our analysis, we also found support for this grouping (see SI Text, Part 5). However, no molecular phylogenetic analysis has ever supported Volitantia; instead, chiropterans generally group with carnivorans and ungulates in Laurasiatheria (31). The result from our analysis of morphological data excluding bats corroborates recent molecular studies that support a relationship between Dermoptera and Scandentia (31) in Sundatheria (29). Either way, the proposed Dermoptera plus Plesiadapiformes plus Euprimates clade [termed Primatomorpha (4)] is not supported by molecular or morphological data. Furthermore, neither the proposed paromomyid–dermopteran clade [Eudermoptera (4)], nor the classification of plesiadapiforms in a modified “Dermoptera” (4) is supported by our analysis of morphological data. Instead, our results are consistent with hypotheses that support a sister-group relationship between a paraphyletic Plesiadapiformes and Euprimates (7, 15, 16, 36). The claim that paromomyids and micromomyids were mitten-gliders was contingent on the conclusion that they were closely related to extant colugos (12). Thus, the cladistic result presented here (Fig. 4) is enough to reject the mitten-gliding hypothesis as originally formulated (12). Additionally, results from functional analyses of new paromomyid and micromomyid plesiadapiform skeletons refute the mitten-gliding hypothesis (see SI Text, Part 2, and its referenced figures and tables).
Time and Place of Origin
Our results bear on the time and place of origin of Primates (sensu lato), Euprimateformes, and Euprimates (Fig. 4). The oldest recognized primate (and euarchontan) is Purgatorius, which dates from the earliest Paleocene or latest Cretaceous (37). The primitive nature of this genus suggests it is not much removed from the common ancestor of Euarchonta or Primates. As such, the fossil record supports an origin of Primates and Euarchonta in the earliest Paleocene or latest Cretaceous, 15–27 million years later than molecular divergence dates (31) and 7–25 million years later than estimates from logistical models (32) (Fig. 4). The origin of Euprimateformes must date back to at least the early Paleocene (based on Pronothodectes and Elphidotarsius). The oldest euprimate is the late Paleocene Altiatlasius, indicating an origin of this group before the early Eocene.
A geographic character was added to the data matrix (see SI Text, Part 8, and its referenced figures and tables) to allow parsimony-based assessments to be made for the places of origin of key groups (38). The place of origin of Primates is “unequivocally” North America in our analysis (Fig. 4). This view is supported by the presence of all of the most primitive forms (e.g., Purgatorius, micromomyids, most microsyopids) on that continent. However, this conclusion is tempered by the recognition that North America is the best sampled continent for the Paleocene, by the discovery of several Asian plesiadapiforms in the last decade (39) and by the location of all extant members of Scandentia and Dermoptera in Asia. We therefore cannot reject the possibility of an Asian origin for Primates. The place of origin of Euprimateformes is reconstructed as being either North America or Asia, depending on the optimization algorithm used. This reflects the North American location of the most primitive members of the sister taxon to this group (Paromomyoidea), and the Asian location of Chronolestes, the most basal plesiadapoid included here. If Azibius is a basal plesiadapoid (8), this would make an African origin for this group also a possibility. Beard (38) reconstructed the place of origin of Euprimates as unequivocally Asia. Our results do not support this view, because the place of origin is equivocal and may be Asia, Africa, or North America. The African location of Altiatlasius, Asian location of Altanius, and the presence of most possible euprimate ancestors in North America makes clear why this issue remains ambiguous. The fossil record of primates does not provide unambiguous support for an “East of Eden” model of mammalian diversification (38).
Discussion and Conclusions
We suggest that the ancestral euarchontan can be reconstructed as a small (20–30 g), arboreal, predominantly insectivorous mammal similar to the extant tree shrew Ptilocercus (30) and to extinct micromomyid plesiadapiforms in postcranial morphology. The radiation of angiosperms in the late Cretaceous–early Tertiary, including a systematic increase in fruit and seed sizes correlated with an increasing proportion of animal-dispersed taxa (40, 41), would have presented early euarchontans with ample opportunities to exploit fruit, flowers, gums, and seeds in the arboreal milieu (27). It is clear that stem primates (“Plesiadapiformes”) radiated explosively in this newly formed adaptive landscape, with even the earliest members being differentiated from insectivores by lower crowned molars with broad talonid basins and more bunodont cusps for increased exploitation of nonleafy plant resources (2) (see SI Text, Part 7, and Fig. 40). Acquisition of grasping hands and feet would have allowed easy access to terminal branches where such resources are typically found. The first euprimates have convergent orbits that are larger relative to skull length and more frontated than those of plesiadapiforms (5, 42) as well as features indicative of specialized leaping (18). These differences from plesiadapiforms could indicate a shift to visually directed predation (5) and/or a change in locomotor mode to include grasp-leaping on small-diameter supports (18). The former explanation is weakened by the inferred omnivorous or herbivorous habits of many early euprimates, even at small body size (43), and the concomitant lack of evidence for a clear transition to more effective predation and greater insectivory at the euprimate node. Because of their implied connection between traits for grasping and either visual predation or leaping, both explanations are weakened by the presence of a divergent hallux bearing a flattened nail in the plesiadapoid C. simpsoni (15), which lacks leaping features and convergent orbits, suggesting that grasping may have arisen independently of these traits. Although absent in certain large-bodied species of Plesiadapis (44), specializations for locomotion on terminal branches have been documented in all other plesiadapiforms, including the more primitive plesiadapid Nannodectes, and likely evolved in the primate lineage in a frugivorous or omnivorous taxon representing the common ancestor of plesiadapoids and Euprimates (16) (Euprimateformes). Thus, the sequence of characters added in early primate evolution (Fig. 5) suggests that primates acquired their suite of diagnostic features through “diffuse coevolution” with angiosperms through the Paleocene and that increasing fruit and seed size at this time might have been central to shaping the origin of the group (27, 41). A key role for coordinated evolution of grasping and leaping features for grasp-leaping (18, 36) or of grasping and visual traits for visual predation (5) is not supported.
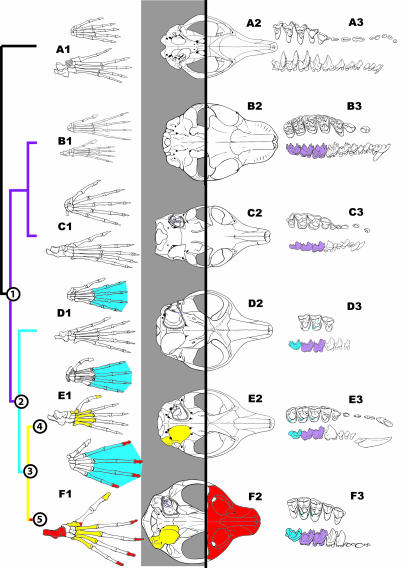
Fig. 5.
Hypothesis of character change in euarchontan evolution. Purple: euarchontan features [Node 1: relatively low crowned molars, entotympanic contribution to the auditory bulla (character 83)]; Blue: primate features [Node 2: increased length of M3 and enlarged M3 hypoconulid (characters 62, 69); presence of a postprotocingulum on the upper molars (character 71); elongate manual phalanges (character 172)]; yellow: euprimateform features [Node 3: relatively short metatarsals, a nail on the hallux (character 129); a petrosal bulla (character 83)]; red: euprimate features [Node 5: elongate tarsals (characters 134, 137); enlarged peroneal process on first metatarsal (character 159); nails on all digits (character 156); forward-facing orbits with a short snout (character 91)]. A, Eutherian Asioryctes; B, Dermopterans Elpidophorus (teeth: B3) and Cynocephalus (cranium and postcrania: B2, B1); C, Scandentian Ptilocercus; D, Primates Purgatorius (teeth: D3), Ignacius (cranium: D2), and D. szalayi (postcrania: D1); E, Plesiadapoids Chronolestes (teeth: E3) and Carpolestes (cranium and postcrania: E2, E1); F, Euprimates Altanius (teeth: F3) and Notharctus (cranium and postcrania: F2, F1).
Plesiadapiforms represent an impressively diverse arboreal radiation that comprises the first 10 million years of known primate evolution. We suggest that Euprimates evolved from within this radiation by at least 62 Mya, sharing a sister relationship with a monophyletic Plesiadapoidea. The first Euprimates do not appear until just before the Paleocene–Eocene boundary (Paleocene–Eocene thermal maximum: PETM) in Africa and possibly Asia, and coincident with the PETM (55 Mya) in North America and Europe. Thus, our result also suggests a euprimate ghost lineage with a hypothesized duration of up to 7 million years spanning the middle–late Paleocene. Given the long history of collecting fossil mammals from rocks of this time period in both North America and Europe, it seems unlikely that this ghost lineage will be recovered from these continents. Instead, it appears more likely that the early evolution of Euprimates occurred on continents with a poorly sampled Paleocene fossil record such as Africa and Asia. Further sampling of Paleocene localities from these areas of the world would serve as a test of the hypotheses presented here.
Methods
A branch and bound search of 173 dental, cranial, and postcranial characters (see SI Text, Parts 5 and 6) was conducted in PAUP* [version 4.0b10 (45)]. The analysis was rooted with Asioryctes, which was chosen as a well preserved primitive eutherian that is clearly not a member of the ingroup. Other possible outgroup choices, such as Apatemyidae and Nyctitheriidae, are not clearly outside the ingroup, and therefore are not appropriate outgroup choices. All characters except characters 55, 62, 71, and 74 (which were treated as ordered) were treated as unordered. All characters were weighted equally. Decay indices were computed in Tree Rot.v2b (46), and bootstrap percentages were computed in PAUP (100 branch and bound replicates).
Supplementary Material
Acknowledgments
We thank P. D. Gingerich, G. F. Gunnell, P. Houde, D. Krause, K. D. Rose, F. S. Szalay, and A. Walker for their support and valuable insights. K. C. Beard, R. D. Martin, E. Seiffert, D. Krause, and six anonymous reviewers critiqued and improved previous versions of this manuscript. Research was supported by grants from the National Science Foundation, Field Museum of Natural History, Yale University, Sigma Xi Scientific Research Society, Natural Sciences and Engineering Research Council (Canada), University of Winnipeg, the Paleobiological Fund, and The Wenner–Gren Foundation for Anthropological Research.
Abbreviation
- UM
University of Michigan Museum of Paleontology.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610579104/DC1.
References
- 1.Gingerich PD. Univ Mich Pap Paleontol. 1976;15:1–141. [Google Scholar]
- 2.Szalay FS. Evolution (Lawrence, Kans) 1968;22:19–36. doi: 10.1111/j.1558-5646.1968.tb03445.x. [DOI] [PubMed] [Google Scholar]
- 3.Beard KC. Nature. 1990;345:340–341. [Google Scholar]
- 4.Beard KC. In: Mammal Phylogeny: Placentals. Szalay FS, Novacek MJ, McKenna MC, editors. New York: Springer; 1993. pp. 129–150. [Google Scholar]
- 5.Cartmill M. Evol Anthropol. 1992;1:105–111. [Google Scholar]
- 6.Rasmussen DT. Am J Primatol. 1990;22:263–277. doi: 10.1002/ajp.1350220406. [DOI] [PubMed] [Google Scholar]
- 7.Silcox MT. A Phylogenetic Analysis of Plesiadapiformes and Their Relationship to Euprimates and Other Archontans. Baltimore: The Johns Hopkins Univ Press; 2001. pp. 1–728. [Google Scholar]
- 8.Tabuce R, Mahboubi M, Tafforeau P, Sudre J. J Hum Evol. 2004;47:305–321. doi: 10.1016/j.jhevol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Bloch JI, Silcox MT. Am J Phys Anthropol. 2001;116:184–198. doi: 10.1002/ajpa.1114. [DOI] [PubMed] [Google Scholar]
- 10.Kay RF, Thewissen JGM, Yoder AD. Am J Phys Anthropol. 1992;89:477–498. [Google Scholar]
- 11.McKenna MC. Folia Primatol. 1966;4:1–25. doi: 10.1159/000155041. [DOI] [PubMed] [Google Scholar]
- 12.Beard KC. In: Primates and their Relatives in Phylogenetic Perspective. MacPhee RDE, editor. New York: Plenum; 1993. pp. 63–90. [Google Scholar]
- 13.Hamrick MW, Rosenman BA, Brush JA. Am J Phys Anthropol. 1999;109:397–413. doi: 10.1002/(SICI)1096-8644(199907)109:3<397::AID-AJPA8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Krause DW. J Hum Evol. 1991;21:177–188. [Google Scholar]
- 15.Bloch JI, Boyer DM. Science. 2002;298:1606–1610. doi: 10.1126/science.1078249. [DOI] [PubMed] [Google Scholar]
- 16.Bloch JI, Boyer DM. Science. 2003;300:741c. [Google Scholar]
- 17.Szalay FS, Drawhorn G. In: Comparative Biology and Evolutionary Relationships of Tree Shrews. Luckett WP, editor. New York: Plenum; 1980. pp. 133–169. [Google Scholar]
- 18.Szalay FS, Dagosto M. Folia Primatol. 1980;34:1–45. doi: 10.1159/000155946. [DOI] [PubMed] [Google Scholar]
- 19.Sargis EJ. J Mammal Evol. 2002;9:137–160. [Google Scholar]
- 20.Silcox MT. J Hum Evol. 2003;44:73–86. doi: 10.1016/s0047-2484(02)0195-1. [DOI] [PubMed] [Google Scholar]
- 21.Gingerich PD. Schmidt B, Sundquist B, Andreasson FP, editors. Early Paleogene Warm Climates and Biosphere Dynamics. 2000:57–59. [Uppsala, Geological Society of Sweden, GFF (Geologiska Foreningens Forhandlingar), Stockholm] [Google Scholar]
- 22.Bloch JI, Boyer DM. In: Paleocene–Eocene Stratigraphy and Biotic Change in the Bighorn and Clarks Fork Basins, Wyoming. Gingerich PD, editor. Ann Arbor, MI: University of Michigan Papers on Paleontology 33; 2001. pp. 185–198. [Google Scholar]
- 23.Gebo DL. Yrbk Phys Anthropol. 2004;47:40–62. doi: 10.1002/ajpa.20154. [DOI] [PubMed] [Google Scholar]
- 24.Thorington RW, Schennum CE, Pappas LA, Pitassy D. J Vert Paleontol. 2005;25:950–961. [Google Scholar]
- 25.Boyer DM, Bloch JI. In: Mammalian Evolutionary Morphology: A Tribute to Frederick S. Szalay. Sargis EJ, Dagosto M, editors. Dordrecht, The Netherlands: Springer; in press. [Google Scholar]
- 26.Gingerich PD. J Dent Res. 1974;53:497. doi: 10.1177/00220345740530025601. [DOI] [PubMed] [Google Scholar]
- 27.Sussman RW, Raven PH. Science. 1978;200:731–736. doi: 10.1126/science.200.4343.731. [DOI] [PubMed] [Google Scholar]
- 28.Bloch JI, Silcox MT. J Hum Evol. 2006;50:1–35. doi: 10.1016/j.jhevol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Olson LE, Sargis EJ, Martin RD. Mol Phylogenet Evol. 2005;35:656–673. doi: 10.1016/j.ympev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Sargis EJ. J Zool London. 2001;253:473–483. [Google Scholar]
- 31.Springer MS, Murphy WJ, Eizirik E, O'Brien SJ. Proc Natl Acad Sci USA. 2003;100:1056–1061. doi: 10.1073/pnas.0334222100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavaré S, Marshall CR, Will O, Soligo C, Martin RD. Nature. 2002;416:726–729. doi: 10.1038/416726a. [DOI] [PubMed] [Google Scholar]
- 33.Gingerich PD, Uhen MD. J Hum Evol. 1994;27:443–445. [Google Scholar]
- 34.de Queiroz K, Gauthier J. Syst Zool. 1990;39:307–322. [Google Scholar]
- 35.Simmons NB. Symp Zool Soc London. 1995;67:27–43. [Google Scholar]
- 36.Szalay FS, Rosenberger AL, Dagosto M. Yrbk Phys Anthropol. 1987;30:75–105. [Google Scholar]
- 37.Lofgren DL. Univ Cal Pub Geol Sci. 1995;140:1–185. [Google Scholar]
- 38.Beard KC. Bull Carnegie Mus Nat Hist. 1998;34:5–39. [Google Scholar]
- 39.Beard KC, Wang J. Ann Carnegie Mus. 1995;64:1–33. [Google Scholar]
- 40.Eriksson O, Friis EM, Lofgren P. Amer Nat. 2000;156:47–58. doi: 10.1086/303367. [DOI] [PubMed] [Google Scholar]
- 41.Tiffney BH. Ann Rev Ecol Evol Syst. 2004;35:1–29. [Google Scholar]
- 42.Ni X, Wang Y, Hu Y, Li C. Nature. 2004;427:65–68. doi: 10.1038/nature02126. [DOI] [PubMed] [Google Scholar]
- 43.Strait SG. J Vert Paleontol. 2001;21:322–334. [Google Scholar]
- 44.Kirk EC, Cartmill M, Kay RF, Lemelin P. Science. 2003;300:741b. doi: 10.1126/science.1082060. [DOI] [PubMed] [Google Scholar]
- 45.Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods) Sunderland, MA: Sinauer; 2003. Version 4. [Google Scholar]
- 46.Sorenson MD. TreeRot, version 2, Program and Documentation. Boston: Boston University; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.