Abstract
After intraperitoneal inoculation with Listeria monocytogenes, γδ T cells appear in the peritoneal cavity preceding the appearance of αβ T cells. Such γδ T cells predominantly express T-cell receptor (TCR)Vγ1/Vδ6, develop through an extrathymic pathway, and contribute to host defence against the bacteria. We have observed a gradual increase in γδ T cells in kidneys of mice after intrarenal inoculation with L. monocytogenes, which resulted in an unusually long-lasting local infection. In this study, we examined the characteristics and the roles of the γδ T cells induced in this model. It was found that these γδ T cells predominantly expressed TCRVγ6/Vδ1 with canonical junctional sequences identical to those expressed on fetal thymocytes. Although depletion of such γδ T cells in vivo did not affect the number of bacteria, it resulted in histologically exacerbated inflammation in the kidneys. These results indicate that a persistent infection with L. monocytogenes in kidneys induces a different kind of γδ T cell from that induced after intraperitoneal infection. The former expresses invariant fetal-type Vγ6/Vδ1+TCR and plays a regulatory role in resolution of inflammation.
Introduction
γδ T cells have limited repertoires of T-cell receptor (TCR) genes, and their developmental pathway or tissue distribution correlates with their TCR repertoires. There are also several lines of evidence indicating an association between function of γδ T cells and their TCR repertoires.
γδ T cells expressing Vγ1 genes are usually paired with Vδ6 or Vδ4, and they have high junctional diversity. They develop either in the thymus or extrathymically and represent a major population of γδ T cells in such lymphoid organs as the spleen and lymph nodes. They are also found in the liver and placenta.1 In the early phase of primary intraperitoneal infection with Listeria monocytogenes, there is an increase in the number of γδ T cells, which play a protective role before αβ T cells appear.2–6 We have recently demonstrated that Vγ1+ T cells were the predominant population of γδ T cells that were induced after intraperitoneal infection with L. monocytogenes and had protective functions against the bacteria.7–9
On the other hand, a predominant induction of Vγ6+ T cells is observed in several situations. Vγ6+ T cells are usually identified as Vγ6/Vδ1+ T cells with canonical junctional sequences, and they develop in the fetal thymus and colonize the mucosal epithelia of the tongue, vagina, uterus, and adult lung.1 Accumulation of Vγ6+ T cells has been observed in the liver during L. monocytogenes infection10 and in the peritoneal cavity during Escherichia coli infection.11,12 They have also been observed in various non-infectious conditions.13–16
We have reported that a local infection with a high dose of L. monocytogenes induced organ-specific autoaggressive responses in some organs.17–24 An intrarenal injection of a high dose of L. monocytogenes induces αβ T cells reactive to kidney antigens.24 In this model, we unexpectedly observed a persistent infection of L. monocytogenes, lasting for more than 1 month in the kidneys of some mice. We also found a striking increase in the number of γδ T cells in the kidneys in the late phase of infection, although both their TCR repertoires and their roles in the persistent infection model of L. monocytogenes remain to be elucidated.
In the present study, we characterized the TCR repertoires of γδ Τ cells accumulated in the kidneys in the late phase of intrarenal infection with L. monocytogenes. We also investigated their roles in the pathogenesis of the persistent infection.
Materials and methods
Mice and microorganisms
Male C3H/He mice were obtained from Japan SLC Inc. (Shizuoka, Japan). They were maintained in specific pathogen-free conditions and were used between 8 and 9 weeks of age. Listeria monocytogenes, strain EGD, were used in all experiments. Bacterial virulence was maintained by serial passages in BALB/c mice. Fresh isolates of L. monocytogenes were obtained from infected spleens, which were grown in tryptic soy broth (Difco, Detroit, MI). They were resuspended in phosphate-buffered saline (PBS) after repeated washing and were stored at −80° in small aliquots until use.
Antibodies and reagents
Hybridomas UC7-13D5 (anti-pan-TCR γδ monoclonal antibody (mAb)) and UC8-1B9 (anti-dinitrophenyl hapten mAb) were generously provided by Dr J. A. Bluestone (Chicago University, Chicago, IL), and 2.11 (anti-TCR Vγ1 mAb) was generously provided by Dr P. Pereira (Institut Pasteur, Paris, France). mAb were prepared from supernatants of hybridoma cells cultured in complete medium. Anti-TCR Vγ1 mAb was conjugated with fluorescein isothiocyanate (FITC; Sigma Chemical Co., St Louis, MO). FITC-conjugated anti-TCR γδ (Cδ) mAb (GL3), anti-TCR Vγ4, anti-TCR Vγ5, anti-TCR Vδ4 and anti-TCR Vδ6·2/6·3 mAb; phycoerithrin (PE)-conjugated anti-TCR αβ and anti-TCR γδ mAb, and allophycocyanin (APC)-conjugated anti-CD3ε mAb were purchased from Pharmingen (San Diego, CA).
Intrarenal infection with L. monocytogenes
Intrarenal infection with L. monocytogenes was performed as described previously24 with minor modifications. Briefly, the right kidneys were exposed through flank incisions under anaesthesia with diethylether. The mice were inoculated in the cortex of the right kidneys with 1 × 103 colony-forming units (CFU) of L. monocytogenes strain EGD in 20 ml of PBS, and then the incisions were sutured. In some experiments, mice were i.p. inoculated with 0·25 mg of anti-TCR γδ mAb in 0·2 ml of PBS on days 7, 14 and 21 after infection with L. monocytogenes to eliminate γδ T cells.
Determination of bacterial growth after intrarenal infection with L. monocytogenes
Bacterial growth in the kidneys and spleens was determined by plating 10-fold serial dilutions of organ homogenates on tryptic soy agar plates. The numbers of colonies were counted after 24 hr of incubation at 37°. The detection limit of this procedure was 102 L. monocytogenes per organ. In order to detect L. monocytogenes in the urine, we used a Listeria-selective agar base to which a Listeria-selective supplement (Oxoid, Hampshire, UK) was added. Urine from each mouse (0·3 ml) was plated onto the Listeria-selective agar plates (each 0·1 ml per a plate), and the numbers of colonies was counted after 24 hr of incubation at 37°.
Flow cytometry and cell sorting
Intrarenal infiltrating lymphocytes were prepared as described previously.24 All samples were preincubated with anti-FcγRII/III antibody (2.4 G2) to block FcγRII/III-mediated binding of mAb. Cells were stained with FITC-, PE-, and APC-conjugated mAbs and were analysed using a FACSCalibur® flow cytometer (Becton Dickinson, Sunnyvale, CA). To purify γδ T cells, the cells were conjugated with anti-FITC Microbeads (Miltenyi Biotec, Auburn, CA) after staining with FITC-conjugated anti-TCR γδ (Cδ) mAb. The labelled cells were sorted using Vario magnetic-activated cell sorting (MACS; Miltenyi Biotec). Sorting procedures were repeated three times. The purity of γδ T cells among CD3+ T cells was more than 96%. For subsequent reverse transcription–polymerase chain reaction (RT–PCR) analysis, all cell staining and sorting procedures were carried out in buffer containing 0·01% sodium azide at 4° to avoid TCR crosslinking and resulting cytokine gene transcription.
RT–PCR analysis
RT–PCR analysis was carried out as described previously21 with minor modifications. In brief, RNA was extracted from the purified γδ T cells from individual mice using TRIZOL®Reagent (Total RNA Isolation Reagent; Life Technologies, Gaithersburg, MD) immediately after cell sorting, and it was reverse-transcribed using Superscript reverse transcriptase (Life Technologies) and random hexamer (Life Technologies). The cDNA was amplified with Vγ/Cγ, Vδ/Cδ, interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), interleukin-4 (IL-4), interleukin-10 (IL-10), or β-actin primers using AmpliTaq® DNA polymerase (Perkin-Elmer, Norwalk, CT) according to the manufacturer's instructions. The primer sequences have been described previously.21 After 30 PCR cycles, a 10-µl aliquot of PCR products was electrophoresed through 1·8% agarose gel and was stained with ethidium bromide. The gel was photographed using a Foto/Analyst image analysis system (Fotodyne, Heartland, WT).
Sequencing
cDNA was amplified with Vγ6/Cγ primers or Vδ1/Cδ primers as described above. The PCR products in gel slices were extracted using Qiaex 2 (Qiagen, Hilden, Germany) and were ligated with a pCRTM2.1 vector using The Original TA Cloning® Kit (Invitrogen, San Diego, CA). They were sequenced by the dideoxy chain termination method with a ABIPrism377 DNA Sequencer (Perkin Elmer) according to the manufacturer's instructions.
Histological examination
The kidney tissue was fixed in formalin, embedded in paraffin, sectioned, and stained with haematoxylin and eosin. The grading of severity of pathological lesions was done by experienced pathologists according to the method of Guze et al.25 Briefly, a section of each kidney was evaluated on a semiquantitative scale of 0–4+: (1) cortical lesions (for example, oedema, tubular atrophy, tubular basement thickening and scars); (2) cortical mononuclear cell infiltrate; (3) papillary lesions (for example, oedema, haemorrhage and sclerosis); (4) subcalyceal mononuclear infiltrate; and (5) calyceal epithelial hyperplasia were evaluated separately. A total score indicative of the overall severity of parenchymal lesions was determined by adding each of the individual scores (maximum 20).
Statistical analysis
Student's t-test was used for the statistical analysis. A P-value < 0·05 was considered to indicate statistical significance.
Results
Persistence of L. monocytogenes in the kidneys of some mice after an intrarenal inoculation
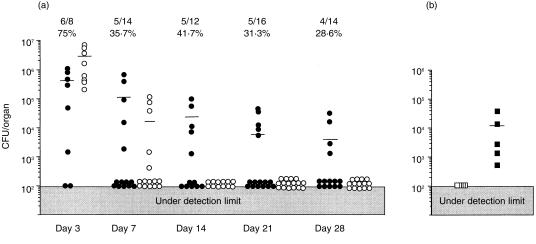
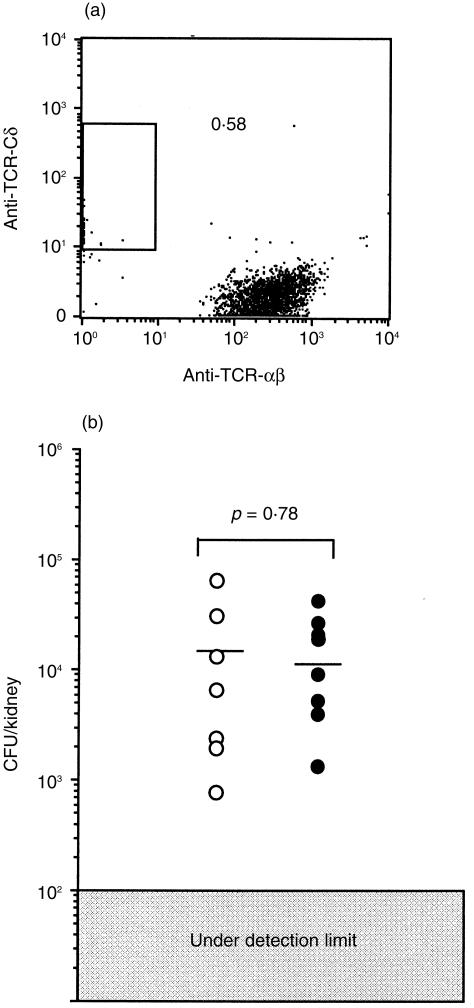
In our previous study, we found a persistent infection of L. monocytogenes in the kidneys of mice that had been injected intrarenally with the bacteria, and we also found that the number of γδ T cells from pooled kidneys markedly increased in the late stage of the infection.24 However, as shown in Fig. 1(a), while the bacteria had been cleared from the spleens of all mice within 2 weeks, about 30% of the mice showed persistent infection with L. monocytogenes. Therefore, a method that enables detection of mice with persistent infection in the kidneys before sacrificing them is needed in order to clarify the relationship between persistent infection and increase in the number of γδ T cells in the kidneys. Interestingly, the percentage of mice with persistent infection in the kidneys remained constant from day 7 after intrarenal injection with L. monocytogenes, suggesting that the fate of infection, i.e. early disappearance or long persistence, is determined at about day 7 after infection. We therefore tried to determine which mice had L. monocytogenes in their kidneys on day 7 after intrarenal injection of L. monocytogenes by examining the urine of the mice using Listeria-selective agar plates. We found that intrarenal persistent infection was present only in the mice whose urine contained Listeria on day 7 (Fig. 1b).
Figure 1.
(a) Kinetics of bacterial growth in the kidneys after intrarenal infection with L. monocytogenes. The mice were injected with 1 × 103 CFU of L. monocytogenes into the right kidneys, and the numbers of bacteria in the kidneys (closed circles) and spleens (open circles) were determined. The small bars represent the mean CFU in each group of mice. The number and the percentage of mice whose kidneys contained bacteria are shown at the top. (b) Numbers of bacteria in the kidneys on day 28 of infection in mice whose urine contained L. monocytogenes on day 7 (closed squares) and in mice whose urine did not contain L. monocytogenes on day 7 (open squares). The figures show the representative data of more than 10 independent experiments that showed nearly the same results.
Increase in number of γδ T cells in the kidneys of mice with persistent L. monocytogenes infection
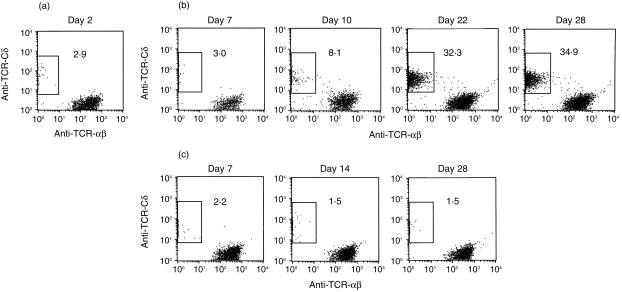
Based on the above result, we examined the numbers of TCR αβ or TCR γδ T cells in the kidneys of mice with and those without persistent infection with L. monocytogenes. The proportion of γδ T cells among CD3+ cells was about 3% on day 2 of infection (Fig. 2a). In the kidneys of mice whose urine contained Listeria on day 7, the percentage of γδ T cells significantly increased from day 10, reaching a plateau level (approximately 30–40%) on day 22, and then remained at almost the same proportion throughout the experimental period (Fig. 2b). In contrast, in the kidneys of mice whose urine did not contain Listeria, the proportion of γδ T cells among CD3+ cells remained less than 3% throughout the experimental period (Fig. 2c). The proportion of γδ T cells among CD3+ cells in spleens remained less than 6% in both groups (data not shown). It was thus thought that the persistence of Listeria elicited infiltration of γδ T cells into the kidneys in the later phase of infection. In the following experiments, we used mice whose kidneys contained L. monocytogenes to investigate the characteristics and the roles of these γδ T cells.
Figure 2.
Kinetics of TCR αβ and γδ T cells in the kidneys after intrarenal injection with L. monocytogenes. (a) Profiles of TCR αβ and γδ T cells from right kidneys of mice on day 2 of infection. The profiles of TCR αβ and γδ expressions in T cells from the right kidneys of mice whose urine contained L. monocytogenes on day 7 of infection (b) and those of mice whose urine did not contain L. monocytogenes on day 7 of infection (c). The analysis gate was set on CD3+ cells. The data shown are representative of the results from individual analyses of 32 mice.
Unchanged number of Vγ1+ T cells in the kidneys during intrarenal persistent infection of L. monocytogenes
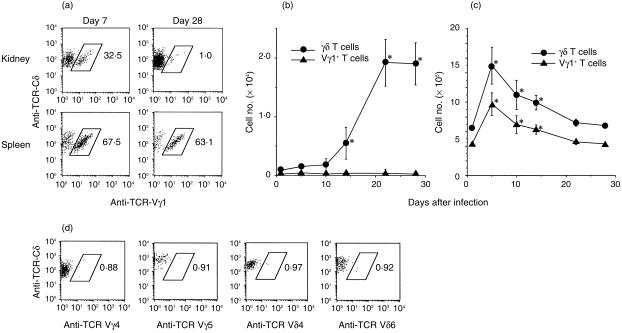
Since we previously reported that intraperitoneal injection with L. monocytogenes predominantly induced Vγ1+ T cells, which have a protective role against the bacteria,9 we next analysed the numbers of Vγ1+ T cells in the kidneys and spleens of mice that had persistent infection with L. monocytogenes in the kidneys. As shown in Fig. 3(a), the proportion of Vγ1+ T cells in the kidneys on day 7 of infection was about 30%, but this proportion had decreased to less than 2% on day 28. The proportion of Vγ1+ T cells in the spleens was higher (around 65%) and remained unchanged throughout the experimental period. More importantly, although the total number of γδ T cells in the kidneys significantly increased from day 10, reaching about 2 × 104 on day 22, the number of Vγ1+ T cells in the kidneys remained at about 2–3 × 102 throughout the experimental period (Fig. 3b). Therefore, more than 98% of γδ T cells that increased in the persistently infected kidneys were not Vγ1+ T cells, which have been shown to play a protective role against L. monocytogenes during intraperitoneal infection.9 On the other hand, Vγ1+ T cells were found to be the predominant population among γδ T cells in the spleen during the experimental period (Fig. 3c).
Figure 3.
Kinetics of TCR γδ T cells and Vγ1+ T cells in the kidneys and spleens of mice with persistently infected kidneys after intrarenal inoculation with L. monocytogenes. (a) Profiles of TCR Vγ1-positive cells from right kidneys and spleens were analysed by gating on both CD3-and TCR-Cδ-positive cells. The numbers of γδ T cells and Vγ1+ cells per kidney (b) and spleen (c) were further calculated based on the number of lymphcytes harvested per mouse and the percentage of γδ T cells or Vγ1+ T cells. Closed circles and triangles represent the data of γδ T cells and Vγ1+ T cells, respectively. *P < 0·05 compared to the data on day 0. (d) Profiles of TCR Vγ4-, Vγ5-, Vδ4- and Vδ6-positive T cells in γδ T cells from the right kidneys on day 28. The data shown are representative of individual analyses of 14 mice.
TCR V region repertoire and junctional sequences of γδ T cells induced by intrarenal persistent infection of L. monocytogenes
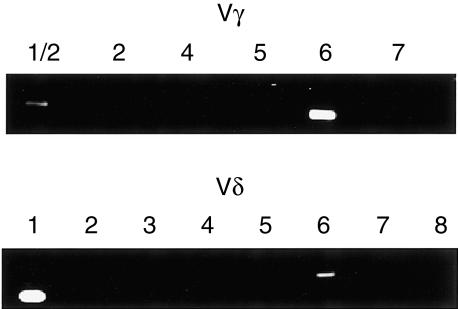
To elucidate the predominant TCR repertoire of γδ T cells in persistently infected kidneys, we first performed flow cytometric analyses using certain Vγ or Vδ chain-specific mAb that are currently available. We examined the expressions of Vγ4, Vγ5, Vδ4 and Vδ6, but all of these subsets were found to be less than 1% of γδ T cells on day 28 of infection (Fig. 3d). Therefore, we carried out a RT–PCR analysis to examine the Vγ and Vδ gene usage of γδ T cells in the kidneys persistently infected with L. monocytogenes. As shown in Fig. 4, γδ T cells derived from a kidney on day 28 of infection expressed only Vγ1, Vγ6, Vδ1, and Vδ6 gene transcripts. Among these, Vγ6 and Vδ1 genes were predominant. We could not detect TCR genes other than Vγ1, Vγ6, Vδ1, and Vδ6 even after 10 more cycles of RT–PCR (data not shown). Furthermore, by a RT–PCR analysis of a T-cell hybridoma (PsA412) that simultaneously expresses Vγ1, Vγ2, Vγ6 as Vγ genes, it was confirmed that these Vγ genes were amplified to almost the same degrees (data not shown). Since the preferential pairing of Vγ6 and Vδ126 and preferential coexpression of Vγ1 and Vδ6 at the clonal level27–29 has been well documented, it was thought that γδ T cells, which increased in kidneys during intrarenal persistent infection with L. monocytogenes, express TCRVγ6/Vδ1 and Vγ1/Vδ6. Together with the results of flow cytometric analyses showing that the proportion of Vγ1+ T cells is less than 2% of the infiltrating γδ T cells in the kidney (Fig. 3b), we conclude that Vγ6/Vδ1+ T cells are the predominant γδ T cells in the kidney persistently infected with L. monocytogenes.
Figure 4.
Vγ and Vδ repertoires of γδ T cells in persistently infected kidneys after intrarenal injection with L. monocytogenes. The lymphocytes from kidneys were prepared from the mice intrarenally inoculated with L. monocytogenes on day 28 of infection. γδ T cells were purified using MACS. The expression of Vγ and Vδ genes was analysed by RT–PCR as described in Materials and Methods.
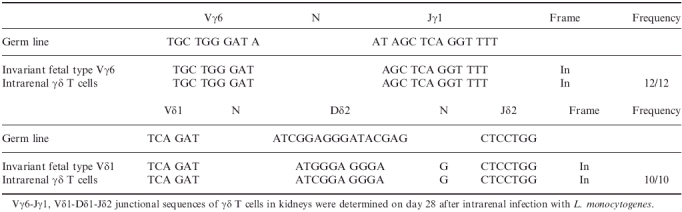
We further analysed the diversity of junctional sequences of Vγ6 and Vδ1 genes (Table 1). It was found that they contain functionally rearranged Vγ6Jγ1Cγ1 and Vδ1Jδ2Cδ transcripts, respectively. Both Vγ6 and Vδ1 transcripts were found to carry canonical junctional sequences; hence, these cells express invariant TCRs. Thus, γδ T cells induced in kidneys persistently infected with L. monocytogenes predominantly express fetal-type invariant Vγ6/Vδ1 TCR, which is apparently different from Vγ1+ T cells, which have high junctional diversity and have been demonstrated to play a protective role in intraperitoneral infection with L. monocytogenes.9
Table 1.
Vγ6-Jγ1, Vδ1-Dδ1-Jδ2 junctional sequences of γδ T cells in kidneys on day 28 after intrarenal infection with L. monocytogenes
Effect of in vivo depletion of γδ T cells on the protection against L. monocytogenes in kidneys
To investigate the role of γδ T cells during intrarenal persistent infection with L. monocytogenes, we depleted γδ T cells by injecting anti-TCRγδ mAb from day 7, just before the number of γδ T cells in the kidney began to increase. Because mAb specific for Vγ6 or Vδ1 are not available, we injected pan-TCRγδ mAb. Control mice were injected with an isotype-matched control mAb. We confirmed that injections of anti-TCRγδ mAb had resulted in nearly complete depletion of γδ T cells in the kidneys with L. monocytogenes on day 28 (Fig. 5a). γδ T cells were also hardly detected in the spleens of these mice (data not shown). To examine both bacterial numbers and histological changes in the same kidney, one half of each kidney was used for counting the number of bacteria, while the other half was used for histological examination.
Figure 5.
Effect of the depletion of γδ+ T cells induced in the late phase of intrarenal infection with L. monocytogenes on bacterial growth. The mice were injected with 1 × 103 CFU of L. monocytogenes into the right kidneys, and mice whose urine contained L. monocytogenes on day 7 were treated with anti-pan-TCR γδ mAb UC7-13D5 or control mAb UC8-1B9 on days 7, 14 and 21. (a) Proportions of TCR αβ and γδ T cells in the right kidney of γδT cell-depleted mice (day 28). The analysis gate was set on CD3-positive cells. (b) The number of bacteria in the kidneys was determined on day 28. Closed circles and open circles represent the data from γδ Τ-cell-depleted mice and control mAb-treated mice, respectively. The small bars represent the mean CFU of each group of mice. The figure shows representative data of three independent experiments.
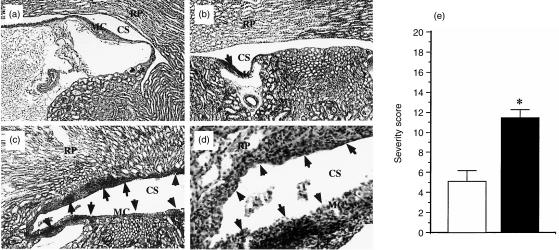
The numbers of bacteria in the kidneys of γδ T cell-depleted mice on day 28 were nearly the same as those in mice treated with control mAb (Fig. 5b). The histology of the kidneys of γδ T-cell-depleted mice was compared with that of the control mice whose kidneys had been confirmed to contain almost the same number of bacteria as that in γδ T-cell-depleted mice. The lesions in the control mAb-treated mice were characterized by localized inflammatory foci containing lymphocytes and a few neutrophils within the transitional cell epithelium and in the subepithelial tissues of minor calices (Fig. 6b). The renal cortex was histologically normal at this phase of infection. In contrast, depletion of γδ T cells resulted in exacerbated cellular response in the kidneys on day 28 (Fig. 6c,d). The lesions in γδ T-cell-depleted mice were abcess-like and were larger than those in the control mice. They showed extensive infiltration of lymphocytes, neutrophils and macrophages with nuclear debris within the transitional cell epithelium and in the subepithelial tissues of minor calices and within the epithelium of renal papillae. Destruction and desquamation of the epithelia were also evident. Even in these mice, the renal cortex was histologically normal. We also estimated the severities of the pathological lesions according to the method of Guze et al.25 (see Materials and Methods). The severity score of γδ T-cell-depleted mice was significantly higher than that of control mice (Fig. 6e). Taken together, these results suggest that the Vγ6/Vδ1+ T cells, which increased in kidneys during persistent infection with L. monocytogenes, do not play a significant role in the eradication of the bacteria but play a regulatory role in the resolution of inflammation.
Figure 6.
Effect of the depletion of γδ T cells induced in the late phase of intrarenal infection with L. monocytogenes on histopathology in the kidneys. A section of a right kidney from a naive mouse (a; ×50), and sections of persistently infected kidney from a mouse on day 28 after intrarenal infection with 1 × 103 L. monocytogenes. (b, c; ×50, d; ×100). The mice were treated with control mAb UC8-1B9 (b) or with anti-pan-TCR γδ mAb UC7-13D5 (c,d) on days 7, 14 and 21 of infection. (Inflammatory foci are shown by arrows. RP, renal papillae; MC, minor carices; CS, pelvicalyceal space.) The data shown are representative of analyses of seven mice in each group (a)–(c). (e) The severity scores of pathological lesions in γδ Τ cell-depleted mice (closed bar) and in control mAb-treated mice (open bar) were calculated according to the method of Glassock et al. (see Materials and methods). *P < 0·05.
Cytokine gene expression of γδ T cells induced by intrarenal persistent infection with L. monocytogenes
To investigate the mechanism involved in the regulatory functions of these γδ T cells, we analysed cytokine gene expression of purified γδ T cells from kidneys persistently infected with L. monocytogenes on day 28 of infection using the RT–PCR method. As a control, we used purified γδ T cells, which play a protective role against L. monocytogenes, derived from peritoneal cavity on day 3 of intraperitoneal infection with L. monocytogenes. As shown in Fig. 7, γδ T cells on day 3 of intraperitoneal infection expressed a significantly high levels of IFN-γ mRNA and low levels of TGF-β and IL-10 mRNA. In contrast, γδ T cells on day 28 of intrarenal infection expressed significantly high levels of TGF-β mRNA and a low level of IFN-γ mRNA. IL-10 and IL-4 mRNA were not detected. As TGF-β has a suppressive effect on immune responses and is produced by several TCR αβ suppressor T cells,30 it is possible that the production of TGF-β is involved in the regulatory functions of γδ T cells induced at the site of persistent infection.
Figure 7.
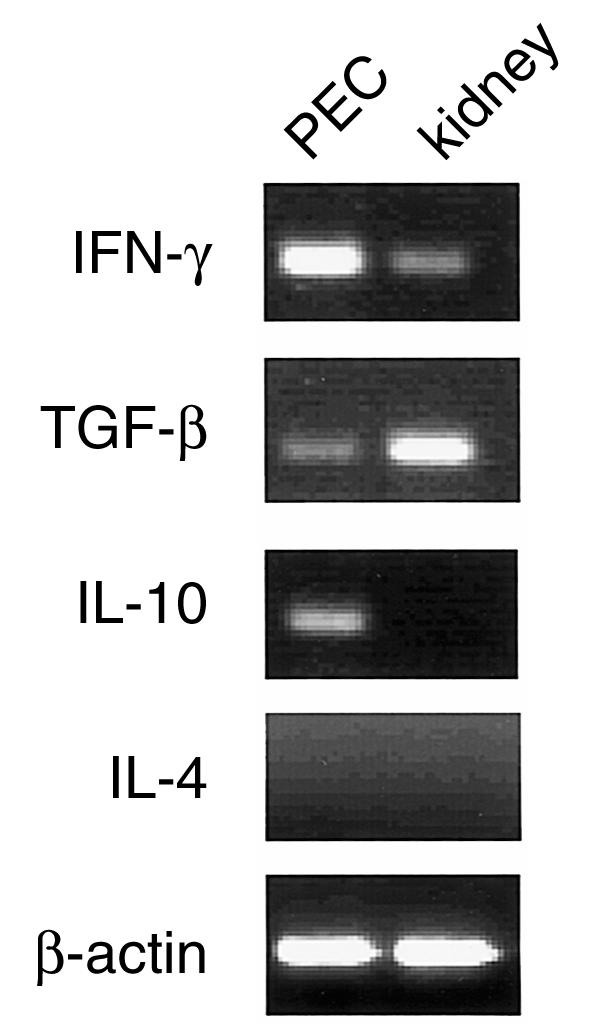
Expression of cytokine mRNA of γδ T cells in the kidneys after intrarenal injection of L. monocytogenes. The lymphocytes were prepared from individual kidneys with persistent infection on day 28 and, as a control, from the peritoneal cavity on day 3 of intraperitoneal infection with 1 × 103 L. monocytogenes. γδ T cells were purified and total RNA was reverse-transcribed into cDNA and amplified by PCR as described in. The purity of the γδ T cells in this experiment was 97·6% and 96·2% of CD3+ cells, respectively. The results are representative of three independent experiments. PEC, peritoneal exudate cells.
Discussion
In the present study, we demonstrated that an intrarenal persistent infection with L. monocytogenes induced γδ T cells, which express invariant fetal-type Vγ6/Vδ1TCR and do not play a significant role in the eradication of Listeria. These γδ T cells were apparently different from the γδ T cells induced in the early phase of intraperitoneal infection with L. monocytogenes, which express Vγ1 and play a protective role against the bacteria.7–9
As shown in Fig. 1, persistent infection with L. monocytogenes was observed in 30% of mice despite our efforts to inject the bacteria into the renal cortex as equally as possible in all mice. We still do not know the precise mechanism that causes the variation in numbers of bacteria in the kidneys. It is possible that there is some instability in the injected site or in the number of injected bacteria at the time of infection due to local blood or urinary flow, and some bacteria might reach the renal pelvis, causing persistent infection. It is also possible that the variation in the responses of other cell populations to the bacteria might cause the variation in the fate of the infection. Nevertheless, there was a clear correlation between persistence of infection and increase in the number of γδ T cells at the later stage of infection, indicating that such γδ cells were induced by the persistent infection.
Preferential induction of Vγ6+ T cells has been shown in various experimental systems associated with bacterial infection. Vγ6/Vδ1+ T cells are induced in the peritoneal cavity during E. coli infection11,12 and in the lungs of mice sensitized with aerosolized mycobacterial antigens.31 Even in the case of infection with L. monocytogenes, Vγ6+ T cells have been reported to increase in the liver10 and testis.20 Importantly, even in several non-infectious experimental systems, an increase in the number of Vγ6/Vδ1+ T cells was observed in inflammatory local lesions such as experimental autoimmune encephalomyelitis14,15 or experimental autoimmune orchitis.16 These findings thus suggest that Vγ6/Vδ1+ T cells are induced by strong inflammatory responses rather than bacterial antigen itself.
In our experimental model of intrarenal infection, there is a possibility that Vγ6/Vδ1+ T cells were induced by autoaggressive inflammatory responses but not by persistent infection, because inflammatory responses to kidney antigen were also induced in mice inoculated with L. monocytogenes in kidneys.24 However, histological features of kidneys in the late phase of infection are apparently different from those of interstitial nephritis induced by autoreactive T cells in the earlier phase, as described in a previous report.24 Furthermore, the histological features of kidneys in the late phase of infection strongly suggest that the bacteria persist in the renal pelvis. Therefore, it is suggested that Vγ6/Vδ1+ T cells were induced by the persistent infection with L. monocytogenes but not by the autoimmune inflammation.
Antigen specificity of fetal-type Vγ6/Vδ1+ T cells has not yet been identified. Hybridomas bearing fetal-type Vγ6/Vδ1 TCR did not respond to mycobacterial heat-shock protein 65, which is a ligand of murine Vγ1/Vδ6+ T cells.32 Hybridoma cells derived from Vγ6/Vδ1+ T cells induced in E. coli infection did not respond to either E. coli or E. coli-derived lipopolysaccharide (LPS) added in vitro in the presence or absence of APC.12 It is thus apparent that Vγ6/Vδ1+ T cells induced in E. coli infection do not recognize E. coli antigen. Since Vγ6/Vδ1+ T cells also increased at the local sites of autoimmune inflammation, it is possible that Vγ6/Vδ1+ T cells recognize an autologous ligand associated with inflammatory host response or that they are induced solely by inflammatory cytokines or chemokines. The fact that the same L. monocytogenes induce γδ T cells expressing different TCR repertoires at infected sites may be due to differences in the expression levels of these inflammatory molecules in different kinds of infected cells.
In order to examine the in vivo role of the γδ T cells, we depleted them by injection of mAb specific for γδ T cells. This treatment was began from day 7 of infection in order to efficiently deplete the γδ T cells since they were found to increase rapidly in number after day 10. We found that the depletion of such γδ T cells exacerbated inflammation in the kidneys persistently infected with L. monocytogenes (Fig. 6). The fact that the numbers of bacteria in the kidneys of γδ T-cell-depleted mice were not significantly different from those of control mice suggested that exacerbated inflammatory lesions were not due to exacerbation of the infection. We injected pan-TCRγδ mAb, because Vγ6 or Vδ1-specific mAbs are unavailable. Therefore, it is possible that very small numbers of γδ T cells other than Vγ6/Vδ1+ T cells in the kidney may be involved in the resolution of inflammation. However, it is more reasonable to assume that such regulatory function is attributable to Vγ6/Vδ1+ T cells, since most of the increased γδ T cells may be Vγ6/Vδ1+ T cells. We could not detect any gene products other than Vγ1, Vγ6, Vδ1, and Vδ6 on day 10 (data not shown) as well as on day 28. Importantly, the number of Vγ1-expressing T cells, which pair with Vδ6, remained at a very low constant level throughout the experimental period, suggesting that this subset of γδ T cells was not affected by the persistent infection.
Interestingly, several studies have shown such regulatory roles of γδ T cells.1,4,33–37 Liver lesions of γδ T-cell-deficient4 or γδ T-cell-depleted mice35 after listerial infection showed atypical lesions characterized by severe abscess formation4 or exacerbated inflammation with severe necrosis.35 Similarly, in Eimeria vermiformis infection, the intestinal lesions of αβ T-cell-deficient mice and γδ T-cell-deficient mice demonstrated distinct phenotypes, revealing the critical role of αβT cells in protective immunity against Eimeria and the important role of γδ T cells as regulators of the host response.36 Furthermore, in Listeria-induced autoimmune orchitis, which induces invariant fetal-type Vγ6/Vδ1+ T cells, depletion of γδ T cells also resulted in exacerbation of inflammation.19 These γδ T cells expressed mRNA of TGF-β and IL-10 in RT–PCR analysis.21 In the present study, we detected a significant level of TGF-β mRNA from γδ T cells induced in the kidneys, suggesting that the invariant fetal-type Vγ6/Vδ1+ T cells contribute to the resolution of inflammation by producing these suppressive cytokines. It is also possible that such γδ T cells may participate in the pathogenesis of various chronic inflammatory lesions. Therefore, it is important to further clarify their functions for understanding and controlling inflammatory disorders.
Acknowledgments
We thank Dr P. Pereira for kindly providing 2.11 hybridoma and Dr J. A. Bluestone for kindly providing UC7-13D5 and UC8-1B9 hybridomas. We also thank Dr G. Matsuzaki for his helpful suggestions, Dr C. Ikebe for performing sequence analysis, and Miss K. Noda for her helpful advice on the histological examination. We also thank Dr B. Quinn and the staff of SES Translation and Proofreading Services (Sapporo, Japan) for checking the English in our manuscript.
Abbreviations
- mAb
monoclonal antibody
- PEC
peritoneal exudate cells
- TCR
T-cell receptor
References
- 1.Born W, Cady C, Jones-Carson J, Mukasa A, Lahn M, O'brien R. Immunoregulatory functions of γδ T cells. Adv Immunol. 1999;71:77–144. [PubMed] [Google Scholar]
- 2.Hiromatsu K, Yoshikai Y, Matsuzaki G, Ohga S, Muramori K, Matsumoto K, Bluestone JA, Nomoto K. A protective role of γ/δT cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992;175:49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas W, Pereira P, Tonegawa S. γ/δ cells. Annu Rev Immunol. 1993;11:637–85. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 4.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SH. Different roles of αβ and γδT cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–6. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 5.Skeen MJ, Ziegler HK. Intercellular interactions and cytokine responsiveness of peritoneal α/β and γ/δ T cells from Listeria-infected mice: synergistic effects of interleukin 1 and 7 on γδ T cells. J Exp Med. 1993;178:985–96. doi: 10.1084/jem.178.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufmann SH. γ/δ and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci USA. 1996;93:2272–9. doi: 10.1073/pnas.93.6.2272. 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuzaki G, Hiromatsu K, Yoshikai Y, Muramori K, Nomoto K. Characterization of T-cell receptor γδ T cells appearing at the early phase of murine Listeria monocytogenes infection. Immunology. 1993;78:22–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Fujise S, Matsuzaki G, Kishihara K, Kadena T, Molina T, Nomoto K. The role of p56lck in the development of γδ T cells and their function during an infection by Listeria monocytogenes. J Immunol. 1996;157:247–54. [PubMed] [Google Scholar]
- 9.Nakamura T, Matsuzaki G, Nomoto K. The protective role of T-cell receptor Vγ1+ T cells in primary infection with Listeria monocytogenes. Immunology. 1999;96:29–34. doi: 10.1046/j.1365-2567.1999.00666.x. 10.1046/j.1365-2567.1999.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roark CE, Vollmer MK, Campbell PA, Born WK, O'brien RL. Response of a γδ+ T cell receptor invariant subset during bacterial infection. J Immunol. 1996;156:2214–20. [PubMed] [Google Scholar]
- 11.Takano M, Nishimura H, Kimura Y, Mokuno Y, Washizu J, Itohara S, Nimura Y, Yoshikai Y. Protective roles of γδ T cells and interleukin-15 in Escherichia coli infection in mice. Infect Immun. 1998;66:3270–8. doi: 10.1128/iai.66.7.3270-3278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuzaki G, Takada H, Nomoto K. Escherichia coli infection induces only fetal thymus-derived γδ T cells at the infected site. Eur J Immunol. 1999;29:3877–86. doi: 10.1002/(SICI)1521-4141(199912)29:12<3877::AID-IMMU3877>3.0.CO;2-C. 10.1002/(sici)1521-4141(199912)29:12<3877::aid-immu3877>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 13.Heyborne KD, Cranfill RL, Carding SR, Born WK, O'brien RL. Characterization of γδ T lymphocytes at the maternal–fetal interface. J Immunol. 1992;149:2872–8. [PubMed] [Google Scholar]
- 14.Olive C. Gamma delta T cell receptor variable region usage during the development of experimental allergic encephalomyelitis. J Neuroimmunol. 1995;62:1–7. doi: 10.1016/0165-5728(95)00081-c. 10.1016/0165-5728(95)00081-c. [DOI] [PubMed] [Google Scholar]
- 15.Olive C. Modulation of experimental allergic encephalomyelitis in mice by immunization with a peptide specific for the γδ T cell receptor. Immunol Cell Biol. 1997;75:102–6. doi: 10.1038/icb.1997.14. [DOI] [PubMed] [Google Scholar]
- 16.Mukasa A, Born WK, O'brien RL. Inflammation alone evokes the response of a TCR-invariant mouse γδ T cell subset. J Immunol. 1999;162:4910–3. [PubMed] [Google Scholar]
- 17.Sanui H, Yoshida S, Himeno K, Nomoto K. Delayed hypersensitivity to syngeneic testicular cells induced by intratesticular bacterial infection in guinea-pigs. Immunology. 1982;46:635–42. [PMC free article] [PubMed] [Google Scholar]
- 18.Sanui H, Yoshida S, Himeno K, Nomoto K. Experimental allergic orchitis induced by unilateral intratesticular bacterial infection in guinea-pigs. Immunology. 1983;49:45–51. [PMC free article] [PubMed] [Google Scholar]
- 19.Mukasa A, Hiromatsu K, Matsuzaki G, O'brien R, Born W, Nomoto K. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of αβ and γδ T cells. J Immunol. 1995;155:2047–56. [PubMed] [Google Scholar]
- 20.Mukasa A, Lahn M, Pflum EK, Born W, O'brien RL. Evidence that the same γδ T cells respond during infection- induced and autoimmune inflammation. J Immunol. 1997;159:5787–94. [PubMed] [Google Scholar]
- 21.Mukasa A, Yoshida H, Kobayashi N, Matsuzaki G, Nomoto K. γδ T cells in infection-induced and autoimmune-induced testicular inflammation. Immunology. 1998;95:395–401. doi: 10.1046/j.1365-2567.1998.00585.x. 10.1046/j.1365-2567.1998.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonoda KH, Matsuzaki G, Nomura A, Yamada H, Hamano S, Nakamura T, Mukasa A, Nomoto K. Macrophages activated by Listeria monocytogenes induce organ-specific autoimmunity. Immunology. 1997;92:274–83. doi: 10.1046/j.1365-2567.1997.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuzaki G, Sonoda KH, Mukasa A, Yamada H, Nakamura T, Ikebe H, Hamano S, Nomoto K. The characterization of testicular cell (TC) -specific T-cell clones induced by intratesticular Listeria monocytogenes infection: TC-specific T cells with atypical cytokine profile transfer orchitis. Immunology. 1997;91:520–8. doi: 10.1046/j.1365-2567.1997.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonoda K, Mukasa A, Matsuzaki G, Nomoto K. The induction of renal autoantigen-specific T cells by a local Listeria monocytogenes infection. Immunology. 1995;86:190–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Guze LB, Guze PA, Kalmanson GM, Glassock RJ. Effect of iron on acute pyelonephritis and later chronic changes. Kidney Int. 1982;21:808–12. doi: 10.1038/ki.1982.103. [DOI] [PubMed] [Google Scholar]
- 26.Ito K, Bonneville M, Takagaki Y, Nakanishi N, Kanagawa O, Krecko EG, Tonegawa S. Different γδ T-cell receptors are expressed on thymocytes at different stages of development. Proc Natl Acad Sci USA. 1989;86:631–5. doi: 10.1073/pnas.86.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'brien RL, Happ MP, Dallas A, Palmer E, Kubo R, Born WK. Stimulation of a major subset of lymphocytes expressing T cell receptor γδ by an antigen derived from Mycobacterium tuberculosis. Cell. 1989;57:667–74. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- 28.O'brien RL, Fu YX, Cranfill R, Dallas A, Ellis C, Reardon C, Lang J, Carding SR, Kubo R, Born W. Heat shock protein Hsp60-reactive γδ cells: a large, diversified T-lymphocyte subset with highly focused specificity. Proc Natl Acad Sci USA. 1992;89:4348–52. doi: 10.1073/pnas.89.10.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roark CE, Vollmer MK, Cranfill RL, Carding SR, Born WK, O'brien RL. Liver γδ T cells. TCR junctions reveal differences in heat shock protein-60-reactive cells in liver and spleen. J Immunol. 1993;150:4867–75. [PubMed] [Google Scholar]
- 30.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-β. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 31.Augustin A, Kubo RT, Sim GK. Resident pulmonary lymphocytes expressing the γ/δT-cell receptor. Nature. 1989;340:239–41. doi: 10.1038/340239a0. [DOI] [PubMed] [Google Scholar]
- 32.Born W, Hall L, Dallas A, Boymel J, Shinnick T, Young D, Brennan P, O'brien R. Recognition of a peptide antigen by heat shock-reactive γδ T lymphocytes. Science. 1990;249:67–9. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- 33.Carding SR, Allan W, Kyes S, Hayday A, Bottomly K, Doherty PC. Late dominance of the inflammatory process in murine influenza by γ/δ+ T cells. J Exp Med. 1990;172:1225–31. doi: 10.1084/jem.172.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterman GM, Spencer C, Sperling AI, Bluestone JA. Role of γδT cells in murine collagen-induced arthritis. J Immunol. 1993;151:6546–58. [PubMed] [Google Scholar]
- 35.Fu YX, Roark CE, Kelly K, Drevets D, Campbell P, O'brien R, Born W. Immune protection and control of inflammatory tissue necrosis by γδ T cells. J Immunol. 1994;153:3101–15. [PubMed] [Google Scholar]
- 36.Roberts SJ, Smith AL, West AB, Wen L, Findly RC, Owen MJ, Hayday AC. T-cell αβ+ and γδ+ deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci USA. 1996;93:11774–9. doi: 10.1073/pnas.93.21.11774. 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D'souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An anti-inflammatory role for γδ T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–21. [PubMed] [Google Scholar]